Abstract
The M-type phospholipase A2 receptor (PLA2R1) was identified recently as a specific target antigen in idiopathic membranous nephropathy. However, the influence of different sample preparation techniques on the immunostaining of PLA2R1 is unclear. Previous studies have identified IgG4 as the dominant subclass of PLA2R1 antibodies. However, it remains unclear whether the IgG subclass profiles of the glomerular immune complexes of PLA2R1-positive and -negative idiopathic membranous nephropathy cases are similar. To address these questions, we conducted the present study of 58 idiopathic membranous nephropathy cases. The PLA2R1 positivity rate for the paraffin-embedded sections was 61 %, whereas that for the frozen sections was 65 %. Nonspecific background staining was observed in the frozen sections. Discrepancies between different sample preparations occurred in three cases (6 %); two cases were PLA2R1-positive in paraffin sections and PLA2R1-negative in frozen sections and one case was PLA2R1-negative in paraffin sections and PLA2R1-positive in frozen sections. Regarding the IgG subclass profile, 89 % of the PLA2R1-positive cases demonstrated the IgG4-dominant/codominant phenotype versus 31 % of the PLA2R1-negative cases (p < 0.001). Clinical characteristics and pathological findings did not significantly differ between PLA2R1-positive and -negative cases. In summary, the PLA2R1 immunofluorescence results were not affected by the different sample preparation techniques, although paraffin-embedded sections were preferred for the histological detection of PLA2R1 because of the nonspecific background staining observed in frozen sections. The observed lower frequency of the non-IgG4-dominant/codominant phenotype in PLA2R1-negative idiopathic membranous nephropathy cases may suggest that there are heterogeneous subgroups of this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Membranous nephropathy (MN), one of the most common causes of nephrotic syndrome in adults, is characterized by the deposition of immune complexes in glomerular subepithelial spaces. Cases without any evidence of systemic disease or specific etiology are termed idiopathic MN (iMN). Patients with an underlying disease, including autoimmune diseases, viral hepatitis, malignancy, or a recent history of drug intake, such as penicillamine, are categorized as secondary MN (sMN) cases [1]. Because treatment strategies differ, it is clinically important to differentiate between iMN and sMN.
The M-type phospholipase A2 receptor (PLA2R1) was identified recently as an autoantigen in most iMN cases [2]. Multiple studies have shown that the frequency of PLA2R1 positivity in patients with iMN ranges from 60 to 80 %, which is in stark contrast to the lower positivity rate (5–25 %) in patients with sMN [3, 4]. Recurrent iMN in post-transplant kidney allografts also have a much higher rate of PLA2R1 positivity in comparison to de novo MN [5, 6], indicating that the PLA2R1 antibody is not involved in the pathogenesis of de novo MN. The results of these studies suggest that the detection of PLA2R1 involvement can serve as a diagnostic hallmark of iMN.
To establish more practical approaches to the application and interpretation of PLA2R1 immunofluorescence studies using histological sections, two major issues have yet to be addressed: detailing the effects of immunostaining by different methodologies and establishing a comprehensive IgG subclass analysis. The protocol for PLA2R1 immunofluorescence studies using paraffin sections was recently established by Larsen et al. [7]. However, adjustment of interpretation of immunostaining is required when different techniques are used. For example, C4d immunostaining of kidney allografts in paraffin sections is less sensitive by approximately one grade level compared with that in frozen sections [8]. However, it is still unclear whether the same holds true for PLA2R1. Regarding the IgG subclass profile, previous studies have demonstrated the IgG4-dominant immunophenotype of PLA2R1-positive iMN based on indirect immunofluorescence tests using either serum samples or formalin-fixed paraffin-embedded sections [7, 9–11]. However, to our knowledge, a comprehensive analysis of the IgG subclass profiles of PLA2R1-positive and -negative iMN cases has not been conducted. Therefore, the question remains unsettled whether PLA2R1-negative iMN consists of immunophenotypes that are similar to those of PLA2R1-positive iMN.
We conducted the present PLA2R1 immunofluorescence study of iMN with two main purposes: (1) to examine whether the interpretation of PLA2R1 immunostaining should be adjusted for the particular technique applied and (2) to determine the proportion of the IgG4-dominant/codominant immunophenotype of both PLA2R1-positive and -negative iMN. In addition, comparisons of other clinicopathological characteristics of PLA2R1-positive versus PLA2R1-negative iMN cases are described.
Materials and methods
Patients
The present study enrolled 58 iMN cases diagnosed pathologically at the Kobe University Hospital, Kobe City Medical Center General Hospital, and Hiroshima Prefectural Hospital from January 2008 to September 2014. Patients were excluded if they had any evidence suggestive of sMN, including autoimmune diseases, viral hepatitis B or C, malignancy, bucillamine administration, and/or sarcoidosis, or if they had undergone hematopoietic stem cell transplantation. Also excluded were patients who had taken immunosuppressive drugs before the renal biopsy was performed. The patient cohort was classified into PLA2R1-positive or -negative groups according to the results using paraffin sections, as described below. From the patient database records, the following clinical and laboratory data were obtained, as recorded at the time of the renal biopsy: age, gender, urine protein to creatinine ratio, serum levels of albumin, creatinine, IgG, IgA, IgM, C3, C4, and CH50. Written informed consent was obtained from the patients for the use of all the specimens. The study was approved by the Ethics Review Board of Kobe University Hospital (No. 1532), Kobe City Medical Center General Hospital (No. 13093), and Hiroshima Prefectural Hospital (No. 131).
Pathological evaluation
All renal biopsy specimens were fixed in formalin and embedded in paraffin, according to standard protocols. The paraffin sections were cut and stained with hematoxylin and eosin, periodic acid-Schiff, Masson trichrome, and periodic acid-methenamine-silver. Histological findings of glomerular changes, such as spike formation, mesangial cell proliferation, endocapillary hypercellularity, and focal segmental glomerulosclerosis, were evaluated.
Direct immunofluorescence studies using frozen sections were performed with fluorescein-conjugated rabbit polyclonal primary antibodies against IgG, IgA, IgM, C1q, and C3. The IgG subclass analysis was performed using antibodies against IgG1, IgG2, IgG3, and IgG4 (Invitrogen, Camarillo, CA, USA), followed by fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Invitrogen). The immunofluorescence intensities were scored according to a semiquantitative five-grade scale that ranged from 0 to 3+ (0, negative; <1+, sparse; 1+, weak; 2+, moderate; and 3+, strong). The IgG4-dominant phenotype was assigned to sections having IgG4 immunofluorescence that was stronger than the other three IgG subclasses; sections were labeled IgG4-codominant if two or three IgG subclasses, including IgG4, had the strongest immunofluorescence intensities [12].
PLA2R1 was detected with an anti-PLA2R1 antibody (Sigma-Aldrich, Tokyo, Japan) in frozen and paraffin-embedded sections. For the frozen sections, the anti-PLA2R1 antibodies (1:100) were applied for 60 min and then incubated with Alexa Fluor 488 Goat anti-rabbit IgG (Invitrogen, 1:100) for 60 min. The procedure used for PLA2R1 immunofluorescence staining of paraffin sections has been described previously [7]. Bright granular PLA2R1 staining on glomerular capillaries was considered positive. In all of the immunofluorescence studies, images were obtained using a conventional immunofluorescence microscope (Olympus, Tokyo, Japan) equipped with a CCD camera and were analyzed using ACT-1 Software (Nikon, Tokyo, Japan).
The ultrastructural findings defining the MN stage were scored based on the modified version of the Ehrenreich and Churg classification described by Huang et al. [12], as follows: score 1, stage I without any spike formation; score 2, stage I–II showing evident segmental spike formation; score 3, stage II showing diffuse spike formation and only a few deposits within the glomerular basement membrane (GBM); score 4, stage II–III in which most deposits were located within the GBM, but numerous subepithelial deposits were noted; and score 5, stage III–IV with intramembranous immune complexes exhibiting partial or diffuse resorption change. The average scores for the PLA2R1-positive and -negative iMN groups were calculated and compared.
Statistical analysis
Data are presented as mean ± standard error. The clinical and pathological parameters were compared using Student’s t tests, Welch’s t tests, and Mann–Whitney U tests. The rate of the IgG4 dominant/codominant phenotype was calculated using Fisher’s exact probability test. The level of significance was set at p < 0.05.
Results
In paraffin sections, there was bright granular PLA2R1 immunofluorescence staining of glomerular capillaries, whereas no significant staining was observed in PLA2R1-negative iMN cases (Fig. 1a, b). Positive glomerular staining of PLA2R1 was also observed in frozen sections (Fig. 1c); however, we noted nonspecific staining in the interstitium and nucleus (Fig. 1c, d). At higher magnification, weak PLA2R1 staining on the glomerular capillaries corresponded to the internal expression of PLA2R1 on podocytes (Fig. 1d, inset). In the present patient cohort, 36 cases were positive for PLA2R1 and 22 cases were negative according to the results of the immunofluorescence study using paraffin sections. Therefore, the PLA2R1 positivity rate in iMN was 62 % when paraffin sections were used for the evaluation. When frozen sections were used, the PLA2R1 positivity rate was 65 % (positive, 31 cases; negative, 17 cases). The results of both immunostaining methods are shown in Table 1. When PLA2R1 was positive using the paraffin sections, the large majority of cases had concordant results in the frozen sections and vice versa (Fig. 2a, f, b, and g). Among the patients for whom the results of both paraffin and frozen section PLA2R1 immunostaining were available, the discrepancy rate was 6 % (3/47 cases). In one case, PLA2R1 was positive in frozen sections and negative in paraffin sections (Fig. 2c, h), whereas in two cases, PLA2R1 was negative in frozen sections and positive in paraffin sections (Fig. 2d, i). In the three iMN cases that were positive for PLA2R1 in paraffin sections, the glomerular immunostaining of PLA2R1 was segmental and weak, which contrasted with the diffuse and strong positivity in frozen sections (Fig. 2e, j). In some paraffin sections, the immunofluorescence intensity of PLAR1 staining differed among glomeruli (data not shown).
PLA2R1 distribution in iMN glomeruli using different staining methodologies. PLA2R1 immunofluorescence images from PLA2R1 (a, c) -positive and (b, d) -negative iMN cases. (a, b) Paraffin sections and (c, d) frozen sections. Note nonspecific PLA2R1 staining of the nucleus and interstitium in frozen sections (c, d). Inset (d) shows internal weak expression of PLA2R1 on glomerular podocytes
Comparison of PLA2R1 immunofluorescence in paraffin and frozen sections from iMN cases. Upper panel (a to e) PLA2R1 immunofluorescence in paraffin sections. Lower panel (f to j) PLA2R1 immunofluorescence in frozen sections. (a, f), (b, g), (c, h), (d, i), and (e, j) are single-case paired images. Both methods yielded matching results in the vast majority of cases. In one case, PLA2R1 was negative in paraffin sections (c) and positive in frozen sections (h). Two cases were PLA2R1-positive in paraffin sections (d) and PLA2R1-negative in frozen sections (i). Three cases had weak and segmental PLA2R1 staining in paraffin sections (e), in stark contrast to diffuse staining in frozen sections (j)
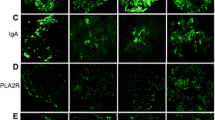
In the present study, 44 cases were eligible for further analysis of the IgG subclass profile. The IgG subclass immunoprofiles of PLA2R1-positive and -negative iMN cases are illustrated in a heat map plot (Fig. 3). Of the PLA2R1-positive iMN cases, 75 % had an IgG4 immunofluorescence score of ≥2+, whereas 13 % of the PLA2R1-negative group had similar scores. Among the cases of PLA2R1-positive iMN, 89 % were categorized into the IgG4 dominant/codominant phenotype, compared to 31 % of the PLA2R1-negative iMN cases (p < 0.001). Figure 4 shows representative immunostaining images for the IgG subclasses of PLA2R1-positive and PLA2R1-negative iMN cases.
Heat map summary of the IgG subclass distributions in PLA2R1-positive and -negative iMN cases. The blue dotted line indicates the threshold for defining the IgG4-dominant/codominant phenotype using immunofluorescence scores for the IgG subclasses. Scores in the PLA2R1-positive iMN cases shifted to the IgG4-dominant/codominant phenotype. In the PLA2R1-negative iMN cases, the non-IgG4 dominant phenotype predominated
The identification of distinct IgG subclass profiles raises the possibility that the PLA2R1-positive status reflects distinct clinicopathological features [13]. Table 2 summarizes the clinical parameters of the PLA2R1-positive and -negative iMN patients. The age of the patients and male to female ratios were almost comparable. The PLA2R1-positive group had slightly increased levels of serum creatinine and urinary protein, but the differences were not significant. Serum immunological tests, including immunoglobulins, CH50, C3, and C4, did not reveal significant differences. Table 3 presents comparisons of light microscopic findings of the glomeruli. Most cases of PLA2R1-positive iMN had apparent spike formation along the glomerular capillary wall, whereas the PLA2R1-negative iMN cases had slightly less frequent spikes on the glomerular tuft. Other glomerular manifestations, such as mesangial cell proliferation, endocapillary hypercellularity, and focal segmental glomerulosclerosis changes, were observed in a minority of cases in both groups. None of the histological parameters of the glomeruli was significantly different. In the immunofluorescence studies, no significant changes in the immunofluorescence scores of the immunoglobulin and complement was observed, irrespective of PLA2R1 positivity on glomeruli (Table 4). The average scores for the ultrastructural stage of the modified Ehrenreich and Churg classification were 3.41 and 2.80 for the PLA2R1-positive and -negative iMN cases, respectively (p = 0.2).
Discussion
In the present study, we conducted a comparative analysis of staining for PLA2R1 of paraffin-embedded versus frozen sections. The concordant results obtained using the two different tissue processing methods indicate that the detection of PLA2R1 protein is not affected by differences in these methods. These results contrast with C4d immunostaining studies of kidney allografts, in which paraffin sections had less sensitivity than frozen sections [14, 15]. In the current study, the interstitium and nucleus demonstrated nonspecific staining in frozen sections. Therefore, the paraffin sections were less problematic for determining the involvement of the PLA2R1 molecule in MN. In cases in which no glomeruli are included in paraffin sections, frozen sections can substitute when determining PLA2R1 staining. The segmental distribution of glomerular PLA2R1 in three cases, as seen on paraffin sections (Fig. 2e, j), can be interpreted as an artifact, because PLA2R1 staining of frozen sections from the same cases was strong and in a global distribution.
The other important finding in our study was the identification of distinct IgG subclass immunophenotypes among PLA2R1-positive and -negative iMN cases. This result can be interpreted in several ways. First, identification of different IgG subclasses of PLA2R1-positive and -negative iMN suggests that iMN consists of immunologically heterogeneous subgroups. Identifying the IgG subclass of glomerular deposits is helpful to distinguish iMN and sMN, because iMN is characterized by the IgG4 dominant/codominant phenotype and sMN comprises various IgG subclasses, including the IgG1/IgG2/IgG3-dominant phenotypes of lupus nephritis [16] and the IgG1/IgG2-dominant phenotype of malignancy-associated MN [17]. The less common IgG4 dominant/codominant phenotype in PLA2R1-negative iMN raises the possibility that PLA2R1-positive and -negative iMN cases have distinct immunological backgrounds. However, serological tests for immunological components such as C3, C4, and CH50 did not significantly differ between these groups. Furthermore, we did not observe any significant changes in the glomerular immunofluorescence scores of the immunoglobulin and complement components. These results argue against the possibility that PLA2R1-positive and -negative iMN represent immunologically distinct entities. Regarding the cytokine responses in iMN, T cells in the peripheral blood preferentially produce Th2 cytokines, such as interleukin (IL)-4 and IL-10, which result in the enhanced production of IgG4 by B cells [18]. From a study on the messenger RNA (mRNA) expression profile in kidney specimens, iMN is characterized by Th2 and Treg immune responses that are manifested by increased expression of IL-4, IL-5, TGF-β, and Foxp3 [19]. A comprehensive analysis of mRNA expression in either serum or biopsy samples may help to clarify the immunological aspects of PLA2R1-positive and -negative iMN.
Secondly, differences in genetic background may contribute to the production of an anti-PLA2R1 antibody that is IgG4-dominant. Recently, Jicheng et al. have reported that individuals carrying high-risk alleles of PLA2R1 and HLA-DQA1 are predisposed to generating the circulating anti-PLA2R1 antibodies of iMN [20]. They also suggested that specific sequence variations in PLA2R1 molecules are involved in the pattern of antigen peptide processing through conformational changes, thus facilitating the production of the anti-PLA2R1 antibody. However, this hypothesis has not been confirmed in evaluations of the antigenic structure of the PLA2R1 molecule using relevant single-nucleotide polymorphisms (SNPs). Future genetic studies are required to address the issue of whether the production of the anti-PLA2R1 antibody in iMN cases is affected by SNPs of a relevant gene locus.
Thirdly, different IgG subclasses may reflect differing activities of the complement system. Previous studies have shown that IgG1 and IgG3 preferentially activate the classical complement pathway, whereas IgG4 has capacity to bind mannose-binding lectin (MBL) and activate the lectin complement pathway [21]. Given the association between the glycosylation status of the IgG subclass molecule and the activation of alternative pathways [22], distinct IgG subclass profiles in PLA2R1-positive and -negative iMN may reflect different proportions of glycosylated IgG molecules. Ma et al. have shown that anti-PLA2R1 IgG4 autoantibodies lack the galactose residue in the terminal position and can bind to C4 through MBL in vitro [23]. Further studies focusing on the question of whether the glycosylation profile of IgG is different between PLA2R1-positive and -negative iMN are needed.
Our study revealed a relatively lower rate of PLA2R1 positivity compared with the previous studies. Studies by Hoxha [10] and Svobodova [3] of paraffin-embedded sections have shown that 84 and 69 %, respectively, of iMN cases were positive for PLA2R1 on glomeruli. In contrast, our study revealed 62 % PLA2R1 positivity in paraffin sections. Using serum samples, a recent Japanese study also showed a lower rate (53 %) of PLA2R1 positivity in iMN [24]. In a recent study on Caucasian patients with biopsy-proven iMN, sequencing of the PLA2R1 gene identified rare variants within the coding region of PLA2R1 which are probably not involved in the pathogenesis of iMN [25]. Therefore, the lower rate of PLA2R1 positivity in the cohort of Japanese iMN patients is probably not due to a genetic variant unique to the Japanese cohort. Early diagnosis of iMN owing to the kidney disease screening program in Japan or unknown environmental and dietary factors may contribute to the low prevalence of anti-PLA2R1 antibody [24], although further studies are required to validate this hypothesis.
The levels of anti-PLA2R1 antibody are associated with higher disease activity in iMN. Oh et al. have reported that proteinuria and hypoalbuminemia were more severe in patients with anti-PLA2R1 than in those without these autoantibodies [26]. More recently, Hoxha has shown that the level of anti-PLA2R1 antibody is an independent risk factor for not achieving remission of proteinuria [27]. Our study did not show significant differences between PLA2R1-positive and -negative iMN regarding clinical manifestations. This discrepancy can be attributed to the different approaches that were used to detect the involvement of the PLA2R1 molecule. In most cases, PLA2R1 positivity of glomerular deposits reflects positive anti-PLA2R1 autoantibodies in the serum of the patient [10]. Considering the various antibody levels in the sera of patients with iMN [26, 27], our study cohort of PLA2R1-positive iMN cases may have also consisted of subgroups that are heterogeneous with regard to their serum autoantibody levels. However, the quantitative data for comparing glomerular PLA2R1 deposition and serum anti-PLA2R1 antibody titers are lacking. Validation studies are required to determine whether high or low serum levels of the anti-PLA2R1 antibody correlate with the immunofluorescence intensity in histological sections after staining for PLA2R1.
Using an Enzyme-Linked Immunosorbent Assay on serum, a level higher than 20 U/ml is considered as positive for anti-PLA2R1 antibody [27]. In contrast, a threshold level for positivity by PLA2R1 immunofluorescence on renal biopsy specimens has not been established. To circumvent overdiagnosis, it is critical to compare the results with a negative control without primary antibody and to include a positive control sample [27].
The most important limitation in our study is the lack of serum anti-PLA2R1 antibody data. Although some studies demonstrated clear correlation of anti-PLA2R1 antibody in serum with the presence of PLA2R1 on glomerular immune deposits [3, 10], quantitative studies comparing serum anti-PLA2R1 antibody level and glomerular PLA2R1 immunofluorescence intensity have not been conducted. To increase the practical importance of glomerular immunofluorescence studies, validation studies will be required using both serum and histological samples.
In summary, our findings demonstrated that the rate of PLA2R1 positivity is equivalent in paraffin-embedded and frozen sections. Because of nonspecific staining of the nucleus and interstitium in frozen sections, the results of our study warrant the use of paraffin sections for PLA2R1 immunostaining. The higher rate of the IgG4 dominant/codominant phenotype indicates that there may be pathogenetically distinct characteristics of PLA2R1-positive and -negative iMN. Further studies using molecular and genetic approaches are required to address the question of whether PLA2R1-positive and -negative iMN have pathogenic mechanism in common.
References
Beck LH Jr, Salant DJ (2010) Membranous nephropathy: recent travels and new roads ahead. Kidney Int 77(9):765–770
Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361(1):11–21
Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H (2013) Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant 28(7):1839–1844
Qin W, Beck LH Jr, Zeng C, Chen Z, Li S, Zuo K, Salant DJ, Liu Z (2011) Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 22(6):1137–1143
Debiec H, Martin L, Jouanneau C, Dautin G, Mesnard L, Rondeau E, Mousson C, Ronco P (2011) Autoantibodies specific for the phospholipase A2 receptor in recurrent and de novo membranous nephropathy. Am J Transplant 11(10):2144–2152
Larsen CP, Walker PD (2013) Phospholipase A2 receptor (PLA2R) staining is useful in the determination of de novo versus recurrent membranous nephropathy. Transplantation 95(10):1259–1262
Larsen CP, Messias NC, Silva FG, Messias E, Walker PD (2013) Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol 26(5):709–715
Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M (2008) Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 8(4):753–760
Dähnrich C, Komorowski L, Probst C, Seitz-Polski B, Esnault V, Wetzels JF, Hofstra JM, Hoxha E, Stahl RA, Lambeau G, Stöcker W, Schlumberger W (2013) Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta 421:213–218
Hoxha E, Kneißler U, Stege G, Zahner G, Thiele I, Panzer U, Harendza S, Helmchen UM, Stahl RA (2012) Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int 82(7):797–804
Cossey LN, Walker PD, Larsen CP (2013) Phospholipase A2 receptor staining in pediatric idiopathic membranous glomerulopathy. Pediatr Nephrol 28(12):2307–2311
Huang CC, Lehman A, Albawardi A, Satoskar A, Brodsky S, Nadasdy G, Hebert L, Rovin B, Nadasdy T (2013) IgG subclass staining in renal biopsies with membranous glomerulonephritis indicates subclass switch during disease progression. Mod Pathol 26(6):799–805
Doi T, Mayumi M, Kanatsu K, Suehiro F, Hamashima Y (1984) Distribution of IgG subclasses in membranous nephropathy. Clin Exp Immunol 58(1):57–62
Seemayer CA, Gaspert A, Nickeleit V, Mihatsch MJ (2007) C4d staining of renal allograft biopsies: a comparative analysis of different staining techniques. Nephrol Dial Transplant 22(2):568–576
Nadasdy GM, Bott C, Cowden D, Pelletier R, Ferguson R, Nadasdy T (2005) Comparative study for the detection of peritubular capillary C4d deposition in human renal allografts using different methodologies. Hum Pathol 36(11):1178–1185
Kuroki A, Shibata T, Honda H, Totsuka D, Kobayashi K, Sugisaki T (2002) Glomerular and serum IgG subclasses in diffuse proliferative lupus nephritis, membranous lupus nephritis, and idiopathic membranous nephropathy. Intern Med 41(11):936–942
Ohtani H, Wakui H, Komatsuda A, Okuyama S, Masai R, Maki N, Kigawa A, Sawada K, Imai H (2004) Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant 19(3):574–579
Kuroki A, Iyoda M, Shibata T, Sugisaki T (2005) Th2 cytokines increase and stimulate B cells to produce IgG4 in idiopathic membranous nephropathy. Kidney Int 68(1):302–310
Ifuku M, Miyake K, Watanebe M, Ito K, Abe Y, Sasatomi Y, Ogahara S, Hisano S, Sato H, Saito T, Nakashima H (2013) Various roles of Th cytokine mRNA expression in different forms of glomerulonephritis. Am J Nephrol 38(2):115–123
Lv J, Hou W, Zhou X, Liu G, Zhou F, Zhao N, Hou P, Zhao M, Zhang H (2013) Interaction between PLA2R1 and HLA-DQA1 variants associates with anti-PLA2R antibodies and membranous nephropathy. J Am Soc Nephrol 24(8):1323–1329
Daha NA, Banda NK, Roos A, Beurskens FJ, Bakker JM, Daha MR, Trouw LA (2011) Complement activity by (auto-) antibodies. Mol Immunol 48(14):1656–1665
Ma H, Sandor D, Beck LH Jr (2013) The role of complement in membranous nephropathy. Semin Nephrol 33(6):531–542
Ma H, Beck LH Jr, Salant DJ (2011) Membranous nephropathy-associated anti-phospholipase A2 receptor IgG4 autoantibodies activate the lectin complement pathway [abstract]. J Am Soc Nephrol 22:62A
Akiyama S, Akiyama M, Imai E, Ozaki T, Matsuo S, Maruyama S (2014) Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol (Epub ahead of print)
Coenen MJ, Hofstra JM, Debiec H, Stanescu HC, Medlar AJ, Stengel B, Boland-Augé A, Groothuismink JM, Bockenhauer D, Powis SH, Mathieson PW, Brenchley PE, Kleta R, Wetzels JF, Ronco P (2013) Phospholipase A2 receptor (PLA2R1) sequence variants in idiopathic membranous nephropathy. J Am Soc Nephrol 24(4):677–683
Oh YJ, Yang SH, Kim DK, Kang SW, Kim YS (2013) Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. Plos One 8(4):e62151
Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA (2014) Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 25(6):1357–1366
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hara, S., Goto, S., Kamiura, N. et al. Reappraisal of PLA2R1 in membranous nephropathy: immunostaining method influence and association with IgG4-dominant phenotype. Virchows Arch 467, 87–94 (2015). https://doi.org/10.1007/s00428-015-1754-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1754-3