Abstract
Background
Membranous glomerulopathy, though typically a disease of adults, does occur in children. Antiphospholipase A2 receptor (PLA2R) autoantibodies have recently been implicated as a causative agent in most cases of adult primary (idiopathic) membranous glomerulopathy. PLA2R staining of renal biopsies in two recent large case series of adults with primary membranous glomerulopathy showed a sensitivity of approximately 75 % for detecting primary membranous glomerulopathy. To our knowledge, this is the largest study of its kind to assess PLA2R staining in a pediatric population.
Methods
Forty-one consecutive cases of pediatric membranous glomerulopathy were identified from our database, and clinical follow-up was performed to confirm primary membranous glomerulopathy. Twenty-two patients met inclusion criteria and are the subject of this report.
Results
Granular, capillary loop immunofluorescence staining for immunoglobulin G (IgG) was present in 100 % of patients, and C3 staining was present in 77 %. PLA2R staining was identified in ten patients, providing a sensitivity of 45 % [confidence interval (CI) 25–67 %]. Bovine serum albumin staining was performed in all PLA2R-negative cases and showed no positivity. Morphologic findings associated with negative PLA2R staining included segmental membranous lesions, mesangial and subendothelial deposits, C1q and “full-house” staining, and lower-stage lesions by electron microscopy. At 38 months’ average follow-up, all patients were still considered as having primary membranous glomerulopathy, with none developing a clinically detectable secondary etiology.
Conclusions
PLA2R staining sensitivity is much lower in the pediatric than the adult primary membranous glomerulopathy population. This finding suggests a more diverse and currently incompletely described set of etiologies for this disease in this group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Membranous glomerulopathy was first described in 1946 by Bell [1] and separated into a unique disease in 1957 by Jones [2]. The presence of glomerular immunoglobulin deposits was discovered by Mellors et al. in 1957, whereas electron microscopic features of the disease were first reported by Movat and McGregor in 1959 [3, 4]. A breakthrough occurred in 1959 when Heymann and colleagues [5] identified an inducible disease (Heymann nephritis) in rats that was virtually identical, both clinically and morphologically, to membranous glomerulopathy in humans. Makker et al. later implicated megalin, a 600-kDa glycoprotein, as the causative antigen [6]. Recently, Beck et al. found that 70 % of adult patients with primary membranous glomerulopathy expressed a specific M-type phospholipase A2 receptor (PLA2R) on podocytes [7]. PLA2R appears to be the predominant causative antigen of primary membranous glomerulopathy in adults [7]. In previous reports, the presence of PLA2R autoantibodies in serum were shown to have a sensitivity of 70–82 %, and tissue staining for PLA2R showed a sensitivity of 74 % [7, 8]. However, in those studies, the majority of patients were adults or older adolescents. Early reports suggest promising serologic tests for diagnosis and monitoring of primary membranous glomerulopathy [7, 9, 10]. Studies in our lab demonstrate the importance and utility of immunofluorescence staining for PLA2R on all newly diagnosed cases of membranous glomerulopathy.
Primary membranous glomerulopathy is a rare entity in children, representing 1–2 % of nephrotic syndrome patients seen in this population [11, 12]. Historically, membranous glomerulopathy in children has been associated with secondary causes, such as systemic lupus erythematosus, hepatitis B infection, congenital syphilis, etc [13, 14]. Recently, Debiec et al. described neutral endopeptidase as a causative alloantibody in neonatal membranous glomerulopathy and bovine serum albumin antibodies as a cause of secondary membranous glomerulopathy in young children [15, 16]. However, there is no information available on a potential role for PLA2R in the pediatric population, and morphologically, there are no findings with a known high sensitivity or specificity for differentiating primary and secondary forms of this disease.
Methods
Patient selection
This study was approved by Schulman institutional review board with data collected according to approved protocols. Our database was searched from 2005 to 2012 for patients aged 17 and younger with membranous glomerulopathy and negative antinuclear antibody (ANA) serology. Forty-one consecutive cases were identified. Clinical follow-up was performed on all patients to determine whether they had an active disease that is a known secondary etiology of membranous glomerulopathy. Either no or inadequate follow-up occurred in 19 patients. Twenty-two patients showed negative serologic testing for ANA, antineutrophil cytoplasmic autoantibodies (ANCA), hepatitis B or C virus (HBV, HCV), sarcoidosis, or syphilis and showed no evidence of neoplasm. All cases had light microscopic, immunofluorescence, and electron microscopic results available.
Routine biopsy processing techniques
Standard renal biopsy processing techniques were used, including light, immunofluorescence, and electron microscopy [17, 18]. All light microscopy samples were stained with hematoxylin and eosin (H&E), Jones methenamine silver, Masson trichrome, and periodic acid-Schiff reagent. All direct immunofluorescence sections were cut at 5 μm and reacted with fluorescein-tagged polyclonal rabbit anti-human antibodies to immunoglobulin (Ig)G, IgA, IgM, C3, C1q, fibrinogen, and κ- and λ-light chains (Dako, Carpenteria, CA, USA) for 1 h, rinsed, and a coverslip was applied using aqueous mounting media. Select cases were stained for fluorescein-tagged polyclonal mouse anti-human antibodies to IgG1, IgG2, IgG3, and IgG4 (Sigma-Aldrich, St. Louis, MO, USA). All PLA2R-negative cases were stained for bovine serum albumin (Life Technologies, Carlsbad, CA, USA). For electron microscopy, thin sections were examined in a Jeol JEM-1011 electron microscope (Jeol, Tokyo, Japan). Photomicrographs were routinely taken at ×5,000, ×12,000, and ×20,000 magnifications. Electron photomicrographs were used to stage the cases of membranous glomerulopathy according to the classification of Ehrenreich and Churg [19]. Electron photomicrographs were also utilized to identify mesangial and subendothelial deposits. For this report, the term “mesangial deposits” refers to deep mesangial deposits within the mesangial matrix and internal to identifiable paramesangial basement membranes [20].
PLA2R immunofluorescence
PLA2R was detected as in our previous study [10] using the routinely prepared, formalin-fixed, paraffin-embedded renal biopsy tissues using rabbit polyclonal anti-PLA2R antibodies (Sigma-Aldrich) at a dilution of 1:50, followed by highly cross-adsorbed Alexa Fluor 488 goat anti-rabbit IgG (Life Technologies, Carlsbad, CA, USA) at a dilution of 1:100. Each case was run with a positive and negative (secondary antibody only) control. The stain was evaluated by standard immunofluorescence microscopy using a Leica L5 filtercube. It was judged to be positive if there was granular capillary loop staining along glomerular capillary loops. Each section was scored on a scale of 0–3+. All PLA2R-stained sections were evaluated by at least two renal pathologists.
Results
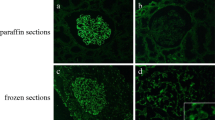
Though 41 consecutive pediatric patients with membranous glomerulopathy were initially identified, follow-up information confirming the primary nature of their disorder was only obtainable for 22 patients. Patient age ranged from 4 to 17 years, with a 1:1 M:F ratio. Average serum creatinine level at time of biopsy was 0.72 mg/dl (0.2–1.3 mg/dl), with average proteinuria of 6.8 gm/day (1.4–18.4 gm/day) (Table 1). Granular capillary loop immunofluorescence staining of glomeruli for PLA2R was present in ten of 22 patients (Fig. 1). Thus, sensitivity of positive PLA2R glomerular staining was 45 % [confidence interval (CI) 25–67 %] for detecting primary membranous glomerulopathy (Fig. 1). All PLA2R-negative biopsies were examined for the presence of antibodies against bovine serum albumin, and all were negative. IgG subclass staining was attempted in all cases. Eight cases had tissue with glomeruli present for evaluation (Table 2). Three of six PLA2R-negative patients showed IgG4 dominance/codominance, whereas both PLA2R-positive patients showed IgG4 dominance/codominance. Of the remaining three PLA2R-negative patients, two showed IgG3 dominance/codominance and one IgG1 dominance. On immunofluorescence, capillary-wall IgG was positive (≥2+) in all patients (two displayed segmental staining); capillary-wall C3 was positive in 77 % (17/22). Kappa and lambda light-chain staining were present in all cases. Capillary-wall IgA and IgM staining were present in 9 % (2/22) and 14 % (3/22), respectively. C1q was present in 18 % (4/22), all of whom were PLA2R negative.
Antiphospholipase A2 receptor (PLA2R) antibody staining pattern in a glomerulus. a Granular capillary-wall 3+ staining for anti-PLA2R antibodies in a glomerulus (PLA2R stain, ×400 original magnification). b Negative staining for anti-PLA2R antibodies in a glomerulus (PLA2R stain, ×400 original magnification)
By electron microscopy, all patients showed subepithelial immune complex deposits, with nine showing stage 1 deposits, four showing stage 2, and nine showing stage 3 [19]. Seven of nine patients with stage 3 deposits showed positivity for PLA2R. Eleven patients (50 %) showed mesangial deposits, 82 % of which (9/11) were also PLA2R negative (Table 3). Follow-up was performed on all cases to ascertain whether the patients had developed any secondary cause of membranous glomerulopathy in the interim. An average of 38 months follow-up was achieved in 17 patients, with all reporting continuation of idiopathic, primary disease and none having developed a secondary etiology (Table 2).
Discussion
We examined the pertinent clinical, morphologic, and PLA2R staining patterns in a group of 22 pediatric primary membranous glomerulopathy patients. In ten patients, PLA2R staining was positive; the remaining 12 showed no staining, providing a sensitivity of 45 % for detecting primary membranous glomerulopathy in the pediatric population. This finding is in contrast to similar studies in primarily adults, where 70–82 % sensitivity was achieved [7, 8, 10, 21]. This suggests that the majority of primary pediatric membranous glomerulopathy patients do not have antibodies to PLA2R antigen as the etiology of their disease. This is critical information for several reasons. First, this suggests that membranous glomerulopathy in the pediatric population has a more diverse set of etiologies than what would be expected from adult studies of this disease and that these etiologies are incompletely described [7, 8, 10]. Also, as better disease monitoring and treatment may one day depend on the underlying autoantibody/etiology in membranous glomerulopathy, a more complete understanding of the different etiologies of this disease is crucial. Lastly, this study suggests that in the majority of pediatric primary membranous glomerulopathy patients, the underlying etiology is currently unknown.
Clinically, we saw similar age and gender distribution, serum creatinine at time of biopsy, and 24-h urine protein levels in both PLA2R-positive and -negative patients. Similarly, by light microscopy no specific features were identified that were more strongly associated with either group. However, by immunofluorescence, C1q staining was identified in four PLA2R-negative patients and was always associated with the presence of mesangial deposits; it was not present in PLA2R-positive patients. Additionally, one patient with C1q staining exhibited full-house staining; however, at 43 months’ follow-up, this patient was still ANA negative and was classified as having primary membranous glomerulopathy. Eleven patients showed mesangial deposits, eight of whom were PLA2R-negative patients; a single patient displayed subendothelial deposits and was PLA2R negative. In adult membranous glomerulopathy, mesangial and subendothelial deposits have been reported as a “soft sign” of secondary etiologies [13]. Also, whereas C1q staining and mesangial deposits are not sensitive or specific for lupus or other autoimmune disease, these findings do raise the possibility that some patients with negative PLA2R staining may represent atypical or early stages of clinically undetectable autoimmune disease. However, follow-up occurred for three of these four patients; none showed progression to a clinically detectable autoimmune disease over an average 35 months.
Patients who showed positivity for IgA and/or IgM in addition to IgG were similar in PLA2R-positive and -negative patients, (four total; two PLA2R +, two PLA2R −); however, all of these patients displayed mesangial deposits. Also, by electron microscopy, nine patients showed stage 3 deposits; seven of them were PLA2R positive. Segmental immune complex deposition was seen in two patients, one male and one female, ages 10 and 17 years, respectively. Both patients showed negative staining for PLA2R, mesangial deposits, and earlier-stage deposits (stage 1 and 2); one showed staining for C1q (1+). On follow-up, neither patient had developed secondary causes for their membranous glomerulopathy.
No consensus exists on the treatment and clinical management of pediatric patients with membranous glomerulopathy. This is likely due to the highly variable prognosis of membranous glomerulopathy and its rare occurrence in children [22–24]. However, Menon et al. suggested a treatment algorithm for membranous glomerulopathy occurring in childhood [22]. They suggest angiotensin blockade for those with subnephrotic proteinuria and steroids for those with nephrotic syndrome. Alkylating agents are used when less aggressive treatment fails [22].
In conclusion, this study suggests that within the pediatric population, primary membranous glomerulopathy has a more diverse set of etiologies than in the adult population, with only 45 % of primary pediatric membranous glomerulopathy showing PLA2R receptor autoantibodies as their causative etiology. Additionally, whereas we found no morphologic finding that was absolutely consistent with PLA2R negativity, we did note frequent association with mesangial and subendothelial immune complex deposits, C1q deposition, lesion segmentality, and lower electron microscopic lesion stage. Lastly, although morphologic findings in some patients with PLA2R-negative primary membranous glomerulopathy are suggestive of underlying secondary etiologies, on follow-up, we found no patient developed clinically identifiable, known secondary etiologies, with all patients still considered to have primary idiopathic membranous glomerulopathy.
References
Bell ET (1950) Renal diseases. Lea & Febiger, Philadelphia
Jones DB (1957) Nephrotic glomerulonephritis. Am J Pathol 33:313–329
Movat HZ, McGregor DD (1959) The fine structure of the glomerulus in membranous glomerulonephritis (lipoid nephrosis) in adults. Am J Clin Pathol 32:100–127
Mellors RC, Ortega LG, Holman HR (1957) Role of gammaglobulins in pathogenesis of renal lesions in systemic lupus erythematosus and chronic membranous glomerulonephriris, with an observation of the lupus erythematosus cell reaction. J Exp Med 1065:191–202
Heymann W, Hackel DB, Harwood S, Wilson SGF, Hunter JL (2000) Production of nephrotic syndrome in rats by Freund’s adjuvants and rat kidney suspensions. J Am Soc Nephrol 10:183–188
Makker SP, Singh AK (1984) Characterization of the antigen (gp600) of heymann nephritis. Lab Investig 50:287–293
Beck LH, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361:11–21
Qin W, Beck LH Jr, Zeng C, Chen Z, Li S, Zuo K, Salant DJ, Liu Z (2011) Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 22:1137–1143
Debiec H, Martin L, Jouanneau C, Dautin G, Mesnard L, Rondeau E, Mousson C, Ronco P (2011) Autoantibodies specific for the phospholipase A2 receptor in recurrent and De Novo membranous nephropathy. Am J Transplant 11:2144–2152
Larsen CP, Messias NC, Silva FG, Messias E, Walker PD (2013) Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor (PLA2R1) staining in renal biopsies. Mod Pathol 26:709–715
Churg J, Habib R, White RH (1970) Pathology of the nephrotic syndrome in children: a report for the International Study of Kidney Disease in Children. Lancet 760:1299–1302
A report for the International Study of Kidney Disease in Children (1978) Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. Kidney Int 13:159–165
Markowitz GS (2001) Membranous glomerulopathy: emphasis on secondary forms and disease variants. Adv Anat Pathol 8:119–125
Beck LH Jr (2012) Childhood membranous nephropathy and dietary antigens. Am J Kidney Dis 59:174–176
Debiec H, Lefeu F, Kemper MJ, Niaudet P, Deschênes G, Remuzzi G, Ulinski T, Ronco P (2011) Early-childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med 364:2101–2110
Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann J, Bensman A, Deschenes G, Ronco PM (2002) Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346:2053–2060
Walker PD, Cavallo T, Bonsib SM (2004) Practice guidelines for the renal biopsy. Mod Pathol 17:1555–1563
Walker PD (2009) The renal biopsy. Arch Pathol Lab Med 133:181–188
Ehrenreich T, Churg J (1968) Pathology of membranous nephropathy. Pathol Annu 2:145–186
Jennette JC, Iskandar SS, Dalldorf FG (1983) Pathologic differentiation between lupus and nonlupus membranous glomerulopathy. Kidney Int 24:377–385
Debiec H, Ronco P (2011) PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med 364:689–690
Menon S, Valentini R (2010) Membranous nephropathy in children: clinical presentation and therapeutic approach. Pediatr Nephrol 25:1419–1428
Waldman M, Austin HA III (2009) Controversies in the treatment of idiopathic membranous nephropathy. Nat Rev Nephrol 5:469–479
Valentini RP, Mattoo TK, Kapur G, Imam A (2009) Membranous glomerulonephritis: treatment response and outcome in children. Pediatr Nephrol 24:301–308
Acknowledgments
The authors thank Robert Yerton, Steve Strong, and Cindy Smith for their valuable technical aid and I-Shen Wen and Tina Priddy for their administrative support. The abstract to this work was presented at the USCAP 102nd Annual Meeting (2013), Baltimore, MD, USA (Mod Pathol 26: 383–396; doi:10.1038/modpathol.2013.16).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cossey, L.N., Walker, P.D. & Larsen, C.P. Phospholipase A2 receptor staining in pediatric idiopathic membranous glomerulopathy. Pediatr Nephrol 28, 2307–2311 (2013). https://doi.org/10.1007/s00467-013-2574-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-013-2574-9