Abstract
In contrast to pancreatic ductal adenocarcinomas (PDAs), intraductal papillary mucinous neoplasms (IPMNs) frequently harbour GNAS mutations. To characterise GNAS-mutated pancreatic carcinomas, we examined mutations of GNAS and KRAS in 290 pancreatic adenocarcinomas and 77 pancreatic intraepithelial neoplasias (PanINs). In 64 % (39/61) of IPMNs and 37 % (11/30) of IPMN-associated adenocarcinomas, a GNAS mutation was found. GNAS mutations were frequent (78 %, 7/9) in mucinous carcinomas, with or without associated IPMN. In contrast, GNAS mutations were rarely observed in PDAs (1 %, 1/88) and PanINs (3 %, 2/77), and not at all in mucinous cystic neoplasms (MCNs) (0/10), neuroendocrine neoplasms (0/52), acinar cell neoplasms (0/16), serous cystadenomas (0/10), and solid-pseudopapillary neoplasms (0/14). We found GNAS mutations in 55/91 IPMNs with or without associated invasive carcinoma, solely in intestinal-type (78 %, 21/27) and gastric-type (62 %, 34/55) IPMNs. Of the IPMN-associated adenocarcinomas, mucinous-subtype tumours harboured GNAS mutations more frequently (83 %, 5/6) than tubular-subtype tumours (25 %, 6/24) (p = 0.02). We separately analysed GNAS in the adenocarcinoma and the IPMN component in the IPMN-associated adenocarcinomas. In all mucinous-subtype tumours, the two components exhibited identical genotypes. In contrast, the two components in 8 of 24 tubular-subtype tumours exhibited different genotypes, indicating intratumour heterogeneity. In conclusion, mucinous carcinomas with or without associated IPMN as well as IPMNs frequently harbour a GNAS mutation, reinforcing the notion that these constitute a spectrum of pancreatic tumours. Clinically and pathologically, these tumours are associated, but GNAS mutation sheds further light on this spectrum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cyclic AMP-mediated signalling pathway is responsible for a wide variety of physiological processes and for maintaining homeostasis in the majority of human cells. The GNAS gene encodes the stimulatory G-protein α-subunit (Gsα) of the heterotrimeric G-proteins. When activated by the exchange of GDP for GTP and the dissociation from G-protein β- and γ-subunits, Gsα stimulates the cyclic AMP-generating enzyme adenylyl cyclase and other effectors. Gsα is turned off by returning to the GDP-bound form through the intrinsic GTPase activity and reassociation with G-protein β- and γ-subunits [1, 2]. Activating GNAS mutations have been described in a wide variety of tumours; early studies identified mutations in pituitary adenomas [3, 4], thyroid adenomas [4] and fibrous dysplasias of bone [5, 6], followed by studies in tumours of the gonads (Leydig cell tumours) [7], soft tissue (intramuscular myxomas) [8], pancreas [9, 10], stomach/duodenum [11], colon [12, 13], bile duct [14, 15], uterine cervix [16] etc. Hot spot missense mutations were noted in codon 201 and codon 227 [2]. These amino acid residues lie in the catalytic domain of the GTPase, and substitution mutations of these codons cause constitutive activation of the Gsα protein and persistent stimulation of their downstream signal transduction [1, 17, 18].
As for pancreatic tumours, two research groups first investigated pancreatic ductal adenocarcinomas (PDAs) and cystic neoplasms, and found recurrent R201H and R201C mutations in intraductal papillary mucinous neoplasms (IPMNs) (41–66 %) but none in PDAs or other cystic neoplasms [9, 10]. A subsequent study reported that pancreatic intraepithelial neoplasias (PanINs), a putative precursor of PDA, harboured infrequent GNAS mutations (10 %) [19]. These results suggest that GNAS mutation is highly correlated with IPMN. However, because GNAS mutation in non-ductal tumours of the pancreas has not been investigated fully, and mutations of GNAS have been documented not only in epithelial tumours but also in endocrine and certain rare tumours in many other organs, investigation of a wide variety of histological types is necessary to address the distribution of GNAS mutation in the pancreas.
IPMN is the most common cystic tumour of the pancreas, characterised by intraductal proliferation of mucinous cells with varying degrees of atypia, leading to cystic dilatation of the pancreatic ducts and the formation of clinically detectable masses. IPMN is considered another putative precursor of invasive adenocarcinoma that develops through the process of multi-step tumourigenesis. Although accumulating evidence suggests that IPMN-associated adenocarcinoma has several biological and prognostic characteristics different from conventional PDA [20, 21], there have been no markers available that can reliably separate IPMN-derived adenocarcinoma from conventional PDA.
In this study, we attempted to specify the distribution of GNAS mutation in the pancreatic tumours and reveal the characteristics of GNAS-mutated carcinomas to obtain insight from a genetic classification of tumours of the pancreas.
Materials and methods
Patients and tissues
We selected 290 surgically resected pancreatic tumours from 284 patients from the database of the Department of Pathology and Molecular Diagnostics, Aichi Cancer Centre Hospital, Nagoya, Japan. The tumours were classified according to the 2010 WHO classification [22]. The cohort consisted of 88 tubular adenocarcinomas, 4 adenosquamous carcinomas, 2 undifferentiated carcinomas, 3 mucinous carcinomas (with no apparent findings of an associated IPMN), 30 IPMN-associated adenocarcinomas (including 6 mucinous subtypes and 24 tubular subtypes), 61 IPMNs, 10 mucinous cystic neoplasms (including 1 with high-grade dysplasia and 9 with low-grade dysplasia), 52 neuroendocrine neoplasms, 16 acinar cell neoplasms (including 15 acinar cell carcinomas and 1 acinar cell cystadenoma), 10 serous cystadenomas, and 14 solid-pseudopapillary neoplasms. In addition, we analysed 77 PanIN lesions (35 of PanIN-1, 25 of PanIN-2, and 17 of PanIN-3) that were microdissected from 57 resected specimens with invasive ductal carcinomas, neuroendocrine neoplasms, mucinous cystadenomas, and serous cystadenomas. Of these, 17 lesions of PanIN-3 were obtained from 14 cases of invasive ductal carcinoma. Each lesion was microdissected from the cut sections at a distance from those containing invasive cancer cells. It is important to distinguish between PanIN, small-sized IPMN, and intraductal extension of invasive carcinoma cells (so-called cancerization of pancreatic duct epithelium). We therefore excluded from our study PanIN lesions occurring in the pancreas along with IPMN or with invasive ductal carcinoma with a carcinoma in situ component extending into the surrounding main pancreatic duct. All tissues were fixed in 10 % formalin and embedded in paraffin. This study was part of a comprehensive research programme of the tissue bank in Aichi Cancer Centre, which has been approved by the institutional review board.
Pathological assessment of IPMN-associated adenocarcinoma and IPMN
As IPMN-associated adenocarcinoma, we only included tumours of which the invasive carcinoma component was in contiguity with the IPMN component. Tumours were excluded from this study if invasive carcinoma nodules and cysts of IPMN were topologically discontinuous. Invasive carcinoma components were subcategorised into 6 mucinous and 24 tubular subtypes. In this study, we included five IPMN-associated adenocarcinomas of which the IPMN component showed low-grade dysplasia, in order to assess the relationship between gene abnormalities in adenocarcinoma and IPMN.
Regarding epithelial subtype of IPMN, we classified all IPMNs into four subtypes (gastric, intestinal, pancreatobiliary, and oncocytic) based on their morphology and immunohistochemical reactivity against antibodies of MUC6, MUC2, MUC5AC, and CDX2, according to previously described criteria [23, 24].
Immunohistochemistry
Immunohistochemical staining was performed using primary antibodies directed against MUC6 (clone CLH5, mouse, 1:200, Novocastra), MUC2 (clone Ccp58, mouse, 1:200, Novocastra), MUC5AC (clone CLH2, mouse, 1:100, Novocastra), and CDX2 (clone DAK-CDX2, mouse, ready to use, Dako). Staining was carried out using an Autostainer Link 48 (Dako, Copenhagen, Denmark) according to the manufacturer’s instruction. The antigens were retrieved by PT Link (Dako) for 30 min in a high-buffer solution (pH 9.0, Dako).
Mutation analysis
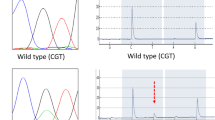
To detect mutations of GNAS and KRAS, we applied direct sequencing and Cycleave PCR methods, as described previously [25, 26]. For direct sequencing of GNAS exon 8, we used the following primer set: 5′-TTCCAAACTACTCCAGACC-3′ as the forward primer and 5′-AAAGGTAACAGTTGGCTTAC-3′ as the reverse primer. For the Cycleave PCR method to detect codon 201 mutations of GNAS, the sequences of the primer set and the probes used were as follows: PCR forward primer, 5′-CAGACCTTTGCTTTAGATTG-3′; PCR reverse primer, 5′-GTAACAGTTGGCTTACTGGA-3′; wild-type probe, 5′-ACGGCAGC-3′; mutant probes, 5′-GACACAGCA-3′ (for the R201C mutation); and 5′-ATGGCAGCG-3′ (for R201H mutation) (the underlines represent codon 201). KRAS mutations were analysed using the Cycleave PCR method, the details of which we described in the previous report [26].
Statistical analysis
The Fisher’s exact test and the Mann–Whitney U test were used to compare categorical data. The unpaired t test was used to compare continuous variables. For survival analysis, the Kaplan–Meier method was used to assess survival time distribution, and the log-rank test was applied using the SYSTAT software (SYSTAT Software, Inc., Richmond, CA, USA). P < 0.05 was considered statistically significant.
Results
GNAS and KRAS mutations in pancreatic tumours and precursor lesions
We examined mutations in GNAS and KRAS in 290 cases of surgically resected pancreatic tumours using Cycleave PCR assay and direct sequencing. It has been reported by us and others that the Cycleave PCR assay has a higher detection sensitivity (that is, samples containing mutant alleles of more than 5 % in the total DNA are sufficient for detection), compared with direct sequencing (mutant alleles need to comprise at least 10–25 % of the total DNA) [25, 27]. Accordingly, all of the lesions microdissected from the formalin-fixed paraffin-embedded tissue sections were analysed by the Cycleave PCR assay, with direct sequencing used to confirm the results of the Cycleave PCR assay, particularly at the initial stage of this study. In IPMN-associated adenocarcinomas, DNA was extracted separately from both adenocarcinoma and IPMN components. The results of the mutation analyses are summarised in Table 1. IPMNs and IPMN-associated adenocarcinomas frequently displayed a GNAS mutation, in 64 % (39/61) and 37 % (11/30) of the cases, respectively. Conversely, GNAS mutation was rarely observed in PDAs (1 %, 1/88), and mutations were not detected in mucinous cystic neoplasms, neuroendocrine neoplasms, acinar cell neoplasms, serous cystadenomas, or solid-pseudopapillary neoplasms. In contrast, KRAS mutations were distributed evenly among PDAs (92 %, 81/88), IPMN-associated adenocarcinomas (77 %, 23/30), cystic neoplasms, including IPMNs (59 %, 36/61), and mucinous cystic neoplasms (40 %, 4/10).
We examined clinicopathological and genetic features of 91 IPMNs with or without an associated invasive carcinoma (Supplementary Table 1). GNAS mutations were more frequently detected in low- and intermediate-grade IPMNs than in high-grade IPMNs (76 % (25/33), 80 % (8/10), and 46 % (22/48), respectively) (for low- and intermediate-grade IPMNs combined versus high-grade IPMNs, p = 0.003). Additionally, GNAS mutations were frequent in intestinal-type IPMNs (78 %, 21/27), followed by gastric-type IPMNs (62 %, 34/55), while GNAS mutations were not detected in the oncocytic and pancreatobiliary types. It is noteworthy that no correlation was observed between the mutation status of GNAS or KRAS and the presence of an associated invasive carcinoma. In total, 92 % (84/91) of IPMNs harboured mutations in either GNAS (27 %, 25/91) or KRAS (32 %, 29/91), or in both genes (33 %, 30/91).
Next, we examined the distribution of gene mutations in IPMNs according to histological grade and epithelial subtype. A combination of low- and intermediate-grade IPMNs, consisting of 37 gastric and 6 intestinal types, was studied, and GNAS mutations were detected in 73 and 100 % of the cases, respectively (Supplementary Table 2). In contrast, high-grade IPMNs, consisting of 18 gastric, 21 intestinal, 2 oncocytic, and 7 pancreatobiliary types, were analysed, and GNAS mutations were found in 39, 71, 0, and 0 % of the cases, respectively. The lower rate of GNAS mutation in high-grade IPMNs compared with low- and intermediate-grade IPMNs appears to be attributed to (1) the rarity of GNAS mutation in oncocytic and pancreatobiliary types, which constituted 19 % of high-grade IPMNs, and (2) the lower incidence of GNAS mutation in gastric-type IPMNs with high-grade dysplasia (39 %, 7/18), compared with those with low- and intermediate-grade dysplasia (73 %, 27/37) (p = 0.01).
We also examined GNAS and KRAS mutations in 77 PanIN lesions, including 35 PanIN-1, 25 PanIN-2, and 17 PanIN-3. The rarity of GNAS mutation (3 %, 2/77) was in marked contrast to the common incidence of KRAS mutation, which was identified in 78 % of the lesions.
To summarise the spectrum of gene mutations among the pancreatic tumours, KRAS mutation was prevalent in ductal neoplasms, including precursor PanINs, whereas IPMN-related neoplasms frequently harboured GNAS mutation.
More frequent GNAS mutation in adenocarcinoma with mucinous features
In IPMN-associated adenocarcinomas, the prevalence of GNAS mutation was different between mucinous and tubular subtypes: almost all mucinous carcinomas showed a GNAS mutation (83 %, 5/6), in contrast to the relatively small proportion of tubular carcinomas (25 %, 6/24) (p = 0.02).
In this study, we included three cases of mucinous carcinoma histologically similar to mucinous-subtype IPMN-associated adenocarcinoma but not accompanied by any discernible IPMN. We designated these cases as mucinous carcinoma without associated IPMN and discussed them separately from mucinous-subtype IPMN-associated adenocarcinoma in order to distinguish the features of IPMN-associated adenocarcinoma more clearly. In doing so, we found that two of three mucinous carcinomas without associated IPMN exhibited GNAS mutations as well.
Table 2 shows clinicopathological characteristics and genetic features of IPMN-associated adenocarcinomas and PDAs in this study. We found that IPMN-associated adenocarcinomas and PDAs differed in several aspects: patients with mucinous-subtype IPMN-associated adenocarcinoma were more likely to be female and presented at an early stage compared with those with PDA. Molecularly, mucinous-subtype IPMN-associated adenocarcinomas were more likely to have mutated GNAS and less likely to have mutated KRAS compared with PDAs. Similarly, tubular-subtype IPMN-associated adenocarcinomas and PDAs showed differences in some features: patients with tubular-subtype IPMN-associated adenocarcinoma were more likely to present at a median of 8 years later, be female, present at an early stage, and have mutated GNAS, compared with those with PDA. These differences in patient characteristics are in agreement with past studies that have shown differences between IPMN-associated adenocarcinomas and conventional PDAs [28–32]. Taken together, these data support the view that IPMN-associated adenocarcinoma is distinct from PDA.
We subsequently focused on the epithelial subtypes of IPMN components in IPMN-associated adenocarcinomas. Mucinous-subtype IPMN-associated adenocarcinomas consisted of IPMNs with intestinal phenotype (5/6) and that with oncocytic phenotype (1/6) (Table 3). Of these, GNAS mutations were detected in all IPMNs with intestinal phenotype (100 %, 5/5). In contrast, tubular-subtype IPMN-associated adenocarcinomas were composed of IPMNs with gastric phenotype (16/24), those with pancreatobiliary phenotype (5/24), and those with intestinal phenotype (3/24). GNAS mutations were identified in three IPMNs with gastric phenotype (19 %, 3/16) and three with intestinal phenotype (100 %, 3/3).
Intratumour genetic divergence in tubular-subtype IPMN-associated adenocarcinoma
Lastly, we examined the gene status of adenocarcinoma and IPMN components in IPMN-associated adenocarcinoma to assess if the components shared the same features. We microdissected multiple portions of IPMN components and examined both GNAS and KRAS genes using the Cycleave PCR assay. Of the 30 IPMN-associated adenocarcinomas, we examined tumours whose invasive portions were more than 5 mm in diameter (n = 23).
Mucinous-subtype IPMN-associated adenocarcinomas showed a consistent result: All cases (6/6, 100 %) displayed an identical mutation pattern of GNAS and KRAS in the mucinous adenocarcinoma and the associated IPMN (Table 3 and Fig. 1). In contrast, tubular-subtype IPMN-associated adenocarcinomas showed an identical gene status in the adenocarcinoma and IPMN components in 9 of 17 cases (Fig. 1), the remaining 8 displaying a discrepant mutation status of GNAS, KRAS, or both genes between components (Supplementary Fig. 1). Among the discrepant tumours, 5 cases displayed different mutations (for example, G12V versus G12D of KRAS in case no. 25), suggesting a collision of a tubular adenocarcinoma and an IPMN. When clinicopathological traits (age, gender, location, macroscopic type of IPMN, and mutation status of adenocarcinoma) were compared, the 8 tumours with a discordant mutation pattern tended to accompany branch-type IPMN, in comparison with the remaining 16 tumours without discordant results (p = 0.02).
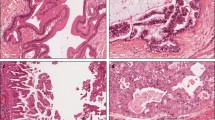
Histology of IPMN-associated adenocarcinoma with concordant genetic status between adenocarcinoma and IPMN components. Mucinous-subtype IPMN-associated adenocarcinoma (a), which consisted of a mucinous carcinoma (b) and an intestinal-type IPMN marked by villous morphology (c) and diffuse and strong CDX2 immunostaining (d), had identical GNAS and KRAS mutations between the components (R201H GNAS; wild-type KRAS). Tubular-subtype IPMN-associated adenocarcinoma (e), consisting of a tubular adenocarcinoma (f) and an intestinal-type IPMN (g) with diffuse and strong immunostaining of CDX2 (h) and MUC2 (i), exhibited shared genetic status of GNAS and KRAS between the components (R201C GNAS; wild-type KRAS). The red marks indicate the microdissected portions
International consensus guidelines for the management of IPMN and MCN of the pancreas have been published recently [33]. The guidelines recommend that IPMN-associated adenocarcinoma be subdivided into carcinoma derived from and concomitant with an IPMN, based on the topological relationship and histological transition between the adenocarcinoma and IPMN components. We compared the result of our mutation analysis with this proposed stratification in order to assess to what extent the mutation pattern was in agreement with the histological evaluation. We classified 5 cases of adenocarcinoma with an associated low-grade IPMN as carcinoma concomitant with IPMN because of a lack of histological transition with a high-grade IPMN component, although both adenocarcinoma and IPMN were topologically contiguous. We found that in a total of 15 IPMN-associated adenocarcinomas with concordant mutational status, the majority were categorised into carcinoma derived from IPMN (14 of 15 cases). One case was carcinoma with concomitant IPMN. As for IPMN-associated adenocarcinoma with discrepant genetic results (n = 8), 4 cases were carcinoma with concomitant IPMN and 2 were carcinoma of undetermined relationship with IPMN. We found 2 IPMN-associated adenocarcinomas with discrepant genetic results that were histologically categorised into carcinoma derived from IPMN (Table 3, Supplementary Fig. 1).
Discussion
We performed mutation analysis of GNAS and KRAS using a series of 290 pancreatic tumours of various histological types and 77 PanINs. This study confirmed previous results that (1) GNAS mutation is commonly detected in IPMNs and rare in PDAs [9, 10, 34], and (2) KRAS is frequently mutated in ductal neoplasms, particularly in the vast majority of ductal adenocarcinomas. In our study, GNAS mutations were prevalent in low- and intermediate-grade IPMNs. In addition, we confirmed the absence of GNAS mutations in mucinous cystic neoplasms and other non-ductal tumours, including neuroendocrine neoplasms, acinar cell neoplasms, serous cystadenomas, and solid-pseudopapillary neoplasms. Taken together, our study suggests that GNAS mutation is a molecular hallmark of IPMN-related neoplasms and plays a role in the tumourigenesis of IPMN during its early stages.
IPMN-associated adenocarcinomas comprise 10–40 % of surgically resected IPMNs [28–30] and account for 1–10 % of resected invasive ductal carcinomas of the pancreas [28, 29, 35]. IPMN-associated adenocarcinoma has been considered to develop via an IPMN to adenocarcinoma sequence. This notion is founded on the histopathological observation that IPMN commonly contains a varying degree of atypia in a single lesion, and that minimally invasive carcinoma is often identified in connection with high-grade IPMN. Several molecular studies have supported the notion by showing that mutations of KRAS, p16, and TP53 genes and epigenetic alteration of various genes accumulate as IPMNs progress from low-grade to high-grade lesions [21]. Furthermore, it has been postulated that IPMN-associated adenocarcinoma is biologically different from conventional PDA. Clinical studies have substantiated this hypothesis: Patients with surgically resected IPMN-associated adenocarcinoma had a better 5-year survival (40–60 %) than those with PDA (10–25 %), although controversy still remains as to whether this favourable outcome is due to its biologic characteristics or to the early-stage manifestation [28–30, 32]. Molecular studies have also supported this notion, by showing that DPC4 expression is absent in half of PDAs, but retained in the majority of IPMN-associated adenocarcinomas [36]. Promoter methylation of several cancer-related genes, including p16, E-cadherin, hMLH1, and MGMT, was reported to be differentially involved in IPMN-associated adenocarcinomas and conventional PDAs [37, 38]. The recent discovery of the recurrent mutation of GNAS in IPMN has paved the way for the evaluation of this hypothesis. In this study, GNAS mutations were seen almost exclusively in IPMN-associated adenocarcinomas as well as IPMNs. This result strongly supports the view that this type of adenocarcinoma develops by multi-step progression of IPMN.
In IPMN-associated adenocarcinomas, two distinct subtypes of invasive adenocarcinomas, mucinous and tubular, have been described. Mucinous-subtype IPMN-associated adenocarcinoma shows a close association with intestinal-type IPMN, characterised by villous morphology and diffuse and strong MUC2 and CDX2 immunostaining. In contrast, tubular-subtype IPMN-associated adenocarcinoma typically develops in association with IPMN of gastric or pancreatobiliary types, both of which are marked by their specific morphology and negative MUC2 and CDX2 immunostaining [39]. Patient prognosis is also different, as mucinous-subtype tumours tend to be more indolent and carry a favourable prognosis, compared with tubular-subtype tumours [28–30, 32]. Based on these results, the terms ‘intestinal and indolent pathway’ and ‘pancreatobiliary and aggressive pathway’ were coined to represent the clinicopathological features of mucinous and tubular subtypes, respectively [20, 39]. In this study, we show the difference between mucinous and tubular subtypes in terms of genetic status of GNAS and KRAS. Specifically, mucinous-subtype IPMN-associated adenocarcinoma was characterised by a high prevalence of GNAS mutation and a strong association with intestinal-type IPMN, which was also characterised by frequent mutation of GNAS. In addition, by demonstrating the identical mutation status between adenocarcinoma and IPMN components, we confirmed that all mucinous-subtype IPMN-associated adenocarcinomas were clonal. Our results are in line with a study by Dal Molin et al., who reported GNAS mutations in all intestinal-type IPMNs (n = 12), although the authors did not examine IPMN-associated adenocarcinomas [40]. In contrast, tubular-subtype IPMN-associated adenocarcinomas appear to be heterogeneous. Tubular-subtype tumours consisted of IPMNs with epithelial subtypes of varying kinds (gastric, pancreatobiliary, and intestinal). Six of 24 tubular-subtype tumours had GNAS mutations in both adenocarcinoma and IPMN components, and this result reliably qualified them as adenocarcinoma that had developed from IPMN. However, in 8 of 24 tubular-subtype IPMN-associated adenocarcinomas, mutation status of the adenocarcinoma was different from that of the IPMN component, indicating genetic divergence of this subtype.
In this study, we distinguished mucinous-subtype IPMN-associated adenocarcinoma from mucinous carcinoma lacking an apparent associated IPMN and examined them separately. Mucinous (or colloid) carcinoma is currently categorised in the 2010 WHO classification as a variant of ductal carcinoma, with a description that ‘it almost always arises in association with an intestinal-type IPMN’. Seidel et al. reported that extensive sampling of colloid carcinomas enabled them to reveal the presence of IPMN in most cases in their study and concluded that colloid carcinoma develops from IPMN [41]. Practically, however, identifying an associated IPMN in a colloid carcinoma tends to be arbitrary among pathologists, particularly in tumours that are exclusively predominated by neoplastic cysts with a pushing border and embedded in dense fibrosis. In our study, a diagnosis of mucinous carcinoma without an associated IPMN was made in three cases. These tumours were large, measuring 50, 50, and 70 mm in diameter and proliferated in such a destructive manner that the associated IPMNs were not clearly discernible. However, our results are in support of the view that although an apparent IPMN component was not identified, mucinous carcinoma is highly linked to IPMN and IPMN-associated adenocarcinoma in terms of GNAS mutation. Because of the limited number of mucinous carcinoma without an associated IPMN analysed in this series, further studies are warranted.
In summary, we confirm that GNAS mutation is almost exclusively confined to IPMN-related tumours, which supports the notion that carcinoma with mucinous features and IPMN, particularly that of intestinal type, constitute a spectrum of pancreatic tumours sharing common pathological and genetic features.
References
Weinstein LS, Liu J, Sakamoto A, Xie T, Chen M (2004) Minireview: GNAS: normal and abnormal functions. Endocrinology 145(12):5459–5464. doi:10.1210/en.2004-0865
O’Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, Gutkind JS (2013) The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer 13(6):412–424. doi:10.1038/nrc3521
Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L (1989) GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 340(6236):692–696. doi:10.1038/340692a0
Lyons J, Landis CA, Harsh G, Vallar L, Grunewald K, Feichtinger H, Duh QY, Clark OH, Kawasaki E, Bourne HR et al (1990) Two G protein oncogenes in human endocrine tumors. Science 249(4969):655–659
Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM (1991) Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 325(24):1688–1695. doi:10.1056/nejm199112123252403
Okamoto S, Hisaoka M, Ushijima M, Nakahara S, Toyoshima S, Hashimoto H (2000) Activating Gs(alpha) mutation in intramuscular myxomas with and without fibrous dysplasia of bone. Virchows Arch 437(2):133–137
Fragoso MC, Latronico AC, Carvalho FM, Zerbini MC, Marcondes JA, Araujo LM, Lando VS, Frazzatto ET, Mendonca BB, Villares SM (1998) Activating mutation of the stimulatory G protein (gsp) as a putative cause of ovarian and testicular human stromal Leydig cell tumors. J Clin Endocrinol Metab 83(6):2074–2078
Delaney D, Diss TC, Presneau N, Hing S, Berisha F, Idowu BD, O’Donnell P, Skinner JA, Tirabosco R, Flanagan AM (2009) GNAS1 mutations occur more commonly than previously thought in intramuscular myxoma. Mod Pathol 22(5):718–724. doi:10.1038/modpathol.2009.32
Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA Jr, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B (2011) Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 3(92):92ra66. doi:10.1126/scitranslmed.3002543
Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N, Shiratori K (2011) Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 1:161. doi:10.1038/srep00161
Matsubara A, Sekine S, Kushima R, Ogawa R, Taniguchi H, Tsuda H, Kanai Y (2013) Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol 229(4):579–587. doi:10.1002/path.4153
Yamada M, Sekine S, Ogawa R, Taniguchi H, Kushima R, Tsuda H, Kanai Y (2012) Frequent activating GNAS mutations in villous adenoma of the colorectum. J Pathol 228(1):113–118. doi:10.1002/path.4012
Nishikawa G, Sekine S, Ogawa R, Matsubara A, Mori T, Taniguchi H, Kushima R, Hiraoka N, Tsuta K, Tsuda H, Kanai Y (2013) Frequent GNAS mutations in low-grade appendiceal mucinous neoplasms. Br J Cancer 108(4):951–958. doi:10.1038/bjc.2013.47
Tsai JH, Yuan RH, Chen YL, Liau JY, Jeng YM (2013) GNAS is frequently mutated in a specific subgroup of intraductal papillary neoplasms of the bile duct. Am J Surg Pathol 37(12):1862–1870. doi:10.1097/PAS.0b013e3182986bb5
Sasaki M, Matsubara T, Nitta T, Sato Y, Nakanuma Y (2013) GNAS and KRAS mutations are common in intraductal papillary neoplasms of the bile duct. PLoS ONE 8(12):e81706. doi:10.1371/journal.pone.0081706
Matsubara A, Sekine S, Ogawa R, Yoshida M, Kasamatsu T, Tsuda H, Kanai Y (2013) Lobular endocervical glandular hyperplasia is a neoplastic entity with frequent activating GNAS mutations. Am J Surg Pathol. doi:10.1097/pas.0000000000000093
Bourne HR, Landis CA, Masters SB (1989) Hydrolysis of GTP by the alpha-chain of Gs and other GTP binding proteins. Proteins 6(3):222–230. doi:10.1002/prot.340060304
Wilson CH, McIntyre RE, Arends MJ, Adams DJ (2010) The activating mutation R201C in GNAS promotes intestinal tumourigenesis in Apc(Min/+) mice through activation of Wnt and ERK1/2 MAPK pathways. Oncogene 29(32):4567–4575. doi:10.1038/onc.2010.202
Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, Goggins M (2012) Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 142(4):730–733.e739. doi:10.1053/j.gastro.2011.12.042
Grutzmann R, Niedergethmann M, Pilarsky C, Kloppel G, Saeger HD (2010) Intraductal papillary mucinous tumors of the pancreas: biology, diagnosis, and treatment. Oncologist 15(12):1294–1309. doi:10.1634/theoncologist. 2010-0151
Hong SM, Park JY, Hruban RH, Goggins M (2011) Molecular signatures of pancreatic cancer. Arch Pathol Lab Med 135(6):716–727. doi:10.1043/2010-0566-ra.1
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system. International Agency for Research on Cancer, Lyon
Furukawa T, Kloppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS, Luttges J, Offerhaus GJ, Shimizu M, Sunamura M, Suriawinata A, Takaori K, Yonezawa S (2005) Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch 447(5):794–799. doi:10.1007/s00428-005-0039-7
Basturk O, Khayyata S, Klimstra DS, Hruban RH, Zamboni G, Coban I, Adsay NV (2010) Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol 34(3):364–370. doi:10.1097/PAS.0b013e3181cf8bb6
Yatabe Y, Hida T, Horio Y, Kosaka T, Takahashi T, Mitsudomi T (2006) A rapid, sensitive assay to detect EGFR mutation in small biopsy specimens from lung cancer. J Mol Diagn 8(3):335–341. doi:10.2353/jmoldx.2006.050104
Hosoda W, Takagi T, Mizuno N, Shimizu Y, Sano T, Yamao K, Yatabe Y (2010) Diagnostic approach to pancreatic tumors with the specimens of endoscopic ultrasound-guided fine needle aspiration. Pathol Int 60(5):358–364. doi:10.1111/j.1440-1827.2010.02527.x
Pao W, Ladanyi M (2007) Epidermal growth factor receptor mutation testing in lung cancer: searching for the ideal method. Clin Cancer Res 13(17):4954–4955. doi:10.1158/1078-0432.ccr-07-1387
Mino-Kenudson M, Fernandez-del Castillo C, Baba Y, Valsangkar NP, Liss AS, Hsu M, Correa-Gallego C, Ingkakul T, Perez Johnston R, Turner BG, Androutsopoulos V, Deshpande V, McGrath D, Sahani DV, Brugge WR, Ogino S, Pitman MB, Warshaw AL, Thayer SP (2011) Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut 60(12):1712–1720. doi:10.1136/gut.2010.232272
Poultsides GA, Reddy S, Cameron JL, Hruban RH, Pawlik TM, Ahuja N, Jain A, Edil BH, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL (2010) Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg 251(3):470–476. doi:10.1097/SLA.0b013e3181cf8a19
Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda Y, Nakaizumi A, Furukawa T, Ban S, Nobukawa B, Kato Y, Tanaka M (2011) Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas 40(4):571–580. doi:10.1097/MPA.0b013e318215010c
Ideno N, Ohtsuka T, Kono H, Fujiwara K, Oda Y, Aishima S, Ito T, Ishigami K, Tokunaga S, Ohuchida K, Takahata S, Nakamura M, Mizumoto K, Tanaka M (2013) Intraductal papillary mucinous neoplasms of the pancreas with distinct pancreatic ductal adenocarcinomas are frequently of gastric subtype. Ann Surg 258(1):141–151. doi:10.1097/SLA.0b013e31828cd008
Koh YX, Chok AY, Zheng HL, Tan CS, Goh BK (2014) Systematic review and meta-analysis comparing the surgical outcomes of invasive intraductal papillary mucinous neoplasms and conventional pancreatic ductal adenocarcinoma. Ann Surg Oncol 21(8):2782–2800. doi:10.1245/s10434-014-3639-0
Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K (2012) International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12(3):183–197. doi:10.1016/j.pan.2012.04.004
Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P, Zamboni G, Maitra A, Salvia R, Hruban RH, Bassi C, Capelli P, Lawlor RT, Goggins M, Scarpa A (2014) Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol 233(3):217–227. doi:10.1002/path.4344
Matsuno S, Egawa S, Fukuyama S, Motoi F, Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K, Nakao A, Hiraoka T, Hosotani R, Takeda K (2004) Pancreatic cancer registry in Japan: 20 years of experience. Pancreas 28(3):219–230
Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, Wilentz RE, Argani P, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH (2000) Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol 157(3):755–761. doi:10.1016/s0002-9440(10)64589-0
Ueki T, Toyota M, Sohn T, Yeo CJ, Issa JP, Hruban RH, Goggins M (2000) Hypermethylation of multiple genes in pancreatic adenocarcinoma. Cancer Res 60(7):1835–1839
House MG, Guo M, Iacobuzio-Donahue C, Herman JG (2003) Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis 24(2):193–198
Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH, Klimstra DS (2004) Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol 28(7):839–848
Dal Molin M, Matthaei H, Wu J, Blackford A, Debeljak M, Rezaee N, Wolfgang CL, Butturini G, Salvia R, Bassi C, Goggins MG, Kinzler KW, Vogelstein B, Eshleman JR, Hruban RH, Maitra A (2013) Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol 20(12):3802–3808. doi:10.1245/s10434-013-3096-1
Seidel G, Zahurak M, Iacobuzio-Donahue C, Sohn TA, Adsay NV, Yeo CJ, Lillemoe KD, Cameron JL, Hruban RH, Wilentz RE (2002) Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol 26(1):56–63
Acknowledgments
The authors thank Chieko Yamada, Noriko Shibata, Ayako Nonaka, and Motoko Nimura for their excellent technical assistance with the molecular genetics. This work was supported in part by a Grant-in-Aid (B-25293090) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosoda, W., Sasaki, E., Murakami, Y. et al. GNAS mutation is a frequent event in pancreatic intraductal papillary mucinous neoplasms and associated adenocarcinomas. Virchows Arch 466, 665–674 (2015). https://doi.org/10.1007/s00428-015-1751-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1751-6