Abstract
Background
The management of intraductal papillary mucinous neoplasms (IPMNs) is mainly based on imaging features and clinical symptoms, and remains challenging.
Objective
The aim of this study was to assess GNAS, RAS family (KRAS, NRAS and HRAS), BRAF, and PIK3CA mutation status in resected IPMNs and correlate it with clinicopathological characteristics and patient survival.
Methods
Overall, 149 consecutive unselected patients who underwent pancreatectomy for IPMNs were included. After dissection from formalin-fixed and paraffin-embedded tumors, GNAS mutational screening was assessed by allelic discrimination using Taqman® probes and confirmed by SNaPshot analysis. RAS family, BRAF, and PIK3CA mutational screening was assessed by high resolution melt and Sanger sequencing.
Results
Gastric- and intestinal-type IPMNs were the most frequent lesions (52% and 41%, respectively). Intestinal-type IPMNs were more frequently associated high-grade dysplasia (49%) and were the only IPMNs associated with colloid-type carcinoma. All pancreatobiliary IPMNs were invasive lesions, located in the main pancreatic duct. GNAS-activating mutations were strongly associated with the intestinal phenotype (p < 10−4), while RAS pathway mutations were not associated with any particular phenotype. Mutations within other members of the epidermal growth factor receptor (EGFR) pathway were very rare (2%). GNAS-mutated IPMNs were rarely invasive (11%) and almost exclusively (83%) of the colloid type. For invasive lesions, multivariate analyses determined that only node negativity was associated with improved cancer-specific survival, but, in univariate analysis, GNAS mutation was associated with prolonged survival.
Conclusion
In patients selected for surgery, GNAS mutation analysis and tumor phenotype can help to better predict patient prognosis. In the near future, a more precise mutational analysis of IPMNs might help to better tailor their management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreatic intraductal papillary mucinous neoplasms (IPMNs) are one of the precursor lesions of pancreatic adenocarcinoma. Histologically, they are categorized according to their localization (main duct, branch duct involvement, or both), their grade of dysplasia (low or high), and their phenotype (gastric, intestinal, pancreaticobiliary, or oncocytic). Main duct localization, high-grade dysplasia, and pancreaticobiliary phenotype have been associated with a worse prognosis,1,2 and their clinical management remains controversial and challenging, mainly based on imaging features and clinical symptoms.3,4 With the increasing number of IPMNs detected, three main questions must be addressed:
To date, despite several national and international guidelines, answers remain elusive.
Somatic activating mutations of the G-protein α-stimulatory subunit (Gsα subunit) encoded by the GNAS gene (GNAS) have been reported in up to 70% of pancreatic IPMNs, with an important discrepancy shown between studies (33–79%).7,8,9,–10KRAS is therefore one of the two most prevalent mutations in these tumors. In this setting, GNAS mutations, known to lead to elevated intracellular cyclic adenosine monophosphate (cAMP) levels and activation of downstream dependent pathways,11 could open new clinical insights into IPMNs. As an example, the IPMN intestinal pattern of differentiation is associated with GNAS mutation,12 underlining the functional consequences of GNAS-activating mutations in the pancreatic tract.
If KRAS mutations are well-documented in IPMNs, the incidence of other gene mutations implicated in the epidermal growth factor receptor (EGFR) pathway has been rarely studied and, overall, the clinical significance of these genetic alterations has been poorly documented. This is of particular interest as several studies have shown that these mutations can be reliably assessed in the cyst liquid acquired during an endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) procedure, or even by collecting the pancreatic juice in the duodenum after secretin stimulation.13,14
In the present study, we examined the mutation status of GNAS, RAS family mutation spectrum (KRAS, NRAS, and HRAS), BRAF, and PIK3CA genes in a large series of consecutive, unselected IPMN patients who underwent pancreatic resection, and correlated mutational status with clinicopathological characteristics and patient survival.
Patients and Methods
Patients
After Institutional Review Board (IRB) approval (IRB 12-055), we reviewed the medical records of 149 consecutive unselected patients who underwent a pancreatic resection for IPMN, between 2007 and 2011, in the Department of Hepatobiliary and Pancreatic Surgery, Beaujon Hospital, Clichy, France. Demographic variables, clinical presentation, intraoperative data, and a definitive pathologic diagnosis were obtained from a prospective database with additional retrospective medical record review. All surgical indications were discussed in a multidisciplinary pancreatic tumor board including surgeons, pathologists, radiologists, and gastroenterologists. Surgical indications were decided according to the most recent guidelines of the International Association of Pancreatology (IAP) for IPMNs.15,16
Tumor Pathology
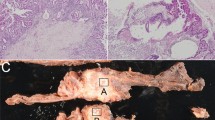
All IPMN cases were reviewed and the diagnosis confirmed by an experienced pathologist in pancreatic pathology (JC). The type of duct involvement was determined by macro- and microscopic examinations, and was classified into main duct, branch duct, or mixed-type IPMNs. Dysplasia was graded as low (previous mild and moderate dysplasia), high-grade dysplasia (carcinoma in situ) and invasive carcinoma, according to World Health Organization criteria17 and the recent Baltimore Consensus.18 Patients with minimally invasive carcinoma as defined by Nara et al.19 were categorized as high-grade dysplasia in view of their comparable prognosis. If an IPMN displayed several grades of dysplasia, the highest grade was recorded for this study. Tumors were classified into four distinct epithelial subtypes, i.e. gastric, intestinal, pancreatobiliary, and oncocytic, on the basis of their epithelial morphology on routine hematoxylin–eosin–safran staining and mucin profile on immunochemistry (MUC1, MUC2 and MUC5AC; BD Bioscience, San Diego, CA, USA; 1/400). Representative pictures of each phenotype are presented in Fig. 1. In the rare cases with two distinct phenotypes (only gastric plus intestinal in this series), the most abundant subtype was recorded. Of note, in all cases, the most abundant subtype always displayed the highest grade of dysplasia. Invasive carcinomas were classified as tubular carcinoma, i.e. as usual classical pancreatic carcinoma or colloid carcinoma in which extracellular mucin comprises at least 80% of the tumor volume.
GNAS Mutational Screening
As previously reported by our group,20 DNA from tumor tissue was extracted after macrodissection from formalin-fixed and paraffin-embedded (FFPE) tumor specimens using a Qiagen® (Courtaboeuf, France) QIAamp FFPE tissue kit according to the manufacturer’s instructions. Briefly, in order to ensure the best macrodissection, the area displaying the highest grade of dysplasia was chosen by an experienced pathologist (JC) on one hematoxylin and eosin slide. The area was marked with an adapted pen and reported on five serial unstained sections of 10 microns each. This area was then scratched with a clean scalpel and put into an Eppendorf tube for DNA extraction (electronic supplementary Fig. 1). Since all GNAS-activating mutations previously described in IPMNs were located in exon 8, codon 201 (GenBank accession no. NM_001077488.2), we consequently limited the mutational analysis to this hot-spot. GNAS status was assessed by allelic discrimination using Taqman® probes and confirmed by SNaPshot analysis (see electronic supplementary Table 1 for primer details). All sequence variants identified were confirmed by two independent experiments.
RAS Mutational Screening
As previously reported,21 the primer sequences used for both high resolution melt (HRM) and Sanger sequencing are shown in electronic supplementary Table 1. The majority of HRM primers were designed to span the entire exons with product sizes under 200 bp. Primers were designed for KRAS (exons 2–4), HRAS (exons 2 and 3), NRAS, (exons 2 and 3), BRAF (exon 15), and PIK3CA (exons 9 and 20). The polymerase chain reaction (PCR) for HRM and Sanger sequencing analysis was performed on a 384-well plate in the presence of the fluorescent DNA intercalating dye LC green (Idaho Technology, Salt Lake City, UT, USA) in a LightCycler480 (Roche Diagnosis, Meylan, France). The reaction mixture in a 15 ml final volume contained LC green, UDP-glycosylase (Roche), and Roche Master Mix (Roche). The cycling and melting conditions were as follows: an initial cycle of 10 min at 40 1C, one cycle of 95 1C for 10 min; 50 cycles of 95 1C for 10 s, 55–65 1C for 10 s, 72 1C for 30 s; one cycle of 97 1C for 1 min; and a melt from 70 1C to 95 1C rising 0.2 1C per second. Depending on the melting temperature, a touchdown approach was used for some primers. All samples were tested in duplicate. The HRM data were analyzed using the Genescan software (Roche). All samples including the wild-type (WT) exons were plotted according to their melting profiles on the differential plot graph. Any difference in the horizon line based on the WT sample was sequenced using Sanger sequencing. The reaction mixture in a total of 50 ml was made using 1 ml of PCR products without first purification, followed by a sequencing reaction with Big Dye Terminator v3.1 (Thermofisher, Courtaboeuf, France) according to the manufacturer’s protocol. The sequencing products were purified with a Sephadex gel (GE Healthcare, Velizy-Villacoublay, France) before running on a 3500 Genetic Analyser (Applied Biosystems, Foster City, CA, USA). The sequencing data were visualized using Finch TV (Geospiza, Inc., Seattle, WA, USA) with detection sensibility of 10% mutated cells.
Statistical Analysis
Values are expressed as median and interquartile range, or percentage, as appropriate. The Chi square or Fisher’s exact tests were used to compare differences in discrete or categorical variables. According to the distribution of variables, the t test or Wilcoxon rank-sum test were used for continuous variables. All preoperative clinical and radiological variables achieving statistical significance at a 0.1 level in univariate analysis were considered for multivariate analysis. A backward variable selection procedure was used to identify the independent predictive factors. Odds ratios (ORs) with 95% confidence intervals (CIs) are reported. Cancer-specific survival was measured from the date of surgery to the date of cancer-related death. Surviving patients were censored at the final follow-up. Cancer-specific survival was estimated using the Kaplan–Meier method, and survival was compared between the two groups using the log-rank test. All tests were two-sided. For all tests, statistical significance was defined as p < 0.05. Data were analyzed using STATA 12 statistical software (StataCorp LP, College Station, TX, USA; 2011. Stata Statistical Software: Release 12).
Results
Patient and Tumor Characteristics
Patient and tumor characteristics according to IPMN phenotype are summarized in Table 1. Briefly, in this cohort of 149 resected lesions, low-grade IPMNs were the most prevalent lesions (n = 78; 52%), with a non-statistically different rate of involvement of the main and branch ducts (n = 84 [56%] vs. n = 65 [44%]). Dysplasia grade was significantly different according to the phenotype (p < 10−4), and, overall, 21% (n = 31) were invasive. Gastric-type IPMNs were the most frequent lesions (n = 77; 52%), mainly of low-grade dysplasia (n = 55; 71%), while intestinal-type IPMNs were almost as frequent (n = 61; 41%), but were more frequently associated high-grade dysplasia (n = 30; 49%). All pancreatobiliary IPMNs were invasive lesions, located in the main pancreatic duct. While the rate of invasive carcinoma was comparable in intestinal and gastric-type IPMNs (n = 8 [13%] vs. n = 14 [18%]), colloid-type carcinoma was only seen in intestinal-type IPMNs (p < 10−4).
GNAS and RAS Pathway Analysis
DNA was available for 135 patients (90.1%) for GNAS analysis, and 117 patients (78.5%) for RAS pathway analysis. Patient and tumor characteristics according to GNAS and RAS mutational status are summarized in Table 1 and electronic supplementary Table 2. Briefly, GNAS-activating mutations were strongly associated with the intestinal phenotype (p < 10−4), while RAS pathway mutations were not associated with a particular phenotype. In addition, IPMNs displaying only the GNAS mutation were almost exclusively of the intestinal phenotype (p < 10−4), and none were of the pancreatobiliary phenotype. Mutations within other members of the EGFR pathway were very rare (NRAS [n = 1] 0.9%; BRAF [n = 1] 0.9%), and mutually exclusive with KRAS mutations. Interestingly, the distribution of dysplasia grade and invasive IPMNs were different according to the GNAS mutational status (p = 0.004) [Table 2]. GNAS WT IPMNs were either of low grade (n = 43; 52%) and mostly of the gastric phenotype, or invasive (n = 25; 30%), while GNAS-mutated IPMNs were rarely invasive (n = 6; 12%) and almost exclusively of the colloid type in these cases, a rare occurrence in GNAS WT invasive IPMNs (n = 2; 8%) [p = 0.001]. In contrast, RAS mutations were not associated with any clinical or pathological variable (Table 2). There was no association between GNAS and KRAS status.
Long-Term Outcome of Intraductal Papillary Mucinous Neoplasms
Four patients deceased during the 90-day postoperative period were excluded from the survival analysis. In the remaining population, after a median follow-up of 104 months (77–123), 16 patients died from pancreatic cancer; the median cancer-specific survival was not reached. The 1-, 3-, and 5-year cancer-specific survival was 98% (94–99), 91% (85–95), and 90% (83–94), respectively. In the 31 patients with invasive cancers, the median survival was 43 months. The 1-, 3-, and 5-year cancer-specific survival was 89% (69–96), 56% (35–72), and 48% (29–65), respectively.
Univariate and multivariate analyses of prognostic factors are shown in Table 3 and Fig. 2. For all resected lesions, the phenotype in univariate analysis correlated with prognosis (Fig. 2a, c, e). In multivariate analyses, only branched-duct lesions and GNAS mutations were significantly associated with improved cancer-specific survival.
Patients’ overall survival according to IPMN characteristics: a grade of dysplasia, b phenotype, c carcinoma phenotype in invasive IPMNs, dGNAS mutation status in all IPMNs, eGNAS mutation status in invasive IPMNs, and eRAS mutation status in all IPMNs. IPMNs intraductal papillary mucinous neoplasms, WT wild-type
For invasive lesions in multivariate analysis, only node negativity was associated with improved cancer-specific survival. In univariate analysis, GNAS mutations were associated with prolonged survival (Fig. 2b), while RAS mutational status did not impact survival. IPMN phenotype was not associated with prognosis, but the colloid cancer phenotype was associated with a non-significant trend of improved cancer-specific survival (Fig. 2f).
Discussion
The present study involves 149 consecutive unselected patients who underwent a pancreatic resection for IPMN, with long-term follow-up (median follow-up of 104 months) and complete pathological analysis, in addition to GNAS and RAS pathway sequencing. First, we confirmed previous pathological observations, i.e. the clinical impact of the IPMN phenotype. Pancreatobiliary IPMNs have an aggressive behavior and are frequently invasive. On the contrary, gastric and intestinal lesions have a more indolent behavior and are less frequently invasive, with colloid-type carcinoma only associated with intestinal-type IPMNs. More interestingly, it is likely that part of these differences are driven by GNAS mutations, present overall in approximately 40% of lesions, and strongly associated with the IPMN intestinal phenotype. In the meantime, RAS pathway mutations, mainly represented by KRAS mutations, were not associated with any significant clinical consequences. Mutations of other members of the EGFR pathway (NRAS, n = 1; BRAF, n = 1) were very rare.
From a pathological point of view, gastric and intestinal IPMNs represent more than 90% of resected IPMNs in the present cohort, and were associated with a significantly better cancer-specific survival than pancreatobiliary IPMNs. These latter are mainly located in the main pancreatic duct, are more frequently invasive, and, when invasive, their long-term survival is comparable with that of pancreatic ductal adenocarcinoma patients because of their ductal differentiation, as previously reported.1,22 Distler et al.1 showed that most cancer recurrences are observed in the pancreatobiliary subtype, i.e. in cancer with a tubular differentiation. Interestingly, at cancer recurrence, patients with an intestinal subtype cancer (i.e. with colloid differentiation) had a significantly better prognosis when compared with the pancreatobiliary subtype. This advocated for a specific biological behavior of pancreatic adenocarcinoma with colloid differentiation,23 either more indolent or more chemosensitive.
The results of our genetic analysis underlined the clinical consequences of GNAS mutations in IPMNs. As previously reported,24,25,–26 and in view of our results, it seems likely that GNAS and KRAS-only mutations define separate progression pathways in IPMN-associated carcinomas. In the present studies, GNAS was strongly associated with intestinal-type IPMNs, and, when invasive, with a colloid carcinoma phenotype. These observations are consistent with recent reports showing the specificity of GNAS-driven IPMN tumorigenesis,26,27,–28 which alters various gene expression, including expression of mucin genes, that may determine the IPMN phenotype.29 In the present series, GNAS mutational status, but not tumor phenotype, lesion size, and IPMN location (branch vs. main/mixed type duct), are independent prognostic factors in resected patients. This might be explained by the fact that GNAS mutational status might represent a good surrogate marker for tumor phenotype, especially intestinal-type IPMNs, and was associated with a favorable long-term evolution. In addition, it was reported that approximately 20% of carcinomas co-arising with IPMNs are not genetically related and are independent events.30 This was not the case with colloid carcinomas in which concordant mutational profiles were observed between the IPMNs and the carcinomas in almost all cases. This suggests that in colloid carcinomas arising in intestinal IPMNs, GNAS mutations are more frequently conserved through tumor progression, and probably correspond to the sequential model described by Omori et al.31 This also suggests that while KRAS mutations may be seen in colloid carcinomas, although at a low frequency compared with ductal adenocarcinoma, the intestinal differentiation program and the carcinogenesis are mainly KRAS-independent. On the other hand, it may be hypothesized that KRAS mutations ‘override’ the GNAS-associated intestinal differentiation program in a subset of IPMN-associated carcinomas. Accordingly, GNAS-only mutations were almost exclusively seen in intestinal IPMNs (35% vs. 6 and 0% in gastric and pancreatobiliary IPMNs, respectively) [Fig. 3]. Finally, as proposed by Omori et al., most carcinomas, whether they are truly independent of the adjacent IPMN (de novo subtype) or have an early clonal relationship (branch-off subtype), have lost the GNAS-bearing clone through expansion of aggressive KRAS-driven clones.
Proposed carcinogenesis pathways in IPMNs. aGNAS mutation, especially when alone, favors an cAMP-driven program toward intestinal differentiation. Progression toward the mucinous type of carcinoma may be KRAS-independent and may rely on other carcinogenesis pathways. bGNAS and KRAS mutations may be heterogeneous in IPMNs, leading to the selection of clones with only KRAS mutation that may possess a growth advantage, explaining the drop in the rate of GNAS mutations between IPMN and IPMN-associated ductal carcinomas. Alternatively, some IPMN-associated carcinomas are in fact not genetically related to the IPMN and are arising de novo. cKRAS-only mutation favors progression toward gastric and pancreaticobiliary-type IPMNs. KRAS mutation together with other (epi)genetic events when associated with GNAS mutation may ‘override’ the later, explaining the GNAS-mutated gastric IPMN. IPMNs intraductal papillary mucinous neoplasms, WT wild-type, mut mutation, PB pancreatobiliary, cAMP cyclic adenosine monophosphate
Cystic pancreatic lesions, especially IPMNs, are diagnosed with an increasing incidence,32 but routine resection of all lesions is no longer advocated. Surgical indications are based on symptoms and risk factors of malignant transformation,3,4 but they remain insufficient, as illustrated by the numerous published and sometimes contradictory guidelines.33 More accurate risk factors than clinical symptoms and radiologic features are urgently needed to best select patients for surgery, ideally before invasive carcinoma appears. Preoperative assessment of the IPMN phenotype or mutational status appears a promising area of research. What the true diagnostic or prognostic added value of histological subtypes and GNAS/KRAS mutations is remains to be more clearly determined. From a clinical point of view, it would be relevant to challenge the predictive value of clinical and radiological factors such as mural nodules, which are now considered reliable predictors of invasive cancer and high-grade dysplasia in IPMNs, as proposed by the 2016 IAP guidelines,34 with the genetic factors assessed in the present work. Unfortunately, our database has not been built to predict IPMN invasiveness, and ‘presence of absence of mural nodules’ has not been captured in our database. Additionally, it seems that a combination of molecular markers, including GNAS and clinical features, improves the classification of pancreatic cysts.35GNAS-only, present in IPMNs,7,10 is a highly specific diagnostic tool, even if its sensibility remains low at approximately 40–60%, and, in view of the present results and others,24 it could also represent an interesting prognostic tool. If preoperative determination of GNAS mutational status by FNA is problematic because of the potential morbidity36 of the procedure, GNAS mutations can also be detected in duodenal collections of secretin-stimulated pancreatic juice,9,13,37 or, even easier, in circulating cell-free DNA isolated from blood samples.38 The present study is the first to investigate, in such a large number of resected IPMNs, the mutational status of the extended RAS family. Unlike in other carcinomas, we found a very low rate of NRAS and HRAS mutations, suggesting that they will have little impact on tumorigenesis, and almost no utility in pancreas-targeted diagnostic panel tools.
We are aware of some limitations of the present study. In the present surgical series, as in all other series, patients have been selected according to clinical and morphological criteria, and our study population does not represent all diagnosed IPMNs, with most of them being indolent.39 Consequently, if GNAS status is a good diagnostic tool, it cannot be used at this moment to tailor management of patients without obvious criteria for surgery.
Conclusions
In patients selected for surgery, GNAS mutation analysis and tumor phenotype help to better predict patient prognosis. GNAS mutation status, tumor size, and IPMN location (branch vs. main/mixed type duct) are independent prognostic factors in resected patients. In the near future, with the diffusion of circulating cell-free DNA isolated from blood samples, a more precise mutational analysis of IPMNs might help to better tailor their management.
References
Distler M, Kersting S, Niedergethmann M, et al. Pathohistological subtype predicts survival in patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg. 2013; 258(2):324–30.
Furukawa T, Hatori T, Fujita I, et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011; 60(4):509–16.
European Study Group on Cystic Tumours of the P. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018; 67(5):789–804.
Tanaka M, Fernandez-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017; 17(5):738–753.
Marchegiani G, Mino-Kenudson M, Ferrone CR, et al. Patterns of recurrence after resection of IPMN: who, when, and how? Ann Surg. 2015; 262(6):1108–14.
Lawrence SA, Attiyeh MA, Seier K, et al. Should patients with cystic lesions of the pancreas undergo long-term radiographic surveillance?: Results of 3024 patients evaluated at a single institution. Ann Surg. 2017; 266(3):536–544.
Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011; 3(92):92ra66.
Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011; 108(52):21188–93.
Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2012; 62:1024–33.
Furukawa T, Kuboki Y, Tanji E, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011; 1:161.
Weinstein LS, Shenker A, Gejman PV, et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991; 325(24):1688–95.
Dal Molin M, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol. 2013; 20:3802–8.
Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2013; 62(7):1024–33.
Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2017; 67:2131–41.
Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006; 6(1–2):17–32.
Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012; 12(3):183–97.
Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004; 28(8):977–87.
Basturk O, Hong SM, Wood LD, et al. A revised classification system and recommendations from the baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015; 39(12):1730–41.
Nara S, Shimada K, Kosuge T, et al. Minimally invasive intraductal papillary-mucinous carcinoma of the pancreas: clinicopathologic study of 104 intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2008; 32(2):243–55.
Parvanescu A, Cros J, Ronot M, et al. Lessons from McCune-Albright syndrome-associated intraductal papillary mucinous neoplasms:: GNAS-activating mutations in pancreatic carcinogenesis. JAMA Surg. 2014; 149(8):858–62.
Cacheux W, Rouleau E, Briaux A, et al. Mutational analysis of anal cancers demonstrates frequent PIK3CA mutations associated with poor outcome after salvage abdominoperineal resection. Br J Cancer. 2016; 114(12):1387–94.
Mino-Kenudson M, Fernandez-del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011; 60(12):1712–20.
Nakata K, Ohuchida K, Aishima S, et al. Invasive carcinoma derived from intestinal-type intraductal papillary mucinous neoplasm is associated with minimal invasion, colloid carcinoma, and less invasive behavior, leading to a better prognosis. Pancreas. 2011; 40(4):581–7.
Tan MC, Basturk O, Brannon AR, et al. GNAS and KRAS mutations define separate progression pathways in intraductal papillary mucinous neoplasm-associated carcinoma. J Am Coll Surg. 2015; 220(5):845–54 e1.
Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004; 28(7):839–48.
Kuboki Y, Shimizu K, Hatori T, et al. Molecular biomarkers for progression of intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2015; 44(2):227–35.
Innamorati G, Valenti MT, Giacomello L, et al. GNAS mutations: drivers or co-pilots? Yet, promising diagnostic biomarkers. Trends Cancer. 2016; 2(6):282–5.
Taki K, Ohmuraya M, Tanji E, et al. GNAS(R201H) and Kras(G12D) cooperate to promote murine pancreatic tumorigenesis recapitulating human intraductal papillary mucinous neoplasm. Oncogene. 2016; 35(18):2407–12.
Komatsu H, Tanji E, Sakata N, et al. A GNAS mutation found in pancreatic intraductal papillary mucinous neoplasms induces drastic alterations of gene expression profiles with upregulation of mucin genes. PLoS ONE. 2014; 9(2):e87875.
Felsenstein M, Noe M, Masica DL, et al. IPMNs with co-occurring invasive cancers: neighbours but not always relatives. Gut. 2018; 67:1652–62.
Omori Y, Ono Y, Tanino M, et al. Pathways of progression from intraductal papillary mucinous neoplasm to pancreatic ductal adenocarcinoma based on molecular features. Gastroenterology. 2019; 156(3):647–61 e2.
Chang YR, Park JK, Jang JY, et al. Incidental pancreatic cystic neoplasms in an asymptomatic healthy population of 21,745 individuals: large-scale, single-center cohort study. Medicine (Baltimore). 2016; 95(51):e5535.
Falconi M, Crippa S, Chari S, et al. Quality assessment of the guidelines on cystic neoplasms of the pancreas. Pancreatology. 2015; 15(5):463–9.
Marchegiani G, Andrianello S, Borin A, et al. Systematic review, meta-analysis, and a high-volume center experience supporting the new role of mural nodules proposed by the updated 2017 international guidelines on IPMN of the pancreas. Surgery. 2018; 163(6):1272–9.
Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015; 149(6):1501–10.
Yoon WJ, Brugge WR. The safety of endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions. Endosc Ultrasound. 2015; 4(4):289–92.
Mori Y, Ohtsuka T, Kono H, et al. A minimally invasive and simple screening test for detection of pancreatic ductal adenocarcinoma using biomarkers in duodenal juice. Pancreas. 2013; 42(2):187–92.
Berger AW, Schwerdel D, Costa IG, et al. Detection of hot-spot mutations in circulating cell-free DNA from patients with intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2016; 151(2):267–70.
Crippa S, Bassi C, Salvia R, et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut. 2017; 66(3):495–506.
Acknowledgment
The authors would like to thank Walid Chemlali for technical assistance, and all the Beaujon and Cochin clinical and technical staff for their helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Sébastien Gaujoux, Alina Parvanescu, Manuella Cesaretti, Caroline Silve, Ivan Bieche, Vinciane Rebours, Philippe Lévy, Alain Sauvanet, and Jérôme Cros have no financial or personal conflicts of interest in relation to this study.
Funding
This work was supported by Fonds d’Aide à la Recherche et à l’Evaluation (FARE) from the Société Nationale Française de Gastro-entérologie (SNFGE).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gaujoux, S., Parvanescu, A., Cesaretti, M. et al. GNAS but Not Extended RAS Mutations Spectrum are Associated with a Better Prognosis in Intraductal Pancreatic Mucinous Neoplasms. Ann Surg Oncol 26, 2640–2650 (2019). https://doi.org/10.1245/s10434-019-07389-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07389-6