Abstract
The role of the microenvironment in high-grade lymphoma is not well defined. In this report, we employ immunohistochemistry to characterise programmed death-1 (PD-1/CD279) and FoxP3 expression in 70 cases of diffuse large B-cell lymphoma (DLBCL). PD-1 is a surface marker characteristic of follicular helper T-cells whilst FoxP3 is characteristic of Tregs. We demonstrate variable infiltration with CD4+ T-cells (<10 to >50 % of all lymph node cells) and PD-1hi cells (0.1 to 1.5 % of all cells). CD4+ T-cells can be distributed in clusters or more diffusely and PD-1hi cells, but not FoxP3+ cells, are found in rosettes around lymphoma cells. Cases with high CD4+ T-cell numbers tended to have higher numbers of both PD-1hi and FoxP3+ cells. Cases with total CD4+ T-cell, PD-1hi and FoxP3+ numbers above the median associate with better clinical outcome. Overall, we demonstrate that infiltration by CD4+ T-cells, including both FoxP3+ and PD-1hi subsets, correlates with prognosis in DLBCL. In distinction to previous reported series, patients (91 %) were treated with rituximab-containing regimens, suggesting that the effects of CD4+ T-cell infiltration are maintained in the rituximab era. This work suggests that determinants of total CD4+ T-cell infiltration, either molecular characteristics of the lymphoma or the patients’ immune system, and not individual T-cell subsets, correlate with clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The high-grade B-cell non-Hodgkin’s lymphoma, diffuse large B-cell lymphoma, DLBCL, is clinically heterogeneous [1–3]. The lymph node microenvironment, which contains T-cells, dendritic cells, stromal cells, basement membrane components, growth factors and chemokines, influences the growth characteristics of lymphoma, and evidence supporting a role for the microenvironment in DLBCL has been obtained from gene expression profiling, which demonstrated that expression of genes representative of the microenvironment determined clinical outcome [4–6]. More recently, expression of only two genes, one of which was stromal cell derived, was found to predict survival [7].
CD4+ T-cells comprise several subsets: TH1, TH2, TH17 and follicular T-cells. Follicular T-cells are, in part, defined by the high expression of PD-1 and comprise both follicular helper (Tfh) T-cells and repressive (Tfr) T-cells [8]. In normal immunity, CD4+ T-cells deliver signals to B-cells through cognate interactions and cytokine production. Indeed production of specific cytokines is one of the defining characteristics of the various T-cell subsets; TH1 cells produce IL-2 and IFNγ, TH2 cells produce IL-4 and IL5 [9,10], TH17 cells produce IL-17 and Tfh cells produce IL-4 and IL-21.
CD4+ T-cell subsets can also be defined by the cytokines required for their differentiation. Differentiation in the presence of IL-12 induces the transcription factor T-bet and leads to TH1 cell production, whereas IL-4 induces GATA-3 in differentiating cells and leads to the production of TH2 cells, and IL-6 and TGFβ induce ROR-γt and TH17 cells. IL-12 produced by dendritic cells [11] together with IL-21 and IL-6 [12], signalling from inducible T-cell co-stimulator (ICOS) and interaction with B-cells, induces BCL6, in primed T-cells to generate Tfh cells [13–15]. A subset of suppressive CD4+ T-cells, Tregs, which are characterised by expression of FoxP3, suppresses autoimmunity and may also reduce the immune response to lymphoma [16].
Tfh cells are required for normal germinal centre development and production of high-affinity antibodies. They are characterised by high expression of PD-1, CXCR5 and ICOS. Tfh cells may be important producers of IL-4 in follicular lymphoma [17], but their role in other lymphomas including DLBCL has been little explored. Treg numbers are elevated in the peripheral blood of patients with a variety of different lymphomas [16], and the number and architecture of FoxP3-expressing cells correlate with clinical outcome in follicular lymphoma [18]. However, in DLBCL, the effects of FoxP3+ cells on survival are controversial. One group suggests that the numbers of FoxP3-expressing cells do not influence clinical outcome [19], whereas others report that increased numbers of FoxP3+ associate with improved survival either regardless of subgroup [20] or in germinal centre B-cell DLBCL only [21].
CD4+ T-cell infiltration correlates with clinical outcome in DLBCL [22,23]. CD4+ T-cells are, therefore, important components of the lymphoma microenvironment, but expression of the newer marker PD-1 in combination with FoxP3 has not previously been investigated in diffuse large B-cell lymphoma.
Materials and methods
Patients
All samples were collected under ethical approval obtained from the Local Research Ethics Committee and the Research and Development Office at the University Hospitals of Leicester NHS Trust and Hammersmith Hospital, London. Samples were anonymised and entered either into the Haematological Malignancy Tissue Bank or the Tonsil and Normal Lymphoid Tissue Resource. A total of 70 patients were analysed (Table 1), 41 male and 29 female. Median age was 67 years, with a range of 30 to 88 years.
Immunohistochemistry
Paraffin-embedded tissue sections underwent deparaffinisation and microwave heat antigen retrieval (700 W for 20 min) in pH 9 tris-EDTA buffer before staining with anti-CD4, anti-FOXP3 and anti-PD1 antibodies (Table 2). Primary antibody was incubated with tissue sections for 3 h at room temperature. The primary antibody was visualised using a Novolink polymer detection system kit (Novocastra). Tissue sections were counterstained with haematoxylin, dehydrated and mounted. Slides were viewed using a Zeiss Axioplan 2 microscope and Axiocam and image capture performed manually with AxioVision Imaging System 4.7. Sections were analysed and quantified utilising ImageJ software [24]. PD1 is expressed at low level on effector CD8+ T-cells [25,26] and pre-germinal centre Tfh [27,28] and at higher level on germinal centre Tfh cells [29,30]. We set an intensity threshold for the definition of high PD-1 expression by utilising PD-1 expression within germinal centres, an easily defined anatomical structure containing Tfh cells, within tonsil sections from normal subjects. Stained sections were imaged and deconvoluted using an ImageJ plug-in (http://www.dentistry.bham.ac.uk/landinig/software/cdeconv/cdeconv.html). An intensity threshold that identified germinal centre PD1hi cells was set. A tonsil section was included with each batch of lymphoma sections stained in order to control for variation in staining. This threshold was applied to lymph node sections stained with PD1. There was little variation in FoxP3 and CD4 staining intensity and we, therefore, designate stained cells as FoxP3+ and CD4+ cells, respectively. Cell numbers were quantified across ten adjoining high power fields (×400 magnification level) in order to produce a representative view of T-cell numbers [31].
Definition of Tfh cells by flow cytometry
We determined Tfh and Treg cells in lymph node sections, and in order to facilitate cell counting, we required a single antibody to identify each of these subsets. We employed nuclear FoxP3 expression to identify Tregs. We have previously evaluated flow cytometry protocols to identify Tfh cells [32], which we define as the CD4+ cells within a gate, including cells possessing a mean fluorescence intensity greater than the 70th centile for both CXCR5 and ICOS.
Statistical analysis
Overall survival was calculated from date of diagnosis and survival curves were constructed using the method of Kaplan and Meier, and log-rank test was used to assess any differences between patient groups (Prism v6.0c, GraphPad Software Inc.). Cox proportional hazards were employed to determine important independent prognostic factors for overall survival (XLSTAT v2014.1.08, Addinsoft).
Results
Tfh cells in lymphoma samples
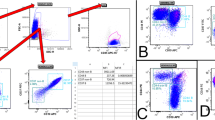
In order to demonstrate Tfh cells in lymphoma lymph nodes, we stained single cell suspensions with anti-CD4, anti-CXCR5 and anti-ICOS and compared to tonsillar cells (Fig. 1). The average number of CD4+CXCR5hiICOShi cells in tonsils (n = 12) was 16.3 %, but in lymphoma lymph nodes the proportion of these cells ranged from 0.7 to 2.8 % (n = 4), comparable to the proportion measured in a reactive lymph node (0.7 %). Combined staining with anti-CXCR5 and anti-ICOS also showed variability in populations other than CXCR5hiICOShi in DLBCL (Fig. 1a). Whilst patients 2 and 3 possessed predominantly CXCR5hiICOSlo cells, the major population of patient 1 was CXCR5loICOSlo. Patient 4 also had a major population of CXCR5loICOSlo cells but, in addition, showed cells with the phenotype CXCR5loICOShi.
CD4+ T-cell and Tfh cell infiltration in lymphoma and non-malignant lymph nodes. a Flow cytometry to demonstrate Tfh cell populations in four samples of DLBCL (identified as patients 1 to 4). b The contour plots show CD4+CXCR5hiICOShi cells from a tonsil sample (left-hand panel) and a reactive lymph node (right-hand panel). In each plot, the Tfh gate is indicated by a box and the number of CXCR5hiICOShi as a proportion of total CD4+ cells (%) is presented. The accompanying pie charts demonstrate the proportion (%) of all tonsil cells that are CD4+ T-cells (black segment) and the proportion of all tonsil cells that are CD4+CXCR5hiICOShi (grey segment)
Therefore, analysis of CXCR5 and ICOS on CD4+ T-cells within DLBCL lymph nodes defined variable sub-populations and a fraction of the CD4+ T-cells demonstrated the immunophenotype of Tfh cells.
PD-1hi cell number and distribution in DLBCL
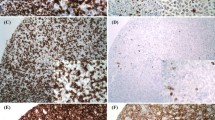
Next we utilised immunohistochemistry to examine the distribution and architecture of CD4+ T-cell infiltration. We employed strong PD1 expression as a marker for Tfh cells and nuclear FoxP3 to identify Tregs (Fig. 2a). Individual cases showed low, intermediate or high infiltration by CD4+ T-cells, which was mirrored by varying levels of FoxP3 and PD1 expression (Fig. 2b). Cases with high CD4+ T-cell infiltration often demonstrated high numbers of both FoxP3+ and PD-1hi cells rather than one sub-type of T-cell predominating, and similarly cases with low CD4+ T-cells showed low numbers of both FoxP3+ and PD-1hi subsets.
Immunohistochemistry for FoxP3 and PD-1 cells. a DLBCL lymph node stained with anti-CD4, anti-FoxP3 and anti-PD1. b Cases of DLBCL, which showed high, intermediate and low infiltration by CD4 T-cells. In each case, the left-hand panel has been stained with anti-FoxP3 and the right-hand panel with anti-PD1
In order to characterise accurately the distribution of T-cells within DLBCL, an entire lymph node section was examined. CD4+ T-cells were found in small clusters (Fig. 3a) or diffusely, but with areas of relatively high and low infiltration (Fig. 3b). CD4+ T-cells were also found “rosetting” lymphoma cells (Fig. 3b, c). Rosettes were only identified in a minority of cases, and there was no clear correlation with clinical characteristics. The cells involved in rosette formation predominantly expressed PD1 (Fig. 3d, e), suggesting that they were Tfh cells. Analysis of sequential sections confirmed that PD1 and not FoxP3-expressing cells formed rosettes around the large lymphoma cells (Fig. 3f). No rosettes of FoxP3-expressing cells were found in any of the cases in this series.
Architecture of PD-1 and FoxP3 infiltration. Low power (original magnification × 25) (LP) and high power (original magnification × 100) (HP) views of DLBCL lymph nodes. a A cluster of CD4+ cells at low power. b Areas of low (to the left of the dashed line) and high infiltration are shown and a rosette of CD4+ cells (dashed circle) surrounding a lymphoma cell. c High power view of the section shown in b to demonstrate the CD4+ cell rosette. d Low power view demonstrating two PD1+ rosettes (dashed circles). e High power view of the same area of the lymph node in d again showing PD1+ rosettes. f Consecutive sections of DLBCL lymph node were stained with anti-FoxP3 and anti-PD1. A large lymphoma cell surrounded by a rosette of PD-1-expressing cells was identified (left-hand panel), and the same large cell was then located in the section stained with anti-FoxP3 (right-hand panel)
FoxP3 and PD1 cell numbers correlate with CD4 T-cell number
In order to understand the role of CD4+ T-cells in DLBCL, we determined CD4+, PD1hi and FoxP3hi cells in 70 cases (see “Materials and Methods”). To avoid problems due to poor representation in one area of the lymph node section, we measured cell numbers in ten separate high power fields. CD4+ T-cell infiltration varied widely (Fig. 4a), and in many cases, the majority of CD4+ T-cells were not accounted for by either FoxP3hi or PD1hi subsets (grey portions of the bars in Fig. 4a). We confirmed the correlation between numbers of FoxP3 and PD1 expressing CD4+ T-cells (Fig. 4b) as well as between total CD4+ T-cells and each of these subsets (Supplemental Fig. 1).
Correlation of total CD4 T-cell numbers with PD-1hi and FoxP3+ cells. a Bar chart to show variation in total CD4+ T-cell infiltration in 70 cases of DLBCL. FoxP3-expressing cells are indicated in white, PD1 cells in black and other CD4+ T-cells in grey. b Correlation between FoxP3 and PD1 expressing cell numbers. The dashed lines indicate the median number of FoxP3 and PD1 cells respectively. c High LDH and advanced clinical stage predict poor clinical outcome in our cases. Patients were separated into groups based on median expression of CD4, FoxP3 or PD1. High FoxP3 (P = 0.0069, logrank test), high PD1 and high total CD4 were associated with improved clinical outcome
To further investigate the clinical usefulness of FoxP3 and PD1 expression in DLBCL, we analysed the effects of these markers on overall survival. High LDH (P = 0.02, log-rank test) and advanced clinical stage were associated with poor outcome, demonstrating that conventional indicators of prognosis associated with the expected effects on survival in our group of patients (Fig. 4b). Next we determined that cases with total CD4+ T-cell number below the median showed significantly worse overall survival than those cases with higher numbers of CD4+ T-cells (P = 0.04, log-rank test). The data are self-consistent; LDH levels were significantly higher (median 610 vs. 239 IU/l; upper limit of normal range 255 IU/l) (P = 0.0014, Mann–Whitney U-test) for those cases with total CD4+ T-cells below the median. Both FoxP3 (P = 0.0001, log-rank test) and PD1 (P = 0.0007, log-rank test) expression levels above the median were associated with better overall survival than those cases with lower expression. Cases with either FoxP3 or PD-1 expression below the median demonstrated LDH levels significantly higher than those cases with higher expression. For FoxP3 cases with lower expression, the median level of LDH was 383 IU/l, whereas for cases with higher expression it was 224 IU/l (P = 0.03, Mann–Whitney U-test); similarly, cases with low PD-1 expression had median LDH level of 369 IU/l versus 226 IU/l for higher expressing cases (P = 0.03, Mann–Whitney U-test).
In univariate analysis, overall survival correlated with PD-1+ (P = 0.0001) and FoxP3+ (P = 0.005) numbers and with total CD4+ (P = 0.007) and LDH (P = 0.001) (Spearman rank correlation). We, therefore, carried out a multivariate analysis (Cox proportional hazards) for overall survival and found that PD-1+ (hazard ratio = 0.469, 95 % confidence interval = 0.016–1.386, P < 0.05), FoxP3+ (HR = 0.735, 95 % CI 0.087–1.619, P < 0.05) and CD4+ (HR = 0.872, 95 % CI 0.619–1.389, P < 0.05) retained independent prognostic significance.
Discussion
T-cells are a component of the lymphoma microenvironment, and CD4+ T-cell infiltration has previously been shown to associate with prognosis in DLBCL [22, 23]. However, since these studies, novel CD4+ expressing T-cell subsets, defined by cytokine, chemokine and transcription factor expression, have been characterised.
Tissue microarrays have been used extensively to analyse lymphomas, and although there are many technical difficulties [33], they have the advantage in that material from a large numbers of cases can be processed in a uniform manner. In order to accomplish this, however, tissue microarrays will utilise a small portion of a lymphoma lymph node, which may not be representative of the whole specimen. This problem may be most significant when analysing infiltrating non-lymphoma cells rather than lymphoma B-cells. Therefore, although we studied a relatively small number of cases, the methodology employed allowed an accurate analysis of infiltrating T-cells and is similar to the methodology used by others to analyse the T-cell compartment in follicular lymphoma [34, 35].
Our data suggest that DLBCL have infiltrating PD-1hi and FoxP3+ cell populations. Although they only form a small proportion of the total CD4+ T-cell population, PD-1hi cells are occasionally found in rosettes surrounding lymphoma cells, but low numbers prevented us from determining associations with clinicopathologic characteristics of DLBCL. Tfh cell rosettes have previously been described in nodular lymphocyte predominant Hodgkin’s lymphoma [36], but not in DLBCL. FoxP3+ cells did not show rosette formation and we, therefore, suggest that this is a feature specific to the PD-1hi subset. PD-1 expressing follicular regulatory T-cells have been described [37], and it is likely that the population that we detect contains both types of cell.
Tregs infiltrate cancers, and mouse models show that they suppress anti-tumour immunity, either through an effect on CD8+ T-cells [38] or on other CD4+ T-cell subsets [39]. Higher Treg infiltration also correlates with improved clinical outcome in follicular lymphoma [18]. There has been less functional work on T-regs in high-grade lymphoma but purified DLBCL cells induced Tregs whilst these T-cells repressed the proliferation of the same lymphoma cells [16], suggesting a possible mechanism underlying their association with higher overall survival. Although work by others raises the question on whether there is a specific function for Tregs in DLBCL, our data (Fig. 4a) suggest that this sub-population of CD4+ T-cells is not specifically increased but that higher numbers of CD4+ T-cells are associated with increased numbers of more than one subset. Interestingly, our work appears to mirror similar studies in follicular lymphoma, which have demonstrated infiltration by both PD-1-expressing cells and FoxP3-expressing cells and that higher numbers of each type of CD4+ T-cell was associated with good clinical outcome [34, 35]. In some of our cases, the majority of CD4+ T-cells did not belong to either Treg or Tfh subsets and may represent CXCR5−CCR7− effector memory T-cells (Tem). Utilising paraffin sections, it is not possible to be more definitive about the nature of the CD4+ T-cells that were neither PD-1 nor FoxP3 expressing because their characterisation requires analysis of cytokine production [10].
There has been little previous work on CD4+ T-cell subsets in DLBCL. Our report is the first to characterise PD-1hi populations in DLBCL, and although FoxP3+ cells have previously been studied, their effects on survival are controversial [19–21]. Such reported variation may reflect differences in anti-FoxP3 antibody specificities, determination of cutoff levels and selection of tumour area for analysis. In addition, two studies [19, 21] did not include any cases treated with rituximab, and therefore our results cannot be directly compared. Only one study included patients treated with RCHOP immunochemotherapy, the current standard of care, but these only comprised 28 % of cases [20]. Our cohort included 91 % of patients who were treated with RCHOP or RPMitCEBO, suggesting that the importance of infiltrating CD4-T-cells to the prediction of survival is maintained in the rituximab era.
Gene expression profiling enables the grouping of DLBCL into prognostically significant categories. Initially, two groups (GC and ABC [2]) have been expanded to include other groups, which may bear the signature of microenvironment genes [5, 40]. Despite initial optimism [41], it appears that immunohistochemistry is not able to reliably predict the subtype of DLBCL [42]. The relationship between T-cell infiltration and prognostic groups defined on the basis of gene expression, therefore, remains an open question. The heterogeneity of DLBCL and the relatively low number of cases reported here warrants independent validation in a larger prospective cohort.
Our observation that total CD4+ T-cell numbers correlate with PD-1hi numbers and that numbers of PD-1hi and FoxP3+ cells, as well as total CD4+ T-cells, associate with improved clinical outcome suggests that the effects of a single CD4+ T-cell subset cannot be taken in isolation. The factors that lead to high CD4+ T-cell infiltration in some cases of DLBCL have not been defined but may represent novel targets for therapy.
References
Shinoda K, Tokoyoda K, Hanazawa A et al (2012) Type II membrane protein CD69 regulates the formation of resting T-helper memory. Proc Natl Acad Sci U S A 109:7409–7414. doi:10.1073/pnas.1118539109
Alizadeh AA, Eisen MB et al (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511. doi:10.1038/35000501
Shipp MA, Ross KN, Tamayo P et al (2002) Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med 8:68–74. doi:10.1038/nm0102-68
Martín P, Gómez M, Lamana A et al (2010) CD69 association with Jak3/Stat5 proteins regulates Th17 cell differentiation. Mol Cell Biol 30:4877–4889. doi:10.1128/MCB.00456-10
Rosenwald A, Wright G, Chan WC et al (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large B-cell lymphoma. N Engl J Med 346:1937–1947. doi:10.1056/NEJMoa032520
Lenz G, Wright GW, Emre NCT et al (2008) Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A 105:13520–13525. doi:10.1073/pnas.0804295105
Alizadeh AA, Gentles AJ, Alencar AJ et al (2011) Prediction of survival in diffuse large B-cell lymphoma based on the expression of 2 genes reflecting tumor and microenvironment. Blood 118:1350–1358. doi:10.1182/blood-2011-03-345272
Ramiscal RR, Vinuesa CG (2013) T-cell subsets in the germinal center. Immunol Rev 252:146–155. doi:10.1111/imr.12031
Shao H, Chung J, Balaj L et al (2012) Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med 18:1835–1840. doi:10.1038/nm.2994
Mosmann TR, Coffman RL (1989) TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7:145–173. doi:10.1146/annurev.iy.07.040189.001045
Schmitt N, Morita R, Bourdery L et al (2010) Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 31:158–169. doi:10.1016/j.immuni.2009.04.016
Nurieva RI, Chung Y, Hwang D et al (2008) Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29:138–149. doi:10.1016/j.immuni.2008.05.009
Johnston RJ, Poholek AC, Ditoro D et al (2009) Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325:1006–1010. doi:10.1126/science.1175870
Nurieva RI, Chung Y, Martinez GJ et al (2009) Bcl6 mediates the development of T follicular helper cells. Science 325:1001–1005. doi:10.1126/science.1176676
Yu D, Rao S, Tsai LM et al (2009) The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31:457–468. doi:10.1016/j.immuni.2009.07.002
Mittal S, Marshall NA, Duncan L et al (2008) Local and systemic induction of CD4 + CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood 111:5359–5370. doi:10.1182/blood-2007-08-105395
Pangault C, Amé-Thomas P, Ruminy P et al (2010) Follicular lymphoma cell niche: identification of a preeminent IL-4-dependent TFH–B cell axis. Leukemia 1–10. doi: 10.1038/leu.2010.223
Lee AM, Clear AJ, Calaminici M et al (2006) Number of CD4+ cells and location of forkhead box protein P3-positive cells in diagnostic follicular lymphoma tissue microarrays correlates with outcome. J Clin Oncol 24:5052–5059. doi:10.1200/JCO.2006.06.4642
Hasselblom S, Sigurdadottir M, Hansson U et al (2007) The number of tumour-infiltrating TIA-1+ cytotoxic T cells but not FOXP3+ regulatory T cells predicts outcome in diffuse large B-cell lymphoma. Br J Haematol 137:364–373. doi:10.1111/j.1365-2141.2007.06593.x
Lee NR, Song EK, Jang KY et al (2008) Prognostic impact of tumor infiltrating FoxP3 positive regulatory T cells in diffuse large B-cell lymphoma at diagnosis. Leuk Lymphoma 49:247–256
Tzankov A, Meier C, Hirschmann P et al (2008) Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica 93:193–200. doi:10.3324/haematol.11702
Ansell SM, Stenson M, Habermann TM et al (2001) CD4+ T-cell immune response to large B-cell non-Hodgkin's lymphoma predicts patient outcome. J Clin Oncol 19:720–726
Lippman SM, Spier CM, Miller TP et al (1990) Tumor-infiltrating T-lymphocytes in B-cell diffuse large cell lymphoma related to disease course. Mod Pathol 3:361–367
Abramoff M, Magalhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 1–7
Kao C, Oestreich KJ, Paley MA et al (2011) Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol 12:663–671. doi:10.1038/ni.2046
Youngblood B, Oestreich KJ, Ha S-J et al (2011) Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity 35:400–412. doi:10.1016/j.immuni.2011.06.015
Lee SK, Rigby RJ, Zotos D et al (2011) B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med 208:1377–1388
McHeyzer-Williams LJ, Pelletier N, Mark L et al (2009) Follicular helper T cells as cognate regulators of B cell immunity. Curr Opin Immunol 21:266–273
Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ (2006) Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol 30:802–810. doi:10.1097/01.pas.0000209855.28282.ce
Wang C, Hillsamer P, Kim CH (2011) Phenotype, effector function, and tissue localization of PD-1-expressing human follicular helper T cell subsets. BMC Immunol 12:53. doi:10.1186/1471-2172-12-53
Rizzardi AE, Johnson AT, Vogel RI et al (2012) Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn Pathol 7:42. doi:10.1186/1746-1596-7-42
Ahearne MJ, Willimott S, Piñon L et al (2013) Enhancement of CD154/IL4 proliferation by the T follicular helper (Tfh) cytokine, IL21 and increased numbers of circulating cells resembling Tfh cells in chronic lymphocytic leukaemia. Br J Haematol 162:360–370. doi:10.1111/bjh.12401
Rimm DL, Nielsen TO, Jewell SD et al (2011) Cancer and Leukemia Group B Pathology Committee guidelines for tissue microarray construction representing multicenter prospective clinical trial tissues. J Clin Oncol 29:2282–2290. doi:10.1200/JCO.2010.33.2023
Carreras J, Lopez-Guillermo A, Fox BC et al (2006) High numbers of tumor-infiltrating FoxP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood 108:2957–2964
Carreras J, Lopez-Guillermo A, Roncador G et al (2009) High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytesare associated with improved overall survival in follicular lymphoma. J Clin Oncol 27:1470–1476
Nam-Cha SH, Roncador G, Sanchez-Verde L et al (2008) PD-1, a follicular T-cell marker useful for recognizing nodular lymphocyte-predominant Hodgkin lymphoma. Am J Surg Pathol 32:1252–1257. doi:10.1097/PAS.0b013e318165b0d6
Chung Y, Tanaka S, Chu F et al (2011) Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med 17:983–988. doi:10.1038/nm.2426
Turk MJ, Guevara-Patiño JA, Rizzuto GA et al (2004) Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med 200:771–782. doi:10.1084/jem.20041130
Sakaguchi S (2004) Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 22:531–562. doi:10.1146/annurev.immunol.21.120601.141122
Lenz G, Wright G, Dave SS et al (2008) Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 359:2313–2323. doi:10.1056/NEJMoa0802885
Hans CP, Weisenburger DD, Greiner TC et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282. doi:10.1182/blood-2003-05-1545
Ott G, Ziepert M, Klapper W et al (2010) Immunoblastic morphology but not the immunohistochemical GCB/nonGCB classifier predicts outcome in diffuse large B-cell lymphoma in the RICOVER-60 trial of the DSHNHL. Blood 116:4916–4925. doi:10.1182/blood-2010-03-276766
Acknowledgments
Work in SDW’s laboratory is supported by the Kay Kendall Leukaemia Fund and the Leicester Haematology Research Fund. MJA was supported by an unrestricted grant from Roche. We also wish to thank the Database Manager, Judy Maynard-Mills, for her assistance with this project. Part of this work was presented as a poster at the American Society of Hematology, San Diego, CA, USA, 2011.
Conflict of interest
We declare that the authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(JPG 166 kb)
Rights and permissions
About this article
Cite this article
Ahearne, M.J., Bhuller, K., Hew, R. et al. Expression of PD-1 (CD279) and FoxP3 in diffuse large B-cell lymphoma. Virchows Arch 465, 351–358 (2014). https://doi.org/10.1007/s00428-014-1615-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1615-5