Abstract
The development of laryngeal squamous cell carcinomas (LSCC) is strongly influenced by the host immune system. Indoleamine 2,3-dioxygenase (IDO) can promote and maintain an immunosuppressive microenvironment which can impede the efficacy of anticancer responses. The purpose of the current study is to investigate the prognostic value of intratumoral IDO expression in LSCC. The expression of IDO protein was retrospectively assessed by immunohistochemistry in 187 LSCC patients. The potential association of tumor IDO expression with clinical parameters and tumor-infiltrating lymphocytes (TILs) was analyzed separately. Survival curves were estimated by the Kaplan–Meier method, and differences between groups were determined by log-rank test. Multivariate logistic regression analysis was performed to determine the independent factors associated with survival. Based on the evaluation score, 90 carcinomas (48.1 %) were identified with high IDO expression and 97 carcinomas (51.9 %) showed low expression. Tumor IDO expression was not associated with clinical stage, presence of metastases, and other clinicopathological parameters. Also, high IDO expression was not correlated with tumor-infiltrating CD3+ and CD8+ TILs. Instead it was positively related with the density of FOXP3+ Tregs. Furthermore, multivariate analysis identified a significant association of overall survival and disease-free survival with tumor IDO status. IDO high expression represents a significant negative prognostic factor in patients with LSCC. Current results provide further support for using IDO as an immunotherapeutic target in LSCC. The precise role of tumoral IDO in human LSCC remains to be elucidated in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immunosuppressive protein indoleamine 2,3-dioxygenase (IDO) is a rate-limiting enzyme in the l-tryptophan-kynurenine metabolic pathway. IDO is widely distributed in mammals and is inducible preferentially by IFN-γ. IDO has also been demonstrated in a wide spectrum of human cancers. Endogenous IDO contributes to maternal tolerance towards the allogeneic fetus and has been implicated as a possible mechanism by which tumors evade immune response [1]. The interaction of tumor cells and immunocompetent cells in the tumor microenvironment exerts an important influence on cancer progression and therapy response. Malignant cells can deploy many different strategies to evade or reduce an immune response. Several intrinsic tumor factors have been linked to these processes, and IDO, one of these factors, has attracted high attention in tumor immunology [2–4].
The role of IDO in tumor-induced tolerance was first described by Uyttenhove et al. in a murine model, in which they showed that IDO expression in cancer cells protects tumors from attack by tumor-associated antigen-specific host cytotoxic T cells [5]. Tryptophan depletion and accumulation of immunomodulatory tryptophan metabolites will specifically affect T cell proliferation, metabolism, and function, which contribute to the immunosuppressive capacities of IDO [6–8]. This suggests that IDO within the tumor microenvironment may modulate the density and/or function of tumor-infiltrating lymphocytes (TILs). These factors can impede the efficacy of anticancer responses and facilitate tumor progression [9].
Laryngeal squamous cell carcinoma (LSCC) constitutes the second most common malignant neoplasm of head and neck tumors. The development of LSCCs is strongly influenced by the host immune system [10]. Accumulating evidence shows a correlation between the expression of IDO and patient outcome in several types of tumor [11–14]. However, publications address the prognostic implication of IDO expression in LSCC. Thus, we investigated the prevalence and localization of tumor IDO expression in 187 LSCC patients, with an emphasis on its prognostic value and clinicopathological relevance. Another purpose was to evaluate the correlation between IDO expression and TIL distribution in the tumor microenvironment.
Materials and methods
Study protocol
This study was a retrospective analysis of patients consecutively collected at Anhui Provincial Hospital and the 3rd Affiliated Hospital of Sun Yat-Sen University, between 1 Jan 2000 and 31 Dec 2005. All patients underwent diagnostic microlaryngoscopy with laryngeal biopsies, upper aerodigestive tract endoscopy, esophagoscopy, neck ultrasonography, contrast-enhanced computerized tomography, and/or magnetic resonance imaging of the head and neck. The inclusion criteria were no history of previous malignancies, primary squamous cell carcinoma of the larynx only, and no previous radio- or chemotherapy. The records of enrolled patients were reviewed and, corresponding paraffin-embedded tissue specimens were retrospectively analyzed. The study protocol was reviewed and approved by the institutional review board of Sun Yat-Sen University.
Data collection
Treatment decision-making was based on clinical stage and on the presence or not of lymph node metastasis at the time of diagnosis. Treatment modalities consisted of surgery including partial laryngectomy (PL) and total laryngectomy (TL), alone or combined with radio-, chemo-, or radiochemotherapy (RC). All data from patients were reviewed by the authors without knowledge of histopathological status.
Data regarding patient demographics, smoking and alcohol history, date of initial diagnosis, and therapy approaches were retrospectively obtained by medical record review. All data from patients were reviewed by the authors without knowledge of histopathological status. An experienced pathologist assessed the immunostained sections for all patients to confirm the histopathological diagnosis, grade of tumor differentiation, and pathological tumor stage. Clinical evaluation included complete examination of the head and neck region by high-resolution computed tomography or magnetic resonance imaging, direct laryngoscopy, pharyngoscopy and esophagoscopy under general anesthesia (with tumor biopsies), chest radiography, and abdominal ultrasonography. T and N categories were retrospectively assigned according to the Union Internationale Contre le Cancer (UICC) classification on the basis of the pathology reports. The clinical staging and the anatomic site of the tumors were assessed according to the UICC tumor node metastasis classification of malignant tumors.
Patient follow-up
This report included follow-up data as of 31 Dec 2010. Follow-up time was measured from the date of diagnosis of LSCC. Follow-up examinations including ENT clinical examination, imaging evaluation, and pathological studies were performed in 3-month intervals during the first 2 years and every 6 months thereafter. Overall survival (OS) and disease-free survival (DFS) were calculated as the period from the date of surgery to the date of death or tumor relapse, respectively.
Immunohistochemistry
Tissue samples from all surgical specimens of the enrolled patients were processed and evaluated in one pathology institute (Department of Pathology, Cancer Center, Sun Yat-Sen University). Formalin-fixed paraffin-embedded tissue specimens were cut into 4-μm sections and mounted on poly-l-lysine-coated slides. For each patient, a representative tissue block containing an adequate amount of tumor cells and non-neoplastic laryngeal tissue was selected. All sections were stained with hematoxylin and eosin and reviewed to confirm the histopathological diagnosis and adequacy of specimens for immunohistochemical analysis.
In brief, sections were deparaffinized in xylene and rehydrated in graded alcohols and water. The slides were then heated in a microwave oven for 10 min in a 10-mM citrate buffer solution at pH 6.0 and cooled to room temperature for 20 min. After quenching endogenous peroxidase activity with 0.3 % hydrogen peroxide (in absolute methanol) for 30 min, the sections were blocked for 2 h at room temperature with 5 % bovine serum albumin (Sigma Chemical Co.). Subsequently, duplicate sections were incubated overnight at 4 °C with specific primary antibodies (listed in Table 1). After several rinses in phosphate-buffered saline (PBS), the sections were incubated in the biotinylated secondary antibody. Then, sections were incubated with streptavidin linked with peroxidase and visualized with 3,3′-diaminobenzidine tetrahydrochloride as the chromogen (Invitrogen, Carlsbad, CA). Slides were rinsed in PBS, exposed to diaminobenzidine, and counterstained with Mayer's hematoxylin. The process consisted of omission of the primary antibody.
Evaluation of immunohistochemical variables
As previously reported [15], staining intensity of IDO and apoptosis-related proteins in tumor cells, not in immune cells or tumor-draining lymph nodes, was graded into three groups: 1 = weak, 2 = moderate, and 3 = strong. Strong intensity corresponded with that in control samples used as standard. Weak intensity was similar to that noted in benign bronchial epithelium. Moderate intensity was classified as a staining intensity between weak and strong. In each case, at least 1,000 cells were counted in ten different areas using the ×40 objective lens. The percentage of the positively stained cancer cells was scored on a continuous scale (0–100 %) and subsequently categorized on a scale of 0–4: 0, none; 1, 1–25 %; 2, 26–50 %; 3, 51–75 %; 4, >75 %. For further analysis, the extent of staining was defined as the product of the categorized percentage and intensity of staining to determine the cutoff value for high protein expression.
The evaluation of TILs was performed by two independent observers in a blinded fashion. Discrepancies in enumeration, within a range of 5 %, were re-evaluated, and a consensus decision was made. To ensure representativeness and homogeneity, ten different high-power fields (HPF, ×400) representing the densest lymphocytic infiltrates were selected for each sample, photographed with a digital camera (Nikon Eclipse 80i, Japan). The number of TILs within the neoplastic nests and immediately adjacent stroma, excluding tumor cells, was counted, and the average in these ten HPF digital images was determined for statistical analysis. No attempt was made to evaluate the various tumor compartments separately (e.g., stroma, tumor cell nests).
Statistical analysis
Given that there are no widely accepted standard cut-points to define the clinical outcome, we selected the median value to be the cutoff for definition of TIL subgroups according to previous reports. All items were treated as dichotomous variables. A comparison between two groups of patients according to IDO status was done using chi-square test or Fisher's exact test as appropriate for categorical data. The correlation of IDO expression with the density of TIL subtypes and the expression of apoptosis-related proteins were examined using Pearson correlation analysis.
Time was defined as the period starting from the date of diagnosis to the date of disease relapse (event) or that of last follow-up visit (censored) for DFS and as the period starting from the date of diagnosis to the date of death (event) or that of last follow-up visit (censored) for OS. Survival curves were estimated by Kaplan–Meier method, and differences in OS and DFS between groups were determined using log-rank test. Cox proportional hazards regression analysis was used to measure the association of clinicopathologic variables to overall survival. In all tests, a P value < 0.05 was considered as significant. All analyses were done with SPSS for Windows 11.0 software package (SPSS Inc., Chicago, IL, USA).
Results
Baseline characters of patients
Within the study period, 216 patients were diagnosed with primary LSCC in two hospitals. Twenty patients were lost during follow-up and nine patients had to be omitted from the final calculation due to incomplete medical records. As a result, 187 patients were included in this retrospective study. The median age was 52.4 years (range, 37 to 85 years) and 54.0 % (n = 101) were over 60 years. The majority of patients were male (95.7 %, n = 179). Most of the patients had been exposed to risk factors such as tobacco smoke (87.2 %, n = 163) and/or alcohol consumption (66.3 %, n = 124).
The clinicopathologic characteristics of the enrolled patients are shown in Table 2. The anatomic subsites of primary lesions were the glottis in 92 cases (49.2 %) and the supraglottis in 65 patients (34.8 %). The pathological classification of the primary laryngeal lesions was T1 in 23 cases (12.3 %), T2 in 42 cases (22.5 %), T3 in 95 cases (50.8 %), and T4 in 27 cases (14.4 %). For the regional lymph nodes, it was N0 in 71 cases (38.0 %), N1 in 62 cases (33.2 %), N2 in 45 cases (24.1 %), and N3 in nine cases (4.8 %). As for histological grade, 83 cases (44.4 %) were well differentiated, while 75 cases (40.1 %) were moderately differentiated. According to the TNM staging system, 20 cases were in stage I, 58 in stage II, 88 in stage III, and 21 in stage IV. Of the 187 patients investigated, 88 (47.1 %) had been treated with surgery alone (PL in 51 cases, TL in 37 cases), 43 (23.0 %) with PL combined with RC, 30 (16.0 %) with TL combined with RC, and 26 (13.9 %) with radio- and/or chemotherapy alone.
IDO expression in LSCC
As previously reported [15], IDO staining resulted in a heterogeneous staining pattern in neoplastic cells and surrounding stroma, and typical staining patterns for other studied genes are shown in Fig. 1. Varying degrees of IDO expression in tumor tissue were detectable in 171 out of 187 cases (91.4 %). The predominant pattern of IDO staining was cytoplasmic without staining of tumor cell membrane. Cytoplasmic IDO-positive staining was most pronounced at the luminal surface of the malignant cells and surrounding tumor stroma (Fig. 1a), which contained a substantial number of IDO-positive cells that we classified as antigen-presenting cells, including macrophages and dendritic cells (data not shown).
Association of IDO expression with clinicopathologic variables
Based on the immunohistochemical evaluation score, the patients were dichotomized in two categories: 90 carcinomas (48.1 %) were identified as highly expressing IDO and 97 carcinomas (51.9 %) as low. Clinicopathologic variables stratified by IDO status are listed in Table 2. The tumor IDO expression was not significantly correlated with histology, clinical stage, nodal status, or tumor differentiation (P > 0.05). Overall, the status of intratumoral infiltration of IDO expression was not associated with any of the selected clinicopathological parameters.
Association of IDO with tumoral lymphocytes infiltration
Immune cells infiltrated tumor tissue in a disseminated manner as scattered solitary cells, as shown by H&E staining, and displayed low level of homogeneity and broad inter-individual differences for stained cell density, the number of CD3+ and FOXP3+ cells varying significantly between samples. CD3 and CD8 immunostaining demonstrated membranous staining in a subset of TIL around the tumor nests (Fig. 1c, d). The median number of CD3+ cells was 40.88 cells/HPF (range 10.39–156.87). The median number of CD8+ cells was 28.10 cells/HPF (range 6.89–98.71). FOXP3 immunostaining demonstrated nuclear staining in a subset of lymphocytes around the tumor tissues (Fig. 1b). The median number of FOXP3+ cells was 13.64 cells/HPF (range 2.64–56.49). By general logical linear regression analysis, no significant correlation was found between the densities of any two types of lymphocytes in the present study (data not shown). As shown in Table 2, tumoral IDO expression was correlated with neither CD3+ nor CD8+ TILs (P > 0.05). Instead it was positively associated with the density of FOXP3+ TILs (P = 0.028).
Prognostic value of tumor IDO expression for overall survival
The follow-up period ranged from 4 to 93 months with a mean of 48.56 months for all patients (SD = 22.8). The mean OS time was 42.08 months for all patients. Mean DFS for the patients included in our study was 21.33 months (range, 2–49 months). At the time of data analysis, a total of 52 (27.8 %) tumors had relapsed. Sixty-nine (36.9 %) patients died of the disease and 12 (6.4 %) died of other causes. For the 106 (56.7 %) surviving patients, 103 (55.1 %) were disease-free and three (1.6 %) were alive with disease.
The association between the density of different lymphocytes types and clinical outcome in LSCC patients has been evaluated in a previous paper. Here we focus on the impact of tumor IDO expression on OS and DFS in LSCC patients. In the entire cohort (n = 187), the 5-year survival was 62.9 % 61/97 (n = 61) in patients with low IDO expression and 50.0 % (n = 45) in patients with high IDO expression (P = 0.032). In a subgroup with 78 early-stage LSCC (defined as clinical stage I and II), the 5-year survival was 67.5 % (n = 27) in patients with low IDO expression and 57.9 % (n = 22) in patients with high IDO expression (P = 0.015). In the subgroup with 109 late-stage LSCC (defined as clinical stage III and IV), the 5-year survival was 59.6 % (n = 34) in patients with low IDO expression and 44.2 % (n = 23) in patients with high IDO expression (P = 0.008).
As shown in Table 3, the mean survival time was 46.38 months for 97 patients with low IDO expression and was 37.44 months for 90 patients with high IDO expression (х 2 = 5.309, P = 0.021). The mean disease-free survival time for patients with low IDO expression was 23.36 versus 19.16 months for patients with high IDO expression (х 2 = 4.484, P = 0.034). The mean survival time of 78 patients with early-stage LSCC was 44.19 months and 40.57 months in 109 patients with late-stage LSCC (х 2 = 1.568, P = 0.366). The mean disease-free survival time was comparable between the early- and late-stage subgroups (21.67 vs. 21.25, P = 0.743). In both early- and late-stage subgroups, the mean survival time for patients with low IDO expression was significantly longer than that in patients with high IDO expression (P < 0.05).
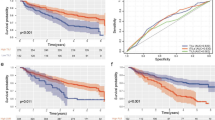
Kaplan–Meier curves were plotted to visualize the influence of IDO expression on survival time in LSCC. Figure 2 illustrates patient survival over time according to IDO distribution in the entire cohort, subgroups with late- and early-stage LSCC, respectively. Tumor expression of IDO had a significant prognostic value for OS and DFS in LSCC (P < 0.05).
Kaplan–Meier survival analysis according to tumor IDO expression of a overall survival and b disease-free survival in entire patients (n = 187), c overall survival and d disease-free survival in subgroups with early-stage LSCC (n = 78), and e overall survival and f disease-free survival in subgroups with late-stage LSCC (n = 109). The log-rank test was used to calculate the P value
IDO expression was included as a covariate for Cox proportional hazards analyses together with clinicopathological and therapy variables, and the significance of the prognostic associations was assessed by multivariate assessment. As shown in Table 4, the results show that clinical stage and presence of metastases are significantly related with OS and DFS. High expression of tumor IDO is an independent predictor for poor outcome for both DFS (HR = 3.973, P = 0.026) and OS (HR = 3.258, P = 0.029). For 161 patients treated with surgery for LSCC, administration of adjuvant chemo- and/or radiotherapy did not significantly improve either DFS (HR = 2.846, P = 0.088) or OS (HR = 2.174, P = 0.170).
Discussion
The present study explores the predictive value of IDO expression in tumor samples of 187 LSCC patients. No apparent association between tumoral IDO expression and clinicopathological parameters was found in our cohort. Instead we identified a significant correlation between IDO expression and the density of FOXP3+ TILs within the tumor microenvironment. Furthermore, IDO overexpression was an independent predictor for poor survival in LSCC patients. This study rendered further evidence for the multiple functions of IDO in the progression of LSCC.
IDO and clinical parameters
As reported, tumor IDO expression has correlated with poor histological differentiation [16] and advanced clinical stage [17] in lung cancer. A recent paper showed that the upregulated expression of IDO in primary breast cancer correlates with an increase of lymph node metastasis [18] and that IDO high immunoreactivity significantly correlated with the frequency of liver metastases in colorectal carcinoma [13]. We did not find tumor IDO expression to be associated with selected clinicopathological parameters in our LSCC cohort. In agreement with our results, IDO expression did not correlate with clinical stage, sex, age, tumor site, tumor size, metastasis, or tumor grade in patients with oral squamous cell carcinomas (OSCC) [19]. The density of IDO-positive cells was not significantly correlated with clinical parameters (tumor invasion depth, node metastasis, and TNM stages) in patients with esophageal squamous cell carcinoma (ESCC) [20]. In contrast, higher IDO expression was closely associated with advanced staging, higher T classification, and more lymph node metastasis in ESCC [21]. These data justify further the evaluation of the expression and potential clinical implication of tumor IDO status in LSCC.
IDO and prognosis in LSCC
Tumoral IDO expression has been shown to correlate with poor clinical prognosis in ovarian carcinoma [14], lung cancer [16, 17], and colon carcinoma [13], but the prognostic value of IDO in head and neck squamous cell carcinoma has not been explored. The current paper is the first to explore the prognostic value of tumor IDO expression in a large cohort of LSCC. IDO high expression has been identified as a significant negative prognostic factor in OSCC patients, especially in those patients undergoing adjuvant radiochemotherapy [19]. The level of IDO expression in ESCC tumor specimens has been associated with disease progression and worse clinical outcome [21]. However, the level of IDO expression did not correlate with the clinical outcomes in ESCC [20]. This inconsistency can be partially attributed to the diverse methods used to detect the enzyme, which may confuse the sources of IDO within the tumor microenvironment. To further examine and confirm the prognostic significance of IDO expression in LSCC, prospective setting, long-term follow-up, and larger sample studies will be required in the future.
IDO and TILs
Although effective antitumor immune responses likely involve many components of the immune system, the presence of TILs within the tumor microenvironment is considered to be an indicator of the host immune response to tumor antigens and is thought to reflect the dynamic process of “cancer immunoediting” [22]. Functional inactivation of tumor-reactive T cells has been analyzed as another important mechanism of tumor immune evasion. In addition, there is accumulating evidence showing a positive correlation between the number of TILs and patient survival in several types of tumor [23–25]. These studies indicate that TILs, in direct contact with tumor cells, can recognize tumor antigens and display tumor-specific cytolytic activity and that their presence correlates with clinical outcome.
Recently, several reports showed that tumoral IDO expression correlated with a significantly reduced number of CD8+ or CD3+ TILs in colorectal cancer [13] and endometrial cancer [26], suggesting an important role of IDO in tumor development and tumor immune function. In ESCC, G. Zhang et al. found that tumoral IDO expression correlated with a reduction in the number of CD8+ TILs in 135 samples [21], but J Liu et al. showed no statistical relation between the density of CD3+ TILs and IDO status in 45 cases [20]. Whether IDO-mediated tryptophan depletion might affect the quantity of local TILs in LSCC is totally unknown. Our results show that low tumor IDO expression is not correlated with the density of CD3+ or CD8+ TILs in the microenvironment.
Another major result of the present paper is the correlation of high FOXP3+ Treg number in the tumor microenvironment with high tumor IDO expression. In agreement with our results, high IDO expression correlated with an increase of FOXP3+ Treg cells in several human tumors, including breast cancer, pancreatic adenocarcinoma, uterine cervical carcinoma, and advanced melanoma [27–31].
Broad evidence implicates IDO and the tryptophan catabolic pathway in the generation of immune tolerance to foreign antigens [32]. In both rodent and human model systems, the correlation between IDO and FOXP3 expression has demonstrated the importance of IDO as a regulator of Tregs function. High levels of Treg cells have been reported in peripheral blood, lymph node, tumor specimens, and ascites of patients with different types of cancer [33]. Although the precise mechanisms by which Tregs suppress immune cell functions remain unclear, Tregs can suppress the activity of cytotoxic T cells through direct cell-to-cell contact or through the release of cytokines. It is has been indicated that the interaction between IDO and Tregs is a mutual effect, in which a high level of IDO promotes the differentiation, activation, and maturation of Tregs. Furthermore, naive CD4+ T cells exposed to IDO during activation are biased to become FOXP3+-inducible Tregs [22]. IDO can also stabilize the suppressive phenotype of Tregs under inflammatory conditions and prevents inflammation-induced reprogramming of Tregs into T-helper-like cells [28]. In summary, elevated IDO activity may lead to impaired antigen-dependent T cell activation and function through, at least partly, induction of the aggregation of Tregs in the cancer microenvironment, thereby contributing to immune tolerance to tumor-associated antigens.
It should be pointed out that the interplay in the tumor microenvironment becomes even more complicated if not only neoplastic epithelium but also cells in the tumor stroma are taken into account as a possible source of IDO production. In particular, recent findings have established that IDO is overexpressed in tumor cells, tumor-infiltrating immune cells, or tumor-draining lymph nodes, where it promotes the establishment of peripheral immune tolerance to tumor antigens. In addition to DCs, other immune cells that may utilize IDO for immunosuppression include macrophages, granulocytes, and neutrophils [32]. Therefore, other factors might participate in the mutual cross-talk between IDO-positive cancer cells and FOXP3+ Tregs and further magnify the immunosuppressive cascade triggered by IDO.
Effects of therapy
In the management of early-stage LSCC, all three modalities—surgery, radiotherapy, and chemotherapy—are used with complementary roles, and LSCC therapy requires an integrated multidisciplinary approach [34]. Few studies compare different available treatment options, and initial treatment combinations can vary significantly between institutions [35, 36]. In this retrospective study on a large, non-selected cohort, the role of adjuvant chemoradiotherapy in the outcome of LSCC patients cannot be assessed. The impact of therapy choice on the outcome of LSCC patients should be investigated in a prospective setting.
Conclusion
In conclusion, upregulation of IDO expression in tumor tissue might inhibit local immune surveillance by favoring amplification and infiltration of FOXP3+ Tregs in the tumor microenvironment, which contributes to a worse prognosis. Although the precise role of tumor IDO in human LSCC remains to be elucidated, our findings provide new evidence of IDO dysregulation in tumor evolution and encourage the development of IDO inhibitors as a new immunoregulatory treatment modality.
Abbreviations
- DFS:
-
Disease-free survival
- ESCC:
-
Esophageal squamous cell carcinoma
- FOXP3:
-
Forkhead box P3
- HPF:
-
High power field
- HNSCC:
-
Head and neck squamous cell carcinomas
- IHC:
-
Immunohistochemistry
- LSCC:
-
Laryngeal squamous cell carcinomas
- OS:
-
Overall survival
- OSCC:
-
Oral squamous cell carcinomas
- PL:
-
Partial laryngectomy
- RC:
-
Radiochemotherapy
- TIL:
-
Tumor-infiltrating lymphocytes
- TL:
-
Total laryngectomy
- Treg:
-
Regulatory T lymphocytes
References
Mellor AL, Chandler P, Lee GK et al (2002) Indoleamine 2,3-dioxygenase, immunosuppression and pregnancy. J Reprod Immunol 57:143–150
Godin-Ethier J, Hanafi LA, Piccirillo CA et al (2011) Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res 17:6985–6991
Prendergast GC, Chang MY, Mandik-Nayak L et al (2011) Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases. Curr Med Chem 18:2257–2262
Johnson BA 3rd, Baban B, Mellor AL (2009) Targeting the immunoregulatory indoleamine 2,3 dioxygenase pathway in immunotherapy. Immunotherapy 1:645–661
Uyttenhove C, Pilotte L, Théate I et al (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9:1269–1274
Funeshima N, Fujino M, Kitazawa Y et al (2005) Inhibition of allogeneic T-cell responses by dendritic cells expressing transduced indoleamine 2,3-dioxygenase. J Gene Med 7:565–575
Sørensen RB, Køllgaard T, Andersen RS et al (2011) Spontaneous cytotoxic T-cell reactivity against indoleamine 2,3-dioxygenase-2. Cancer Res 71:2038–2044
Mellor AL, Keskin DB, Johnson T et al (2002) Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol 168:3771–3776
Liu X, Newton RC, Friedman SM et al (2009) Indoleamine 2,3-dioxygenase, an emerging target for anti-cancer therapy. Curr Cancer Drug Targets 9:938–952
Allen CT, Judd NP, Bui JD et al (2012) The clinical implications of antitumor immunity in head and neck cancer. Laryngoscope 122:144–157
Pan K, Wang H, Chen MS et al (2008) Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol 134:1247–1253
Riesenberg R, Weiler C, Spring O et al (2007) Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Cancer Res 13:6993–7002
Brandacher G, Perathoner A, Ladurner R et al (2006) Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res 12:1144–1151
Okamoto A, Nikaido T, Ochiai K et al (2005) Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res 11:6030–6039
Liu H, Zhang T, Li X et al (2008) Predictive value of MMP-7 expression for response to chemotherapy and survival in patients with non-small cell lung cancer. Cancer Sci 99:2185–2192
Engin AB, Ozkan Y, Fuchs D et al (2010) Increased tryptophan degradation in patients with bronchus carcinoma. Eur J Cancer Care (Engl) 19:803–808
Suzuki Y, Suda T, Furuhashi K et al (2010) Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 67:361–365
Yu J, Sun J, Wang SE et al (2011) Upregulated expression of indoleamine 2,3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin Dev Immunol. doi:10.1155/2011/469135
Laimer K, Troester B, Kloss F et al (2011) Expression and prognostic impact of indoleamine 2,3-dioxygenase in oral squamous cell carcinomas. Oral Oncol 47(5):352–357
Liu J, Lu G, Tang F et al (2009) Localization of indoleamine 2,3-dioxygenase in human esophageal squamous cell carcinomas. Virchows Arch 455:441–448
Zhang G, Liu WL, Zhang L et al (2011) Involvement of indoleamine 2,3-dioxygenase in impairing tumor-infiltrating CD8 T-cell functions in esophageal squamous cell carcinoma. Clin Dev Immunol. doi:10.1155/2011/384726
Elkord E, Alcantar-Orozco EM, Dovedi SJ et al (2010) T regulatory cells in cancer: recent advances and therapeutic potential. Expert Opin Biol Ther 10:1573–1586
Zhang L, Conejo-Garcia JR, Katsaros D et al (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348(3):203–213
Vesalainen S, Lipponen P, Talja M et al (1994) Histological grade, perineural infiltration, tumourinfiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer 30:1797–1803
Ropponen KM, EskelinenMJ LPK et al (1997) Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 182:318–324
Ino K, Yamamoto E, Shibata K et al (2008) Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res 14:2310–2317
Rudensky AY (2011) Regulatory T cells and Foxp3. Immunol Rev 241:260–268
Fallarino F, Grohmann U (2011) Using an ancient tool for igniting and propagating immune tolerance: IDO as an inducer and amplifier of regulatory T cell functions. Curr Med Chem 18:2215–2221
Sun J, Yu J, Li H et al (2011) Upregulated expression of indoleamine 2,3-dioxygenase in CHO cells induces apoptosis of competent T cells and increases proportion of Treg cells. J Exp Clin Cancer Res. doi:10.1186/1756-9966-30-82
Munn DH (2011) Indoleamine 2,3-dioxygenase. Tregs and cancer. Curr Med Chem 18:2240–2246
Nakamura T, Shima T, Saeki A et al (2007) Expression of indoleamine 2,3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci 98(6):874–881
Chen W, Liang X, Peterson AJ et al (2008) The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol 181:5396–5404
Katz JB, Muller AJ, Prendergast GC (2008) Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev 222:206–221
Silver CE, Beitler JJ, Shaha AR et al (2009) Current trends in initial management of laryngeal cancer: the declining use of open surgery. Eur Arch Otorhinolaryngol 266:1333–1352
Mendenhall WM, Mancuso AA, Hinerman RW et al (2007) Multidisciplinary management of laryngeal carcinoma. Int J Radiat Oncol Biol Phys 69:S12–S14
Marioni G, Marchese-Ragona R, Cartei G et al (2006) Current opinion in diagnosis and treatment of laryngeal carcinoma. Cancer Treat Rev 32:504–515
Acknowledgments
We gratefully thank Dr. Jinghui Hou and his colleagues (Department of Pathology, Cancer Center, Sun Yat-Sen University) for technical assistance in pathological evaluations.
Conflict of interest
All other authors indicated no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, J., Liu, H., Hu, Y. et al. Tumoral indoleamine 2,3-dioxygenase expression predicts poor outcome in laryngeal squamous cell carcinoma. Virchows Arch 462, 73–81 (2013). https://doi.org/10.1007/s00428-012-1340-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-012-1340-x