Abstract
Alterations in the p16/cyclinD1/Rb and ARF/Mdm2/p53 pathways are frequent events in the pathogenesis of squamous cell carcinomas. Different mechanisms of p16 regulation have been described for penile carcinomas so far. Therefore, expression of p16 and p53 was immunohistochemically detected with monoclonal antibodies in 52 primary invasive penile squamous cell carcinomas. The carcinomas were analyzed for allelic loss (LOH) in p16 INK4A and p53, as well as for mutations in the p16 INK4A and the p53 gene. In addition, we examined the promoter status of p16 INK4A by methylation-specific PCR. The presence of human papilloma virus (HPV) 6/11, HPV 16 and HPV 18 DNA was analyzed by PCR. Data were compared to clinical data. Concerning p16, 26 (50%) tumors showed positive immunohistochemistry, 32 (62%) tumors showed allelic loss and 22 tumors (42%) showed promoter hypermethylation. All tumors with negative p16 immunohistochemistry showed LOH near the p16 INK4A locus and/or hypermethylation of the p16 INK4A promoter. HPV 16 DNA was detected in 17 tumors, ten of them with positive p16 immunostaining. The remaining seven tumors with negative p16 staining showed allelic loss and/or promoter hypermethylation. Evidence of lymph node metastasis was significantly associated with negative p16 immunohistochemistry as well as with combined LOH and promoter hypermethylation (p = 0.003 and p = 0.018, respectively). Allelic loss around p53 was found in 22 tumors (42%), and seven mutations of the p53 gene could be demonstrated in our tumors. No correlations could be found between any p53 alteration and clinical parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tumor suppressor genes p16 INK4A and p53 play an essential role in prevention of malignant transformation of cells and of tumor progression. Especially the expression of p16 increases at an early stage of tumorigenesis. The tumor suppressor p16 induces cell cycle arrest and prevents cell division by inhibition of the cyclin-dependent kinases CDK4 and CDK6 and inhibition of CDK-mediated phosphorylation of the retinoblastoma (Rb) gene [1].
Expression of p16 increases in reaction to DNA damage including UV damage, oxygen radicals, ionizing radiation, and activating mutations of oncogenes (e.g. RAS) [1, 2]. A close association of oncogenic stress by human papilloma virus (HPV) infection and increased p16 expression has been described for several tumor entities, including squamous cell carcinomas of the head and neck region and of the cervix uteri [3–5].
Since the presence of HPV DNA is found in 15–71% of penile carcinomas [6–8], depending on detection method and geographical location, a possible role of p16 alterations and HPV was discussed for the carcinogenesis of penile cancer. Due to the low incidence of penile carcinomas in North America and Western Europe, there is scant information about the oncogenesis and tumor progression of this tumor. The limited studies of p16 INK4A showed conflicting results so far. Overexpression as well as loss of p16 INK4A tumor suppressor gene has been described in penile carcinoma [9]. The prognostic implications of this phenomenon in patients with penile carcinoma as well as whether all HPV-related tumors show that alterations in the p16INK4a pathway are pending issues that remain unclear. Solving these issues will also permit a better estimation of the putative benefits of HPV vaccination in men.
Besides positive and negative regulation of p16INK4A expression, genetic and epigenetic alteration of the p16 INK4A gene has been described. Loss of heterozygosity (LOH) at chromosome 9p21, including the p16 and ARF region, is a frequent event in several tumor entities and has been described before for penile carcinomas [10]. Point mutations of p16 INK4A and—more frequently—silencing of the p16 INK4A gene by hypermethylation of the promoter have been reported for many human cancers [11, 12]. Therefore, we examined the mechanisms of p16 regulation in penile squamous carcinoma (PSCC), including HPV analysis. Since regulation of the tumor suppressor gene p53 is closely related to p16 (ARF and p21 pathways) and alterations of p53 have been described for penile cancer, we additionally investigated p53 expression and genetic alterations in the p53 gene area.
Furthermore, we analyzed clinical data and examined the prognostic role of p16 and p53 alterations for lymph node metastases and prognosis in penile cancer.
Materials and methods
Patients
Fifty-two patients (median patient age, 59.8 years, range 22–89 years) with invasive PSCC treated at the University Hospital of Greifswald, Helios Hospital Schwerin and University Hospital of Essen were available for investigation. Follow-up data were available from all but three patients. Median follow-up was 44.2 months (range, 2–105). Patient data and tissues were obtained and used after advice from the Medical Ethics Committee of the University of Greifswald and University of Essen in accordance with the declaration of Helsinki and the International Conference of Harmonisation—Good Clinical Practice. The anonymity of the patients investigated was preserved corresponding to rules of data protection of the Human Medical Faculty Greifswald and the Human Medical Faculty Essen.
The samples from 52 patients comprised 15 pT1 (28.8%), 24 pT2 (46.2%), 12 pT3 (23.1%), and 1 pT4 (1.9%) tumors. Lymph node metastases were diagnosed in 32 patients (61.5%). Forty-one tumors were conventional squamous cell carcinoma (SCC). Moreover, four patients with basaloid variants of SCC, three patients with verrucous variants, and one patient each with a papillary, a condylomatous, and two sarcomatoid variants of SCC were included in this study. Lymphadenectomy was done within 2–4 weeks after operation of the primary tumor according to the recommendations of the EAU Guidelines. Dynamic sentinel lymph node dissection was done in three patients; all other patients underwent modified or radical lymphadenectomy depending on the extension of lymph node metastasis. Six elderly patients underwent radiotherapy for histologically confirmed inguinal lymph node metastases. Due to micrometastasis or pathological preparations, enough material for DNA extraction was available only in ten metastases.
Representative tumor tissue samples with adjacent non-neoplastic tissue allowing sufficient classification and grading were obtained during surgery and subjected to 4% buffered formalin. For each patient, DNA was extracted from tumor tissue samples as well as non-neoplastic tissue. Pathological classification was done on hematoxylin and eosin stained specimen. All specimens underwent additional independent histopathological review (CK, EW). Tumors were staged according to the 2002 TNM system, histological grading was classified as follows: grade 1 (G1), well-differentiated; grade 2 (G2), moderately differentiated; and grade 3 (G3), poorly differentiated [13]. The tumors were additionally histologically subtyped according WHO classification [14].
Immunohistochemistry
Immunohistochemistry was done on paraffin sections (4 μm). After blocking of endogenous peroxidase activity (Peroxydazed 1, Biocarta, Hamburg, Germany, 5 min 22°C), sections were subjected to heat antigen retrieval (microwave oven, 700 W, 20 min) using 10 mM citrate buffer (pH 6.0). After cooling in citrate buffer and subsequent washings in phosphate-buffered saline (PBS), immunohistochemistry was performed using a staining kit (Biocarta). Application of blocking solution (Biocarta, 10 min) was followed by overnight incubation with monoclonal antibodies against p16 (Novocastra, UK) and p53 (Dako, Denmark) at 4°C. After two washes in PBS (2 × 5 min), antibody binding was achieved using the 4plusTM Universal Immunoperoxidase Detection System (Biocarta). Sections were incubated with secondary antibody (20 min, 22°C) and washed in PBS (2 × 5 min). Slides were subjected to streptavidin–horseradish peroxidase (HRP) solution (Biocarta, 10 min). Visualization was performed after washing in PBS (2 × 5 min) with 0.1% diamino-benzidine (Sigma) in PBS/0.01% H2O2 (3–8 min). Slides were counterstained with hematoxylin, dehydrated and mounted in Neo-mount (Merck, Darmstadt, Germany). Control reactions were done by (1) using PBS instead of primary antibody and (2) replacing the primary antibodies with a monoclonal antibody (β1-integrin, Biomol, Hamburg, Germany) not reactive in paraffin sections. Photographs were taken on an Olympus Bx50 microscope equipped with an Olympus DP 10 digital camera.
The percentage of p16- and p53-positive tumor cells was evaluated by three independent observers (CK, EW, and BK), and intensity was measured by percentage of stained tumor cells. According to previous studies, expression of p16 was classified as negative (<5% of the tumor cells positive) or positive (>5% of the tumor cells positive), while the expression of p53 was classified as weak expression (<20% of the tumor cells positive) or overexpression (>20% of tumor cells positive) [15–17].
DNA extraction
For DNA extraction, hematoxylin and eosin stained slides were carefully inspected by light microscopy to identify areas that carry a sufficient amount (at least 3 mm2) of tumor tissue measured by a scaled optical adjustment. This same area was then identified on the unstained 10 μm dewaxed, rehydrated, and air-dried tissue section, which was fixed in an optical installation allowing the separate isolation of predominantly tumor tissue without adherent non-tumorous structures under microscopical control with a cannula (used for intravenous injections). DNA isolation from paraffin-embedded tissue was performed as previously described [18, 19] with the High Pure PCR Template Preparation Kit (Roche Molecular Biochemicals, Mannheim, Germany) and the innuPrep DNA Minikit (Analytik Jena, Jena, Germany). Quality of DNA was assessed by agarose gel electrophoresis.
Human papilloma virus DNA
The amplification of HPV6/11, HPV16, and HPV18 DNA was detected in a multiplex PCR with primer sequences as described by Sotlar et al. [20] with fluorescence-labeled primers. The products were analyzed on an ABIPrism310 Genetic Analyzer. To prove the presence of amplificable DNA in the extractions, all of them were amplified with primers for the human beta-globin gene.
LOH analysis
The following nine microsatellite loci were studied: D9S1604, D9S1748, D9S161, D9S286, D9S162, and D9S171 (9p21, covering the p16 INK4A region) and D17S513 (17p13), D17S786, and D17S952 (17p, around p53). Primer sequences and cytogenetic locations were obtained from Ensembl Data Base (http://www.ensembl.org). PCR amplification was performed in multiplex assays with fluorochrome-labeled primers (6-FAM, JOE, or TAMRA) in 12.5-μl sample volumes with 2–5 ng of genomic tumor or nonneoplastic DNA as template in 15 mM Tris/HCl, 50 mM KCl, with 200 μM dNTPs, 1.5 mM MgCl2, 0.1 nM primers, and 1 U HotStart Taq Polymerase (Applied Biosystems, Darmstadt, Germany). An initial denaturation and activation step of 8 min at 95°C was followed by 30–35 cycles of 1 min at 95°C, 1 min at 55–58°C, and 2 min at 72°C, and a 30 min final elongation step at 72°C. PCR products were analyzed on an ABI310 genetic analyzer (Applied Biosystems, Darmstadt, Germany) with ROX-labeled internal lane standard. All PCR assays were repeated at least once. LOH was scored if one allele was >90% decreased in tumor DNA when compared with the same allele in normal control DNA in both PCR assays.
Sequencing analysis
Mutation analysis of p16 INK4A was carried out with primers as described before [21]. For mutation analysis of p53, the exons 5, 7, and 8 were amplified using primers as described by Yano et al. [22]; for exon 6, we designed new primers as follows: forward, 5′CCAGGCCTCTGATTCCTCA and reverse, 5′TTAACCCCTCCTCCCAGAGA.
PCR amplification was performed in 12.5-μl sample volumes with 2–5 ng of genomic tumor or nonneoplastic DNA as template in 15 mM Tris/HCl, 50 mM KCl, with 200 μM dNTPs, 1.5 mM MgCl2, 0.1 nM primers, and 1 U HotStart Taq Polymerase (Applied Biosystems). An initial denaturation and activation step of 8 min at 95°C was followed by 30–35 cycles of 1 min at 95°C, 1 min at 55–62°C, and 2 min at 72°C, and a 30-min final elongation step at 72°C. Sequencing was always carried out in both directions with the BigDye sequencing kit (Applied Biosystems). Each polymorphism or mutation was verified by a second sequencing reaction of an independent amplification product.
Methylation analysis
Methylation analysis of p16 INK4A promoter was performed on tumor DNA and nonneoplatic DNA as described by Liu et al. in a two-stage methylation specific PCR [23]. Bisulfite treatment was done with the EpiTect Biosulfite kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. PCR products were evaluated on silver-stained polyacrylamide gels.
Statistical analysis
Descriptive statistical data were reported in terms of mean values and standard deviation. The chi-square test was used to examine the association among p16 and p53 expression and genomic alteration and clinical parameters. A p < 0.05 was considered statistically significant.
Results
All 52 primary tumors and ten metastases were investigated for expression of p16 and p53, presence of HPV DNA, LOH in microsatellite markers surrounding the genes p16 INK4A and p53, as well as promoter methylation of the p16 INK4A gene (Table 1).
p16INK4A
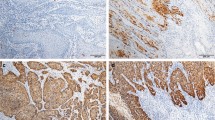
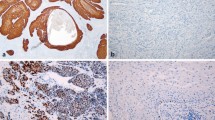
Overexpression of p16 was found in 26 primary tumors (50%), including all four basaloid SCCs as well as two verrucous SCCs (Fig. 1a). In contrast, one half of conventional PSCCs, the condylomatous, papillar, and sarcomatoid variants expressed no p16 (Fig. 1b).
Promoter hypermethylation of the p16 INK4A gene was observed in 22 primary penile carcinomas (42%) and seven metastases, and loss of heterozygosity in at least one microsatellite marker around this gene was detected in 32 primary tumors (62%) and eight metastases (Fig. 2a), while 16 tumors (31%) did not show loss of heterozygosity or promoter methylation. Mutations were evident in only five tumors. None of these genetic aberrations could be found in non-neoplastic DNA.
Electropherogram of microsatellite loci in a penile primary tumor and the corresponding normal tissue. The y-axis represents the peak height in fluorescence units. The arrows mark the lost alleles. a D9S161 and D9S171 show LOH in the primary tumor. b D17S952 shows LOH, whereas D17S513 is not informative
Negative p16 protein expression status was detected significantly more often in those penile carcinoma in which a methylation of the p16 INK4A promoter or loss of heterozygosity in at least one microsatellite marker around the p16 INK4A gene occurred (p < 0.0001), independent of the detection of HPV DNA. All tumors with negative p16 immunohistochemistry showed LOH near the p16 INK4A locus and/or hypermethylation of the p16 INK4A promoter.
p53
Overexpression of p53 was found in 29 of 50 primary carcinomas (58%; in two primary carcinomas, no analysis of p53 was possible due to material shortage) and four metastases (Fig. 1c), whereas weak expression of p53 was detected in the remaining 21 carcinomas (Fig. 1d). LOH in at least one microsatellite marker surrounding the p53 gene was found in 22 primary tumors (44%), including the sarcomatoid, condylomatous, papillar, and one verrucous subtype as well as 46% of conventional SCCs, and four metastases (Fig. 2b). Four primary carcinomas displayed five missense mutations in the p53 gene (Fig. 3). An additional missense mutation was detected in a skin metastasis, one nonsense mutation in a lymph node metastasis. There was neither a significant association between genetic alteration of p53 and p53 expression nor between p16 and p53 expression in tumors of our study. Moreover, we were not able to show a correlation between LOH in the 9p21 area (ARF region) and the expression of p53 in penile carcinomas of our study.
Human papilloma virus DNA
HPV DNA was detected in 20 tumors, in detail 17 HPV16 (33%), one HPV18 (2%), and two HPV6/11 (4%). Ten HPV 16 positive tumors showed a positive p16INK4A staining. The remaining seven tumors with negative staining showed allelic loss and/or promoter hypermethylation (p = 0.035). The HPV 18 positive tumor was found to have negative p16INK4A staining and allelic loss and promoter hypermethylation. Positive p16INK4A staining and no allelic loss/promoter hypermethylation were observed in both HPV 6/11 positive tumors.
There was no association between the presence of HPV DNA and expression of p53 (eight p53 negative tumors with HPV16 vs. ten p53 positive tumors with HPV16; p = 0.77), but 56% of the tumors with positive p16 staining and no HPV-DNA showed a p53 expression.
Correlation with clinical data
Negative p16 immunohistochemistry was significantly associated with the occurrence of lymph node metastasis (p = 0.003). Loss of heterozygosity around p16 INK4A and promoter hypermethylation could be found more frequently in carcinomas with metastasis (Table 2). A combination of LOH and promoter hypermethylation was also significantly associated with lymph node metastases. Patients with positive p16 immunohistochemistry showed a better prognosis, but the Kaplan–Meier analysis failed to show statistical significance (p = 0.381). There was no significant association between p16 expression and local tumor stage (T stage).
Analyses of p53 expression, LOH, or mutations in the p53 locus and clinical data failed to show a statistical significance (data not shown).
Discussion
The overall HPV DNA detection rate in the primary tumors of this study is 35.8%, which is in line with results from other working groups regarding European countries [7, 9, 24, 25]. The HPV DNA detection rate depends on the sensitivity of the detection method and on the number of HPV subtypes investigated. Given that only four HPV genotypes were investigated in this study, we cannot rule out either an HPV infection by other genotypes or absence of viral infection in our HPV negative cases. In accordance with data from Stankiewicz et al. [26], none of the verrucous carcinomas displayed an HPV infection.
Overexpression of p16 was found in several squamous cell carcinomas of different origins. While p16 overexpression is essentially associated with the presence of HPV-DNA in nearly 100% of cervical carcinomas, a relevant part of head and neck carcinomas and vulvar carcinomas shows p16 overexpression in the absence of HPV infection [27–29]. Our results show that overexpression of p16 is a frequent event also in penile carcinomas, although not necessarily associated with the presence of HPV DNA. Several other pathways have to be taken into consideration, since we found p16- and p53-positive carcinomas without the presence of HPV-DNA. Overexpression of p16 is also frequently seen in tumors, which are unrelated to HPV [30], and has even been described in HPV-independent squamous dysplasias [31].
Recently, a variety of studies focused on p16 alterations in penile cancer, but with different emphases. Ferreux found an overexpression of p16 in 29% of penile carcinomas, especially in connection with HPV infection [9]. Prowse et al. detected p16 overexpression in 46% of penile SCCs, which was significantly associated with HPV infection [24]. In contrast, Senba et al. described p16 overexpression in an equal amount of HPV-positive and HPV-negative penile carcinomas from Kenya [32].
Interestingly, all HPV16 positive tumors in our study showed a positive p16 immunoreaction, except those with allelic loss in the p16 INK4A region and/or promoter hypermethylation. HPV DNA-positive but p16-negative tumors have been described for head and neck tumors, but the pathogenesis remains unclear so far [33].
Our results propose at least two important ways of p16 inactivation since all p16 negative tumors showed LOH in the p16 INK4A gene locus and/or promoter hypermethylation. A significant role of LOH at chromosome 9p21 was reported for penile carcinomas in an earlier study [10]. Allelic loss in the p16 INK4A region was found in over 60%, and promoter hypermethylation was evident in over 40% of the tumors of our study. Yanagawa et al. reported p16 INK4A methylation in 26% of penile carcinomas, exclusively in those without HPV DNA [8]. Ferreux et al. found p16 INK4A methylation in 17% of tumors, only in HPV negative ones with the exception of two tumors [9]. In agreement with data from Ferreux et al., 75% of penile carcinomas of this study with p16 INK4A promoter methylation displayed no p16 staining. The crucial role of p16 INK4A alterations is underlined by the significant association of LOH and promoter hypermethylation with the occurrence of lymph node metastasis.
Inactivation of the tumor suppressor p16 INK4A seems to play an important role in tumor progression and dissemination [34]. In accordance to squamous cell carcinomas of other origins, we were able to demonstrate a more aggressive behavior of p16-negative tumors. Besides its impact in suppression of cell division, p16 seems to play a major role in suppression of lymphangiogenesis and lymphatic metastasis, since in vivo re-expression of p16 inhibited the process of lymphangiogenesis in pancreatic cancer [35].
The tumor suppressor gene p53 is the second important key holder in the cell cycle that is, like p16, connected to the oncoproteins of HPV-DNA. Furthermore, a close connection of the ARF/MDM2/p53 and the p16INK4/RB pathways is known. ARF, a part of the INK4a/ARF/INK4b locus at 9p21, acts “upstream” of p53 by antagonizing the p53-negative regulator MDM2 [2]. Expression of p53 is closely related to p16 expression (p21 pathway), and expression of both tumor suppressor genes is partly influenced by the same regulators [1, 2]. Since strong expression of p53 has been shown to be associated with metastasis and poor prognosis in penile carcinomas [36, 37], we additionally examined the expression of p53, searched for allelic losses and mutations in the p53 gene, and compared p53 expression to HPV status, LOH in 9p21, and p16 expression.
There are slightly more data regarding the overexpression of p53 than the expression status of p16. However, the results vary greatly between 26% and 91% [8, 32, 36, 38, 39]. Moreover, correlations between p53 overexpression and HPV status or p53 mutations also display a very heterogenous picture. Gentile et al. reported that all HPV-positive penile carcinomas had an overexpression of p53 [39], whereas Yanagawa found p53 staining in 67% of HPV-positive and 48% of HPV-negative tumors [8] and Levi et al. in a quarter of HPV-positive and HPV-negative carcinomas [38]. In this study, p53 overexpression was seen in about one third of penile tumors with and without HPV DNA. There was no significant difference in p53 expression in HPV-DNA positive or negative tumors. Regulation of p53 expression remains a multifactor process. We showed that p53 mutations play a minor role in penile cancer, while LOH in the p53 locus is frequently found. Allelic loss and mutations are also only one possibility for p53 alteration in penile cancer, since we did not found a significant association between p53 expression status and genetic alterations.
There was no significant association between allelic loss in the ARF gene locus and expression of p53 in penile carcinomas, demonstrating once more that ARF-mediated activation is only one pathway in p53 regulation. On the other hand, there may be HPV-independent ways of coactivation of p53 and p16, since over 50% of HPV-negative tumors with positive p16 expression showed also p53 activation.
Surprisingly, we did not found a significant association of p53 expression and lymph node metastasis in the patients of our study. This may be a result of the limited number of patients, but it underlines the problems of the prognostic role of immunohistochemical detection in clinical praxis [40].
Our data underline the important role of alterations of tumor suppressor genes p16 in carcinogenesis, invasion, and dissemination of penile carcinomas. We were able to demonstrate that promoter hypermethylation and allelic loss are the most important ways for p16 inactivation in penile cancer. Re-expression of p16 may be a future target in new anti-neoplastic treatment regimens. Due to the limited number of patients in the study, the preliminary results should be confirmed in a larger study.
References
Kim WY, Sharpless NE (2006) The regulation of INK4/ARF in cancer and aging. Cell 127:265–275
Sherr CJ (2000) The Pezcoller lecture: cancer cell cycles revisited. Cancer Res 60:3689–3695
zur Hausen H (2009) Papillomaviruses in the causation of human cancers—a brief historical account. Virology 384:260–265
Munoz N, Bosch FX, de Sanjose S, Tafur L, Izarzugaza I, Gili M, Viladiu P, Navarro C, Martos C, Ascunce N et al (1992) The causal link between human papillomavirus and invasive cervical cancer: a population-based case–control study in Colombia and Spain. Int J Cancer 52:743–749
Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, Sasaki C, Joe J, Camp RL, Rimm DL, Psyrri A (2006) Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 24:736–747
Rubin MA, Kleter B, Zhou M, Ayala G, Cubilla AL, Quint WG, Pirog EC (2001) Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol 159:1211–1218
Lont AP, Kroon BK, Horenblas S, Gallee MP, Berkhof J, Meijer CJ, Snijders PJ (2006) Presence of high-risk human papillomavirus DNA in penile carcinoma predicts favorable outcome in survival. Int J Cancer 119:1078–1081
Yanagawa N, Osakabe M, Hayashi M, Tamura G, Motoyama T (2008) Detection of HPV-DNA, p53 alterations, and methylation in penile squamous cell carcinoma in Japanese men. Pathol Int 58:477–482
Ferreux E, Lont AP, Horenblas S, Gallee MPW, Raaphorst FM, von Knebel DM, Meijer CJLM, Snijders PJF (2003) Evidence for at least three alternative mechanisms targeting the p16INK4A/Cyclin D/Rb pathway in penile carcinoma, one of which is mediated by high-risk human papillomavirus. J Pathol 201:109–118
Poetsch M, Schuart B-J, Schwesinger G, Kleist B, Protzel C (2007) Screening of microsatellite markers in penile cancer reveals differences between metastatic and non-metastatic carcinomas. Mod Pathol 20:1069–1077
Forbes S, Clements J, Dawson E, Bamford S, Webb T, Dogan A, Flanagan A, Teague J, Wooster R, Futreal PA, Stratton MR (2006) Cosmic 2005. Br J Cancer 94:318–322
Esteller M, Corn PF, Baylin SB, Herman JG (2001) A gene hypermethylation profile of human cancer. Cancer Res 61:3225–3229
Sobin LH, Wittekind C (2002) TNM classification of malignant tumours, 6th edn. Wiley-Liss, New York
Eble JN, Sauter G, Epstein JI, Sesterhenn IA (2004) World Health Organization classification of tumours. Pathology and genetics of tumours of the urinary system and male genital organs. IARC, Lyon, pp 280–290
Hendricksen K, Moonen PM, der Heijden AG, Witjes JA (2006) False-positive lesions detected by fluorescence cystoscopy: any association with p53 and p16 expression? World J Urol 24:597–601
Kong CS, Balzer BL, Troxell ML, Patterson BK, Longacre TA (2007) p16INK4A immunohistochemistry is superior to HPV in situ hybridization for the detection of high-risk HPV in atypical squamous metaplasia. Am J Surg Pathol 31:33–43
Stelow EB, Shaco-Levy R, Bao F, Garcia J, Klimstra DS (2010) Pancreatic acinar cell carcinomas with prominent ductal differentiation: mixed acinar ductal carcinoma and mixed acinar endocrine ductal carcinoma. Am J Surg Pathol 34:510–518
Poetsch M, Dittberner T, Woenckhaus C (2001) PTEN/MMAC1 in malignant melanoma and its importance for tumour progression. Cancer Genet Cytogenet 125:21–26
Poetsch M, Zimmermann A, Wolf E, Kleist B (2005) Loss of heterozygosity occurs predominantly, but not exclusively in the epithelial compartment of pleomorphic adenoma. Neoplasia 7:688–695
Sotlar K, Diemer D, Dethleffs A, Hack Y, Stubner A, Vollmer N, Menton S, Menton M, Dietz K, Wallwiener D, Kandolf R, Bültmann B (2004) Detection and typing of human papillomavirus by E6 nested multiplex PCR. J Clin Microbiol 42:3176–3184
Poetsch M, Lorenz G, Kleist B (2002) Detection of new PTEN/MMAC1 mutations in head and neck squamous cell carcinomas with loss of chromosomes 10. Cancer Genet Cytogenet 132:20–24
Yano M, Hamatani K, Eguchi H, Hirai Y, MacPhee DG, Sugino K, Dohi K, Itamoto T, Asahara T (2007) Prognosis in patients with hepatocellular carcinoma correlates to mutations of p53 and/or hMSH2 genes. Eur J Cancer 43:1092–1100
Liu Y, Lan Q, Siegfried JM, Luketich JD, Keohavong P (2006) Aberrant promoter methylation of p16 and MGMT genes in lung tumors from smoking and never-smoking lung cancer patients. Neoplasia 8:46–51
Prowse DM, Ktori EN, Chandrasekaran D, Prapa A, Baithun S (2008) Human papillomavirus-associated increase in p16INK4A expression in penile lichen sclerosus and squamous cell carcioma. Br J Dermatol 158:261–265
Pascual A, Pariente M, Godinez JM, Sánchez-Prieto R, Atienzar M, Segura M, Poblet E (2007) High prevalence of human papillomavirus 16 in penile carcinoma. Histol Histopathol 22:177–183
Stankiewicz E, Kudahette SC, Prowse DM, Ktori E, Cuzick J, Ambroisine L, Zhang X, Watkin N, Corbishley C, Berney DM (2009) HPV infection and immunochemical detection of cell-cycle markers in verrucous carcinoma of the penis. Mod Pathol 22:1160–1168
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PF, Peto J, Meijer CJ, Munoz N (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12–19
Singhi AD, Westra WH (2010) Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer 116:2166–2173
van de Nieuwenhof HP, van Kempen LC, de Hullu JA, Bekkers RL, Bulten J, Melchers WJ, Massuger LF (2009) The etiologic role of HPV in vulvar squamous cell carcinoma fine tuned. Cancer Epidemiol Biomarkers Prev 18:2061–2067
Mulvany NJ, Allen DG, Wilson SM (2008) Diagnostic utility of p16INK4a: a reappraisal of its use in cervical biopsies. Pathology 40:335–344
Galmiche L, Coste-Burel M, Lopez P, Phillippe HJ, Laboisse C (2006) The expression of p16INK4a is not correlated with HPV status in CIN 1. Histopathology 48:767
Senba M, Buziba N, Mori N, Wada A, Irie S, Toriyama K (2009) Detection of human papillomavirus and cellular regulators p16INK4a, p53, and NF.kappaB in penile cancer cases in Kenya. Acta Virol 53:43–48
Smith EM, Rubenstein LM, Hoffman H, Haugen TH, Turek LP (2010) Human papillomavirus, p16 and p53 expression associated with survival of head and neck cancer. Infect Agent Cancer 5:4
Karamitopoulou E, Zlober I, Koumarianou A, Patsouris ES, Peros G, Lugli A (2010) Expression of p16 in lymph node metastases of adjuvantly treated stage III colorectal cancer patients identifies poor prognostic subgroups: a retrospective analysis of biomarkers in matched primary tumor and lymph node metastases. Cancer 116:4474–4486
Schulz P, Scholz A, Rexin A, Hauff P, Schirner M, Wiedenmann B, Detjen K (2008) Inducible re-expression of p16 in an orthotopic mouse model of pancreatic cancer inhibits lymphangiogenesis and lymphatic metastasis. Br J Cancer 99:110–117
Lopes A, Bezerra AL, Pinto CA, Serrano SV, de Mello CA, Villa LL (2002) p53 as a new prognostic factor for lymph node metastasis in penile carcinoma: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. J Urol 168:81–86
Martins AC, Faria SM, Cologna AJ, Suaid HJ, Tucci S Jr (2002) Immunoexpression of p53 protein and proliferating cell nuclear antigen in penile carcinoma. J Urol 167:89–93
Levi JE, Rahal P, Sarkis AS, Villa L (1998) Human papillomarvirus DNA and p53 status in penile carcinomas. Int J Cancer 76:779–783
Gentile V, Vicini P, Giacomelli L, Cardillo MR, Pierangeli A, Degener AM (2006) Detection of human papillomavirus DNA, p53 and ki67 expression in penile carcinomas. Int J Immunopathol Pharmacol 19:209–215
Goebell PJ, Groshen SG, Schmitz-Drager BJ (2010) p53 immunohistochemistry in bladder cancer—a new approach to an old question. Urol Oncol 28:377–388
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poetsch, M., Hemmerich, M., Kakies, C. et al. Alterations in the tumor suppressor gene p16 INK4A are associated with aggressive behavior of penile carcinomas. Virchows Arch 458, 221–229 (2011). https://doi.org/10.1007/s00428-010-1007-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-010-1007-4