Abstract
The immunohistochemical expression of phosphorylated (activated) Akt (pAkt) in 50 advanced gastric carcinomas has been analyzed and the results correlated with age, sex, location in the stomach, histotype, stage, survival, mitotic and apoptotic index, some cell cycle regulators (cyclin D1, cyclin E, p34/cdc2, p27/kip1), and cell proliferation. There was a statistically significant direct correlation between pAkt expression (both cytoplasmatic and nuclear) and depth of infiltration of the tumor, number of infiltrated lymph nodes and p34/cdc2 expression, and between prevalently nuclear pAkt and cyclin D1 and cyclin E. Conversely, there was a significant inverse correlation between nuclear pAkt and apoptotic index and between cytoplasmatic and nuclear pAkt and patient survival. No correlation was found between pAkt and sex, age, tumor location, histotype, mitotic index, and cell proliferation. These findings suggest that pAkt may be considered an indicator of tumor progression and patient survival in gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is one of the most common malignancies worldwide that originates through a multi-stage process involving multiple interacting genetic and environmental factors. It is known that chronic gastric infection due to Helicobacter pylori is associated with a six-fold greater risk of stomach cancer, although the molecular mechanism by which H. pylori predisposes to cancer is not well established [1]. As other cancers, gastric carcinoma develops through the accumulation of several genetic alterations, such as the inactivation of tumor suppressor genes and/or the activation of oncogenes [2, 3], however, the underlying critical molecular mechanism of its progression is largely not yet understood. It has recently been demonstrated that the proto-oncogene Akt/protein kinase B plays an important role in neoplastic transformation. Overexpression of Akt has an anti-apoptotic effect in many cell types, resulting in resistance to, or delay of, cell death. Akt regulates cell survival through the phosphorylation of downstream substrates that directly or indirectly control the apoptotic machinery [4–6]. Immunohistochemical studies using specific antibodies against the activated form, phosphorylated Akt (pAkt), have shown that Akt activity is detectable in many tumors, such as myeloma and breast, colon, ovary, prostate, lung, kidney, and pancreatic cancers [7–9]. Activated Akt has been functionally linked to a poor prognosis in many cancers [10, 11] and it has been shown that it can promote resistance to chemo- and radiotherapy [12–14]. It has also recently been demonstrated that pAkt expression is associated with increased resistance to multiple chemotherapeutic agents in gastric cancer patients [15].
No gene mutation of Akt has been reported in cancer, even though its amplification has been found in many tumors [16–18]. The centrality of Akt to gastric physiology and neoplastic transformation has been suggested by the cloning of this oncogene from a gastric adenocarcinoma in which the gene was amplified and by its potent promitogenic action on gastric cancer cells in vitro [18, 19]. pAkt protein modulates the function of numerous substrates related to the regulation of cell proliferation, such as cyclin-dependent kinase inhibitors, p21/Waf1/Cip and p27/Kip1 [20, 21], and cyclin D1 and cyclin E [5]. p27/Kip1 is a cyclin-dependent kinase inhibitor that mainly binds cyclin D1/cdk4 and cyclin E/cdk2 complexes, thus blocking the G1/S transition necessary for cell cycle progression [22]. p27/Kip1 expression is mainly regulated through degradation by ubiquitin-dependent proteolysis and the protein level is up-regulated under stress conditions, leading to cell cycle inhibition and apoptosis [22, 23]. Additional roles for p27/Kip1 have also been proposed recently, including tumor suppression [24], regulation of cell migration [25], and mitosis [26]. It has been shown that Akt phosphorylates p21/Waf1 and p27/Kip1 and inhibits their anti-proliferative effects [27–29]. In particular, phosphorylated p27/Kip1 is exported from the nucleus to the cytoplasm and degraded by an ubiquitin system and is therefore no longer capable of inhibiting the activation of cyclin/cdk complexes.
In the present study, we examined pAkt by immunohistochemistry in a series of advanced gastric carcinomas and correlated its expression with clinicopathological parameters, mitotic and apoptotic index, cell cycle regulators (p27/Kip1, cyclin D1 and cyclin E, p34/cdc2) and cell proliferation (Mib1), with the aim of evaluating whether the expression of pAkt may be related to tumor progression and considered a prognostic indicator in gastric cancer.
Materials and methods
Patients
50 cases of advanced gastric cancer were analyzed. Patients had undergone curative resection (R0 gastrectomy with D2 lymphadenectomy) in the Division of Surgical Oncology, Siena University Hospital, Italy, between 1994 and 1997, and had been diagnosed at the Division of Pathological Anatomy and Histopathology, Siena University Hospital, Italy. None of the patients underwent pre- or post-operative chemotherapy or radiotherapy. Patients were followed up from the date of surgery to death or to 31 December 2005 (minimum follow-up period—5 years). The age of the patients (34 males and 16 females) ranged from 34 to 83 years. Tumor localization was in the gastric cardias (seven cases), gastric body (14 cases), and antrum (29 cases).
Histopathology
Samples of the surgical specimens were immediately fixed in 10% buffered formalin for 24 h and then processed for routine paraffin embedding. Five-micrometer-thick sections were stained with hematoxylin–eosin (HE) to determine the stage (TNM) [30] and the histotype according to Lauren [31].
According to the TNM classification, 23 cases (46%) were T2, 26 (52%) T3, and one case (2%) T4. Sixteen cases (32%) were negative for lymph node invasion and 34 (68%) were positive. In the positive cases, 12 (35%) were N1, eight (24%) were N2, and 14 (41%) were N3.
At the time of the study, further HE sections were examined to confirm stage and histotype: 31 cases (62%) were intestinal and 19 (38%) diffuse. Contiguous sections were stained with Azur A to count mitoses and apoptoses. Mitotic index (MI) and apoptotic index (AI) were calculated as the percentage of cancer cells in mitosis or apoptosis in 15–20 randomly selected fields at 400× [32].
Immunohistochemistry
Five-micrometer-thick sections were deparaffinized, rehydrated, immersed in citric acid buffer (pH6.0) and incubated in a 750 W microwave oven twice for 5 min. Primary monoclonal antibodies were used for phosphorylated Akt (Ser-473, New England Biolabs, Ipswich, MA, USA), Ki67/Mib1 (clone SP6, Neomarkers, Fremont, CA, USA), cyclin D1 (clone SP4, NeoMarkers, Fremont, CA, USA), cyclin E (clone CYE05, NeoMarkers, Fremont, CA, USA), p27/Kip1 (clone DCS-F2F6, NeoMarkers, Fremont, CA, USA) and p34/cdc2 serine/threonine kinase/cdk AB1 (clone A 17.1.1, NeoMarkers, Fremont, CA, USA). All slides were incubated with the primary antibody for 1 h and with the secondary antibody (Ultra Vision Large Volume Detection System, anti-polyvalent, HRp, Lab Vision, Fremont, CA, USA) for 20 min. The binding reaction was detected using 3,3′-diaminobenzidine (DAB; DAKO Corporation, Carpinteria, CA, USA) and slides were then counterstained with hematoxylin.
In each case, ten high power (400×) fields were selected and at least 1,000 cells were evaluated. pAkt staining was detected in the cytoplasm (pAkt) or in the nuclei (pAktn) of cancer cells. pAkt expression was considered nuclear (pAktn) when nuclear staining was prevalent. For Mib1, cyclin D1 and cyclin E, the localization had to be exclusively nuclear; for p27/Kip1, both nuclear and cytoplasmatic localization were considered for positivity; for p34/cdc2 the localization had to be cytoplasmatic.
Expression of pAkt, pAktn, and p34/cdc2 was graded on a semiquantitative scale, ranging from 0 to 3 (for pAkt and pAktn: 0 = 0, 1 ≤ 15%, 2 = 15–30%, 3 > 30%; for p34/cdc2: 0 = 0, 1 ≤ 25%, 2 = 25–50%, 3 > 50%). Cancers were also classified as pAkt/pAktn negative (<10% positive cells) or pAkt/pAktn positive (>10% positive cells).
For other markers, the positivity was calculated as the percentage of positive cancer cells out of the total amount of cancer cells. The staining patterns of cyclin D1 and cyclin E were classified as follows: negative (none or <5% of cells positive) or positive (>5% of cells positive). p27/Kip1 immunostaining was considered positive if 20% or more of cancer cells were stained.
Reproducibility
The reproducibility of all parameters was assessed by two independent observers in six cases, by repeating counts seven times. The correlation coefficient within and between the two observers was greater than 0.9 for all the parameters.
Statistics
Qualitative data were expressed as frequencies, organized into contingency tables and evaluated using the chi-square test to verify the dependence of observations on two or more categorical variables (Pearson’s chi-square test or the Fisher exact test). Since the Kolmogorov–Smirnov normality test applied to the sample data determined that the quantitative variables could not be considered Gaussian distributed, all statistical analyses were carried out using non-parametric tests. The Mann–Whitney U test was used to compare data using quantitative variables. Survival curves were estimated using the Mantel–Cox test and differences between survival curves were determined using the log-rank test. The results were considered statistically significant for p < 0.05.
Statistical analysis was performed using the SPSS 10.0 statistical software (SPSS, Chicago, IL, USA).
Results
Correlation of pAkt/pAktn expression with clinicopathological parameters
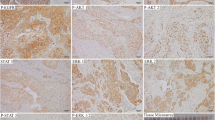
The data related to clinicopathological parameters are summarized in Table 1. pAkt immunostaining was detected in 34 (68%) of the 50 cases analyzed, 17 of which (50%) showed prevalently nuclear positivity (Fig. 1). Among these 34 positive cases, 21 were intestinal type cancers (61.8%) and 13 diffuse type cancers (38.2%). No significant correlation was found between pAkt and pAktn expression and sex, age, tumor location, and Lauren histotype (p > 0.05).
On the other hand, a statistically significant direct correlation was found between pAkt and pAktn expression and depth of infiltration (p = 0.032); patients with high expression of pAkt and pAktn were prevalently allocated in categories T3–T4.
There was also a significant direct correlation between pAkt and pAktn expression and the number of infiltrated lymph nodes (respectively p = 0.011 and p = 0.025; Fig. 2). More than 80% of pAkt/pAktn positive cases had lymph node metastases and 50% of them had more than 15 positive lymph nodes (category N3).
pAkt and pAktn expression had a significantly negative correlation with survival rate (respectively p = 0.039 and p = 0.011; Fig. 3). Five-year survival probability was 18% in patients with high pAkt/pAktn expression vs. 58% in negative cases.
Cumulative survival in relation to pAkt positivity. The difference between the pAkt-negative cases (broken line) and pAkt-positive cases (solid line) is statistically significant (log-rank test, p = 0.039): number of relapsing (total) patients in pAkt negative = 16(34), number of relapsing (total) patients in pAkt positive = 13(16)
Correlation between pAkt/pAktn expression, cell proliferation and apoptosis
pAktn was found to be inversely correlated with apoptotic index (AI; p = 0.043; Fig. 4), while no significant correlation was found between pAkt expression and AI, and between pAkt and pAktn expression and mitotic index (MI) and Mib1 positive cells (p > 0.05; not shown).
p27/kip1 was positive in 19 (38%) of the 50 cases analyzed. Nuclear immunolocalization was detected in most of the cases (68.4%), while only 31.6% of them showed cytoplasmatic localization. Cases with high pAkt and pAktn expression showed higher values of p27/kip1 and of p27 cytoplasmatic overexpression, but the differences were not significant (p > 0.05).
Positive expression of cyclin D1 was detected in 12 of the 50 cases (24%) and of cyclin E in eight cases (16%). A significant correlation was found between pAktn and cyclin D1 (p = 0.018) and cyclin E (p = 0.046). High pAktn expression was associated with a strong positivity for cyclin D1 and cyclin E.
As regards p34/cdc2 expression, 26 cases out of 50 (52%) showed positivity in less than 25% of cells, 22 cases (44%) positivity in 25% to 50% of cells, and two cases (4%) showed positivity in more than 50% of cells. A significant direct correlation (p = 0.019) was found between pAkt and p34/cdc2 expression
Discussion
Akt modulates the function of numerous substrates related to the regulation of cell proliferation and apoptosis and is putatively involved in the development of some cancers. An elevated Akt activation, the phosphorylated Akt (pAkt), has been demonstrated in various malignancies [33] and often correlated with tumor progression, such as in colon (using western blotting and immunohistochemistry) [34], prostate [35], lung [36], and thyroid cancer [37]. It has been shown that Akt activation in cancer cells increases the motility required for tissue invasion and metastases [38] and is consequently related to poor prognosis in many cancers [10, 11, 39, 40]. pAkt can promote resistance to chemo- and radiotherapy in many tumors [12–14] and it has been found to be significantly correlated with cancer progression, cell proliferation and angiogenesis, as well as with chemoresistance in gastric cancer [32, 41–44]. The results of Han et al. [42] are obtained by using immunohistochemistry, western blotting, and polymerase chain reaction (PCR) analyzed on gastric cancer cell lines.
In the present study, pAkt was expressed in 34 (68%) of the 50 tumors analyzed, 50% of which showed a prevalently nuclear localization. pAkt/pAktn positivity was found to be directly correlated with the number of infiltrated lymph nodes and depth of tumor invasion of stomach wall, while it was inversely correlated with patient survival rate. Our data are in agreement with a previous study, reporting that Akt activation is related to a poor outcome in gastric cancer [41]. We also analyzed the relationship between pAkt expression and apoptotic index, because Akt has been reported to be a signal transduction protein capable of controlling the balance between cell survival and apoptosis [5, 45]. It is known that phosphorylation of Akt is promoted by phosphatidylinositides converted by PI3K products at the cytoplasmatic level. Phosphorylated Akt delivers anti-apoptotic survival signals by phosphorylating Bad and activating caspase-9 at the cytoplasmatic level [46, 47], as well as by inhibiting the function of some nuclear proteins at the nuclear level, such as forkhead transcription factors, which are involved in the apoptotic response. Akt-dependent phosphorylation of forkhead proteins leads to their exclusion from the nucleus and to loss of transcription targets such as Bim and Fas-ligand in the nucleus [6]. In the present study, we found that high expression of pAktn is correlated with a low apoptotic index, confirming that a nuclear localization of pAkt is necessary to negatively control the apoptotic response in cancer cells.
The Akt pathway has also been implicated in altering p27/Kip1 activity. The cyclin-dependent kinase inhibitor p27/kip1 is a putative tumor suppressor factor in human cancer. It has been demonstrated that the serine/threonine kinase Akt regulates cell proliferation in cancer by preventing p27/kip1-mediated growth arrest. p27/kip1 phosphorylation, induced by pAkt, causes retention of p27/kip1 in the cytoplasm and, thus, prevents cell cycle arrest induced by p27/kip1. Phosphorylated p27/kip1 accumulates in the cytoplasm of cancer cells in coincidence with Akt activation and is subsequently degraded by the ubiquitin system [5, 21, 29]. In this way, the growth inhibitory properties of p27/kip1 are functionally inactivated and the proliferation of cancer cells is sustained. Although we did not find a statistically significant correlation between pAkt/pAktn and p27/Kip1 expression in our study, we observed that cases with high pAkt/pAktn expression had a high level of cytoplasmatic p27/kip1 expression. This result is in agreement with previous studies [5, 27, 29] performed on breast cancer, in cell culture, by site-directed mutagenesis and transfection, by Akt immunoblotting activation, as well as by immunohistochemistry, BrdU incorporation, and indirect immunofluorescence.
pAkt is also implicated in controlling cyclin D1 and cyclin E activity, thus, contributing to the regulation of cell cycle progression. pAkt prevents the degradation of cyclins D1 and E, which is induced by an ubiquitin-dependent proteolysis pathway, because it inactivates GSK3, which is necessary for their phosphorylation, translocation from nucleus to cytoplasm and degradation [5]. Both cyclin D1 and cyclin E have been found to be deregulated and overexpressed in various types of cancers and seem to play an important role in the progression and biological behavior of cancer. Overexpression of both these cyclins has also been found in gastric cancer, but their prognostic value is still controversial. For cyclin D1, no significant correlation has been found between its immunohistochemical expression and any of the more frequently considered clinicopathological parameters [48–50]. On the other hand, cyclin E overexpression was found to be directly correlated with the number of infiltrated lymph nodes and the low incidence of T1 and stage I tumors, as well as with p53 overexpression in early gastric cancer [49, 51, 52]. On the basis of these findings, it may be argued that cyclin E overexpression is a useful prognostic indicator in gastric carcinoma, quite the contrary to cyclin D1. In this study, a significant correlation was found between pAktn and both cyclin D1 and cyclin E expression, suggesting that the pAkt may promote also gastric cancer progression by controlling cyclins D1 and E, which are two critical players in the cell cycle.
In conclusion, our preliminary results indicate that pAkt controls the activation of p27/kip1, cyclins D1 and E and apoptosis, which play an important role in the cell cycle. Hence, it is not surprising that pAkt may be correlated with cancer progression and patient survival in gastric cancer, as it may be in other malignancies. However, to reach a conclusion, our results need to be confirmed in larger series of cases.
References
Axon A (2002) Review article: gastric cancer and Helicobacter pylori Alimet. Pharmacol Ther 16(4):83–88
Saegusa M, Takano Y, Kamata Y et al (1996) Bcl-2 expression and allelic loss of the p53 gene in gastric carcinomas. J Cancer Res Clin Oncol 122:427–432
Endoh Y, Sakata K, Tamura G et al (2000) Cellular phenotypes of differentiated-type adenocarcinomas and precancerous lesions of the stomach are dependent on the genetic pathways. J Pathol 191:257–263
Fresno Vara JA, Casado E, de Castro J et al (2004) PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 30:193–204
Chang F, Lee JT, Navolanic PM et al (2003) Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia 17:590–603
Wang R, Brattain MG (2006) AKT can be activated in the nucleus. Cell Signaling 18:1722–1731
Roy HK, Olusola BF, Clemens DL et al (2002) AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis 23:201–205
Altomare DA, Tanno S, De Rienzo A et al (2003) Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem 88:470–476
Alkan S, Izban KF (2002) Immunohistochemical localization of phosphorylated AKT in multiple myeloma. Blood 99:2278–2279
Yamamoto S, Tomita Y, Hoshida Y et al (2004) Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res 10:2846–2850
Ermoian RP, Furniss CS, Lamborn KR et al (2002) Dysregulation of PTEN and protein kinase B is associated with glioma histology and patient survival. Clin Cancer Res 8:1100–1106
Clark AS, West K, Streicher S et al (2002) Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther 1:707–717
Brognard J, Clark AS, Ni Y et al (2001) Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 61:3986–3997
Tanno S, Yanagawa N, Habiro A et al (2004) Serine/threonine kinase AKT is frequently activated in human bile duct cancer and is associated with increased radioresistance. Cancer Res 64:3486–3490
Oki E, Baba H, Tokunaga E et al (2005) Aky phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int J Cancer 117:376–380
Bellacosa A, de Feo D, Godwin AK et al (1995) Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer 64:280–285
Cheng JQ, Ruggeri B, Klein WM et al (1996) Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA 93(8):3636–3641
Staal SP (1987) Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA 84:5034–5037
Rusnak DW, Lackey K, Affleck K et al (2001) The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther 1:85–94
Diehl JA, Cheng M, Roussel MF et al (1998) Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Gen Dev 12:3499–3511
Liang J, Zubovitz J, Petrocelli T et al (2002) PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 8:1153–1160
Philipp-Staheli J, Payne SR, Kemp CJ (2001) p27(Kip1): regulation and function of a haplo-insufficient tumor suppressor and its misregulation in cancer. Exp Cell Res 264:148–168
Pagano M, Tam SW, Theodoras AM et al (1995) Role of the ubiquitin–proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269:682–685
Fero M, Randel E, Gurley KE et al (1998) The murine gene p27Kip1 is haplo-insufficient for tumor suppression. Nature 396:177–180
Besson A, Gurian-West M, Schmidt A et al (2004) p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev 18:862–876
Nakayama K, Nagahama H, Minamishima YA et al (2004) Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell 6:661–672
Liang J, Slingerland JM (2003) Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2:339–345
Shin I, Yakes FM, Rojo F et al (2002) PKB/Akt mediates cell-cycle progression by phosphorylation of p27 (kip1) at threonine 157 and modulation of its cellular localization. Nat Med 8:1145–1152
Viglietto G, Motti ML, Bruni P et al (2002) Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med 8:1136–1144
Greene FL, Page DL, Fleming ID et al (eds) (2002) American Joint Committee on Cancer Staging Manual, 6th ed. Springer, New York
Lauren T (1965) The two histologic main types of gastric carcinoma: diffuse and so called intestinal type. Acta Pathol Microbiol 64:31–49
Vindigni C, Miracco C, Spina D et al (1997) Cell proliferation, cell death and angiogenesis in early and advanced gastric cancer of intestinal type. Int J Cancer 74:637–641
Cicenas J (2008) The potential role of Akt phosphorylation in humane cancers. Int J Biol Markers 23:1–9
Itoh N, Semba S, Masafumi I et al (2002) Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 94:3127–3134
Liao Y, Grobholz R, Abel U et al (2003) Increase of AKT/PKB expression correlates with Gleason pattern in human prostate cancer. In J Cancer 107:676–680
Okudela K, Hayashi H, Ito T et al (2004) K-ras gene mutation enhances motility of immortalized airway cells and lung adenocarcinoma cells via Akt activation: possible contribution to non-invasive expansion of lung adenocarcinoma. Am J Pathol 164:91–100
Vasko V, Saji M, Hardy E et al (2004) Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet 41:161–170
Grille SJ, Bellicosa A, Upson J et al (2003) The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res 63:2172–2178
Schmitz KJ, Otterbach F, Callies R et al (2004) Prognostic relevance of activated Akt kinase in node-negative breast cancer: a clinicopathological study of 99 cases. Mod Pathol 17:15–21
Horiguchi A, Oya M, Uchida A et al (2003) Elevated Akt activation and its impact on clinicopathological features of renal cell carcinoma. J Urol 169:710–713
Murakami D, Tsujitani S, Osaki T et al (2007) Expression of phosphorylated Akt (pAkt) in gastric carcinoma predicts prognosis and efficacy of chemotherapy. Gastric Cancer 10:45–51
Han Z, Wu K, Shen H et al (2008) Akt1/protein kinase Bα is involved in gastric cancer progression and cell proliferation. Dig Dis Sci 53:1801–1810
Lee BL, Kim WH, Jung J, Cho SJ et al (2008) A hypoxia-independent up-regulation of hypoxia-inducible factor-1 by AKT contributes to angiogenesis in human gastric cancer. Carcinogenesis 29:44–51
Yu HG, Ai YW, Yu LL et al (2008) Phosphoinositide 3-kinase/Akt pathway plays an important role in chemoresistance of gastric cancer cells against etoposide and doxorubicin induced cell death. Int J Cancer 122:433–443
Kobayashi I, Semba S, Matsuda Y et al (2006) Significance of Akt phosphorylation on tumor growth and Vascular Endothelial Growth Factor expression in human gastric carcinoma. Pathobiology 73:8–17
Cardone MH, Roy N, Stennicke HR et al (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321
Brunet A, Bonni A, Zigmond MJ et al (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868
Takano Y, Kato Y, Masuda M et al (1999) Cyclin D2, but not cyclin D1, overexpression closely correlates with gastric cancer progression and prognosis. J Pathol 189:194–200
Aoyagi K, Koufuji K, Yano S et al (2000) Immunohistochemical study on the expression of cyclin D1 and E in gastric cancer. Kurume Med J 47:199–203
Chetty R, Sitti CW (2003) Cyclin E immunoexpression in gastric cancer does not correlate with clinicopathological parameters. Histopathology 42:66–69
Jiaqing L, Hokita S, Xiangming C et al (1998) Role of cyclin E and p53 expression in progression of early gastric cancer. Gastric Cancer 1:160–165
Bani-Hani KE, Almasri NM, Khader YS et al (2005) Combined evaluation of expressions of cyclin E and p53 proteins as prognostic factors for patients with gastric cancer. Clinical Cancer Research 11:1447–1453
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cinti, C., Vindigni, C., Zamparelli, A. et al. Activated Akt as an indicator of prognosis in gastric cancer. Virchows Arch 453, 449–455 (2008). https://doi.org/10.1007/s00428-008-0676-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-008-0676-8