Abstract
Intrahepatic nerves serve important metabolic, sensory and motor functions. Their ontogeny in human liver has not been elucidated. We aimed to characterise the ontogeny of human intrahepatic innervation, to assess its relationship with biliary structures and to examine the distribution and nature of peptidergic nerves during development. We used immunohistochemistry on archival normal human liver tissue from 63 fetuses [8–40 gestational weeks (gw)] and 10 adults with antibodies to pan-neural markers and neuropeptides. Few nerve fibers appeared in portal tracts at 8 gw. Their density increased gradually from 12 gw and reached adult levels at 32–33 gw. Rare intra-acinar nerves, restricted to periportal areas, appeared at 40 gw. Galanin-, somatostatin- and calcitonin-gene-related peptide-positive nerve fibers were noted only in portal tracts from 22, 26 and 32 gw, respectively. In human adult liver, dense portal and intra-acinar neural supply was observed. Human fetal liver contains a neural network distributed mainly in portal tracts with a density that increases progressively towards term. Intra-acinar innervation appears at term, suggesting that is not required for normal liver function during development, while peptidergic nerves are important for intrauterine liver functions. Developmentally regulated expression of galanin and somatostatin may play a role in liver morphogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver of most mammalian species, including man, is richly innervated by afferent and efferent sympathetic and parasympathetic fibers. Efferent nerve fibers are implicated in the regulation of hepatic hemodynamics [16, 20], microvasculature [35] and carbohydrate and lipid metabolism [16, 26], while afferent nerve fibers may serve sensory functions, such as ionoreception, baroreception, osmoregulation and control of metabolic receptors [2, 8]. Recent studies have shown that sympathetic nerves may regulate liver repair by modulating hepatic stellate cells and hepatic epithelial progenitors [23–25]. The latter may also be activated by stimulation of the vagal nerve [6] and may receive nerve endings [7]. The putative anatomical “niche” of hepatic progenitor cells in human liver lies in the canal of Hering, which conducts bile from canaliculi to terminal bile ducts in portal tracts [28]. It is not as yet clear whether intrahepatic nerves exert their influence on hepatic progenitor cells through direct nerve–progenitor cell contact, nerve contact with intermediate cell effectors or through diffusion of neurotransmitters.
Portal tract innervation is remarkably consistent across mammalian species [4, 13–15, 20] with adrenergic nerves forming an intrinsic plexus around the walls of portal tract blood vessels and less frequently in relation to bile duct radicles [4]. The latter, including bile ductules and canals of Hering, are the site of close nerve–cholangiocyte contact [36].
The extent and acinar distribution of intra-acinar nerve fibers is very variable and shows significant differences between species. While rat and mouse livers have only limited intrasinusoidal innervation, the livers of man and guinea pig show rich intra-acinar innervation [3, 4, 13, 14, 19, 20]. In human adult liver, afferent and efferent nerve aminergic and peptidergic fibers are distributed unevenly in the acini running along the sinusoidal walls, predominating in the periportal region [29] but also extending to the terminal hepatic venules [4]. Nerve fibers are identified within the space of Disse, between hepatocytes and in close contact with perisinusoidal, endothelial and Kupffer cells [3, 4, 20].
Neuropeptide Y (NPY)-, substance P- and somatostatin-containing nerve fibers are demonstrated in portal tracts and along sinusoidal walls in close contact with hepatocytes and hepatic stellate cells [1, 4, 12, 34]. Vasoactive intestinal peptide (VIP)- and calcitonin-gene related peptide (CGRP) nerve fibers are mainly detected around portal blood vessels and may co-exist in cholinergic and sensory nerves [20, 33]. Nerves containing glucagon, glucagon-like peptide, neurotensin and galanin are found mainly in portal tracts [20, 11]. Cholinergic nerves are restricted in portal tracts with only a few fibers reaching periportal hepatocytes and sinusoids [3, 20].
Few studies have been conducted on the ontogeny of liver innervation [8]. In developing mice and rats, intrahepatic NPY-ergic nerve fibers first appear at the late fetal period, increase in density with age and reach adult levels at 2 weeks postnatal [9, 10]. In infant guinea pig liver (3 and 7 days postnatal), the distribution and density of NPY-ergic fibers is similar to that observed in the adult [9]. The ontogeny of human intrahepatic innervation has not been studied extensively, and the anatomical relationship of nerve fibers with bile ducts and possibly with proximal biliary structures (bile ductules and canals of Hering), a putative hepatic progenitor cell “niche”, has not been assessed during liver development. Our group has already published a preliminary study in a small number of second- and third-trimester fetuses, showing the presence of nerve fibers in portal tracts from 20 gestational weeks (gw), reaching adult levels at 32 gw. Intrasinusoidal innervation was detected in human fetal liver only at term [31].
The present immunohistochemical study defines the ontogeny of human intrahepatic innervation using more detail in a large number of cases, including first-trimester fetuses, to assess the distribution and the nature of peptidergic nerves in human fetal liver and to examine the relationship of intrahepatic nerve fibers with biliary structures during development.
Materials and methods
Our material consisted of archival normal liver tissue from 63 human fetuses (gestational ages, 8–40 weeks) and 10 adults (normal areas from surgical liver specimens). The fetal material was obtained from therapeutic and spontaneous abortions; no fetal malformations or other pathology was detected at autopsy. According to the autopsy protocols at that time, one tissue block from the right lobe of the liver was collected per case. Tissue blocks from first-trimester cases, however, may have included part of other lobes and the hilum. Normal adult liver tissue was obtained from surgical specimens of liver resection for benign tumour distant from the neoplasm. The study was performed in accordance with National Bioethical Standards and ethical standards of the Helsinki Declaration of World Medical Association (2000) and with appropriate consent for use of anonymised archival tissue for research.
Tissue blocks were fixed in either Bouin’s fixative or 10% buffered formalin (with mercuric chloride post-fixation) and then were routinely paraffin-embedded. Approximately 5 μm serial sections were cut from each block and were mounted onto poly-l-lysine- (Sigma, St. Louis, MO, USA) coated slides. The peroxidase–antiperoxidase immunohistochemical technique with antibodies specific for “pan-neural” markers and neuropeptides (Table 1) was performed. All steps were carried out in a 0.25 M phosphate buffer saline (PBS, pH = 7.4) at 25°C. After deparaffinization and rehydration in a series of graded ethanols, sections were incubated in 0.9% hydrogen peroxide (H2O2, Sigma) in methanol for 30 min in the dark, to inactivate endogenous peroxidase, and then were rinsed in tap and subsequently in distilled water. After 4 × 4 min treatment with citric acid 0.001 M in a microwave oven (800 W), sections were rinsed in distilled water, PBS and then incubated for 20 min in 20% normal rabbit serum (NRS, Dako A/S Denmark) or 20% normal swine serum (NSS, Dako, Denmark) in PBS. Sections were then incubated for 1 h at room temperature with the primary antibodies diluted in 0.25 M PBS containing 0.5% NRS or NSS (dilutions shown in Table 1). After thorough rinse with PBS, the sections were incubated with the secondary antibody (rabbit anti-mouse or swine anti-rabbit), diluted in PBS (1:200 for monoclonal antibodies and 1:300 for polyclonal antisera) for 30 min at room temperature. The sections were then washed in PBS and incubated with the peroxidase–antiperoxidase complex (Dako), diluted 1:100 in PBS, for 25 min. Following rinses with PBS, sections were incubated with a solution of 3,3′diaminobenzidine tetrahydrochloride (DAB) 25 g/100 ml in PBS (Sigma) containing 80 μl H2O2 (Sigma). Nickel enhancement was applied in some experiments. Sections were then washed in tap water, counterstained with haematoxylin for 15 s, dehydrated in graded ethanols, cleared in xylene, mounted with dibutyl polystyrene xylene (DPX) (BDH, England) and coverslipped. Negative control sections were used for each case in every immunostaining run, consisting of a duplicate section from each case treated as above with the exception that the incubation with the primary antibody was omitted. Nerve fibers in normal colonic wall tissue sections were used as positive control. Where applicable, distinction between immunopositive fibers and cells was based on known differences in their shape and size aided by the identification of nucleus in the latter.
The density of immunostained nerve fibers was scored semi-quantitatively with a scale of «−» to «+++» as previously described in studies on the ontogeny of solid-organ intraparenchymal innervation [32]. Briefly, «+++» corresponds to the highest density of portal or intra-acinar nerves, «++» refers to an intermediate nerve fiber density, «+» is used when only few immunopositive nerve fibers are recognized, and «−» shows that there are no immunostained nerve fibers. The slides were scored separately by two pathologists (D.G.T., J.M.). There were no significant differences between observers’ scoring results.
Results
The topography and density of the intrinsic innervation of human fetal and adult liver is summarised in Table 2, while the distribution and density of peptidergic nerves is shown in Table 3. There were no significant differences in the density of immunostained nerve fibers between cases within in each of the time periods shown.
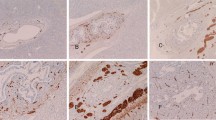
The distribution of neurone-specific enolase (NSE)-, neurofilament-, S-100 protein-, protein gene product 9.5 (PGP9.5)-, neural cell adhesion molecule (N-CAM)- and synaptophysin-positive nerve bundles in the portal tracts of fetal liver appeared similar to that observed in the human adult liver, i.e. many fibers were identified in close apposition to hepatic artery branches, fewer were related to portal vein branches, and only occasional fibers were detected close to bile duct radicles (Fig. 1). However, the latter were observed more frequently in fetal liver than in adult liver, at least until the 32nd gestational week. Occasional PGP9.5-positive fibers were detected close to the ductal plate, and in some first- and early second-trimester fetuses, scattered neurofilament- and N-CAM-positive cells were identified in close proximity to the ductal plate. Rare S100- and PGP9.5-immunoreactive fibers were identified in the walls of large hepatic veins at 29–32 gw, while there was no immunoreactivity for any marker within the walls of terminal hepatic veins. The density of the portal nerve bundles increased with the gestational age and reached adult levels at 32nd–33rd gestational week.
Fetal liver, portal tract. a 20 gw, NSE-positive nerve fibers (arrows), DAB chromogen, ×100. b 29 gw, NSE-positive nerve fibers. Few nerve fibers are noted in the vicinity of a bile duct (arrow), DAB, ×200. c 29 gw, PGP9.5-positive nerve bundles. Some nerve fibers are shown close to the interlobular bile duct (arrow), nickel-DAB chromogen, ×200. d 29 gw, S-100-positive nerve bundles and fibers in a large portal tract. In the rectangle, a bile ductule is shown, nickel-DAB, ×200. e Magnification of the area in rectangle of d. An S-100-positive nerve fiber (thin arrow) is present in close apposition to the wall of the bile ductule (Bd), while a small nerve bundle is noted in the vicinity (thick arrow), nickel-DAB. f 33 gw, S-100-positive nerve fibers (arrows), probably originating from the nerve bundle showing at the upper right, in the wall of a terminal hepatic artery, nickel-DAB, ×400. PV Terminal portal vein branch, HA terminal hepatic artery branch, BD interlobular bile duct
Few neurofilament-positive nerve fibers were observed in the portal tracts of 8–10 gw fetal liver, while all other “pan-neural” markers failed to identify intrahepatic nerve fibers at this stage. In a single case at 8 gw, sections included the hilum where the neurofilament-positive nerve fibers were most abundant. The density of nerves in portal tracts increased from 12th gestational week onwards and were identified with all the pan-neural markers used. Greater numbers of nerve fibers were detected using PGP9.5 than any of the other markers. There were no differences in immunostaining intensity with these markers between portal tracts of different sizes.
Intra-acinar nerve fibers, positive only for S-100 protein and PGP9.5, first appeared in human fetal liver only at the 40th gestational week, and they were restricted in periportal areas (acinar zone 1). In adult liver, dense intra-acinar innervation was identified using all “pan-neural” markers. Most intrasinusoidal nerve fibers were positive to PGP9.5, while lesser numbers were detected using the other markers. N-CAM detected the fewest intra-acinar nerve fibers. Occasional PGP9.5-positive nerve fibers were identified within the walls of terminal hepatic vein radicles.
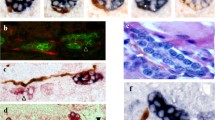
Peptidergic nerves in human fetal liver were identified in portal tracts from 22 gw (Fig. 2). Intrasinusoidal peptidergic nerves fibers were not identified in fetal liver at any gestation. In detail, galaninergic intrahepatic nerve fibers were distributed mainly around hepatic arteries in portal tracts from 22nd gestational week, and they were present until term. However, they were absent from all adult liver cases. Galanin-positive nerve fibers were also noted close to portal vein branches and occasionally in close apposition to interlobular bile ducts in contact with their basement membrane (Fig. 2a,b). CGRP-ergic nerves were detected in low density in the portal tracts of human fetal liver from 32nd gestational week until term (Fig. 2c). Somatostatinergic nerves were detected in portal tracts only between the 26th and 33rd gestational weeks with a distribution similar to that of galaninergic nerves (Fig. 2d–f). A few somatostatinergic nerve fibers were identified around portal bile duct radicles until the 29th week of gestation (Fig. 2d,e). NPY-containing nerves were not demonstrated in any of the human fetal liver cases examined. In contrast, in human adult liver, NPY-ergic nerves were the only peptidergic nerves identified in portal tracts and acini, forming an extensive intrahepatic network.
Fetal liver, portal tract. a 32 gw, Galaninergic nerve fibers with characteristic portal distribution: dense innervation of terminal hepatic arteries, fewer nerve fibers in relation to terminal branch of portal vein and interlobular bile duct, DAB chromogen, ×400. b Magnification of the area in rectangle of a. Galanin-positive nerve fibers (arrows) attached to bile duct wall but not crossing the basement membrane or in between cholangiocytes. c 32 gw, CGRP-immunopositivity (arrow) between two terminal hepatic artery branches in portal tract, DAB, ×200. d 29 gw, somatostatin-positive nerve fibers, DAB, ×200. e Magnification of the area in rectangle of d. Somatostatin-positive nerve fiber in close apposition to the interlobular bile duct not extending between cholangiocytes. f 32 gw, somatostatinergic nerve: dense innervation of the terminal hepatic artery branch (arrow). Notice absence of immunopositive nerve fibers close to the bile duct radicle (arrowhead), DAB, ×200. PV Terminal portal vein branch, HA terminal hepatic artery branch, BD interlobular bile duct
Discussion
We studied the development of intrahepatic innervation in human fetuses using immunohistochemistry with antibodies to pan-neural markers and neuropeptides. We have previously shown that among the pan-neural markers, antibodies to PGP9.5 and S-100 are the most effective in identifying intra-acinar nerve fibers [18]. In the present study, the density of PGP9.5-positive nerve fibers was greater than that of S-100-positive fibers. This is not unexpected as S-100 is reportedly present in the cytoplasm of Schwann cells and not in neural cell axoplasm. Electron microscopic studies have shown that intrasinusoidal nerve fibers are only partially covered by Schwann cell processes [17].
The distribution of intrahepatic innervation in normal human adult tissues in our study was similar to that previously reported [1, 3, 4, 11, 15, 19–21, 29, 33], showing a rich neural supply of portal tracts, mainly around hepatic artery and portal vein branches with less in relation to intralobular bile ducts. PGP9.5-, NSE-, synaptophysin, S-100 and N-CAM-immunoreactive nerve fibers were identified in hepatic acini, along sinusoidal walls, in the space of Disse and in the walls of a few terminal hepatic veins. The intra-acinar distribution of nerve fibers was denser in zone 1 in accordance with previous studies [22, 29]. The close relationship of intra-acinar nerve fibers with hepatocytes may reflect their influence in the regulation of carbohydrate and lipid metabolism [26], while nerve fiber distribution in portal tracts is likely related to their hemodynamic actions [20]. Their presence in the space of Disse appears to be related to their contact with hepatic stellate cells [34] which are thought to be involved in the regulation of hepatic sinusoidal microcirculation by contraction and relaxation [35]. It is known that human and rat hepatic stellate cells, which play significant role in tissue remodelling, production of extracellular matrix components and response to acute liver necrotic injury, express neurotrophins and neurotrophin receptors [5]. Close contact of N-CAM-positive nerve endings with N-CAM-immunoreactive hepatic stellate cells has been observed in human liver [22]. In human liver disease, alterations in the distribution of intrahepatic nerves have been reported. These may play a role in the metabolic disturbances observed in the final stages of chronic liver disorders and in the alterations of intrahepatic blood flow in cirrhosis which may be related to the development of portal hypertension [18].
Zanchi et al. [36] reported close contact between immunoreactive nerve fibers and cholangiocytes of inter-lobular bile ducts and proximal biliary structures (bile ductules and canals of Hering) in human normal adult liver using S-100 immunohistochemistry. We observed occasional nerve fibers in close contact with interlobular bile duct basement membranes in human fetal liver and less often in adult liver, but we did not observe nerve fibers crossing through the basement membrane and located between cholangiocytes. Terada and Nakanuma [30] detected nerve fibers in the walls of large-, medium-sized and septal bile ducts and surrounding peribiliary glands in normal human adult liver. In these branches of the biliary tree, some nerve fibers were seen in contact with epithelial cells. However, only sparse nerve fibers were detected around interlobular bile ducts and bile ductules [30]. This difference may be attributed to the fact that Zanchi et al. [36] examined many serially sectioned levels of the same portal tracts using computer-generated three-dimensional reconstructions and thus have examined in more detail the particular area of interest detecting the terminal location of some nerve fibers between cholangiocytes at the interface with the bile duct lumen. Recent studies have suggested a role for hepatic innervation in liver regeneration and hepatic epithelial progenitor cell modulation supporting the search for a possible anatomical link between intrahepatic nerves and the putative hepatic progenitor cell “niche” in the canals of Hering [6, 7, 22, 23]. It is still unclear, however, whether the cholangiocytes of the canal of Hering or other cells in the same location represent the progenitor cell compartment of the human liver [28].
The N-CAM- and neurofilament-positive cells close to the ductal plates of some first- and second-trimester fetuses could represent progenitor cells which are known to show neuroendocrine features including N-CAM immunoreactivity [27]. N-CAM is a cell-adhesion molecule with a significant regulatory role during development mainly through cell–cell and cell–matrix interactions. N-CAM may modulate the extension of hepatic progenitor cells through the extracellular matrix during fetal life by interacting with hepatic stellate cells, which also express N-CAM [22], and by binding to matrix components like heparan sulphate [27].
We have shown that human fetal liver is relatively poorly innervated during the first two trimesters of gestation with a neural network established in portal tracts, mainly around hepatic artery and portal vein branches and less often in relation to bile ducts. The relative paucity of nerve fibers in relation to ductal plates suggests that they are unlikely to play a major role in their remodelling during development. The density of nerve fibers in portal tracts reaches adult levels between 32nd and 33rd gestational weeks. Intra-acinar nerve fibers are scarce in the human liver before birth suggesting that during human fetal life, direct innervation of hepatocytes and intrasinusoidal innervation is not required for normal liver function in utero. Peptidergic nerves were detected in human fetal liver as early as the 22nd gestational week and were localised in portal tracts mainly around hepatic arteries, in lesser extent close to portal vein branches and less often in relation to bile duct radicles. Some neuropeptides, such as galanin and somatostatin, were expressed only transiently during development. The latter suggests that fetal intrahepatic peptidergic nerves may play important roles in hepatic functions peculiar to life in utero and related to morphogenesis.
In human fetal liver, as in the mammalian adult liver, somatostatinergic nerves and CGRP-ergic nerves are thought to represent afferent sensory fibers [2]. NPY is present in sympathetic efferent nerves to the adult liver and may co-operate with noradrenaline in regulating hepatic blood flow [9, 15]. We have not observed NPY-ergic nerve fibers in human fetal liver. Ding et al. [9] have shown that NPY-immunoreactive fibers appear in the developing mouse liver only in late gestation and that they are present in the liver of neonatal guinea pigs both in portal tracts and in intra-acinar locations. We have shown that in human adult liver, NPY-ergic nerves are the most abundant peptidergic nerve fibers and are distributed in portal tracts in relation to blood vessels and in the acinus along sinusoids, in accordance with previous results of our group and others [1, 4, 9, 12, 21].
In human normal adult liver, many other types of immunoreactive peptidergic nerves may be detected including glucagon-, glucagon-like peptide-, somatostatin-, VIP, neurotensin-, gastrin/cholecystokinin C-terminus-, substance P- and serotoninergic nerve fibers [1, 11, 20, 33]. However, differences exist between studies on the intra-acinar distribution of VIP- and CGRP-ergic nerve fibers and the presence of galaninergic nerves. We have not observed somatostatinergic, galaninergic and CGRP-ergic nerve fibers in human adult liver. The disparities in the immunohistochemical results between studies may be attributed to the diversity in the specificity of the antibodies used, to differences in the methods applied and in the fixation of the specimens.
In conclusion, we showed that the human fetal liver is relatively poorly innervated during the first two trimesters of gestation with a neural network distributed mainly in portal tracts. There is a progressive increase in the density of intrahepatic nerve fibers towards term. The human adult liver receives dense portal and intra-acinar innervation in contrast to fetal liver where intra-acinar innervation appears only at term, suggesting that during development, intrasinusoidal nerves are not required for normal hepatic function. During the third trimester, a rich peptidergic neural network is identified within portal tracts with a transient expression of galanin, somatostatin and CGRP. In human adult liver, NPY-ergic nerve fibers dominate peptidergic innervation in both portal tracts and acini. The differences observed in peptidergic innervation between fetal and adult liver suggest that the fetal intrahepatic peptidergic network may play important role in liver morphogenesis and in hepatic functions peculiar to life in utero.
References
Akiyoshi H, Gonda T, Terada TA (1998) Comparative histochemical and immunohistochemical study of aminergic, cholinergic and peptidergic innervation in rat, hamster, guinea pig and human livers. Liver 18:352–359
Berthoud HR (2004) Anatomy and function of sensory hepatic nerves. Anat Rec 280A:827–835
Bioulac-Sage P, Lafon ME, Saric J, Balabaud C (1990) Nerves and perisinusoidal cells in human liver. J Hepatol 10:105–112
Burt AD, Tiniakos D, MacSween RNM, Griffiths MR, Wisse E, Polak JM (1989) Localization of adrenergic and neuropeptide tyrosine-containing nerves in the mammalian liver. Hepatology 9:839–845
Cassiman D, Denef C, Desmet VJ, Roskams T (2001) Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology 33:148–158
Cassiman D, Libbrecht L, Sinelli N, Desmet V, Denef C, Roskams T (2002) The vagal nerve stimulates activation of the hepatic progenitor cell compartment via muscarinic acetylcholine receptor 3. Am J Pathol 161:521–530
Cassiman D, Sinelli N, Bockx I, Vander Borght S, Petersen B, De Vos R, van Pelt J, Nevens F, Libbrecht L, Roskams T (2007) Human hepatic progenitor cells express vasoactive intestinal peptide receptor type 2 and receive nerve endings. Liver Int 27:323–328
Delalande JM, Milla PJ, Burns AJ (2004) Hepatic nervous system development. Anat Rec 280A:848–853
Ding WG, Kitasato H, Kimura H (1997) Development of neuropeptide Y innervation in the liver. Microsc Res Tech 15:365–371
El-Salhy M, Grimelius L, Emson PC, Falkmer S (1987) Polypeptide YY- and neuropeptide Y-immunoreactve cells and nerves in the endocrine and exocrine pancreas of some vertebrates: and onto- and phylogenetic study. Histochem J 19:111–117
El-Salhy M, Stenling R, Grimelius L (1983) Peptidergic innervation and endocrine cells in human liver. Scand J Gastroenterol 28:809–815
Feher E, Fodor M, Gorcs T, Feher J, Vallent K (1991) Immunohistochemical distribution of neuropeptide Y and catecholamine synthesizing enzymes in nerve fibers of the human liver. Digestion 50:194–201
Feher E, Fodor M, Feher J (1992) Ultrastructural localisation of somatostatin- and substance P immunoreactive nerve fibers in the feline liver. Gastroenterology 102:287–294
Goehler LE, Sternini C, Brecha NC (1988) Calcitonin gene-related peptide immunoreactivity in the biliary pathway and liver of the guinea pig: distribution and co-localization with substance P. Cell Tissue Res 253:145–150
Goehler LE, Sternini C (1991) Neuropeptide Y immunoreactivity in the mammalian liver: pattern of innervation and coexistence with tyrosine hydroxylase immunoreactivity. Cell Tissue Res 265:287–295
Jungermann K, Stumpel F (1999) Role of hepatic, intrahepatic and hepatoenteral nerves in the regulation of carbohydrate metabolism and hemodynamics of the liver and intestine. Hepatogastroenterol 46(suppl 2):1414–1417
Lafon ME, Bioulac-Sage P, LeBail B (1991) Nerves and perisinusoidal cells in human liver. In: Wisse E, Knook DL, McCuskey RS (eds) Cells of the hepatic sinusoid. Kupffer Cell Foundation, Rijswick, pp 230–234
Lee JA, Ahmed Q, Hines JE, Burt AD (1992) Disappearance of hepatic parenchymal nerves in human liver cirrhosis. Gut 33:87–91
Lin YS, Nosaka S, Amakata Y, Maeda T (1995) Comparative study of the mammalian liver innervation: an immunohistochemical study of PGP 9.5, dopamine-beta-hydroxylase and tyrosine hydroxylase. Comp Biochem Physiol A Physiol 110:289–298
McCuskey RS (2004) Anatomy of efferent hepatic nerves. Anat Rec 280A:821–826
Μiyazawa Y, Fukuda Y, Imoto M, Koyama Y, Nagura H (1988) Immunohistochemical studies on the distribution of nerve fibers in chronic liver diseases. Am J Gastroenterol 83:1108–1114
Nakatani K, Seki S, Kawada N, Kobayashi K, Kaneda K (1996) Expression of neural cell adhesion molecule (N-CAM) in perisinusoidal stellate cells of human liver. Cell Tissue Res 283:159–165
Oben JA, Roskams T, Yang S, Lin H, Sinelli N, Li Z, Torbenson M, Huang J, Guarino P, Kafrouni M, Diehl AM (2003) Sympathetic nervous system inhibition increases hepatic progenitors and reduces liver injury. Hepatology 38:664–673
Oben JA, Diehl AM (2004) Sympathetic nervous system regulation of liver repair. Anat Rec 280A:874–883
Oben JA, Roskams T, Sinelli N, Li Z, Torbenson M, Yang S, Lin H, Smedh U, Moran TH, Thomas SH, Diehl AM (2004) Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut 53:438–445
Püschel GP (2004) Control of hepatocyte metabolism by sympathetic and parasympathetic hepatic nerves. Anat Rec 280A:854–867
Roskams T, Cassiman D, De Vos R, Libbrecht L (2004) Neuroregulation of the neuroendocrine compartment of the liver. Anat Rec 280A:910–923
Saxena R, Theise N (2004) Canals of Hering: recent insights and current knowledge. Semin Liver Dis 24:43–48
Scoazec JY, Racine L, Couvelard A, Moreau A, Flejou JF, Bernuau D, Feldmann G (1993) Parenchymal innervation of normal and cirrhotic human liver: a light and electron microscopic study using monoclonal antibodies against neural cell-adhesion molecule. J Histochem Cytochem 41:899–908
Terada T, Nakanuma Y (1989) Innervation of intrahepatic bile ducts and peribiliary glands in normal human livers, extrahepatic biliary obstruction and hepatolithiasis: an immunohistochemical study. Hepatology 9:141–148
Tiniakos D, Tiniakos G, Burt AD (1994) Peptidergic innervation of human fetal liver (abstract). J Hepatol 21(suppl 1):S73
Tiniakos D, Anagnostou V, Stavrakis S, Karandrea D, Agapitos A, Kittas C (2004) Ontogeny of intrinsic innervation in the human kidney. Anat Embryol 209:41–47
Ueno T, Inuzuka S, Torimura T, Sakata R, Sakamoto R, Gondo K, Aoki T, Tanikawa K, Tsutsumi V (1991) Distribution of substance P and VIP in the human liver. Am J Gastroenterol 86:1633–1637
Ueno T, Tanikawa K (1996) Intralobular innervation and lipocyte contractility in the liver. Nutrition 13:141–148
Ueno T, Bioulac-Sage P, Balabaud C, Rosenbaum J (2004) Innervation of the sinusoidal wall: regulation of the sinusoidal diameter. Anat Rec 280A:868–873
Zanchi A, Feldman H, Reidy J, Qualter J, Theise N (2005) Innervation of a intraorgan hepatic progenitor cell “niche” in normal human liver (abstract). Hepatology 42(suppl 1):279A
Acknowledgements
This study was funded in part by “Kapodistrias” Research Program, Special Accounts Research Fund no 70/4/6549, University of Athens, Greece. The antibodies to somatostatin, NPY, galanin and CGRP were kindly supplied by Professor J.M. Polak, Imperial College, University of London, UK.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiniakos, D.G., Mathew, J., Kittas, C. et al. Ontogeny of human intrahepatic innervation. Virchows Arch 452, 435–442 (2008). https://doi.org/10.1007/s00428-007-0569-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0569-2