Abstract
The aim of the study was to investigate nerve fibers (NF) in human fetal livers. An immunohistochemical study was performed. NF were classified into portal tract innervation (PoI) and parenchymal innervation (PaI). The hilum area showed many Pol NF at 7 GW, and NF increased with gestational week (GW). Direct innervations to biliary epithelium were recognized. In large portal tracts, a few NCAM-positive mesenchymal cells were seen at 8 GW and many mesenchymal cells were noted around 12 GW. Apparent NF emerged around 15 GW, and NF increased with GW. Many NF plexuses were seen in 30–40 GW. In small portal tracts, no NF were seen in 7–10 GW. A few NCAM-positive mesenchymal cells emerged in 11 GW, and they increased thereafter. Apparent NF were seen around 20 GW and NF increased with GW. At term (40 GW), PoI NF were still immature. Ductal plate (DP) was positive for NCAM, NSE, chromogranin and synaptophysin, and direct innervations to DP were seen. The direct innervations to developing bile ducts and peribiliary glands were also seen. PaI NF were first seen at 21 GW and was consistent until 40 GW in which a few NF were seen in PaI. These observations suggest that PoI NF arise from committed portal mesenchyme. PaI NF are very immature at 40 GW. There are direct innervations to bile ducts, peribiliary glands, portal veins, hepatic arteries, and DP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mammalian liver is innervated by sympathetic and parasympathetic nerve systems (El-Salhy et al. 1983; Μiyazawa et al. 1988; Goehler et al. 1988; Burt et al. 1989; Feher et al. 1991, 1992; Goehler and Sternini 1991; Lee et al. 1992; Lin et al. 1995; Jungermann and Stumpel 1999; McCuskey 2004; Berthoud 2004; Oben and Diehl 2004; Püschel 2004; Roskams et al. 2004; Akiyoshi 1989; Scoazec et al. 1993; Nakatani et al. 1996; Ueno et al. 1991, 2004; Cassiman et al. 2001, 2002, 2007; Oben et al. 2003, 2004; Ueno and Tanikawa 1996; Bioulac-Sage et al. 1990; Ding et al. 1997; Delalande et al. 2004; Tiniakos et al. 2008). Both the autonomous nerve systems have afferent and efferent nerve fibers (NF) (El-Salhy et al. 1983; Μiyazawa et al. 1988; Goehler et al. 1988; Burt et al. 1989; Feher et al. 1991, 1992; Goehler and Sternini 1991; Lee et al. 1992; Lin et al. 1995; Jungermann and Stumpel 1999; McCuskey 2004; Berthoud 2004; Oben and Diehl 2004; Püschel 2004; Roskams et al. 2004; Akiyoshi 1989; Scoazec et al. 1993; Nakatani et al. 1996; Ueno et al. 1991, 2004; Cassiman et al. 2001, 2002, 2007; Oben et al. 2003, 2004; Ueno and Tanikawa 1996; Bioulac-Sage et al. 1990; Ding et al. 1997; Delalande et al. 2004; Tiniakos et al. 2008). The NF within liver may be myelinated or non-myelinated. The NF are classified by neurotransmitters into aminergic, cholinergic, peptidergic, and nitrergic NF. Some NF are sensory, while others act as motor NF influencing blood flow. The innervation of the mammalian liver is largely classified as portal tract innervation (PoI) and parenchymal innervation (PaI). The PoI is composed of NF located within the portal tracts. The PoI NF are particularly dense around the portal veins and bile ducts, suggesting PoI NF play a role of portal blood flow and biliary functions. There are no significant differences in PoI NF among species. In contrast, the PaI is composed of NF present in the hepatic parenchyma. The Pal NF are associated with innervation of hepatocytes, sinusoidal endothelium, hepatic veins, and probably stellate cells. There are significant differences in PaI NF among species. For example, no PaI was seen in rat and mouse, but well-developed PaI was present in guinea pig and human. Both Pal and PoI have an important role of fat and carbohydrate metabolisms, vascular dilations and contraction, control of portal venous blood flow, and control of biliary tree functions (El-Salhy et al. 1983; Μiyazawa et al. 1988; Goehler et al. 1988). Resent evidence suggests that hepatic stem/progenitor cells (HSPC) (Cassiman et al. 2001, 2002, 2007; Oben et al. 2003) and hepatic stellate cells (HSC) (also called fat-storing cells or Ito cells) (Oben et al. 2004; Ueno and Tanikawa 1996; Ueno et al. 2004; Bioulac-Sage et al. 1990) are directly innervated. Now, it is thought that a significant percentage of hepatocellular carcinoma and intrahepatic cholangiocarcinoma are derived from HSPC, namely stem cell cancers. HSPC also play an important role as a source providing cholangiocytes and hepatocytes under various hepatobiliary disorders. The innervation of HSPC implies that HSPC are controlled by autonomic nervous system (ANS) (Cassiman et al. 2001, 2002, 2007; Oben et al. 2003). Cassiman et al. (2002) showed that vagal nerve stimulates activation of the HSPC via muscarinic acethylcholine receptor type 3. HSC also play an important role in control of sinusoidal blood flow, producing collages, and fat and vitamin deposition. The innervation of hepatic stellate cells implies that sinusoidal blood flow, liver fibrosis, and fat deposition were controlled by ANS. Oben et al. (2004) demonstrated that sympathetic neurotransmitters activate HSC and convert then into myofibroblasts producing collagens. The degree of PaI and PoI, in particular PaI, is different from a species to another (El-Salhy et al. 1983; Μiyazawa et al. 1988; Goehler et al. 1988; Burt et al. 1989; Feher et al. 1991, 1992; Goehler and Sternini 1991; Lee et al. 1992; Lin et al. 1995; Jungermann and Stumpel 1999; McCuskey 2004; Berthoud 2004; Oben and Diehl 2004; Püschel 2004; Roskams et al. 2004; Akiyoshi 1989; Scoazec et al. 1993; Nakatani et al. 1996; Ueno et al. 1991, 2004 ; Cassiman et al. 2001, 2002, 2007; Oben et al. 2003, 2004; Ueno and Tanikawa 1996; Bioulac-Sage et al. 1990; Ding et al. 1997; Delalande et al. 2004; Tiniakos et al. 2008). The NF are associated with mast cells, and neurotransmitters released from NF can induce liver fibrosis (Cassiman et al. 2001, 2002, 2007; Oben et al. 2004). It is also suggested that mast cells themselves are associated with liver fibrosis. These studies of liver innervation have been performed in animal models, and the studies of humans are relatively scant. The human adult livers have well-developed PoI and PaI. Innervation of HSPC and HSC has been suggested to be present in adult human livers. In human liver diseases, the PaI is decreased in chronic hepatitis and Pal disappears in cirrhosis (Lee et al. 1992; Ueno and Tanikawa 1996), while PoI is not decreased but is conversely increased (Μiyazawa et al. 1988; Ueno et al. 1991). However, the development of NF in human fetal livers has rarely been reported.
Materials and methods
The author recently collected 32 human fetal livers. The livers were abortions (spontaneous and artificial), intrauterine fetal death, and autopsies. The gestational week (GW) of the 32 fetal livers was as follows: 7, 8, 9 (n = 2), 10 (n = 3), 11 (n = 2), 12 (n = 3), 13 (n = 2), 14 (n = 2), 15 (n = 2), 16 (n = 2), 17, 18, 19, 21, 23, 24, 26, 29, 30, 36, 38, and 40 GW. The sex was unclear. Informed consent was obtained from each mother. The fetal liver specimens thus obtained were immediately fixed in formalin and embedded in paraffin. Many 3-μm-thin sections were cut, and they were subjected to hematoxylin and eosin (HE) stain and immunohistochemical analysis.
A preliminary immunohistochemical study was performed to select the antibodies appropriate for identifying NF. Five control fetal liver specimens were immunohistochemically stained for a panel of antibodies for chromogranin (clone DAK-A3, Dako Corp, Glostrup, Denmark), synaptophysin (polyclonal, Dako), NSE (polyclonal, Dako), NCAM (clone MOC-1), PgP9.5 (polyclonal, Dako), serotonin (clone 5HT-H209, Dako), S100 protein (polyclonal, Dako), neuropeptide Y (clone 3B5, Abnova Corp), vasoactive intestinal polypeptide (VIP) (polyclonal, IBL, Tokyo, Japan), substance P (polyclonal, LBL, Tokyo, Japan), calcitonin gene-related peptide (polyclonal, ImmunoStar Corp), myelin basic protein (polyclonal, Dako), neurofilament protein (clone 2F11, Dako), N-cadherin (clone 6G11, Dako), gastric (polyclonal, Dako), glucagon (polyclonal, Dako), somatostatin (polyclonal, Dako). As a result, immunostainings of NCAM and NSE most clearly identified NF. Therefore, the author used NCAM and NSE for the study in addition to chromogranin and synaptophysin, both of which are the most sensitive and specific markers of neuroendocrine features. NCAM (CD56) is intercellular adhesion molecule and is normally found in neurons, astrocytes, Schwann cells, myoblasts, and NK lymphocytes. NCAM is now recognized as a pan-neuroendocrine antigen, although, like NSE, the specificity for neuroendocrine marker is relatively weak, compared to chromogranin and synaptophysin. NSE is preferentially found in neurons and neuroendocrine cells. Thus, NSE is used as a pan-neuroendocrine marker. However, unfortunately NSE is also detected in several other cell types of normal and neoplastic cells. Thus, the specificity of NSE for pan-neuroendocrine marker is relatively low, compared to chromogranin and synaptophysin. In other staining with more specific NF markers including PgP9.5, VIP, chromogranin, synaptophysin, NF were not labeled occasionally when HE staining clearly depicts NF, indicating such markers had less sensitivity than did NSE and NCAM. This may be due to reduction in antigenicity during the prefixation in human materials in these stainings. Therefore, the author decided to investigate the materials by immunohistochemical procedures using NCAM and NSE.
An immunohistochemical study was performed with the use of Dako Envision method (Dako Corp) as previously described (Terada and Nakanuma 1989; Terada et al. 2002; Terada and Kawaguchi 2005). The antibodies used were as follows: anti-NCAM (MOC-1, Dako Corp, dilution = 1:200), anti-NSE (polyclonal, Dako Corp, dilution = 1:100), anti-chromogranin (clone DAK-A3, Dako Corp, dilution = 1:100), and anti-synaptophysin (polyclonal, Dako Corp, dilution = 1:150). Microwave pretreatment was performed by each immunohistochemical run. In this study, the term “immature” implies that NF are still underdeveloped in morphology, compared to NF of adult and postnatal children livers, which was deduced by the author’s experience (Terada and Nakanuma 1989) and immunostains of 5 normal adult livers.
Results
The most useful antigen for highlighting NF was NCAM, followed in order by NSE, synaptophysin, and chromogranin. Although NCAM can label NK/T cells and HSC, the distinction from NF was very easy. As mentioned earlier, the stainings of chromogranin, synaptophysin, PgP9.5, and VIP, etc. occasionally missed the NF apparent in HE stainings.
Basically, NF were present in portal tracts = portal tract innervation (PoI) and parenchyma = parenchymal innervation (PaI). No innervation was seen in the hepatic veins from 7 to 40 GW. The Pol was classified into hepatic hilum, large portal tract, and small portal tract.
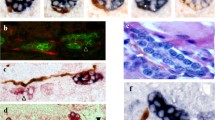
The hepatic hilum area already showed many POI NF in 7 GW (Fig. 1a). At the 7 GW, the PoI NF were evenly present in the hilum portal tract, but NF were relatively dense around the portal veins, bile duct, and hepatic arteries (Fig. 1a). Direct innervations of NF to the portal veins, bile ducts, and hepatic arteries were occasionally seen already in 7 GW. At 16 GW, the hilum NF increased in number and were numerous (Fig. 1b), and they tended to accumulate around the hilum portal structures such as bile ducts, peribiliary glands (PG) (Terada and Nakanuma 1993b), portal veins, hepatic arteries, and peribiliary capillary plexus (PCP) (Terada and Nakanuma 1993a; Terada et al. 1989). The NF increased in number with GW. At 20–30 GW, NF became numerous and large NF appeared. These NF were distributed evenly in the hilum portal area, but they are particularly dense around the hilum portal structures such as bile duct, PB (Terada et al. 1987; Terada and Nakanuma 1987, 1988), portal veins, hepatic arteries, and PCP. At 40 GW, numerous and large, giant, bizarre NF emerged (Fig. 1c) and various NF plexuses around the portal structures were seen. However, the NF status was still immature and did not reach the adult level. At 30–40 GW, NF formed plexuses around the hilum portal structures. Thus, the NF formed the peribiliary NF plexus (Fig. 1d), periportal vein NF plexus, perihepatic artery NF plexus (Fig. 1e), PG NF plexus (Fig. 1f), and peri-PCP plexus. Direct innervations of NF to the portal structures were seen; the NF was in direct continuity with the epithelial cells of bile ducts and PG, smooth muscle cells of the media of the portal veins and hepatic arteries, and capillary of PCP. Interestingly, direct innervations to ductal plate (DP) were occasionally seen in the hilum lesions at 7–15 GW.
Ontogeny of nerve fibers (NF) in hilar area in human fetal livers. The hilar area already shows many NF in 7 GW (a). NF were dense around the portal veins, bile duct, and hepatic arteries (a). Direct innervations or direct touching of NF to the portal veins, bile ducts, and hepatic arteries were occasionally seen already in 7 GW. At 16 GW, the numerous NF are seen in the hilar area (b). At 40 GW, numerous and large, giant, bizarre NF emerge (c). However, the NF status was still immature and does not reach the adult level (c). At 30–40 GW, the NF formed the peribiliary NF plexus (d), periportal vein NF plexus, perihepatic artery NF plexus (e), peri-peribiliary glands NF plexus (f), and peri-peribiliary capillary plexus (PCP). Immunostaining for NCAM: a ×40. b ×20. c ×20. d ×80. e ×40. f ×50
In large portal tracts, only a few round NCAM-positive NSE-positive NF-like round bodies were already seen at 7 GW (Fig. 2a). These NCAM- and NSE-positive bodies were round and did not resemble NF but were very similar to portal mesenchymal cells. In fact, they were the same except for NSE or NCAM positivity (Fig. 2b). At 8 or 9 GW, a small number of NCAM- and NSE-positive NF-like round bodies were found in the large portal tracts. Again, the NCAM- and NSE-positive round bodies did not take a shape of NF but were almost identical to portal mesenchymal cells except for positive neuron markers (Fig. 2d). At 11 GW, many NCAM- and NSE-positive round bodies, large NF-like structures, and fibrilar NF were noted. Apparent NF emerged around 13 GW (Fig. 2b), and NF increased in number with GW. At 18 GW, much more NF emerged, and the NF tended to be localized around the structures such as bile ducts, PG, portal veins, hepatic arteries, and PCP. Direct contact innervations of these structures were frequently seen. At 24 GW, the NF were forming immature peribiliary NF plexus (Fig. 2c), periportal vein NF plexus (Fig. 2d), perihepatic artery NF plexus (Fig. 2e), PG NF plexus (Fig. 2f), and peri-PCP NF plexus (Fig. 2g). Interestingly, innervation of DP, and anlagen of cholangiocytes, was seen in 8–15 GW. At 26–38 GW, numerous NF were seen, and there were immature NF plexus including peribiliary NF plexus, periportal vein NF plexus, perihepatic artery NF plexus, periglandular NF plexus, and pericapillary NF plexus. The NF of these NF plexuses showed direct innervations or direct attachments to the epithelial cells of intrahepatic bile duct and intrahepatic PG and the medial smooth muscle cells of portal veins and hepatic arteries. No direct innervations to endothelial cells of portal veins and hepatic arteries were seen. The NF of peri-PCP did not show direct innervations to the endothelium of the PCP vessels. At 40 GW, numerous NF were seen. The plexus formations were well developed. However, the NF and NF plexus were as yet immature and did not reach the level of the NF of adult livers.
Ontogeny of nerve fibers (NF) of the large portal tract in human fetal livers. In large portal tracts, only a few round NCAM-positive NSE-positive NF-like round bodies are already seen at 7 GW (a). These NCAM- and NSE-positive bodies are round and did not resemble NF but were very similar to portal mesenchymal cells. They increased with age (b), and at 24 GW, the NF were forming immature peribiliary NF plexus (c), periportal vein NF plexus (d), perihepatic artery NF plexus (e), and peri-PCP NF plexus (f). Immunostaining for NCAM: a ×100. b ×200. c ×100. d ×200. e ×100. f ×150
In small portal tracts, no NF were seen in 7–10 GW. One or two NSE- and NCAM-positive round stromal cells emerged in 11 and 12 GW, and they increased thereafter; many NCAM- and NSE-positive NF-like round cells were seen in 14 GW (Fig. 3a). Apparent NF were seen around 20 GW and the NF increased with GW. At 35 GW, many NF were seen. Although at 40 GW NF were located in any areas in the portal tracts, they were accentuated around the developing structures of intrahepatic bile ducts (Fig. 3b), portal veins (Fig. 3c), hepatic arteries (Fig. 3d), and PCP (Fig. 3e). However, this accentuation was mild, and no apparent NF plexuses were seen. The NF at 40 GW were immature and did not reach the adult level. No PG were seen in small portal tracts. Quite interestingly, direct touching innervation to DP is frequently seen (Fig. 3f) at 10–18 GW.
Ontogeny of nerve fibers (NF) of the small portal tract in human fetal livers. In small portal tracts, no NF were seen in 7–10 GW. A few NSE- and NCAM-positive round stromal cells emerged in 11 and 12 GW (b), and they increased thereafter; many NCAM- and NSE-positive NF-like round cells were seen in 14 GW (a). Apparent NF were seen around 20 GW (d) and the NF increased with GW. At 35 GW, many NF were seen (e). Although at 40 GW NF were located in any area in the portal tract (f), they were accentuated around the developing structures of intrahepatic bile ducts (b), portal veins (c), hepatic arteries (d), and peribiliary capillary plexus (e). Interestingly, direct touching innervation (open arrows) to DP (black arrows) is frequently seen (f) at 10–18 GW. Immunostaining for NCAM: a–f ×200
Pal was first seen in 21 GW; only a few NF were seen in the hepatoblasts (Fig. 4a). The degree PaI was consistent until 40 GW (Fig. 4b). At 40 GW, a small amount of Pal NF were seen in the periportal parenchyma. Pericentral or perivenular parenchyma and hepatic veins were free from NF.
Some cells of DP were positive for NCAM, NSE, chromogranin, and synaptophysin (Fig. 5a–d). Interestingly, portal tracts showed unidentified cells positive for the four neuroendocrine antigens. These four neuroendocrine molecules were negative in remodeling DP, remodeled DP, and immature bile ducts.
Expression of neuroendocrine antigen in the ductal plate (DP). Some cells of the DP were positive for NCAM (a), NSE (b), chromogranin (c), and synaptophysin (d). Unidentified cells within portal mesenchyme were positive for NCAM (a) and synaptophysin (e, arrows). a, b, c, d, e immunostains. All, ×400
Discussion
The origin of NF of the human liver is not known. In embryonic livers of rodents, CGRP-positive mesenchymal cells are thought to the precursor of NF in portal tract (Berthoud 2004; Delalande et al. 2004). The present study showed that the hilum area was already PoI innervated at 7 GW. At 40 GW, apparent large and bizarre NF were noted. The NF in the hepatic hilum portal tract are thought to be derived from ectodermal ANS. However, in large and small portal tracts, NCAM-/NSE-positive round bodies were first seen in portal mesenchyme. These bodies increased in number with GW and transformed into NF. The NCAM-/NSE-positive round bodies have no characters of NF, but they were very similar to portal mesenchymal cells expert for NCAM/NSE reactivity. Although the origin of the NCAM-/NSE-positive round bodies cannot be determined, the author thinks that they are derived from portal mesenchyme. The presence of small portal tracts without NF at early stages (7–10 GW) supports this suggestion. It is unlikely that neuroectodermal ANS entered deep into the small portal tracts from the hepatic hilum. If such entrance of ectodermal ANS occurs, the authentic NF, not NCAM-positive round bodies, should be seen. Thus, the NCAM-/NSE-positive round bodies are strongly suggested to have arisen from portal mesenchymal stromal cells. Similar phenomena are seen in the extra- and intrahepatic bile ducts. The extrahepatic bile duct arises directly from embryonic forgot as the hepatopancreatic bud, while intrahepatic bile ducts are derived from the ductal plate which was thought to original from hepatoblasts just adjacent to the portal mesenchyme. The portal mesenchyme is marvelous tissue and appears to have many functions, which are not yet unraveled. For example, the portal mesenchyme induces the neighboring hepatocytes to DP, which is a group of progenitor cells of cholangiocytes. DP is interesting fetal liver structures with potential multifunctional capacities. The innervation of DF in the present study may show that NF influence the DP development and differentiation.
The present study revealed that PoI NF increased with GW. The increase in PoI NF seems to be not NF elongation but NF proliferation. This point should be elucidated by, for example, double staining of Ki-67 and NCAM. The authors recent study revealed high proliferative activity (high Ki-67 labeling index) in the portal parenchyma (unpublished). If NF proliferate in human fetal life, the mechanism of NF proliferation should be performed. In general, it is commonly thought that NF, like muscles, do not proliferate. However, the author demonstrated high number of NF in various chronic hepatobiliary diseases, hepatolithiasis, and intrahepatic cholangiocarcinoma stroma and demonstrated high expression of nerve growth factor in hepatolithiasis (Terada and Nakanuma 1989). These findings apparently indicate that adult NF can proliferate. Taken together, the NF in fetal and adult life seem to do proliferate. The mechanisms of NF proliferation during human liver ontogeny should be elucidated.
The present study demonstrated that NF proliferate with GW and produce NF plexus around the portal structures. Peri-biliary NF plexus directly innervated the biliary epithelial cells. This phenomenon was already observed in adult normal livers and hepatolithiasis (Terada and Nakanuma 1989). Thus, the innervations of the developing bile ducts by the NF plexus may play a certain role in bile duct functions such as contraction, bile secretion, absorption, and mucins secretion. The present study demonstrated that NF proliferate and form NF plexus around the portal veins and hepatic arteries. The NF plexus innervated the muscles of the media of the portal vein and hepatic arteries. These innervations may control portal and arterial blood flow by contraction and dilation of the innervated muscles. The NF also formed plexus around intrahepatic peribiliary glands, in which direct innervations of the glandular epithelium were seen. Intrahepatic peribiliary glands have many important roles such as mucin secretion, bile absorption, local immune defense, endocrine features, serous secretion, neuroendocrine features, and secretion of pancreatic digestive enzymes. Thus, it is conceivable that the NF plexus that innervate the intrahepatic peribiliary glands controls these glandular functions. The present study also showed that the proliferated NF formed a plexus around PCP. PCP plays an important role in the blood supply to the portal mesenchyme in infant or portal tracts in postnatal period. It may also play an important role as bypasses of portal blood flows. Thus, it seems that the proliferated peri-PCP NF plexus controls these functions. In the small portal tracts, there were no well-developed NF plexus, but proliferated NF were accentuated around the bile ducts, portal veins, hepatic arteries and PCP, and direct innervations to these portal structures were frequently seen. Thus, the proliferated NF may control bile duct functions and blood flow of the portal veins, hepatic arteries, and PCP within small portal tracts. The NF also influence the function of portal mesenchyme in large and small portal tracts in addition to the hilum portal tracts.
With regard to PaI innervation, the present study showed PaI first appeared at 21 GW. The PaI of the present human study persisted without changes until term (40 GW). Unfortunately, the present study did not examine PaI after birth. Tiniakos et al. first observed PaI at 40 GW in human NF ontogeny. The difference may be due to different antibodies used or different techniques. The author and others observed PaI in postnatal and adult human livers (El-Salhy et al. 1983; Μiyazawa et al. 1988; Goehler et al. 1988; Burt et al. 1989; Feher et al. 1991, 1992; Goehler and Sternini 1991; Lee et al. 1992; Lin et al. 1995; Jungermann and Stumpel 1999; McCuskey 2004; Berthoud 2004; Oben and Diehl 2004; Püschel 2004; Roskams et al. 2004; Akiyoshi 1989; Scoazec et al. 1993; Nakatani et al. 1996; Ueno et al. 1991, 2004; Cassiman et al. 2001, 2002, 2007; Oben et al. 2003, 2004; Ueno and Tanikawa 1996; Bioulac-Sage et al. 1990; Ding et al. 1997; Delalande et al. 2004; Tiniakos et al. 2008). In my experience, PaI in humans reached the adult level around 15 years of age. In any way, the present study and the study of Taniakos showed that PaI in the human fetal liver is still underdeveloped, and the PaI development of the human livers mainly occurs at postnatal life.
It is interesting that the DP was directory innervated in the present study. The DP is a single or double layered structures with features of cholangiocytes (Terada and Nakanuma 1994, 1995a, b; Terada et al. 1995, 1997, 1998). DP has been thought to lead to intrahepatic bile ducts. Recently, it has been suggested that HSPC and HSC are innervated because these cells have receptors of neurotransmitters and also because direct observation of the innervation was made (Cassiman et al. 2001, 2002, 2007; Oben et al. 2003, 2004; Ueno and Tanikawa 1996; Ueno et al. 2004; Bioulac-Sage et al. 1990). The HSPC are located in the Herring ductile just adjacent to the hepatocytes in human adult livers. The direct innervations of the DP and intrahepatic bile ducts by NF in the large and small portal tracts in human fetal livers seem novel and important findings. The development of HSPC and HSC has not been investigated. The present data and the author’s other data may indicate that DP gives rise to HSPC and the HSPC are innervated in the fetus life. It may be possible that the development of HSPC is controlled by NF. The NCAM, chromogranin, and synaptophysin are among the markers of HSPC. In the present study, some cells of DP were labeled by these molecules, indicating that the DP contains many HSPC and give rise to HSPC. The direct innervation of ductal plate positive for HSPC antigens indicate that HSPC are innervated and are under the controls of autonomous NF. Thus, the DP innervation seems to be indirect evidence for the innervation of HSPC.
NCAM can detect HSPC and HSC (Nakatani et al. 1996). However, the immunohistochemical analysis of NCAM in the present study showed only PaI NF and probable NK lymphocytes. No apparent HSC were found by NCAM immunostaining in the present study. The development of HSC is unknown. Because HSC play an important role in the sinusoidal blood flow, fat and vitamin deposition, and hepatic fibrosis fibrogenesis by transformation to myofibroblasts, and others, its development should be elucidated. The present study suggests that HSC development was not seen in the fetal life but in postnatal life. However, since DP showed NCAM and other stellate cell markers in the current study, it seems possible that DP may be a candidate of source of HSC because human DP can differentiate into hepatocytes. Thus, the present study showed positive expression of markers of both HSPC and HSC in the human DP, suggesting that the human DP may give rise to HSPC and HSC. It is also possible that human DP which contains HSC markers can differentiate into HSC. DP was directly innervated in the present study. The HSC innervations are well explained by these phenomena. In any way, the present study suggests that the DP, NF, and HSC are related association structures, as is the case with DP, NF, and HSPC complexes. Much more studies of the two important cells of HSPC and HSC in association with NF are mandatory. It is plausible that HSC are derived from portal mesenchyme, because the portal mesenchyme has a capacity to differentiate into many cells types as shown in the present study. The portal mesenchyme is a multipotent tissue that merits much more studies.
It was interesting that DP showed cells immunoreactive for NCAM, synaptophysin, and chromogranin. These data show that human DP has neuroendocrine features and lineage, and give rise to neuroendocrine cells in the postnatal livers. In fact, fetal livers of late gestational area and adult livers are known to contain endocrine cells (Terada et al. 1997). These antigens are known to be expressed in HSPC and HSC. The data suggest that HSPC arise from DP in fetal period. It is also possible that HSC are derived at least in part from the DP. Further studies of the development of HSPC and HSC are mandatory.
Finally, the present study showed innervation of DP, bile duct, peribiliary glands, portal veins, and hepatic arteries in the human fetal life. Such NF may be directly innervated by nerve endings to these structures. These data suggest that NF play an important role by controlling blood flow of portal veins and hepatic arteries and biliary duct functions in fetal livers in which NF was as yet immature. The adult liver has fully developed NF, and the NF systems play an important role in controlling the portal and arterial blood flow, bile duct function, peribiliary glands functions, HSC control, and hepatic stem cell control. However, although the autonomic nerve fibers in the liver have important role, the importance appears low. For example, denervation of the liver NF by vagotomy, operation, and transplantation cause no significant problems in the liver functions. These indicate that the autonomous nerve system is not so important with regard to lifestyles and life expectancy.
References
Akiyoshi H (1989) Ultrastructure of cholinergic innervation in the cirrhotic liver in guinea pigs. Neurohistochemical and ultrastructural study. Virchow Arch B 57:81–90
Berthoud HR (2004) Anatomy and function of sensory hepatic nerves. Anat Rec 280A:827–835
Bioulac-Sage P, Lafon ME, Saric J, Balabaud C (1990) Nerves and perisinusoidal cells in human liver. J Hepatol 10:105–112
Burt AD, Tiniakos D, MacSween RNM, Griffiths MR, Wisse E, Polak JM (1989) Localization of adrenergic and neuropeptide tyrosine-containing nerves in the mammalian liver. Hepatology 9:839–845
Cassiman D, Denef C, Desmet VJ, Roskams T (2001) Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology 33:148–158
Cassiman D, Libbrecht L, Sinelli N, Desmet V, Denef C, Roskams T (2002) The vagal nerve stimulates activation of the hepatic progenitor cell compartment via muscarinic acetylcholine receptor 3. Am J Pathol 161:521–530
Cassiman D, Sinelli N, Bockx I et al (2007) Human hepatic progenitor cells express vasoactive intestinal peptide receptor type 2 and receive nerve endings. Liver Int 27:323–328
Delalande JM, Milla PJ, Burns AJ (2004) Hepatic nervous system development. Anat Rec 280A:848–853
Ding WG, Kitasato H, Kimura H (1997) Development of neuropeptide Y innervation in the liver. Microsc Res Tech 15:365–371
El-Salhy M, Stenling R, Grimelius L (1983) Peptidergic innervation and endocrine cells in human liver. Scand J Gastroenterol 28:809–815
Feher E, Fodor M, Gorcs T, Feher J, Vallent K (1991) Immunohistochemical distribution of neuropeptide Y and catecholamine synthesizing enzymes in nerve fibers of the human liver. Digestion 50:194–201
Feher E, Fodor M, Feher J (1992) Ultrastructural localisation of somatostatin- and substance P immunoreactive nerve fibers in the feline liver. Gastroenterology 102:287–294
Goehler LE, Sternini C (1991) Neuropeptide Y immunoreactivity in the mammalian liver: pattern of innervation and coexistence with tyrosine hydroxylase immunoreactivity. Cell Tissue Res 265:287–295
Goehler LE, Sternini C, Brecha NC (1988) Calcitonin gene-related peptide immunoreactivity in the biliary pathway and liver of the guinea pig: distribution and co-localization with substance P. Cell Tissue Res 253:145–150
Jungermann K, Stumpel F (1999) Role of hepatic, intrahepatic and hepatoenteral nerves in the regulation of carbohydrate metabolism and hemodynamics of the liver and intestine. Hepatogastroenterology 46(suppl 2):1414–1417
Lee JA, Ahmed Q, Hines JE, Burt AD (1992) Disappearance of hepatic parenchymal nerves in human liver cirrhosis. Gut 33:87–91
Lin YS, Nosaka S, Amakata Y, Maeda T (1995) Comparative study of the mammalian liver innervation: an immunohistochemical study of PGP 9.5, dopamine-beta-hydroxylase and tyrosine hydroxylase. Comp Biochem Physiol A Physiol 110:289–298
McCuskey RS (2004) Anatomy of efferent hepatic nerves. Anat Rec 280A:821–826
Μiyazawa Y, Fukuda Y, Imoto M, Koyama Y, Nagura H (1988) Immunohistochemical studies on the distribution of nerve fibers in chronic liver diseases. Am J Gastroenterol 83:1108–1114
Nakatani K, Seki S, Kawada N, Kobayashi K, Kaneda K (1996) Expression of neural cell adhesion molecule (N-CAM) in perisinusoidal stellate cells of human liver. Cell Tissue Res 283:159–165
Oben JA, Diehl AM (2004) Sympathetic nervous system regulation of liver repair. Anat Rec 280A:874–883
Oben JA, Roskams T, Yang S et al (2003) Sympathetic nervous system inhibition increases hepatic progenitors and reduces liver injury. Hepatology 38:664–673
Oben JA, Roskams T, Sinelli N et al (2004) Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut 53:438–445
Püschel GP (2004) Control of hepatocyte metabolism by sympathetic and parasympathetic hepatic nerves. Anat Rec 280A:854–867
Roskams T, Cassiman D, De Vos R, Libbrecht L (2004) Neuroregulation of the neuroendocrine compartment of the liver. Anat Rec 280A:910–923
Scoazec JY, Racine L, Couvelard A, Moreau A, Flejou JF, Bernuau D, Feldmann G (1993) Parenchymal innervation of normal and cirrhotic human liver: a light and electron microscopic study using monoclonal antibodies against neural cell-adhesion molecule. J Histochem Cytochem 41:899–908
Terada T, Kawaguchi M (2005) Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med 271:271–275
Terada T, Nakanuma Y (1987) Solitary cystic dilation of the intrahepatic bile duct: morphology of two autopsy cases and a review of the literature. Am J Gastroenterol 82:1301–1305
Terada T, Nakanuma Y (1988) Morphological examination of intrahepatic bile ducts in hepatolithiasis. Virchows Arch 413:167–176
Terada T, Nakanuma Y (1989) Innervation of intrahepatic bile ducts and peribiliary glands in normal livers, extrahepatic biliary obstruction and hepatolithiasis: an immunohistochemical study. J Hepatol 9:141–148
Terada T, Nakanuma Y (1993a) Development of human peribiliary capillary plexus: a lectin-histochemical and immunohistochemical study. Hepatology 18:529–536
Terada T, Nakanuma Y (1993b) Development of human intrahepatic peribiliary glands: histological, keratin immunohistochemical and mucus histochemical analyses. Lab Invest 68:261–269
Terada T, Nakanuma Y (1994) Profiles of expression of carbohydrate chain structures during human intrahepatic bile duct development and maturation: a lectin-histochemical and immunohistochemical study. Hepatology 20:388–397
Terada T, Nakanuma Y (1995a) Expression of pancreatic enzymes (α-amylase, trypsinogen and lipase) during human liver development and maturation. Gastroenterology 108:1236–1245
Terada T, Nakanuma Y (1995b) Detection of apoptosis and expression of apoptosis-related proteins during human intrahepatic bile duct development. Am J Pathol 146:67–74
Terada T, Nakanuma Y, Ohta G (1987) Glandular elements around the intrahepatic bile ducts in man: their morphology and distribution in normal livers. Liver 7:1–8
Terada T, Ishida F, Nakanuma Y (1989) Vascular plexus around intrahepatic bile ducts in normal livers and portal hypertension. J Hepatol 8:139–149
Terada T, Okada Y, Nakanuma Y (1995) Expression of matrix proteinases during human intrahepatic bile duct development: a possible role in biliary cell migration. Am J Pathol 147:1207–1213
Terada T, Kitamura Y, Nakanuma Y (1997a) Normal and abnormal development of the intrahepatic biliary system: a review. Tohoku J Exp Med 181:19–32
Terada T, Kitamura Y, Ohta T, Nakanuma Y (1997b) Endocrine cells in hepatobiliary cystadenoma and cystadenocarcinoma. Virchows Arch 430:37–40
Terada T, Ashida K, Kitamura Y et al (1998) Expression of E-cadherin, alpha-catenin and beta-catenin during human intrahepatic bile duct development. J Hepatol 28:263–269
Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y (2002) Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int 52:740–746
Tiniakos DG, Mathew J, Kittas C, Burt AD (2008) Ontogeny of human intrahepatic innervation. Virchows Arch 452:435–442
Ueno T, Tanikawa K (1996) Intralobular innervation and lipocyte contractility in the liver. Nutrition 13:141–148
Ueno T, Inuzuka S, Torimura T et al (1991) Distribution of substance P and VIP in the human liver. Am J Gastroenterol 86:1633–1637
Ueno T, Bioulac-Sage P, Balabaud C, Rosenbaum J (2004) Innervation of the sinusoidal wall: regulation of the sinusoidal diameter. Anat Rec 280A:868–873
Conflict of interest
The author has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terada, T. Ontogenic development of nerve fibers in human fetal livers: an immunohistochemical study using neural cell adhesion molecule (NCAM) and neuron-specific enolase (NSE). Histochem Cell Biol 143, 421–429 (2015). https://doi.org/10.1007/s00418-014-1286-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-014-1286-y