Abstract
Non-sebaceous lymphadenoma (NSL) is a rare, recently described, benign salivary gland tumor characterized by a dense lymphoid infiltrate and absence of sebaceous differentiation. To our knowledge, only seven previous cases have been reported. In this paper, we describe an additional example of NSL along with an extensive analysis of its keratin (CK) profile. The patient was a 50-year-old woman presenting with a slowly growing painless mass in the right parotid gland. The tumor was encapsulated and measured 3 × 2 × 2 cm. Microscopically, the tumor comprised islands of epithelial cells with centrally located duct-like structures within a dense lymphoid stroma. Immunohistochemically, the tumor regularly expressed CKs 7, 8/18, and 19, which are typical for columnar differentiation and CKs 17 and 5/6, which are most typically expressed in basal cells of complex epithelia. CK14 was only expressed in rare scattered cells and eventually in groups of cells. The expression of CK10/13, which correlates with squamous differentiation, was negative. Additionally, immunostaining for smooth muscle actin, vimentin, and S-100 was also performed. The immunohistochemical findings in the neoplastic epithelial component of our case suggest a differentiation of “intercalated duct phenotype” without myoepithelial cell participation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-sebaceous lymphadenoma (NSL) is a rare, recently described, benign salivary gland tumor [7]. In 1991, Auclair et al. [5] first described some cases of cystadenoma with a prominent lymphoid element that were named cystic lymphadenoma. Then, in a review paper, they used the term lymphadenoma to describe some salivary gland adenomas accompanied by a prominent lymphoid component that lacked characteristic features of specific adenoma types [6]. Subsequently, this entity was included by the same authors as a variant of sebaceous lymphadenoma (“lymphadenoma that lacks sebaceous differentiation”) in the 1996 Armed Forces Institute of Pathology fascicle on salivary gland tumors [12]. To the best of our knowledge, thereafter, only seven cases of NSL have been reported in the medical literature [8, 9, 15, 17, 20]. In the 2005 World Health Organization histological classification of salivary gland tumors, NSL is classified as a lymphadenoma, together with sebaceous lymphadenoma [7]. Herein, we report a case of NSL arising from the parotid gland along with an extensive analysis of its keratin (CK) profile because previous immunophenotypic studies of NSL have been rather limited in scope.
Clinical history
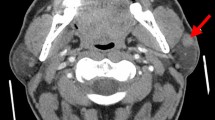
The patient, a 50-year-old woman, had a slowly growing painless mass in her right infra-auricular area of 1 month’s duration. She was otherwise well with no significant past medical history. The tumor was surgically excised with the safe margins of the adjacent non-tumorous tissues. There were no signs of recurrence 11 months after surgery.
Materials and methods
The surgical specimen was fixed in formalin, routinely processed, with representative tissue sections being embedded in paraffin and stained with hematoxylin and eosin (H and E). Immunohistochemical stains were performed in selected blocks. Staining using a standard streptavidinbiotin method was performed for keratin 7 (CK7, Dako, 1:100), keratin 8/18 (CK8/18, Dako, 1:160), keratin 19 (CK19, Dako, 1:50), keratin 5/6 (CK5/6, Dako, 1:50), keratin 14 (CK14, Dako, 1:100), keratin 10/13 (CK10/13, Dako, 1:50), S-100 (Dako, polyclonal, 1:200), smooth muscle actin (SMA, Dako, 1:400), and vimentin (Neomarker, 1:100). Appropriate positive and negative controls were included.
Results
The tumor was 3 × 2 × 2 cm in size, well-circumscribed without signs of necrosis or hemorrhage. It had a soft consistency and was white to gray in color on cut section. Microscopically, the tumor was composed of islands of epithelial cells in which centrally located duct-like structures with eosinophilic secretions were frequently seen, accompanied by a dense lymphoid stroma (Fig. 1). No sebaceous glands were identified. There was no evidence of cytological atypia or abnormal mitotic activity. The lymphoid stroma showed follicle formation with germinal centers, but no subcapsular sinus was seen.
Immunohistochemically, CK7 and CK8/18 were expressed in almost all tumor cells with stronger immunoreactivity in inner cells compared with outer cells (Fig. 2a,b). CK19 was mainly found in luminal cells (Fig. 2c). CK17 was positive in most of the tumor cells, but negative in some luminal cells (Fig. 2d). CK5/6 was present in the periphery of epithelial component (Fig. 3a), and CK14 was found in rare scattered cells and eventually in groups of cells (Fig. 3b). Anti-S100 protein antibodies revealed weak staining of the luminal cells of epithelial component and strong positivity in the cells with elongated projections irregularly distributed throughout both the neoplastic epithelial component and the lymphoid stroma (Fig. 3c). Only a few tumor cells scattered among the neoplastic epithelial component were positive to vimentin, whereas the stromal cells showed diffuse immunoreactivity (Fig. 3). CK10/13 and SMA were negative in all tumor cells.
a Intense expression of CK7 by the tumor cells (Strep.; original magnification ×200). b CK8/18 staining with strong expression in the tumor cells (Strep.; original magnification ×200). c Internal cells showing strong positive to CK19 compared with outer cells of the epithelial component (Strep.; original magnification ×200). d Tumoral islands showing diffuse positivity to CK17. Note non-immunoreactivity in some luminal cells (arrows; Strep.; original magnification ×200)
a Tumoral islands showing positivity of the peripheral cells to CK5/6 (Strep.; original magnification ×200). b CK14 is expressed by a few cells (Strep.; original magnification ×200). c S-100 staining of the luminal cells of epithelial component and cells with elongated projections throughout both the neoplastic epithelial component and the lymphoid stroma (Strep.; original magnification ×200). d Only scattered epithelial cells showing positivity to anti-vimentin antibody, while the stroma demonstrating diffuse immunoreactivity (Strep.; original magnification ×400)
Discussion
NSL is a rare benign tumor of salivary gland origin. To the best of our knowledge, only eight cases, including the present case, have been reported in the medical literature [8, 9, 15, 17, 20]. All cases have exclusively occurred in the parotid gland with male predilection. Patients typically present with a painless mass, and these tumors rarely recur. The tumor, which is well circumscribed and encapsulated, was composed of epithelial component within a background of lymphocytes and lymphoid follicles, which is similar to sebaceous lymphadenoma but lacks sebaceous differentiation. The epithelial component can take the form of anastomosing trabeculae or solid tubules surrounded by basement membrane-like material or cystically dilated glands filled with proteinaceous materials [7, 17]. The current case is similar to previous observations, and it is the second case reported occurring in woman.
A prominent lymphoid element that accompanies epithelial neoplasms of the salivary glands is not infrequent and can occur among a variety of salivary gland tumors, such as Warthin’s tumor, sebaceous lymphadenoma, NSL, lymphoepithelial carcinoma, acinic cell carcinoma, mucoepidermoid carcinoma, etc [6]. The lymphoid element in such lesions often should be regarded as a required element in diagnosis. However, this feature may result in the misinterpretation of malignant tumors of the parotid as benign neoplastic or non-neoplastic lesions and of primary benign and malignant tumors as carcinomas that have metastasized to intra-glandular lymph nodes. The most important differential diagnosis of the present case is lymphoepithelial carcinoma. Lack of mitotic activity, lack of invasive growth with desmoplastic stroma, and presence of overt ductal differentiation in lymphadenoma can set it apart from lymphoepithelial carcinoma. In addition, metastatic carcinoma and malignant lymphoma should be considered [6, 17].
The lymphoid component in NSL is generally considered to represent tumor-associated lymphoid proliferation [5, 7, 17], but the histogenesis of the neoplastic epithelial component is unclear. Immunohistochemical labeling with antibodies for various antibodies such as cytokeratin subtypes, S-100, vimentin, or SMA is an important technique used to better understand the histogenesis of normal and pathological epithelial tissues and has helped to characterize different salivary gland tumors [1–4, 11, 13, 14, 16, 18, 19, 21, 25–29]. The expression of CKs 7, 8, 18, and 19 is typical for columnar differentiation of epithelia [10, 25]. In the current study, CKs 7 and 8/18 were positive in almost all epithelial neoplastic cells, while the staining intensities were higher in inner cells compared with outer cells. CK19 mainly expressed in luminal cells. On the other hand, immunoreactivity with antibodies to CKs 5/6 and 17, which are most typically expressed in basal cells of complex epithelia [10, 25], was present mainly in the outer cells. Negative staining results were obtained with antibody to CK10/13, the expression of which correlates with squamous differentiation of epithelia [10, 16, 25, 27]. CK14, which is an indicator of the basal cells in the keratinized epithelium and also has been identified in basal cells of the excretory ducts [3, 4, 10, 13, 18], stained only rare tumor cells in the present case. This expression pattern for keratin in our case indicates the presence of dual luminal cell and abluminal cell differentiation and suggests that this tumor mirrors what might be expected of a tumor with differentiation toward that of an intercalated duct [3, 4, 13, 14, 18, 28]. Ma et al. [17] reported three cases of NSL and the immunohistochemical reactivities of these cases, in which abluminal cells were usually predominant. Bos et al. [8] described a case of NSL with CK5/6, CK10/13, and CK14 expression in most tumor cells and mild expression of columnar type cytokeratins. The expression of cytokeratin profile in our case is different from that in previous studies because of its obvious columnar cell differentiation, which may be due to the different degrees of differentiation of the tumor cells.
Immunohistochemical profile of neoplastic myoepithelial cells is a perplexing issue [24]. The expression of SMA, which is an indicator of myoepithelial cell differentiation [2, 4, 18, 24], was not detected in our case. Vimentin and S-100, although nonspecific, in conjunction with cytokeratins, have been demonstrated to be sensitive markers of neoplastic myoepithelial cells [1, 4, 24]. However, immunoreactivity for vimentin was seen in single scattered tumor cells in our case, which probably reflects the loss of cellular adhesion under inflammatory conditions [18, 19]. Previous studies have demonstrated S-100 protein is not a specific marker for myoepithelial cells [21]. Our study revealed that S-100 weakly expressed in luminal cells. We assumed that this reactivity is related to the intercalated duct origin of tumor cells and the S-100 positive cells with elongated projections in the epithelial element belong to the Langerhans cells just as that in the lymphoid stoma, similar to previous study in basal cell adenoma and Warthin’s tumor [21, 26, 29]. These findings point to the absence of myoepithelial cell participation within this neoplasm and are in agreement with previous study by Ma et al. [17].
Although NSL has been considered to be a distinctive entity, the exact morphologic and nosologic relationships to other salivary gland tumors are not clearly established. Without lymphoid stroma, the hitherto reported cases of NSL would be easily recognized as basal cell adenoma or cystadenoma [17]. Given that sebaceous differentiation is a rather common finding in normal salivary gland and can be seen in many known benign and malignant salivary gland tumors, a relationship between sebaceous lymphadenoma and NSL is suggested [15]. Sebaceous lymphadenoma is thought to be related to Warthin’s tumor, as both can have overlapping histological features, both are purported to arise from parotid lymph node salivary duct inclusions with concomitant oncocytic and sebaceous metaplasia, both can present with or without associated lymph node architecture, and synchronous presentation of both was reported in literature [23]. Similarities between NSL and Warthin’s tumor have been shown in previous studies [5, 15, 17]. The present case reveals a similar cytokeratin profile to Warthin’s tumor [25], which is also devoid of myoepithelial cell differentiation [22, 24]. Therefore, further studies would be necessary to elucidate the pathogenesis of prominent lymphoid proliferation in salivary gland adenomas and the relationships among those adenomas with prominent lymphoid proliferation.
In conclusion, the authors presented a case of NSL with immunohistochemical investigation. The immunohistochemical findings in the neoplastic epithelial component of our case suggest a differentiation of “intercalated duct phenotype” without myoepithelial cell participation. More cases are required to better understand this entity.
References
Araújo VC, Araújo NS (1990) Vimentin as a marker of myoepithelial dells in salivary gland tumors. Eur Arch Otorhinolaryngol 247:252–255
Araújo VC, Carvalho YR, Araújo NS (1994) Actin versus vimentin in myoepithelial cells of salivary gland tumors. A comparative study. Oral Surg Oral Med Oral Pathol 77:387–391
Araújo VC, Sousa SOM (1996) Expression of different keratins in salivary gland tumours. Oral Oncol Eur J Cancer 32B:14–18
Araújo VC, Souza SOM, Carvalho YR, Araújo NS (2000) Application of immunohistochemistry to the diagnosis of salivary gland tumors. Appl Immunohistochem Mol Morphol 8:195–202
Auclair PL, Ellis GL, Gnepp DR (1991) Other benign epithelial neoplasms. In: Ellis GL, Auclair PL, Gnepp DR (eds) Surgical pathology of the salivary glands. Saunders, Philadelphia, pp 252–268
Auclair PL (1994) Tumor-associated lymphoid proliferation in the parotid gland. A potential diagnostic pitfall. Oral Surg Oral Med Oral Pathol 77:19–26
Barnes L, Eveson JW, Reichart P, Sidransky D (2005) Tumors of the salivary glands. World Health Organization classification of tumors. Pathology and genetics of head and neck. IARC, Lyon, p 269
Bos I, Meyer S, Merz H (2004) Lymphadenoma of the parotid gland without sebaceous differentiation. Immunohistochemical investigations. Pathologe 25:73–78
Chang JY, Hsiao CH (2004) Lymphadenoma lacking sebaceous differentiation in the parotid gland. J Formos Med Assoc 103:459–462
Chu PG, Weiss LM (2002) Keratin expression in human tissues and neoplasms. Histopathology 40:403–439
Crivelini MM, Souza SOM, Araújo VC (1997) Immunohistochemical study of acinic cell carcinoma of minor salivary gland. Oral Oncol 33:204–225
Ellis GL, Auclair PL (1996) Benign epithelial neoplasms. In: Rosai J, Sobin LH (eds) Tumors of the salivary glands. Atlas of tumor pathology, third series. Armed Forces Institute of Pathology, Washington, DC, pp 135–136
Foschini MP, Marucci G, Eusebi V (2002) Low-grade mucoepidermoid carcinoma of salivary glands: characteristic immunohistochemical profile and evidence of striated duct differentiation. Virchows Arch 440(5):536–542
Gustafsson H, Kjorell U, Eriksson A, Virtanen I, Thornell L-E (1988) Distribution of intermediate filament proteins in developing and adult salivary glands in man. Anat Embryol 178:243–251
Kwon GY, Kim EJ, Go JH (2002) Lymphadenoma arising in the parotid gland: a case report. Yonsei Med J 43:536–538
Loyola AM, Souza SOM, Araújo NS, Araújo VC (1998) Study of minor salivary gland mucoepidermoid carcinoma differentiation based on immunohistochemical expression of cytokeratins, vimentin, and muscle-specific actin. Oral Oncol 34:112–118
Ma J, Chan JKC, Chow CW, Orell SR (2002) Lymphadenoma: a report of three cases of an uncommon salivary gland neoplasm. Histopathology 41:342–350
Martins MD, Cavalcanti de Araújo V, Raitz R, Soares de Araújo N (2002) Expression of cytoskeletal proteins in developing human minor salivary glands. Eur J Oral Sci 110:316–321
Morinaga S, Nakajima T, Shimosato Y (1987) Normal and neoplastic myoepithelial cells in salivary glands: an immunohistochemical study. Hum Pathol 18:1218–1226
Musthyala NB, Low SE, Seneviratne RH (2004) Lymphadenoma of the salivary gland: a rare tumour. J Clin Pathol 57:1007
Ogawa I, Nikai H, Takata T, Miyauchi M, Ito H, Ijuhin N (1990) The cellular composition of basal cell adenoma of the parotid gland: an immunohistochemical analysis. Oral Surg Oral Med Oral Pathol 70(5):619–626
Prasad AR, Savera AT, Gown AM, Zarbo RJ (1999) The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med 123(9):801–806
Rund CR, Spies JA (2005) A neck mass in a 57-year-old man. Arch Pathol Lab Med 129(7):e171–e172
Savera AT, Zarbo RJ (2004) Defining the role of myoepithelium in salivary gland neoplasia. Adv Anat Pathol 11:69–85
Schwerer MJ, Kraft K, Baczako K, Maier H (2001) Cytokeratin expression and epithelial differentiation in Warthin’s tumour and its metaplastic (infarcted) variant. Histopathology 39:347–352
Segami N, Fukuda M, Manabe T (1989) Immunohistological study of the epithelial components of Warthin’s tumor. Int J Oral Maxillofac Surg 18:133–137
Sobral AP, Loducca SV, Kowalski LP, Santos IR, Almeida OP, Araújo NS, Araújo VC (2002) Immunohistochemical distinction of high-grade mucoepidermoid carcinoma and epidermoid carcinoma of the parotid region. Oral Oncol 38(5):437–440
Su L, Morgan P, Harrison DL, Waseem A, Lanes B (1993) Expression of keratin mRNAs and proteins in normal salivary epithelia and pleomorphic adenomas. J Pathol 171:173–181
Takahashi H, Tsuda N, Tezuka F, Okabe H (1986) An immunoperoxidase investigation of S-100 protein in the epithelial component of Warthin’s tumor. Oral Surg Oral Med Oral Pathol 62(1):57–62
Acknowledgements
The authors thank Mrs. Li Yuan and Mr. Xiong Shichun for their skilled technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, S., Chen, X., Wang, L. et al. Non-sebaceous lymphadenoma of the salivary gland: case report with immunohistochemical investigation. Virchows Arch 450, 595–599 (2007). https://doi.org/10.1007/s00428-007-0393-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0393-8