Abstract
Non-sebaceous lymphadenoma is a sporadic benign tumor of salivary glands. Histopathologic and immunohistochemical properties, diagnostic criteria, and theories for the histologic origin of the disease have been defined and well-discussed in the literature. However, none of the cases showed malignant transformation to date. We reported a case of 54 years old female patient with a right preauricular mass. Magnetic resonance imaging demonstrated a 2 cm, well-defined contrast-enhanced mass in the right parotid gland. Fine needle aspiration cytology was undiagnostic but suspicious for malignancy. Total parotidectomy with facial nerve preservation was done. In the histopathological examination, non-sebaceous lymphadenoma regions and malignant cells with abundant cytoplasm, large vesicular nuclei, and prominent nucleoli, which occupied approximately 70% of the mass, were seen. The diagnosis was undifferentiated carcinoma arisen from non-sebaceous lymphadenoma. Adjuvant radiotherapy was given. No recurrence was detected during ten months of follow-up. This case is the first case of a malignancy developed from non-sebaceous lymphadenoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-sebaceous lymphadenoma (NSL) is an extremely rare benign tumor of salivary glands, which was first defined as cystadenoma by Auclair at al. [1] in 1991 [2, 3]. Then the term was replaced with lymphadenoma, which shows prominent lymphoid components with epithelial nests and no sebaceous differentiation [4]. In 1996, the Armed Forces Institute of Pathology fascicle on salivary gland tumors recognized the NSL as a variant of sebaceous lymphadenoma (SL) [2, 3]. Finally, this entity has taken its place as a subtype of lymphadenomas since the third edition in 2005 of the World Health Organisation Classification of Tumors [5]. On the other hand, undifferentiated carcinoma (UC) of the salivary glands is also a rare malignant tumor that contains very poorly differentiated cells, and the diagnosis is made by excluding other poorly differentiated tumors [6].
Since 1991, only 45 NSL cases have been published in the English literature apart from the ones Auclair et al. [1, 4] described [2, 3, 15,16,17,18,19, 7,8,9,10,11,12,13,14]. None of these cases showed a recurrence, metastasis to distance organs, or malignant transformation. We reported for the first time a case of UC of a parotid gland which has been arisen from NSL. Moreover, we have reviewed all the accessible cases of NSL in English literature.
Case Report
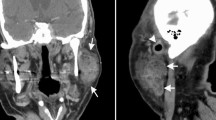
A 54 years old female patient was admitted to our clinic with a right preauricular mass for 12 months, which was enlarged in the last three months. She had no accompanying symptoms or disease. On physical examination, a two centimeter (cm) in diameter, fixed and rubbery mass was palpated anterior to right tragus. There was no sign of facial nerve paralysis. Systemic physical examination showed no enlargement of any lymph node at any lymphoid tissue region. Contrast-enhanced magnetic resonance imaging (cMRI) of head and neck region demonstrated that a 2.1 × 1.8 × 1.1 cm, well-defined mass in the superior part of the right parotid gland which was hypointense in T1-weighted and hyperintense in T2-weighted images. The lesion displayed intense gadolinium enhancement. (Fig. 1) In the inferior part of the gland, an oval-shaped 5.4 mm solid lesion was seen and considered as a lymph node. Fine needle aspiration cytology (FNAC) revealed large, polygonal, and cytolised clusters of cells with a lack of cytoplasm. The nature of these cells could not be differentiated. Because of the suspicion of malignancy, total parotidectomy was selected as a surgical procedure. The facial nerve was protected.
In the histopathological examination of the total parotidectomy specimen, the tumor has two components somewhat intermingled with each other in a lymphocyte-rich stroma. The first component was composed of irregular groups of epithelial cells with small-sized cyst formations. The BerEP4 staining applied to all tissue blocks, and it showed no sebaceous differentiation. These areas are considered as non-sebaceous lymphadenoma regions. (Figs. 2 and 3) The approximately 70% of the mass consisted of the malignant cells with abundant cytoplasm, large vesicular nuclei, prominent nucleoli, and approximately 70% Ki-67 proliferation rate. (Fig. 4) Lymphoepithealial carcinoma, carcinoma metastasis to intraparotidal lymph node, and UC or squamous cell carcinoma arose from NSL were included in the differential diagnosis. The lymphoepithelial carcinoma was excluded by Ebstein-Barr Virus (EBV) negativity by immunohistochemistry and in-situ hybridization methods. (Fig. 5a) The carcinoma metastasis was excluded by the lack of the elements of lymph node microanatomy in the lymphoid tissue harboring the tumor and no evidence of primary. P63 staining displayed no squamous cell differentiation. (Fig. 5b) Therefore, the diagnosis was UC arisen from NSL.
There was a consensus of adjuvant radiotherapy to the right parotid and neck area in the tumor board of our clinic. The patients received 6400 cGy, 6000 cGy, and 5600 cGy dose of radiation to right parotid, upper neck, and lower neck area, respectively. The patient had no adverse events. No recurrence was detected during ten months of follow-up.
The patient gave informed consent for publication.
Literature Review
We included all case reports and series of NSL, which are indexed in PubMed in the English language, into this review. There were 17 papers and 45 cases. The most extensive series of NSL has belonged to Seethala et al. [18] with 11 cases. Weiler et al. [10] have the second-largest series to date with nine cases. The most recent case of NSL was presented by Yamanaka H. et al. [15] in March 2019. All cases were summarized in Table 1.
A total of 44 patients was reported. One of them showed two different focuses of NSL in the same parotid gland. Therefore, forty-five cases were counted. Among them, twenty-five were female, and nineteen were male. The mean age was 52.82 ± 18.39 (10–80). There were only four children whose ages were between ten and fifteen years. Presenting symptoms of the disease was a painless and slowly growing mass in the parotid or neck region in most of the patients. Tumors were sited mostly in the parotid gland, in 40 of all the cases. Two were in the submandibular gland, and two were in the cervical lymph node. There was only one case that the tumor has arisen from the left lacrimal gland, which was diagnosed incidentally.[7] The average of greatest tumor size was 24.36 ± 14.48 mm (6–80). Treatment of choice was the surgical excision of the tumor in 34 of the 45 cases. Although the treatment method was not clear in the Seethala’s [18] publication, it was probably surgical excision because the histopathological investigation of the cases were mentioned. The knowledge of the postoperative follow-up period was available in 17 cases. Among them, the mean follow-up period was 27.3 ± 15.7 months (5–40). None of the reported cases showed malignant transformation or distant metastasis until now.
Discussion
Auclair et al. [4] published an article to demonstrate the different parenchymal parotid tumors that contain lymphoid tissue in 1994. One of the benign lesions was described as lymphadenoma that shows no sebaceous differentiation. The tumor consisted of a well-bounded proliferation of epithelial and lymphoid elements. Because the epithelial component of the tumor can vary in size and shape, it resembles other tumors of parotid gland such as; mucoepidermoid carcinoma (MEC) and benign lymphoepithelial lesion (BLEL). However, NSL lacks intermediate cell and invasion that distinguish it from MEC. Additionally, its closely packed appearance and well-defined ductal structure are not seen in BLEL.
Know GY et al. [16] have mentioned two alternative theories for the histological origin of NSL. One of them claims that the tumor arises from intra- or periparotid lymph nodes. The second theory is reactive lymphoid proliferation secondary to the neoplastic epithelial component, which was defined by Auclair et al. [4] A fibrous capsule and subcapsular sinus structure were seen in Know and colleagues’ [16] reported cases that supported the first theory. However, nodal capsules and sinuses were not seen in the cases reported by most of the authors [2, 3, 8, 11, 13, 14, 17, 20]. Therefore, it is claimed that the lymphoid elements of the tumor as a result of the tumor-associated reaction [3]. On the other hand, Seethala and associates [18] reported 4 cases of NSL had intranodal origin, two in intraparotid lymph node, two in cervical lymph node. At the same time, Weiler et al. [10] indicated a clear hilus structure with embryonic salivary inclusions in four of their nine cases. Additionally, secondary follicles and lymph vessels within marginal sinus structures were shown in all of their cases. All these findings support the second theory. They explained the absence of hilus structure in 5 of their cases by the effacement effect of the expanding tumor. Surprisingly, Pau et al. [7] presented a case of NSL in the lacrimal gland in 2018 for the first time. They demonstrated neither subcapsular sinus structure nor any pre-existing lymph node suggesting finding. Furthermore, they pointed out that the lacrimal glands generally do not have intraglandular lymph nodes.
Additionally, Ma et al. [3] described a detailed differential diagnosis of NSL. In contrast to NSL, sebaceous lymphadenoma (SL) shows mostly squamous cell lining rather than a basaloid cell in epithelial nests. Also, sebaceous differentiation is the main difference between them. Rare or absent papillary formation and no oncocytic epithelial lining make us differentiate NSL from Whartin’s tumor. Metastatic carcinoma from an epithelial origin shows nuclear atypia, invasive growth, and presence of sinuses, while NSL does not have these features. They also pointed out that the lymphoepithelial carcinoma, which shows mild cellular atypia, makes the diagnosis very difficult. Besides the lack of mitotic activity and invasive growth, the presence of ductal differentiation demonstrated by epithelial membrane antigen immunostaining and EBV negativity would make us more consider to NSL. In our case, nuclear atypia, invasive growth, mitotic activity were present. Thus, we included the metastatic carcinoma and lymphoepithelial carcinoma into our consideration. Nevertheless, there were not microanatomical structures of a lymph node and EBV positivity.
Dardick and associates [13] described diagnostic criteria for NSL based on previous studies.[3, 4, 11, 12, 16, 17, 19] These are ‘no sebaceous differentiation’, ‘nononcocytic epithelium’, ‘solid, glandular or cystic epithelial nests’, ‘predominant lymphocytic component with or without germinal centers’ and ‘well-defined tumor mass with a lack of nodal capsule or subcapsular sinusoids’. The last criteria may be modified considering the probability of salivary inclusions in the intraparotid lymph nodes and cervical lymph nodes close to salivary glands. [10, 18]
In order to make the diagnosis more accurate, Yang et al. [17] and Gallego et al. [14] investigated the immunohistochemical properties of the NSL. The epithelial component of the tumor showed dual luminal and abluminal cell differentiation. Their common findings were CK5/6 expression from the outer cell of epithelial nests. CK7 was significantly expressed in most of the cells of the tumor, while CK 14 was expressed in only a small group of cells. Gallego and his collegues [14] have also performed flow-cytometry for the first time, and it was indicated that the tumor cells were DNA diploid as the other benign salivary gland tumors are. Chang et al. [12] have also found a normal diploid karyotype in their case.
Castelino-Prabhu et al. [9] have suggested cytological diagnostic features for NSL. In their case, FNAC gave hypercellular, closely arranged basaloid cell fragments. These cells had small, uniform, round to oval nuclei without nucleoli. Atypia, mitosis, and necrosis were not seen. While lymphocytes were noted in the background, neither hyaline globules nor myoepithelial cells were documented.
Seethala et al. [18] reported the largest case series of lymphadenoma (LAD) until now. In their paper, 33 cases of LAD were presented, twenty-two were SL, eleven were NSL. According to the presence of comorbidities of their cases, they suggested that altered immune status may be a predisposing factor for LAD, although it could be coincidental explaining with the advanced age of most patients. They also investigated possible viral etiology of LAD and demonstrated no correlation between EBV, Human Papilloma Virus, Human Herpes Virus-8, and LAD. Malignant transformation has never occurred in cases of NSL in the literature, while Mayorga et al. [21] and Seethala et al. [18] reported SL cases that showed carcinomatous change or association.
We present a case of NSL that showed malignant transformation to UC for the first time in the literature. In light of this case, possible malignant transformation should be kept in mind in the NSL cases.
References
Auclair PL, Ellis GLGD. Other benign epithelial neoplasms. In: Auclair PL, Ellis GL, editors. Surgical pathology of the salivary glands. Philadelphia: Saunders; 1991.
Ishii A, Kawano H, Tanaka S, Yamamoto Y, Nakamoto T, Hirose Y et al. Non-sebaceous lymphadenoma of the salivary gland with serous acinic cell differentiation, a first case report in the literature. Pathol Int. 2013;66(5):272–76.
Ma J, Chan JKC, Chow CW, Orell SR. Lymphadenoma: a report of three cases of an uncommon salivary gland neoplasm. Histopathology 2002;41:342–50.
Auclair PL. Tumor-associated lymphoid proliferation in the parotid gland: a potential diagnostic pitfall. Oral Surg Oral Med Oral Pathol. 1994;77(1):19–26.
Barnes L, Eveson JW, Reichart PSD. Pathology and genetics of head and neck. In: Barnes L, Eveson JWR, Sidransky D, editors. World Health Organization classification of tumours. Lyon: IARC Press, 2005.
Hatta C, Terada T, Okita J, Kakibuchi M, Kubota A, Sakagami M. Clinicopathological study of undifferentiated carcinoma of the parotid gland. Auris Nasus Larynx 2003;30(3):273–277.
Pau M, Brcic L, Seethala RR, Klein-Theyer AK, Magyar M, Reinbacher KE et al. Non-sebaceous lymphadenoma of the lacrimal gland: first report of a new localization. Virchows Arch. 2018;473(1):127–130.
Liu G, He J, Zhang C, Fu S, He Y. Lymphadenoma of the salivary gland: report of 10 cases. Oncol Lett. 2014;7(4):1097–1101.
Castelino-Prabhu S, Li QK, Ali SZ. Nonsebaceous lymphadenoma of the parotid gland: Cytopathologic findings and differential diagnosis. Diagn Cytopathol. 2010;38(2):137–140.
Weiler C, Agaimy A, Zengel P, Zenk J, Kirchner T, Ihrler S. Nonsebaceous lymphadenoma of salivary glands: Proposed development from intraparotid lymph nodes and risk of misdiagnosis. Virchows Arch. 2012;460(5):467–472.
Musthyala NB, Low SE, Seneviratne RH. Lymphadenoma of the salivary gland: a rare tumour. J Clin Pathol. 2004;57(9):1007.
Chang KTE, Chadha NK, Leung R, Shago M, Phillips MJ, Thorner PS. Lymphadenoma: case report of a rare salivary gland tumor in childhood. Pediatr Dev Pathol. 2010;13(4):331–337.
Dardick I, Thomas MJ. Lymphadenoma of parotid gland: two additional cases and a literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2008;7(4):1097–1101.
Gallego L, Junquera L, Fresno MF. Non-sebaceous lymphadenoma of the parotid gland: immunohistochemical study and DNA ploidy analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2009;107(4):555–558.
Yamanaka H, Hasegawa H, Tanaka M, Matsuzaki H, Oshima T, Sugitani M. Parotid gland non-sebaceous lymphadenoma: a case report. Turkish Arch Otorhinolaryngol. 2019;57(1):42–45.
Kwon GY, Kim EJ, Go JH. Lymphadenoma arising in the parotid gland: a case report. Yonsei Med J. 2002;43(4):536–538.
Yang S, Chen X, Wang L, Zhang J. Non-sebaceous lymphadenoma of the salivary gland: case report with immunohistochemical investigation. Virchows Arch. 2007;450(5):595–599.
Seethala RR, Thompson LDR, Gnepp DR, Barnes EL, Skalova A, Montone K et al. Lymphadenoma of the salivary gland: clinicopathological and immunohistochemical analysis of 33 tumors. Mod Pathol. 2012;25(1):26–35.
Bos I, Meyer S, Merz H. Lymphadenom der Glandula parotis ohne Talgdrüsendifferenzierung. Pathologe 2004;25:73–8. https://doi.org/10.1007/s00292-003-0644-7.
Mori D, Akashi M, Shibaki M, Koike E, Miyazaki J. Nonsebaceous lymphadenoma in the parotid gland: true neoplastic or reactive? A report of two cases. Int J Surg Pathol. 2013;21:509–513.
Mayorga M, Fernández N, Fernando Val-Bernal J. Synchronous ipsilateral sebaceous lymphadenoma and acinic cell adenocarcinoma of the parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:593–596.
Funding
No financial supporter of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kara, H., Sönmez, S., Bağbudar, S. et al. Malignant Transformation of Parotid Gland Non-sebaceous Lymphadenoma: Case Report and Review of Literature. Head and Neck Pathol 14, 1123–1128 (2020). https://doi.org/10.1007/s12105-020-01133-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-020-01133-3