Abstract
Although the frequent occurrence of meniscal degeneration is a well-known fact and its consequences include rupture and even loss of the meniscus, the pathogenetic factors are established insufficiently. Because complement factors and leukocytes are present in synovial fluid, we tried to detect complement deposits and macrophages in menisci, which displayed degenerative changes. We therefore performed a retrospective analysis by immunohistochemical staining of C4d and CD68 in meniscal tissue derived from patients (n=15) who underwent meniscectomy because of meniscal tears and from three autopsy cases (n=3). In this study, focal C4d deposits in the meniscal extracellular matrix in areas of mucoid degeneration or fibrillation were demonstrated for the first time, while no C4d deposits in the avascular zone of menisci without signs of degeneration or injury could be detected. In addition, colocalization of C4d and CD68+ cells was found at sites of meniscal tissue disintegration in five cases. These results represent the first evidence for an involvement of complement and macrophages in meniscal tissue disintegration, indicating a complement mediated reaction at the site of tissue alteration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meniscal degeneration (MD) is a common disorder of the fibrous cartilage of the human knee joint. Although the first descriptions of characteristic histopathological changes in MD are nearly 30 years old, studies concerning the pathogenesis are scarce. All we know today about MD almost exclusively derives from diagnostic imaging techniques [14] and conventional HE or EvG staining, while no immunohistochemical marker for MD is available.
Histomorphologically, three different variances of MD have been described: calcification, acellular hyalin degeneration, and mucoid or myxoid degeneration [5, 6]. The latter is the most frequent degenerative lesion and can further be subdivided into intrameniscal stromal mucoid degeneration (mD) and cystic parameniscal degeneration.
In the past, mD has usually been considered to be the result of endogenous or exogenous trauma [15, 19, 20]. In addition, synthesis of mucopolysaccharides after malnutrition of the meniscal tissue was suspected to be of pathogenetic relevance [19]. Stromal mD was even considered to represent a physiological condition in the meniscus [5]. Furthermore, vascular disturbance/posttraumatic hematoma due to trauma and accumulation of proteoglycans after altered mechanical strains were accused to give rise to degenerative lesions [7, 11, 12]. Some studies demonstrated MD to be age- and stress-related [8, 10, 18].
Although trauma has been repeatedly accused over the last decades to be the source of MD, Weber [21] questioned the existence of isolated traumatic injury and stated that the conventional criteria to distinguish between traumatic and degenerative meniscal lesions were not longer necessary.
These conflicting concepts can be brought together by considering the basic distinction between primary [8] and secondary MD. According to Aufdermaur [2], secondary degenerative lesions are massive and show only few changes due to repair. Especially in cases with preceding primary degeneration, trauma may aggravate degenerative lesions [4].
So apart from mechanical factors, are there any additional factors, which are involved in the development, maintenance, or even aggravation of degenerative lesions? Are there any signs of an immunoresponse at sites of MD?
Because complement factors and leukocytes are present in synovial fluid [3], we tried to detect complement deposits and macrophages by immunohistochemical staining of C4d and CD68 in menisci, which displayed degenerative changes.
Materials and methods
Medial menisci, obtained from 15 patients (n=15, 6 women and 9 men, ages 13–68, average age 41 years) who underwent meniscectomy because of meniscal tears were selected for this study. In addition, three medial menisci (n=3) of the right knee, which did not display macroscopic signs of degeneration or injury, were obtained from autopsy cases (comparison group, average age 62 years). These menisci were removed totally and triangular slices were cut to evaluate both the vascular and the avascular zones of the tissue.
Histology
After fixation in formalin (4%), the meniscal tissue was embedded in paraffin. The microtomed sections of 1–3 μm width were stained with hematoxylin and eosin. In addition, an EvG staining was performed and an immunohistochemical staining was carried out using antibodies to C4d and CD68.
After the tissue sections were deparaffinized, epitopes were demasked (pressure cooker, citrate buffer, pH 6). Staining was performed using commercially available rabbit polyclonal antibody against C4d (Biomedica, Austria) and CD68, which was appropriately diluted (1:10).
Antibody binding was detected by using the labeled streptavidin–biotin method (LSAB-Kit+, DAKO, Denmark). Peroxidase-linked streptavidin binds to biotin, which again is linked to the secondary antibody. DAB (3,3-diaminobenzidine; DAKO, Denmark) served as a chromogen for the reaction with peroxidase. Endogenous peroxidase was blocked by H2O2. In a final step, nuclear counterstaining with hematoxylin according to Mayer was performed. Successful IH was controlled using tonsil tissue treated as described above; negative controls were obtained by omitting the primary antibody. Immunostainings were evaluated by two pathologists (V.K. and A.D.).
Five selected cases additionally underwent IH double staining to validate colocalization of macrophages and C4d deposits. The EnVision Doublestain System (DAKO, Denmark) was used according to the manufacturer’s instructions: after epitope demasking (pressure cooker, citrate buffer, pH 6) anti-C4d antibody (polyclonal, Biomedica, Austria) and anti-CD68 antibody (DAKO, Denmark) were successively added. DAB (C4d) and Fast Red (CD68) were used as chromogens. Tonsil tissue served as a positive control; negative controls were obtained by omitting the primary antibody.
Results
Hematoxylin–eosin
Comparison group
Although only menisci without macroscopic signs of tears and degeneration were selected from the comparison group, two of three menisci (n=3) histologically showed discrete signs of mD and two displayed slight fibrillation of the extracellular matrix. Proliferating chondrocytes were seen in all cases. The vascular zone presented with several small vessels, fibrous tissue, and fat.
Meniscal degeneration
Thirteen of 15 menisci (n=15) displayed discrete to severe mD. Menisci without signs of mD belonged to a 22-year-old man and an 18-year-old woman. Fibrillation of the extracellular matrix was seen in 14 of 15 patients, the meniscus without fibrillation deriving from a 19-year-old man. Tears of the meniscal tissue could be demonstrated in 14 patients, four with sharp edges, three with smooth tear contours, and six displaying both features. Smooth tear edges were in all cases accompanied by deposits of fibrin. Fourteen menisci showed proliferating chondrocytes. Small areas of fibrous cartilage necrosis were only seen in three cases.
C4d
Comparison group
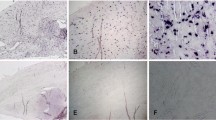
No C4d deposits were detected within the extracellular matrix of the avascular zone of the meniscal tissue; only one case showed weak focal staining in a small area with signs of mD. Linear C4d deposits were seen along the meniscal surface (Fig. 1a,b), while the vascular zone presented with positive perivascular and endothelial C4d staining.
Meniscal degeneration
Twelve menisci (n=15) displayed diffuse C4d deposits in the extracellular matrix with strong relation to areas of mD or fibrillation, partially in the vicinity of meniscal tears (Fig. 2a,b). No correlation between deposition of C4d and the age of traumatic changes could be demonstrated. In addition, as it was seen in the comparison group, linear C4d deposits were detected along the meniscal surface, surrounding vessels, and along endothelial cells in the vascular zone.
CD68
Comparison group
CD68+ cells were predominantly located in the vicinity of vessels in the vascular zone. No macrophages were detected in the extracellular matrix of the avascular zone.
Meniscal degeneration
Twelve menisci (n=15) showed CD68+ macrophages, while the distribution pattern differed. If the examined tissue specimen included the vascular zone of the meniscus, CD68+ cells could be demonstrated in the vicinity of blood vessels. Regarding the avascular zone, macrophages were in some cases located directly beyond the meniscal surface. Three menisci presented with solitary CD68+ macrophages distributed diffusely in the extracellular matrix, while five menisci displayed colocalization of mD/tissue fibrillation, C4d deposits, and macrophages.
C4d/CD68
Five menisci, which presented with macrophages in areas, which also showed C4d deposits in IH single staining, were selected for IH double staining of CD68/C4d. As expected, colocalization of macrophages and C4d deposits was clearly detectable (Fig. 3).
Discussion
Although histopathology of bradytrophic tissue has not received much attention in the past, some studies just recently were able to highlight its importance and amend the diagnostic repertoire [9, 13]. In this study, focal C4d deposits in the meniscal extracellular matrix of areas of mD or fibrillation were demonstrated for the first time. Because no intrameniscal deposits in menisci without signs of degeneration or injury could be detected, these complement deposits specifically trace disintegration of fibrocartilage. In addition, colocalization of C4d and CD68+ cells could be demonstrated in these lesions, indicating that C4d deposits are not an unspecific sign of inflammation but reflect a complement mediated reaction at the site of tissue alteration.
As up to now, only histomorphological descriptions of degenerative meniscal changes, based on conventional staining methods like HE and EvG staining, exist; these results represent the first immunohistochemical evidence for an involvement of complement and macrophages in MD.
Degeneration of the meniscal tissue is subdivided into primary and secondary changes [1, 2, 8], depending on preexisting conditions like trauma or biomechanically defective positions of the joint. Beyond this, three morphologically different types can be distinguished: calcification, acellular hyalin degeneration, and mucoid or myxoid degeneration [5, 6]. mD is the most frequent form and, depending on the location of the degenerative lesions, is again divided into intrameniscal stromal mD and cystic parameniscal degeneration [5, 6].
Although the frequent occurrence of MD is a well-known fact and its consequences include rupture and even loss of the meniscus, the pathogenetic factors are established insufficiently.
Besides endogenous and exogenous trauma [14, 19, 20], posttraumatic changes like hematoma or vascular disorders [7], synthesis of mucopolysaccharides arising from nutritional deficiencies, and accumulation of proteoglycans after altered mechanical strains [11, 12] are suspected to be the source of MD. While an aggravating effect of traumatic changes on preexisting MD is well accepted [4], the existence of isolated traumatic ruptures of unaltered meniscal tissue is doubted [21].
In this study, C4d deposits in areas of mD or fibrillation were demonstrated for the first time, indicating complement activation within this lesions, possibly resulting in perpetuation of preexisting tissue disintegration.
Activation of the complement cascade by immunocomplexes via the classical pathway results in enzymatic cleavage of the complement component C4, whose final split product is C4d [16]. As C4d exposes a thioester group, which leads to the formation of a covalent bond to the closest protein or carbohydrate near the site of C4 activation, it is not as transient as other complement fragments and can therefore be detected much easier by immunohistochemistry.
The fact that degenerative lesions with C4d deposits were seen in unvascularized areas raises the question of from what kind of source complement factors derive. On the one hand, complement may originate from the serum, invading the extracellular matrix in the course of trauma. On the other hand, complement factors could derive from inside the joint because they have been demonstrated in synovial fluid [3].
Although colocalization of C4d and CD68+ macrophages suggests that complement activation is involved in the development or aggravation of MD because macrophages are known to be activated by the formation of immunocomplexes by IgG and complement components (C5a) [17] and are capable of synthesizing complement factors (C4 and C3), the functional relevance of C4d deposits at sites of MD remains to be elucidated. Measurement of intraarticular complement turnover could supply further evidence for an involvement of complement activation in MD.
Nevertheless, complement deposits and macrophages were demonstrated for the first time in MD, indicating that apart from mechanical mechanisms, complement mediated processes may be involved.
Taken together, detection of C4d deposits could serve as an immunohistochemical marker for disintegration of fibrocartilage, providing an auxiliary tool for the histopathological diagnosis of MD.
References
Andreesen M (1956) Praktische Erfahrung bei der Begutachtung von Meniskusschäden. Hefte Unfallheilkd 52:214
Aufdermaur M (1971) Die Bedeutung der histologischen Untersuchung des Kniegelenksmeniskus. Schweiz Med Wochenschr 101:1405–1441
Brodeur JP, Ruddy S, Schwartz LB, Moxley G (1991) Synovial fluid levels of complement SC5b-9 and fragment Bb are elevated in patients with rheumatoid arthritis. Arthritis Rheum 34(12):1531–1537
Burri C (1976) Meniscusverletzungen. Hefte Unfallheilkd 128:73
Ferrer-Roca O, Vialalta C (1980) Lesions of the meniscus. I. Macroscopic and histologic findings. Clin Orthop 146:289–300
Ferrer-Roca O, Vialalta C (1980) Lesions of the meniscus. II. Horizontal cleavages and lateral cysts. Clin Orthop 146:301–307
Kleinberg S (1938) Cysts of external semilunar cartilage: report of three cases. Arch Surg 37:827–834
Könn G (1985) Möglichkeiten und Grenzen der histologischen Altersbestimmung von Zusammenhangstrennungen des Kniegelenkmeniskus. Unfallchirurgie 88:1
Krenn V, Morawietz L, Burmester GR, Haupl T (2005) Synovialitis score: histopathological grading system for chronic rheumatic and non-rheumatic synovialitis. Z Rheumatol 64(5):334–342
Lindström A (1950) Trauma and ganglia of the semilunar cartilages of the knee. Acta Orthop Scand 23:237–246
McDevitt CA, Muir H (1976) Biochemical changes in the cartilage of the knee in experimental and natural osteoarthritis in the dog. J Bone Joint Surg Br 58:94–101
McDevitt CA, Webber RJ (1990) The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop 252:8–18
Morawietz L, Gehrke T, Classen RA, Barden B, Otto M, Hansen T, Aigner T, Stiehl P, Neidel J, Schroder JH, Frommelt L, Schubert T, Meyer-Scholten C, Konig A, Strobel P, Rader ChP, Kirschner S, Lintner F, Ruther W, Skwara A, Bos I, Kriegsmann J, Krenn V (2004) Proposal for the classification of the periprosthetic membrane from loosened hip and knee endoprotheses. Pathologe 25(5):375–384
Richter J, Grifka J, Fisseler-Eckhoff A, Müller KM, Kramer J (1996) Ultrasound morphologic criteria in evaluating meniscus changes—an experimental study. Z Orthop Ihre Grenzgeb 134(2):137–143
Romanini L, Calvisi V, Collodel M, Masciocchi C (1988) Cystic degeneration of the lateral meniscus. Pathogenesis and diagnostic approach. Ital J Orthop Traumatol 14:493–500
Sacks SH, Chowdhury P, Zhou W (2003) Role of complement system in rejection. Curr Opin Immunol 15(5):487–492
Shushakova N, Skokowa J, Schulman J, Baumann U, Zwirner J, Schmidt RE, Gessner JE (2002) C5a anaphylatoxin is a major regulator of activating versus inhibitory Fcgammas in immune complex-induced lung disease. J Clin Invest 110:1823–1830
Slany A (1941) Autoptische Reihenuntersuchungen an Kniegelenken mit besonderer Berücksichtigung der Meniskuspathologie. Arch Orthop Unfallchir 41:256
Smillie IS (1978) Surgical pathology of the menisci. In: Smillie IS (ed) Injuries of the knee joint. Churchill Livingstone, Edinburgh, pp 83–111
Walter JB, Talbot IC (1996) Connective tissue: its normal structure and the effects of disease. In: Walter JB, Talbot IC (eds) General pathology. Churchill Livingstone, Edinburgh, pp 103–116
Weber M (1994) Die Beurteilung des Unfallzusammenhangs von Meniskusschäden. Orthopäde 23:171–178
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dankof, A., Krenn, V. C4d deposits mark sites of meniscal tissue disintegration. Virchows Arch 449, 230–233 (2006). https://doi.org/10.1007/s00428-006-0221-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-006-0221-6