Abstract
The purpose of the study is to highlight oncocytic modifications in rectal adenocarcinomas and evaluate a possible correlation with preoperative radiochemotherapy (RCT). Twenty-eight cases of advanced rectal carcinoma, treated preoperatively by 5-fluorouracil (200–225 mg/m2) and 44–46 Gy in 22–23 fractions, were studied. All patients underwent biopsy before RCT. Surgery was performed within 6 weeks after RCT. In all cases oncocytic modifications were searched for on hematoxylin and eosin (H&E) and at immunohistochemistry using an antimitochondrial antibody. In addition, in two cases, both pre- and post-RCT tissues were examined at electron microscopy. All tumors were adenocarcinomas. In pre-RCT biopsies, oncocytic changes were difficult to find on H&E, while the antimitochondrial antibody strongly stained numerous neoplastic cells (mean 48.4%). In post-RCT surgical specimens, oncocytic changes were detected in 24 out of 28 cases on H&E and the antimitochondrial antibody stained most of the residual neoplastic cells (mean 76.7%). Ultrastructural examination revealed large and bizarre mitochondria inside tumor cells both in pre- and post-RCT tissues. In conclusion, the present data suggest that rectal adenocarcinomas are “mitochondrion-rich” tumors. After preoperative RCT, residual neoplastic cells acquire a definite oncocytic phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the first definition given by Hamperl in 1894 [12], oncocytes are traditionally described as cells with abundant, granular, and eosinophilic cytoplasm on the basis of optical microscopic appearance at hematoxylin and eosin (H&E). Immunohistochemical and ultrastructural studies revealed that the cytoplasm of these cells is mainly composed of mitochondria [3, 7, 31]. Furthermore, many tumors present focal oncocytic differentiation, however, in oncocytomas or oncocytic tumors (both benign and malignant lesions), at least 75% of the constituent neoplastic cells are oncocytes [40]. Immunohistochemical examination with antimitochondrial monoclonal antibody provides helpful evaluation for diagnosis [26, 41]. Oncocytomas are diffusely distributed in various organs, although the most frequent sites of origin are thyroid, salivary glands, and kidney [39]. To the best of our knowledge, rare carcinomas with oncocytic changes were described in the gastrointestinal tract [25], although only one oncocytoma arising in the rectum was reported [27].
During histological evaluation of tumors from patients treated with preoperative radiochemotherapy (RCT) for locally advanced rectal carcinoma, we noted a peculiar differentiation toward an oncocytic phenotype. Thus, the aim of the present study is to consider oncocytic differentiation in colorectal adenocarcinoma and to evaluate the possible correlation between oncocytic differentiation and response to preoperative RCT.
Materials and methods
Patients
From January 2000 to February 2005, a total of 28 cases of rectal carcinoma (20 men, eight women) were preoperatively treated with concomitant radiotherapy and chemotherapy at the Department of Oncological Sciences, Bellaria Hospital, University of Bologna (Italy). The clinical assessment and the tumor stage prior to RCT were estimated by physical examination, colonoscopy with rectal biopsy, endorectal ultrasound, abdominopelvic computed tomography (CT) scan, and chest X-ray. Radiotherapy consisted of an external beam irradiation given by means of three orthogonal fields (posterior–anterior, left lateral, right lateral) with a total dose of 44–46 Gy, subdivided in 22–23 fractions, and distributed in the course of about 1 month. Simultaneously (for approximately 32–33 days), patients were treated with 5-fluorouracil (5FU) (200–225 mg/m2) by continuous intravenous infusion. Four to 6 weeks after completion of RCT, patients underwent either an anterior resection (20 patients), or abdominoperineal resection (six patients); an anterior resection with left hemicolectomy and an abdominoperineal resection with radical cystectomy were also performed (one patient each). Surgical specimens were evaluated for residual disease, depth of tumor penetration, and lymph node metastasis according to the tumor-node-metastasis staging [35]. Pathological stage after RCT (tumor classification after neoadjuvant therapy) was then compared with the clinical stage as previously assessed.
Control cases
As controls, 14 surgical specimens of colon adenocarcinoma (four ascending colon, five transverse colon, five sigmoid colon) not treated with preoperative RCT were retrieved from the Anatomic Pathology files of University of Bologna, at Bellaria Hospital, for study and search for oncocytic differentiation.
Histology and immunohistochemistry
All tissues were fixed in 4% buffered formalin and paraffin embedded according to a routine procedure. From each block, sections were stained with H&E and with antimitochondrial antibody (BioGenex, clone 113-1, dilution 1:500). The processing was carried out using the Ventana BenchMark automated stainer.
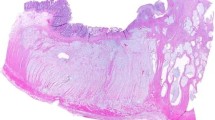
On H&E, oncocytic differentiation was diagnosed when cells had intense and granular cytoplasmic eosinophilia and nuclei showed granular chromatin and prominent nucleoli.
Finely granular cytoplasmic immunostaining observed in stromal fibroblasts and in nonneoplastic epithelial cells was considered as internal control. Positive oncocytic counting was performed both on the preoperative small biopsies and in the surgical specimens containing residual cells within fibrous tissues. We decided to count 200 consecutive cells to obtain a quantitative evaluation of the immunohistochemical results.
Ultrastructure
In two cases, ultrastructural investigation was performed, both pretreatment biopsy and posttreatment surgical specimen. Tissues were retrieved from paraffin blocks. Samples were deparaffined; rehydrated; fixed in phosphate-buffered 2.5% glutaraldehyde and 1% osmium tetroxide; dehydrated in alcohol and propylene oxide; and embedded in Epon 812. Thin sections were stained with uranyl acetate and Reynolds’ lead citrate and observed in a Phillips CM 10 TEM.
Results
Tumor stage and pathologic response
The total of 28 patients was composed of 20 males and eight females, with a median age at surgery of 66 years.
The pretreatment clinical stage, evaluated by endorectal ultrasound and abdominopelvic CT scan revealed: (1) five stage T2 tumors, 17.9%; (2) 21 stage T3 tumors, 75%; and (3) two stage T4 tumors, 7%. Possible lymph node metastasis was found in 15 (53.6%) cases. All T2 tumors were located very close to the anorectal junction, and preoperative RCT was performed in the attempt to reduce the size, avoiding abdominoperineal resection.
After RCT, pathological examination of surgical specimens detected: (1) one stage T0 tumor, 3.6%; (2) three stage T1 tumors, 10.7%; (3) ten stage T2 tumors, 35.7%; (4) 13 stage T3 tumors, 46.4%; and (5) one stage T4 tumor, 3.6%. Histological staging performed on the surgically resected specimen showed complete regression in one (3.6%) patient and down-staging in 13 (46.4%) patients.
Morphologic analysis before and after treatment
Pretreatment biopsies
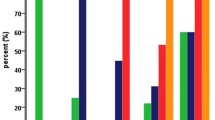
Twenty-seven patients were diagnosed with invasive, conventional type, adenocarcinoma (nine well-differentiated, 15 moderately differentiated, and three poorly differentiated) and one was diagnosed with mucinous adenocarcinoma. On H&E, neoplastic cells with oncocytic differentiation were detectable in 16 cases where they composed 3 to 20% (mean, 5.3%) of the neoplastic population (Fig. 1). Antimitochondrial antiserum strongly stained 5 to 100% (mean, 48.4%) of the neoplastic cells (Fig. 2).
Posttreatment surgical specimens
Twenty-four patients were diagnosed with invasive, conventional type adenocarcinoma (15 moderately differentiated, two well-differentiated, five poorly differentiated, and two with scattered neoplastic cells only) and three were diagnosed with mucinous adenocarcinoma. In one case, a complete pathologic response was documented. Neoplastic cells with oncocytic differentiation were detectable on H&E in 24 cases where they constituted 10 to 100% (mean, 54.4%) of the residual neoplastic population. Antimitochondrial-positive neoplastic cells varied from 5 to 100% (mean, 76.7%). The two cases with scattered residual neoplastic cells exhibited a definite oncocytic differentiation on H&E and a strong immunoreactivity to antimitochondrial antiserum (Figs. 3 and 4).
Ultrastructure
Ultrastructural examination revealed similar alterations in mitochondrial morphology, both in pretreatment biopsies and in posttreatment surgical specimens. Mitochondria were numerous, closely packed, large, swollen, and bizarre in shape; nucleoli were prominent. The same alterations were more pronounced and appeared more evident in surgical specimens after RCT (Fig. 5).
Control cases
Oncocytic modifications were identifiable on H&E in 14 cases. Oncocytic cells composed 5 to 30% of neoplastic cells (mean 10.7%). Immunoreactivity for antimitochondrial antiserum was present in all cases with the percentage of positive cells varying from 20 to 80% (mean 64.3%).
Additional modifications observed in posttreatment surgical specimens
In surgical specimens of post-RCT rectal carcinomas, eight cases (28.6%) showed mucinous modifications and two cases acquired a definite mucinous phenotype. The case diagnosed as mucinous adenocarcinoma in pretreatment biopsy did not show features of regression or modifications of histotype after RCT.
Clinical correlations
The percentage of mitochondrial antigen positive neoplastic cells detected in pretreatment biopsies was correlated to the tumor stage reduction induced by RCT. Tumor stage reduction was evaluated by the comparison between pretreatment clinical staging and posttreatment pathological staging. Data collected are illustrated in Fig. 6. Correlation index calculated for the two variable groups was 0.03896. Statistical analysis did not show any statistically significant correlation.
Discussion
In the present study, a series of preoperative RCT led to a stage reduction of the tumor in 13 (46.4%) out of 28 patients. Complete regression was achieved in only one case (3.6%) while 14 (50%) out of 28 patients with rectal adenocarcinoma were not affected by preoperative treatment. These data are consistent with previously published series where a complete response after RCT was observed in only 5 to 10% of the cases [11, 29, 42, 43]. Preoperative RCT can reduce tumor mass in rectal carcinomas and improve surgery efficacy, although the magnitude of this benefit is relatively small. Understanding the reasons for partial response to preoperative RCT is of utmost importance and numerous studies have already addressed this issue [2, 8, 9, 14, 19, 20, 23, 30, 44]. It was shown that radiotherapy may induce changes at the genetic level [21, 22], particularly in those genes regulating proliferation, apoptosis, and tissue repair. This might explain the morphological modifications observed in tissues after RCT.
Regarding the colorectal site, many authors have analyzed morphological features following RCT or radiotherapy alone. Extensive fibrosis, mucous lakes, thickening of the intima, and media layers of blood vessels are well-documented features [4, 45]. Nagtegaal et al. [22] documented mucinous changes in irradiated rectal carcinomas. Increased number of endocrine cells following RCT in rectal cancer were also reported [33, 32]. Similar aspects were also observed in our cases.
To the best of our knowledge, oncocytic modifications after RCT in colorectal adenocarcinomas were never extensively investigated, although tumor epithelial cells with marked eosinophilic cytoplasm were described [17, 32]. Even after short-term preoperative radiotherapy (25 Gy in 5–7 days), epithelial cells in the crypts displayed an intensive eosinophilic cytoplasm [15]. It is possible to hypothesize that the intense cytoplasmic eosinophilia previously identified is comparable to the oncocytic modifications observed here.
The present cases show that colorectal adenocarcinomas are characterized by numerous mitochondrion-rich neoplastic cells. These cells, even if not clearly recognizable on H&E, became more evident after immunostaining with antimitochondrial antiserum. In the pretreatment biopsies, a mean of 48.4% of antimitochondrial antibody positive cells was observed. This finding was confirmed in surgical specimens of the 14 untreated colon adenocarcinomas (“control cases”) where a mean of 64.3% cells positive to antimitochondrial antiserum was detected. Thus, colorectal adenocarcinomas may be considered “mitochondrion-rich” tumors [3, 5].
True oncocytic modifications became evident in surgical specimens of rectal adenocarcinomas after RCT where a mean of 54.4% of neoplastic cells showed the typical features of oncocytes on H&E and a mean of 76.7% of neoplastic cells was strongly positive to antimitochondrial immunostaining. In surgical specimens from two cases that underwent a marked reduction of neoplastic population, the residual neoplastic cells were oncocytic.
Oncocytic modifications due to radiotherapy were described in the literature in various organs. Busuttil [1] described similar alterations in 48 specimens of salivary glands after irradiation. In seven irradiated adenomas of a pituitary gland, diffuse fibrosis was found and some irradiated pituitary cells showed cytoplasmic immunoreactivity for mitochondrial protein, mimicking oncocytes [24]. Oncocyte differentiation was also observed after metyrapone therapy in adrenal cortex [38]. Oncocytic modifications observed and related abundance in mitochondria may represent one of the factors responsible for resistance to RCT.
The mechanism of mitochondriogenesis is a complex phenomenon resulting in the synthesis of membrane phospholipids, DNA replication and expression of the mitochondrial genome and of genes encoding for mitochondrial proteins [46].
Possible mechanisms for oncocytic cell increase might be: (1) differentiation of epithelial neoplastic cells toward an “oncocyte-appearance” phenotype and (2) selective proliferation of oncocytes within tumor cells due to their resistance to RCT.
Neoplastic cells may acquire an oncocytic phenotype after radiation therapy as a response to injury (so-called “secondary oxyphilia”) [36]. It was observed that ionizing radiation and other oxidants increase mitochondrial gene expression and its activity. After irradiation, expression of mitochondrially encoded genes, mitochondrial membrane potential, and ATP production were enhanced in a cell line derived from glioblastoma [10]. Furthermore, the viability of this cell line after exposure to irradiation was high, suggesting a possible protective role against additional oxidative damage. Studies conducted with cationic hexakis (2-methoxyisobutylisonitrile)-technetium-99 m (99mTc-MIBI), an agent that accumulates in mitochondria of various cells and tissues of high mitochondrial metabolic activity, have shown an increase of mitochondrial membrane potentials and mitochondrial metabolic activity in cancer cell lines after irradiation [6]. Mitochondria may be metabolically activated after RCT and become larger and swollen, determining an oncocytic appearance on H&E.
On the other hand, oncocytic differentiation detected in pretreatment biopsies may be responsible for resistance to RCT itself. This was observed in carcinomas of the thyroid exhibiting oncocytic appearance that are less responsive to radioactive iodine [18]. Homoplasmic mitochondrial DNA (mtDNA) somatic mutations were detected in colorectal cancer [28]. Experimental studies have shown that damage to mtDNA and to the mitochondrial respiratory chain may change the response of tumor cells to anticancer drugs [3, 47]. The antioxidant enzyme manganese superoxide dismutase (MnSOD), located in mitochondria, seemed to be one of the most effective enzymes in protecting cells against ionizing radiation damage [37]. Overexpression of MnSOD improves the survival of cells exposed to gamma radiation [13]. Mitochondrial somatic mutations in the D-loop region—a noncoding sequence of the mitochondrial genome—was described in colorectal cancer not only as potential tumor marker and an independent prognostic factor, but as a factor of resistance to adjuvant chemotherapy with 5FU, suggesting a central role of the mitochondria in drug resistance [16].
In conclusion, we demonstrated that colorectal adenocarcinomas are mitochondrion-rich tumors. Although oncocytic features were difficult to identify on H&E, immunohistochemical and ultrastructural studies revealed their abundance of mitochondria. After preoperative RCT, residual neoplastic cells of rectal adenocarcinomas exhibited a definite oncocytic phenotype, as evaluated by both H&E and immunohistochemistry along with ultrastructure. The oncocytic component and mitochondria enrichment may be considered to be among the factors responsible for the resistance to preoperative RCT.
References
Busuttil A (1977) Irradiation-induced changes in human salivary glands. Clin Otolaryngol Allied Sci 2:199–206
Chapet O, Romestaing P, Mornex F, Souquet JC, Favrel V, Ardiet JM, D’Hombres A, Gerard JP (2005) Preoperative radiotherapy for rectal adenocarcinoma: which are strong prognostic factors? Int J Radiat Oncol Biol Phys 61:1371–1377
Damiani S, Eusebi V, Losi L, D’Adda T, Rosai J (1998) Oncocytic carcinoma (malignant carcinoma) of the breast. Am J Surg Pathol 22:221–230
Dworak O, Keilholz L, Hoffmann A (1997) Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 12:19–23
Foschini MP, Macchia S, Losi L, Dei Tos AP, Pasquinelli G, Di Tommaso L, Del Duca S, Roncaroli F, Dal Monte PR (1998) Identification of mitochondria in liver biopsies. A study by immunohistochemistry, immunogold and Western blot analysis. Virchows Arch 433:267–273
Furuta M, Nozaki M, Kawashima M, Iimuro M, Okayama A, Fukushima M, Natsui S, Souma R, Jinnai M (2004) Monitoring mitochondrial metabolism in irradiated human cancer cells with 99mTc-MIBI. Cancer Lett 212:105–111
Ghadially FN (1988) Ultrastructural pathology of the cell and matrix, 3rd edn, vol 1. Butterworth, London, UK, pp 260–265 (Chapter 3)
Ghadimi BM, Grade M, Difilippantonio MJ, Varma S, Simon R, Montagna C, Füzesi L, Langer C, Becker H, Liersch T, Ried T (2005) Effectiveness of gene expression profiling for response prediction of rectal adenocarcinomas to preoperative chemoradiotherapy. J Clin Oncol 23:1826–1838
Giralt J, Eraso A, Armengol M, Rosselló J, Majó J, Ares C, Espin E, Benavente S, de Torres I (2002) Epidermal growth factor receptor is a predictor of tumor response in locally advanced rectal cancer patients treated with preoperative chemotherapy. Int J Radiat Oncol Biol Phys 54:1460–1465
Gong B, Chen Q, Almasan A (1998) Ionizing radiation stimulates mitochondrial gene expression and activity. Radiat Res 150:505–512
Hahnloser D, Nelson H, Gunderson LL, Hassan I, Haddock MG, O’Connell MJ, Cha S, Sargent DJ, Horgan A (2003) Curative potential of multimodality therapy for locally recurrent rectal cancer. Ann Surg 237:502–508
Hamperl H (1931) Beiträge zur normalen und pathologischen Histologie menschlicher Speicheldrüsen. Z Mikrosk Anat Forsch 27:1–55
Hirose K, Longo DL, Oppenheim JJ, Matsushima K (1993) Overexpression of manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anticancer drugs, and ionizing radiation. FASEB J 7:361–368
Komuro Y, Watanabe T, Tsurita G, Muto T, Nagawa H (2005) Evaluating the combination of molecular prognostic factors in tumor radiosensitivity in rectal cancer. Hepatogastroenterology 52:666–671
Leupin N, Curschmann J, Kranzbuhler H, Maurer C, Laissue J, Mazzucchelli L (2002) Acute radiation colitis in patients treated with short-term preoperative radiotherapy for rectal cancer. Am J Surg Pathol 26:498–504
Lièvre A, Chapusot C, Bouvier AM, Zinzindohoué F, Piard F, Roignot P, Arnould L, Beaune P, Faivre J, Laurent-Puig P (2005) Clinical value of mitochondrial mutations in colorectal cancer. J Clin Oncol 23:3517–3525
Mai KT, Carnat T (2004) Mucinous and immunohistochemical changes of colonic adenocarcinoma secondary to chemo-radiotherapy. Histopathology 45:91–93
Máximo V, Sobrinho-Simões M (2000) Hurtle cell tumours of the thyroid. A review with emphasis on mitochondrial abnormalities with clinical relevance. Virchows Arch 437:107–115
McLeod HL, Murray GI (1999) Tumour markers of prognosis in colorectal cancer. Br J Cancer 79:191–203
Myerson RJ, Singh A, Birnbaum EH, Fry RD, Fleshman JW, Kodner IJ, Lockett MA, Picus J, Walz BJ, Read TE (2001) Pretreatment clinical findings predict outcome for patients receiving preoperative radiation for rectal cancer. Int J Radiat Oncol Biol Phys 50:665–674
Nagtegaal ID, Gaspar CGS, Peltenburg LTC, Marijen CAM, Kepiteijn E, van de Velde CJH, Fodde R, van Krieken JHJM (2005) Radiation induces different changes in expression profiles of normal rectal tissue compared with rectal carcinoma. Virchows Arch 446:127–135
Nagtegaal ID, Gaspar CGS, Marijen CAM, van de Velde CJH, Fodde R, van Krieken JHJM (2004) Morphological changes in tumour type after radiotherapy are accompanied by changes in gene expression profile but not in clinical behaviour. J Pathol 204:183–192
Nehls O, Klump B, Holzmann K, Lammering G, Borchard F, Gruenagel HH, Gaco V, Gregor M, Porschen R (1999) Influence of p53 status on prognosis in preoperatively irradiated rectal carcinoma. Cancer 85:2541–2548
Nishioka H, Hirano A, Haraoka J, Nakajima N (2002) Histological changes in the pituitary gland and adenomas following radiotherapy. Neuropathology 22:19–25
Papotti M, Cassoni P, Taraglio S, Bussolati G (1999) Oncocytic and oncocytoid tumors of the exocrine pancreas, liver, and gastrointestinal tract. Semin Diagn Pathol 16:126–134
Papotti M, Gugliotta P, Forte G, Bussolati G (1994) Immunocytochemical identification of oxyphilic mitochondrion-rich cells. Appl Immunohistochem 2:261–267
Piscitelli D, Ingravallo G, Resta L, Fiore MG, Maiorano E (2003) Oncocytic adenocarcinoma of the rectum with diffuse intra-luminal microcalcification: the first reported case. Virchows Arch 443:579–582
Polyak K, Li Y, Zhu H, Lengauer C, Willson JK, Markowitz SD, Trush MA, Kinzler KW, Vogelstein B (1998) Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet 20:291–293
Rödel C, Grabenbauer GG, Matzel KE, Schick C, Fietkau R, Papadopoulos T, Martus P, Hohenberger W, Sauer R (2000) Extensive surgery after high-dose preoperative chemoradiotherapy for locally advanced recurrent rectal cancer. Dis Colon Rectum 43:312–319
Rödel C, Grabenbauer GG, Papadopoulos T, Bigalke M, Günther K, Schick C, Peters A, Sauer R, Rödel F (2002) Apoptosis as a cellular predictor for histopathologic response to neoadjuvant radiochemotherapy in patients with rectal cancer. Int J Radiat Oncol Biol Phys 52:294–303
Roth SI, Olen E, Hansen LS (1962) The eosinophilic cells of the parathyroid (oxyphilic cells), salivary (oncocytes), and thyroid (Hürtle cells) glands. Light and electron microscopic observations. Lab Invest 11:933–941
Shia J, Guillem JG, Moore HG, Tickoo SK, Qin J, Ruo L, Suriawinata A, Paty PB, Minsky BD, Weiser MR, Temple LK, Wong WD, Klimstra DS (2004) Patterns of morphologic alterations in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol 28:215–223
Shia J, Tickoo SK, Guillem JG, Qin J, Nissan A, Hoos A, Stojadinovic A, Ruo L, Wong WD, Paty PB, Weiser MR, Minsky BD, Klimstra DS (2002) Increased endocrine cells in treated rectal adenocarcinomas. A possible reflection of endocrine differentiation in tumor cells induced by chemotherapy and radiotherapy. Am J Surg Pathol 26:863–872
Singh KK, Russel J, Sigala B, Zhang Y, Williams J, Keshav KF (1999) Mitochondrial DNA determines the cellular response to cancer therapeutic agents. Oncogene 18:6641–6646
Sobin LH, Wittekind Ch (eds) (2002) TNM classification of malignant tumours. Wiley, New York
Sobrinho-Simões M, Máximo V, Vieira de Castro I, Fonseca E, Soares P, Garcia-Rostan G, Cardoso de Oliveira M (2005) Hürtle (oncocytic) cell tumors of thyroid: etiopathogenesis, diagnosis and clinical significance. Int J Surg Pathol 13:29–35
Sun J, Chen Y, Li M, Ge Z (1998) Role of antioxidants enzymes in ionizing radiation resistance. Free Radic Biol Med 24:586–593
Suvarna SK, Stephenson TJ (2001) Oxyphilic metaplasia of adrenal cortex secondary to metyrapone therapy. Histopathology 39:546–547
Tallini G (1998) Oncocytic tumours. Virchows Arch 433:5–12
Tallini G, Carcangiu ML, Rosai J (1992) Oncocytic neoplasm of the thyroid gland. Acta Pathol Jpn 42:305–315
Tickoo SK, Amin MB, Linden MD, Lee MW, Zarbo RJ (1997) Antimitochondrial antibody (113-1) in the differential diagnosis of granular cells tumors. Am J Surg Pathol 21:922–930
Valentini V, Morganti AG, De Franco A, Coco C, Ratto C, Battista Doglietto G, Trodella L, Ziccarelli L, Picciocchi A, Cellini N (1999) Chemoradiation with or without intraoperative radiation therapy in patients with locally recurrent rectal carcinoma: prognostic factors and long term outcome. Cancer 86:2612–2624
Vermaas M, Ferenschild FT, Nuyttens JJ, Marinelli AW, Wiggers T, van der Sijp JR, Verhoef C, Graveland WJ, Eggermont AM, de Wilt JH (2005) Preoperative radiotherapy improves outcome in recurrent rectal cancer. Dis Colon Rectum 48:918–928
Willett CG, Warland G, Coen J, Shellito PC, Compton CC (1995) Rectal cancer: the influence of tumor proliferation on response to preoperative irradiation. Int J Radiat Oncol Biol Phys 32:57–61
Wittekind C, Tannapfel A (2003) Regressionsgrading des präoperativ-radiochemotherapierten Rektumkarzinoms. Eine Bestandsaufnahme. Pathologe 24:61–65
Wrutniak-Cabello C, Casas F, Cabello G (2001) Thyroid hormone action in mitochondria. J Mol Endocrinol 26:67–77
Xu R, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P (2005) Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res 65:613–621
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ambrosini-Spaltro, A., Salvi, F., Betts, C.M. et al. Oncocytic modifications in rectal adenocarcinomas after radio and chemotherapy. Virchows Arch 448, 442–448 (2006). https://doi.org/10.1007/s00428-005-0137-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-005-0137-6