Abstract
Oncocytic cell tumors (OCTs) of the thyroid include oncocytic cell adenomas (OCAs) and oncocytic cell carcinomas (OCCs). Oncocytic variant of papillary carcinoma (OVPC) has also been described. These tumors may present similar diagnostic problems as their non-oncocytic counterparts, in both conventional histology and fine-needle aspiration biopsies. Several markers were shown able to distinguish benign from malignant thyroid follicular tumors, galectin-3 and HBME-1 being the most promising ones. Controversial data have been reported on their discriminatory potential in the small series of OCTs so far analyzed. We aimed to assess the role of galectin-3 and HBME-1 in a large series of 152 OCTs (including 50 OCAs, 70 OCCs and 32 OVPCs). The expression of PPARγ protein was also evaluated. Using a biotin-free detection system, the sensitivity of galectin-3 was 95.1%, while that for HBME-1 was nearly 53%. The combination of galectin-3 and HBME-1 increased the sensitivity up to 99%. However, for both markers, the specificity was 88%, lower than that reported for non-oncocytic follicular tumors. PPARγ protein overexpression was absent in all OCAs tested and present in only 10% of OCCs, confirming previous reports on the low prevalence of PAX8-PPARγ translocations in OCT and ruling out its role as a potential diagnostic marker of malignancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oncocytic cell tumors (OCTs) of the thyroid include adenomas and carcinomas of follicular origin characterized by a predominant (usually more than 75% of the tumor area) population of eosinophilic mitochondrion-rich cells [18, 31]. They may be separated into oncocytic cell adenomas (OCAs) and oncocytic cell carcinomas (OCCs). An oncocytic variant of papillary carcinoma (OVPC) has also been described [13, 27]. Their biological behavior has been the matter of long-standing controversies. Originally, these tumors, also referred to as Hürthle cell or oxyphilic tumors, were all considered potentially malignant, based on the reported higher aggressive behavior than their conventional non-oncocytic follicular counterparts [3, 32]. More recently [6, 23], it became evident that the clinical outcome of OCTs was by no means different from that of the corresponding non-oncocytic tumors, irrespective of them being benign (adenomas) or malignant (well or poorly differentiated carcinomas). Nevertheless, their recognition is an important task for pathologists, since oncocytic cells may also be found in non-neoplastic conditions (goiter, thyroiditis). In addition, as in the case of non-oncocytic minimally invasive follicular carcinomas, the differential diagnosis of oncocytic adenomas from carcinomas may not be easily performed in all cases, and it is, in any case, impossible in cytological material from fine-needle aspiration (FNA) biopsies.
Several markers have been proposed, in both surgical and FNA cytological specimens, to distinguish follicular adenomas from carcinomas, including the oncocytic cell types. The most promising appeared to be galectin-3, cytokeratin 19, the mesothelial cell marker HBME-1 and thyroperoxidase [1, 4, 5, 8, 10, 11, 12, 22, 24, 25, 26, 28, 29]. In all published series, however, the number of OCTs studied was generally low. For galectin-3 and HBME-1 in particular, controversial data have been reported, indicating that the discriminatory potential of such markers in OCT was of limited value [14, 17]. To this purpose, we aimed to validate the role of galectin-3 and HBME-1 in a large series of 152 OCT, which included 50 OCAs, 70 OCCs and 32 OVPCs. In addition, we searched for PPARγ protein overexpression in all cases of OCA and OCC. PPARγ protein was found to be expressed by follicular carcinoma nuclei but not by adenomas [20], as a consequence of the PAX8-PPARγ translocation t(2;3)(q13;p25). This finding was not confirmed in subsequent reports and the specificity of this marker is rather poor [15]. Moreover, a very low rate of overexpression in OCC has been described in previous reports [20], thus excluding an involvement of PAX8-PPARγ translocation in the pathogenesis of this tumor type.
We here show that using a biotin-free detection system, galectin-3 alone is capable of correctly identifying 95% of malignant OCTs, and the association of galectin-3 and HBME-1 increases the sensitivity up to 99%.
Materials and methods
Case selection
We retrospectively analyzed 152 thyroid OCTs (including 50 OCAs, 70 OCCs and 32 OVPCs) and 34 non-neoplastic, oncocytic, cell-rich control cases (including 19 cases of nodular goiter with oncocytic changes and 15 cases of Hashimoto’s thyroiditis). Tumor samples were collected from the pathology files of the Department of Pathology, University of Turin, from 1974 to 2003. All tumors were classified according to widely accepted histological criteria [13, 27], and a diagnosis of oncocytic cell neoplasm was made when more than 75% of the cells had oncocytic features. Diagnostic criteria of malignancy discriminating OCC from OCA were complete capsular penetration and/or invasion of blood vessels within the capsule or external to it. OVPC were diagnosed in the presence of the characteristic nuclear features of papillary carcinoma, irrespective of their growth pattern (either papillary or follicular).
Immunohistochemical analysis
Immunohistochemistry was performed on paraffin-embedded sections using a standard manual biotin-free immunoperoxidase procedure with monoclonal antibodies against human galectin-3 (clone 9C4, diluted 1:200; Novocastra, Newcastle, UK), HBME-1 (clone HBME-1, diluted 1:50, DakoCytomation, Glostrup, Denmark), and PPARγ (clone E-8, 1:300; Santa Cruz Biotechnology, Santa Cruz, CA). For all antibodies tested, antigen-retrieval treatment (three 3-min passages in citrate buffer, pH 6.0) was performed, and then immune complexes were detected with the EnVision system (DakoCytomation) to prevent endogenous biotin activity, and visualized by diaminobenzidine precipitation. Slides were then counterstained with Mayer’s hematoxylin and mounted.
Scoring of staining and statistical data
All cases were evaluated by three independent investigators, blind with respect to the histological diagnosis. Cytoplasmic galectin-3 and HBME-1-positive cases were scored as + (≤10%), ++ (11–50%) and +++ (>50%), as previously reported [1, 14]. Nuclear staining for PPARγ was scored as weak (+), moderate (++), or strong (+++), and as focal (<50% of the tumor cells positive) or diffuse (>50% of tumor cells), as proposed by Nikiforova et al. [20]. According to these latter authors, in the final interpretation of the results, only cases with diffuse (>50% of tumor cells) and strong (+++) staining were considered positive (being correlated to the presence of the translocation at a molecular level).
Sensitivity, specificity, and diagnostic accuracy were assessed for each antibody tested, considered separately or in combination. Sensitivity was defined as true positive/(true positive+false negative) and specificity as true negative/(true negative+false positive). Diagnostic accuracy was defined as (true positive+true negative)/(true positive+false positive+true negative+false negative).
Results
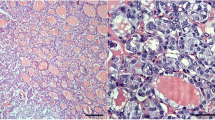
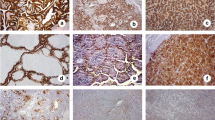
The immunohistochemical expression of galectin-3 and HBME-1 is summarized in Table 1. Among 50 cases of OCA, 44 were negative for galectin-3. In 6 cases (12%), a focal reactivity (score +) was observed. Accurate histological revision failed to detect in these latter cases the presence of capsular penetration or vascular invasion, or nuclear features suggestive of a follicular variant of papillary carcinoma. Cytoplasmic galectin-3 immunostaining was restricted to small clusters of tumor cells located beneath the tumor capsule. Cytoplasmic galectin-3 was expressed in 66 of 70 (94.3%) OCCs (Fig. 1) and in 31 of 32 (96.9%) OVPCs (Fig. 2). The immunoreactivity was typically strong and diffuse in cases of OVPC; while, in cases of OCC, it was mostly located at the periphery of the nodule and particularly in areas of capsular or vascular invasion, although some diffusely positive cases have been observed.
A case of oncocytic cell carcinoma (a), angioinvasive (top right) diffusely expressing cytoplasmic and nuclear galectin-3 in tumor cells and also in a blood vessel (top right; b). Tumor cells also co-express HBME-1 (c), with the typical endoluminal reinforcement (arrowheads) and PPARγ proteins (d). a Hematoxylin and Eosin, ×100, bar 100 μm. b Immunoperoxidase, ×100, bar 100 μm. c Immunoperoxidase, ×200, bar 50 μm. d Immunoperoxidase ×400, bar 25 μm
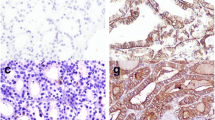
HBME-1 reactivity was detected in 6 of 50 OCAs (12%), 26 of 70 OCCs (37.1%) (Fig. 1) and 28 of 32 (87.5%) OVPCs. HBME-1 pattern of immunostaining was diffuse in most cases, while in some cases a clustered reactivity was observed either in the center or in the periphery of the nodule (in contrast with that of galectin-3). With respect to “false-positive” OCAs, 4 cases were reactive for both galectin-3 and HBME-1 (Fig. 3). Concerning “false-negative” OCCs, interestingly, 3 of the 4 galectin-3-negative cases were positive for HBME-1. All cases of hyperplastic nodular goiters with oncocytic changes were unreactive for both galectin 3 and HBME-1. Of 15 cases of Hashimoto’s thyroiditis, 14 showed a moderate (score ++) to strong (score +++) immunoreactivity for both galectin-3 and HBME-1 in clusters of oncocytic epithelial cells within lymphocytic infiltrates.
Immunostaining with PPARγ antibody was performed on 120 oncocytic thyroid tumors, comprising 50 OCA and 70 OCC (Table 1). Seven carcinomas (10%), being all but one minimally invasive, showed a strong and diffuse nuclear staining. In 6 cases (5 OCCs and 1 OCA), a weak to moderate nuclear staining (either focal or diffuse) was observed; since, according to the literature data [9, 20], only the strong diffuse nuclear staining is correlated with the presence of the PAX8-PPARγ rearrangement detected by RT-PCR, these latter cases were considered as negative.
Data on sensitivity, specificity and diagnostic accuracy of the three antibodies tested are reported in Table 2. Sensitivity ranged from 10% for PPARγ antibody to 95.1% for galectin-3. Although sensitivity for HBME-1 was very low (52.9%) in the present series of OCTs, the combination of galectin-3 and HBME-1 was shown to increase the general sensitivity up to 99%. Specificity was 88% for both galectin-3 and HBME-1, and slightly decreased using a combination of the two. A strong and diffuse PPARγ reactivity confirmed to be 100% specific of carcinomas. Finally, galectin-3 gained the highest diagnostic accuracy (92.8%) used alone or in combination with HBME-1, while HBME-1 alone showed a diagnostic accuracy of 64.5%, due to its low sensitivity.
Discussion
In this study, we have shown that galectin-3 is a useful marker of oncocytic carcinomas, having a sensitivity of 95.1%. Its combined use with HBME-1, however, increased sensitivity to 99%, suggesting that their association in a panel of markers is optimal for better characterizing OCCs in histological and, possibly, cytological specimens. These findings confirm the reported role of galectin-3 and HBME-1 when employed separately in conventional non-oncocytic tumors [1, 22] and also expand their usefulness for OCTs. Actually, the potential role of galectin-3 as a marker of malignancy in OCT as been questioned by some authors. In fact, a few recent papers suggested that galectin-3 immunodetection is not restricted to malignant tumors, but is also observed in adenomas [16, 17, 19].
A possible explanation for such reported discrepancies in OCT comes from the knowledge that oncocytic cells are rich in endogenous biotin and galectin-3 immunocytochemistry (as well as HBME-1) and may provide false-positive results in OCT using biotin-based detection systems, especially when heat-induced antigen retrieval methods (required in the case of galectin-3 immunocytochemistry) are employed. Therefore, as correctly pointed out by some authors [12, 30], galectin-3 immunodetection may be a useful adjunct to distinguish benign from malignant thyroid tumors, only if used in a biotin-free detection system.
Moreover, different technical procedures used to reveal galectin-3 may also explain controversial data from the literature. In some recent reports based on real-time reverse-transcription polymerase chain reaction (RT-PCR) methods [7], high levels of galectin-3 mRNA were detected also in benign thyroid nodules. In our opinion, methods aimed to localize the molecule in the tissue, such as immunohistochemistry, seem to be more reliable in interpreting the final results, the cytoplasmic fraction of galectin-3 being the only specific sign of malignant transformation [2]. PCR-based techniques and in situ hybridization, although highly sensitive, can distinguish neither the cytoplasmic from the nuclear galectin-3 localization nor (with regard to PCR) the cell type expressing galectin-3 mRNA [4]. Since it is well known that macrophages and endothelial cells produce relatively large amounts of galectin-3 in physiological conditions, such cell types are probably present in the tissue submitted to PCR analysis, irrespective of the benign or malignant nature [2]. In addition, although the immunohistochemical expression of galectin-3 was found to correlate with mRNA expression [10], it is not completely understood how mRNA transcription is regulated in benign conditions.
Our data on 152 cases of OCTs, under strictly controlled technical conditions, showed a galectin-3 immunoreactivity in 95.1% malignant OCTs (including OCC and OVPC cases) and in 12% of OCAs, values that are consistent with those observed in non-oncocytic follicular and papillary tumors. Conversely, lower sensitivity, specificity and, therefore, diagnostic accuracy (52.9%, 88% and 64.5%, respectively) were observed for HBME-1, as also pointed out by other authors [14] with special reference to OVPC cases. Our data suggest a limited diagnostic role of HBME-1 used alone in the diagnosis of OCC (since only 37.1% of cases were positive), its sensitivity in OVPC being slightly below that of galectin-3 used alone. Nevertheless, HBME-1 was able to recognize three of the four galectin-3-negative OCCs. Although this result has poor diagnostic utility due to the low HBME-1 specificity (being positive in 12% of OCAs), it suggests that an alternative phenotype is probably present in this small group of tumors. The presence of two alternative and non-overlapping pathogenetic pathways of tumorigenesis in follicular carcinoma have already been postulated by some authors [21], the presence of the PAX8-PPARgamma translocation being most frequently associated with a galectin-3-positive/HBME-1-negative immunophenotype and the presence of RAS mutations associated with a galectin-3-negative/HBME-1-positive immunophenotype. These data indirectly indicate that, in the diagnosis of follicular tumors in general, a panel of markers including at least galectin-3 and HMBE-1 would probably increase the sensitivity of an immunohistochemical approach up to nearly 100%.
A separate comment is deserved for “false-positive” OCAs, i.e., four cases positive for both galectin-3 and HBME-1 and two cases positive for one of the two markers, each. They may represent, on the one hand, true false-positive cases by immunohistochemistry, but on the other may reflect lesions having a biologically malignant potential in the absence of morphological signs of invasive growth (as already described in conventional follicular adenomas) [1]. As opposed to molecular markers (such as PAX8-PPARγ gene fusion product), which possess oncogenic implications and therefore are an early event in malignant transformation, galectin-3 and HBME-1 regulation in morphologically benign tumors, in tumors of uncertain malignant potential, as well as in early malignant lesions is poorly understood. In this respect, we were able to confirm the presence of immunoreactivity for both galectin-3 and HBME-1 in clusters of epithelial follicular cells with oncocytic changes in the setting of Hashimoto’s thyroiditis. These markers may be upregulated due to reactive processes rather than neoplastic transformation. The exact interactions between lymphoid and follicular epithelial cells, as well as the processes leading to oncocytic transformation in Hashimoto’s thyroiditis, are far to be understood. However, the positive cells often present a mild to moderate degree of atypia, with nuclear features resembling those of papillary carcinoma, although clear molecular markers of oncogenic transformation, typical of papillary carcinoma, such as RET/PTC translocation, have not been definitely identified. Therefore, further studies are required to better understand the biological significance of such morphological and immunophenotypical features.
With regard to PAX8-PPARγ gene fusion protein, we have investigated a large series of 120 cases by means of immunohistochemistry, to validate previous reports, based on small number of cases, which failed to detect PPARγ abnormalities in OCT (either by RT-PCR or immunohistochemistry). Since a good correlation has been reported between the molecular detection of PAX8-PPARγ translocation and a strong and diffuse PPARγ protein overexpression, only immunohistochemistry was employed (also to check the possible applications of PPARγ as a diagnostic marker of OCC). Our data confirmed that PPARγ protein over-expression occurred in a very small number of OCCs, all being OCA negative. These results support the hypothesis that OCCs may follow distinct molecular pathways from those of non-oncocytic follicular carcinomas [20].
In conclusion, the present data confirm a high sensitivity and diagnostic accuracy of galectin-3 immunohistochemistry in the diagnosis of malignant OCT, at variance with HBME-1, which had a much lower sensitivity and specificity in OCT than that reported for non-oncocytic follicular tumors (an exception being OVPC). However, the combined use of these two markers was able to improve the sensitivity up to 99%, and therefore a panel including galectin-3 and HBME-1 is recommended in the diagnosis of OCT, although the low specificity of HBME-1 has to be considered when dealing with galectin-3-negative/HBME-1-positive OCT cases. Such a panel of markers is probably the best choice in FNA cytological diagnosis of OCT, to obtain the highest sensitivity in selecting presumably malignant cases to be sent to the surgeon.
References
Bartolazzi A, Gasbarri A, Papotti M, Bussolati G, Lucante T, Khan A, Inohara H, Marandino F, Orlandi F, Nardi F, Vecchione A, Tecce R, Larsson O, Thyroid Cancer Study Group (2001) Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet 357:1644–1650
Bartolazzi A, Papotti M, Orlandi F (2003) Methodological considerations regarding the use of galectin-3 expression analysis in preoperative evaluation of thyroid nodules. J Clin Endocrinol Metab 88:950
Carcangiu ML, Bianchi S, Savino D, Voynick IM, Rosai J (1991) Follicular Hurthle cell tumors of the thyroid gland. Cancer 68:1944–1953
Cheung CC, Ezzat S, Freeman JL, Rosen IB, Asa SL (2001) Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol 14:338–342
De Micco C, Ruf J, Chrestian MA, Gros N, Henry JF, Carayon P (1991) Immunohistochemical study of thyroid peroxidase in normal, hyperplastic, and neoplastic human thyroid tissues. Cancer 67:3036–3041
Evans HL, Vassilopoulou-Sellin R (1998) Follicular and Hurthle cell carcinomas of the thyroid: a comparative study. Am J Surg Pathol 22:1512–1520
Feilchenfeldt J, Totsch M, Sheu SY, Robert J, Spiliopoulos A, Frilling A, Schmid KW, Meier CA (2003) Expression of galectin-3 in normal and malignant thyroid tissue by quantitative PCR and immunohistochemistry. Mod Pathol 16:1117–1123
Fonseca E, Nesland JM, Hoie J, Sobrinho-Simoes M (1997) Pattern of expression of intermediate cytokeratin filaments in the thyroid gland: an immunohistochemical study of simple and stratified epithelial-type cytokeratins. Virchows Arch 430:239–245
French CA, Alexander EK, Cibas ES, Nose V, Laguette J, Faquin W, Garber J, Moore F Jr, Fletcher JA, Larsen PR, Kroll TG (2003) Genetic and biological subgroups of low-stage follicular thyroid cancer. Am J Pathol 162:1053–1060
Gasbarri A, Martegani MP, Del Prete F, Lucante T, Natali PG, Bartolazzi A (1999) Galectin-3 and CD44v6 isoforms in the preoperative evaluation of thyroid nodules. J Clin Oncol 17:3494–3502
Henry JF, Denizot A, Porcelli A, Villafane M, Zoro P, Garcia S, De Micco C (1994) Thyroperoxidase immunodetection for the diagnosis of malignancy on fine-needle aspiration of thyroid nodules. World J Surg 18:529–534
Herrmann ME, LiVolsi VA, Pasha TL, Roberts SA, Wojcik EM, Baloch ZW (2002) Immunohistochemical expression of galectin-3 in benign and malignant thyroid lesions. Arch Pathol Lab Med 126:710–713
LiVolsi VA (1990) Surgical pathology of the thyroid. Saunders, Philadelphia
Mai KT, Bokhary R, Yazdi HM, Thomas J, Commons AS (2002) Reduced HBME-1 immunoreactivity of papillary thyroid carcinoma and papillary thyroid carcinoma-related neoplastic lesions with Hurthle cell and/or apocrine-like changes. Histopathology 40:133–142
Marques AR, Espadinha C, Catarino AL, Moniz S, Pereira T, Sobrinho LG, Leite V (2002) Expression of PAX8-PPAR gamma 1 rearrangements in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab 87:3947–3952
Martins L, Matsuo SE, Ebina KN, Kulcsar MA, Friguglietti CU, Kimura ET (2002) Galectin-3 messenger ribonucleic acid and protein are expressed in benign thyroid tumors. J Clin Endocrinol Metab 87:4806–4810
Nascimento MC, Bisi H, Alves VA, Longatto-Filho A, Kanamura CT, Medeiros-Neto G (2001) Differential reactivity for galectin-3 in Hürthle cell adenomas and carcinomas. Endocr Pathol 12:275–279
Nesland JM, Sobrinho Simoes M, Holm R, Sambade MC, Johannessen JV (1985) Hurthle-cell lesions of the thyroid: a combined study using transmission electron microscopy, scanning electron microscopy, and immunohistochemistry. Ultrastruct Pathol 8:269–290
Niedziela M, Maceluch J, Korman E (2002) Galectin-3 is not an universal marker of malignancy in thyroid nodular disease in children and adolescents. J Clin Endocrinol Metab 87:4411–4415
Nikiforova MN, Biddinger PW, Caudill CM, Kroll TG, Nikiforov YE (2002) PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol 26:1016–1023
Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW 2nd, Tallini G, Kroll TG, Nikiforov YE (2003) RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab 88:2318–2326
Orlandi F, Saggiorato E, Pivano G, Puligheddu B, Termine A, Cappia S, De Giuli P, Angeli A (1998) Galectin-3 is a presurgical marker of human thyroid carcinoma. Cancer Res 58:3015–3020
Papotti M, Torchio B, Grassi L, Favero A, Bussolati G (1996) Poorly differentiated oxyphilic (Hurthle cell) carcinomas of the thyroid. Am J Surg Pathol 20:686–694
Papotti M, Volante M, Saggiorato E, Deandreis D, Veltri A, Orlandi F (2002) Role of galectin-3 immunodetection in the cytological diagnosis of thyroid cystic papillary carcinoma. Eur J Endocrinol 147:515–521
Raphael SJ, McKeown-Eyssen G, Asa SL (1994) High-molecular-weight cytokeratin and cytokeratin-19 in the diagnosis of thyroid tumors. Mod Pathol 7:295–300
Raphael SJ (2002) The meanings of markers: ancillary techniques in diagnosis of thyroid neoplasia. Endocr Pathol 13:301–311
Rosai J, Carcangiu ML, De Lellis RA (1992) Tumors of the thyroid gland. In: Atlas of tumor pathology. Armed Forces Institute of Pathology, Washington, pp 161–182
Sack MJ, Astengo-Osuna C, Lin BT, Battifora H, LiVolsi VA (1997) HBME-1 immunostaining in thyroid fine-needle aspirations: a useful marker in the diagnosis of carcinoma. Mod Pathol 10:668–674
Saggiorato E, Cappia S, De Giuli P, Mussa A, Pancani G, Caraci P, Angeli A, Orlandi F (2001) Galectin-3 as a presurgical immunocytodiagnostic marker of minimally invasive follicular thyroid carcinoma. J Clin Endocrinol Metab 86:5152–5158
Saggiorato E, Aversa S, Deandreis D, Arecco F, Mussa A, Puligheddu B, Cappia S, Conticello S, Papotti M, Orlandi F (2004) Galectin-3: presurgical marker of thyroid follicular epithelial cell-derived carcinomas. J Endocrinol Invest (in press)
Tallini G (1998) Oncocytic tumors. Virchows Arch 433:5–12
Watson RG, Brennan MD, GoellnerJR, van Heerden JA, McConahey WM, Taylor WF (1984) Invasive Hurthle cell carcinoma of the thyroid: natural history and management. Mayo Clinic Proc 59:851–855
Acknowledgements
The work was supported by grants from the Italian Ministry of University and Education (ex 60% to M.P.), from the Regione Piemonte (D.D. no.173 dated October 30, 2003, to F.O.), and The Compagnia di San Paolo “Progetto Speciale Oncologia”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Volante, M., Bozzalla-Cassione, F., DePompa, R. et al. Galectin-3 and HBME-1 expression in oncocytic cell tumors of the thyroid. Virchows Arch 445, 183–188 (2004). https://doi.org/10.1007/s00428-004-1074-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-004-1074-5