Abstract
The high-mobility group protein HMGI(Y) is a member of a family of non-histone chromosomal proteins, which have been implicated in the regulation of inducible gene transcription, integration of retroviruses into chromosomes and induction of neoplastic transformation and metastatic progression in cancer cells. The human trophoblast is a tissue that shares proliferation capacity and invasiveness with neoplastic tissues, but in which these processes are tightly regulated. In the present study, we analyzed the expression of HMGI(Y) in the human placenta using immunohistochemistry. We found expression of HMGI(Y), with nuclear localization, in the villous cytotrophoblast (vCT), which is a highly proliferative cell type. In contrast, the majority of the nuclei of the villous syncytiotrophoblast, a terminally differentiated tissue, was negative. Interestingly, expression of HMGI(Y) was strongest in anchoring villi at the implantation site and in extravillous (intermediate) trophoblast (EVT) invading the maternal decidua. As vCT cells differentiate to become EVT, the HMGI(Y) protein appears to switch from a nuclear to a cytoplasmic localization. Expression of HMGI(Y) in isolated trophoblast populations in primary cell culture was also confirmed using Western-blot analysis. This study shows for the first time expression and localization of HMGI(Y) in the subpopulations of placental tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The high mobility group I (HMG I) proteins are a family of non-histone chromatin-associated proteins [5, 14, 22, 31]. HMGI and HMGY are isoforms derived from the same gene, HMG I(Y), located on chromosome 6 (6p21) and generated by alternative splicing of the pre-mRNA. They differ by 11 amino acids present in HMGI but deleted in HMGY [11, 15, 16]. Mammalian HMGI/Y proteins function as architectural transcription factors, which participate in multi-protein activating structures on target gene promoters and which have been shown to be implicated in various cellular processes, including regulation of inducible gene expression, integration of retroviruses into chromosomes and induction of neoplastic transformation and metastatic promotion of cancer cells [20, 26].
HMGI(Y) proteins are highly expressed during early stages of embryonic development, and their gene expression is either completely silent or markedly (approximately 200-fold) reduced in adult tissues [7, 27, 35]. As for neoplastic transformation, the first evidence for an oncogenic potential of HMGI(Y) proteins and their causal involvement in tumorigenesis came from the analysis of sporadic chromosomal translocations in human tumors [17], showing that HMGI(Y) is the target of 6p21.3 rearrangements in various benign mesenchymal tumors. Similarly, rearrangements on human chromosome 12q15, an event frequently found in benign solid tumors, were shown to also involve a high-mobility group protein, HMGI-C [29].
The trophoblast is the first tissue to differentiate in the mammalian conceptus, and its normal development and specific properties are crucial for both implantation and further survival of the embryo. Furthermore, the placenta is unique in its ability to proliferate and invade another tissue in a fashion similar to malignant tumors, but is tightly controlled both spatially and temporally. It is, thus, a very interesting model for the study of molecular mechanisms involved in these processes and for differentiating them from those implicated in tumor progression.
During development of the human placenta, the cytotrophoblast (CT) proliferates and gives rise to the differentiated syncytiotrophoblast (ST) on the villous surface and to the extravillous (intermediate) trophoblast (EVT), which invades the maternal tissues and provides the anchoring of the placenta and the conceptus at the maternal–fetal interface [30].
The present study was designed to investigate the expression pattern of HMGI(Y) in the human placenta using immunohistochemistry. Furthermore, expression was investigated in isolated placental cells in primary culture using Western-blot analysis.
Materials and methods
Tissue collection
The tissue material was selected following histological review from the files of the Department of Gynecopathology, University Hospital Eppendorf, Hamburg. Only normal placentae were included in this study (for the second-trimester cases, these represented late abortions, i.e., for psychiatric indications. Cases with chromosomal abnormalities were not included). For immunohistochemistry, specimens that had been routinely fixed in 4% buffered formalin and embedded in paraffin were used. A total of 20 samples were analyzed, including 7 first-trimester, 6 second-trimester and 7 third-trimester samples.
Immunohistochemistry
Serial sections of 4–6 µm were cut from the paraffin blocks and mounted on 3-amino propyl tri-ethoxysilone (APES)-coated slides, deparaffinized in xylene and rehydrated in graded alcohol to TBS (Tris buffered saline; 50 mM Tris, 150 mM NaCl, pH 7.4). The slides were microwaved for 5×2 min in 10 mM citrate, pH 6.0. After cooling down for 20 min, the slides were washed in TBS, blocked for 20 min at room temperature with normal rabbit serum (Dako, Glostrup, Denmark), diluted 1:20 in TBS and incubated overnight at 4°C with HMGI(Y) affinity-purified goat polyclonal antibody (N-19; Santa Cruz Biotechnology) diluted 1:25–1:50. Omission of the first antibody was used for negative controls. Slides were reacted with biotin-labeled corresponding secondary antibody, incubated with preformed avidin biotinylated enzyme complex (ABC)-complex (Vectastain; Vector Laboratories, Burlingame, CA, USA) and detected with diaminobenzidine (DAB) kit (Vectastain; Vector Laboratories, Burlingame, CA, USA). The slides were counterstained with hemalaun and mounted with glycerin/gelatin.
Isolation of invasive and non-invasive trophoblast populations
Cultures of first-trimester invasive and non-invasive trophoblast populations were established and characterized as reported previously [1]. Briefly, eight to ten placentae (5–12 weeks) obtained after legal termination of pregnancy were washed in sterile phosphate buffer saline (s-PBS), and areas rich in chorionic villi were selected and minced between scalpel blades and were subjected to three sequential 10-min treatments with 0.125 % trypsin and 0.2 mg/ml DNAse I (Boehringer Mannheim, Germany) in s-PBS containing 5 mM MgCl2. Cells released from each 10-min step were pooled and filtered through two layers of muslin, resuspended in 70% percoll (Pharmacia, Uppsala, Sweden) at a density of 2×105 cells/ml, and put under 20 ml of 25% percoll. Next, 10 ml s-PBS was put on top of the 25% percoll and a gradient was established by centrifuging for 20 min at 800×g. Cells from the middle band (density 1.048–1.062 g/ml) of the gradient were pooled, washed in s-PBS and seeded at a density of 1×106 cells/ml of keratinocyte growth medium (KGM) supplemented with 10% fetal calf serum (FCS).
Cells were identified as trophoblast using immunocytochemical staining with monoclonal antibodies to cytokeratin (Dako-CK, MNF 116 and 35BH11; 1:100). Their functional ability to produce hormones and the 92-kDa and 72-kDa gelatinases were described previously [1].
In vitro invasion assay
The invasive characteristics of the EVT cells were determined by an in vitro matrigel invasion assay as described [1] using transwells with a polycarbonate filter of 2.5 cm diameter and 8 µm pore size. The upper surface of the filter was coated with Matrigel (Collaborative Research, Bedford, MA; dilution 1:20 with KGM). The bottom chamber was filled with 3 ml of KGM containing 10% FCS. Trophoblast cells labeled for 72 h with 10 µci/ml H3-thymidine in KGM were trypsinized, washed and resuspended at a density of 1.0×105 cells/ml KGM containing 10% FCS, and 2 ml of the labeled cell suspension was added to the upper well of the transwell chamber. After 72 h of incubation, the cells from the lower wells were harvested and used for Western-blot analysis.
Western-blot analysis of human placental tissue and trophoblast cells in primary culture
Extraction of proteins from endometrial tissue, HeLa cells (used as a positive control) and villous and EVT cells was carried out in PBS in the presence of 1% NP40 and protease inhibitors as previously described [2]. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed in a 7.5% polyacrylamid gel under reducing conditions, applying 50 µg of each sample of the concentrated protein extract. After electrophoretic transfer to nitrocellulose and blocking in TBS containing 5% bovine serum albumin (BSA) for 2 h, the anti-HMGI(Y) antibody (same as used for immunohistochemistry) was added (dilution 1:1000) and incubated overnight at 4 C. Detection was carried out with peroxidase.
Results
Immunohistochemical localization of HMGI(Y) in the human placenta
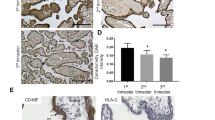
Representative results of immunohistochemical detection of HMGI(Y) in placental structures are shown in Fig. 1. As can be observed, HMGI(Y) is expressed in the villous CT (panels A–D), where it has a nuclear localization. The nuclei of the terminally differentiated villous ST, however, were mostly HMGI(Y) negative. Also, expression of HMGI(Y) can be observed in some nuclei of villous core cells. As can be seen in panels B, C and D, expression of HMGI(Y) is strongest in anchoring villi at the implantation site and, specifically, in EVT invading the maternal decidua. Interestingly, in these cells, expression appears to switch from the nuclear pattern observed for CT (also the CT of the anchoring villi show this nuclear pattern) to a cytoplasmic localization. This pattern of expression is consistently observed in placenta samples from the first and second trimesters.
Immunohistochemical localization of high-mobility group protein [HMGI(Y)] in the human placenta. A Chorionic villi showing nuclear expression of HMGI(Y) in cells of the villous cytotrophoblast. In contrast, most nuclei of the syncytiotrophoblast are HMGI(Y)-negative (×400). B Nuclear expression in the villous cytotrophoblast and almost absent expression in the villous syncytiotrophoblast. Additionally, strong, predominantly cytoplasmic/membranous expression pattern in the extravillous trophoblast invading the maternal decidua (×400). C Anchoring villus showing nuclear expression of HMGI(Y) in the cytotrophoblast and mostly membranous/cytoplasmatic expression in the emerging extravillous trophoblast invading the maternal decidua (×200). D Higher magnification of C showing a predominantly nuclear expression of HMGI(Y) in the proximal extravillous trophoblast and mostly cytoplasmatic/membranous expression in the deeper (interstitial) extravillous trophoblast invading the maternal decidua and maternal vessels (×400). E Both nuclear and cytoplasmatic/membranous expression of HMGI(Y) in extravillous trophoblast of the chorion laeve (×400). F Predominantly cytoplasmatic/membranous expression of HMGI(Y) in the endovascular extravillous trophoblast (×400). VL vascular lumen
Expression of HMGI(Y) is also seen in the EVT of the chorion laeve (panel E) and in the amniotic epithelial cells. Cytoplasmic expression of HMGI(Y) is also observed in cells of the decidualized endometrium and in endometrial glands of pregnancy endometrium as well as in glandular epithelium of normal non-pregnant endometrium (not shown).
Western-blot analysis of human trophoblast cells in primary culture
As can be seen in Fig. 2, Western-blot analysis with the same anti-HMGI(Y) antibody as has been employed for immunohistochemistry shows strong HMGI(Y) expression in the positive controls (Endo=endometrium and HeLa cells). From the trophoblast cell populations in primary culture, the invasive trophoblast (EVT) shows strong expression, followed by villous CT. Very weak/absent detection is observed in villous ST, corresponding to the observations made by analyzing the immunohistochemistry data, where expression was also strongest in EVT, while villous ST was frequently negative.
Discussion
In the present study, we used immunohistochemistry to investigate the expression pattern of the high-mobility group protein HMGI(Y) in the human placenta. In addition, HMGI(Y) expression was analyzed using Western blot on placental tissue and isolated placental cells in primary culture.
As shown in Fig. 1, HMGI(Y) is strongly expressed in the human placenta in both villous and extravillous trophoblast, with different subcellular localization patterns. In the villous cytotrophoblast (vCT), expression is strictly nuclear, with a granular appearance, indicating association with chromatin. The CT is a highly proliferative tissue, in which HMGI(Y) could be implicated in regulating proliferation, as has been shown to be the case for other rapidly dividing cells [20]. In the terminally differentiated villous syncytiotrophoblast (vST), the majority of nuclei were negative, indicating that HMGI(Y) expression is probably shut down during differentiation of CT into ST, similar to later embryonic developmental stages [7, 27, 35]. Aside from differentiating into ST, the CT also differentiates into EVT. The EVT can be further divided into proximal EVT originating from the anchoring villi, deep interstitial EVT invading the decidual stroma and the myometrium, endovascular trophoblast, which assumes endothelium-like characteristics, and "intermediate-like" EVT of the chorion laeve [6, 8, 36]. Interestingly, we observed very strong expression of HMGI(Y) in the EVT. Cytoplasmatic expression of HMGI(Y) was also found in the deeper (interstitial) EVT invading the maternal decidua, and the maternal vessels (endovascular trophoblast), as well as in the EVT of the chorion laeve. As found by others (A. Flohr, personal communication) and confirmed in this study, cytoplasmatic HMGI(Y) expression is also found in glandular epithelia of both cycling and pregnant endometrium and in decidual cells.
Regarding the possible roles of HMGI(Y) in the human placenta, these could obviously include regulation of cell proliferation in the CT and proliferation stop correlated with differentiation in the ST. In a previous study, we demonstrated that the proliferative CT expressed the cell cycle promoter cyclin E but not the cell cycle inhibitor p27, while ST showed an inverse expression pattern [3]. While expression of p27 is regulated mostly at the post-transcriptional level, expression of cyclin E is transcriptionally regulated and might be influenced by HMGI(Y). One could speculate that this could also be the case for the EVT, where expression of both proliferation-inducing molecules, such as cyclin E [3], adhesion molecules, such as CEACAM1 [4], or of invasion-promoting enzymes, such as matrix-metallo proteinase 9 (MMP-9) could be influenced by HMGI(Y). In this line of thought, one should notice that HMGI(Y) was shown to modulate expression of adhesion molecules, such as E-selectin [19, 34], which can function as a ligand for CEACAM1 [18], which is specifically expressed by EVT [4]. Also, junB and fra-1, members of the AP-1 transcription factor family, have been shown to be positively regulated by HMGI(Y) [33], and the MMP-9 promoter has been shown to carry AP-1 responsive and be regulated by AP-1 transcription factors [12, 28]. Furthermore, in invasive breast cancer cells (MDA-MB 231), which highly express HMGI(Y), treatment with an inhibitor of MMP-9 resulted in a reduction of both invasive capability of the cells in vitro and reduced expression of HMGI(Y), indicating the existence of a link among invasiveness, expression of HMGI(Y) and MMP-9 [21]. Since EVT cells invading the maternal decidua also use MMP-9 as a main matrix-degrading enzyme [25], this observation might apply to EVT as well. Furthermore, in mice lacking junB, lethality due to defects in placental development was observed, along with a downregulation of MMP-9 expression [13], and, as mentioned, junB has, in another context, been shown to be under the influence of HMGI(Y) [33].
However, the cytoplasmatic localization of HMGI(Y) in the deeper EVT indicates that it might also play roles different from those previously described. It is thus possible that it might be associated with proteins localized to the cytoplasm, or that it might be secreted and act as an extracellular ligand, as has recently been shown to be the case for HMGB1 [23]. HMG-B1 (also called amphoterin) has been shown to be secreted by neurons and be retained associated with their plasma membranes at the leading edge of migration [10] and with promoter cell migration of vascular smooth muscle cells and fibroblasts [9, 23]. It is possible that HMGI(Y) might play a similar role, for instance, in the migration of EVTs, yet data supporting this hypothesis are lacking to date. Furthermore, HMGB1 appears to play a role in the activation of extracellular proteases, which are important for tissue invasion. It has been shown to bind to several components of the plasminogen activation system and enhance activation of tissue plasminogen activator [24] and also to activate MMP-2 and MMP-9 [23, 32], which play an important role in trophoblast invasion.
The present study shows, to our knowledge, for the first time the expression pattern of HMGI(Y) in the cellular populations of the human placenta and its nuclear localization in villous cytotrophoblast and cytoplasmatic localization in EVT cells. Further studies will now be necessary to investigate the new possible functions of this molecule in both the process of trophoblast proliferation, differentiation and invasion and in the related process of tumor invasion and metastasis.
References
Aboagye-Mathiesen G, Zdravkovic M, Toth FD, Ebbesen P (1997) Effects of human trophoblast-induced interferons on the expression of c-fms/CSF-1R, EGF-R and c-erbB2 in invasive and non-invasive trophoblast. Placenta 18:155–161
Bamberger AM, Riethdorf L, Nollau P, Naumann M, Erdmann I, Götze J, Brümmer J, Schulte HM, Wagener C, Löning T (1998) Dysregulated expression of CD66a (BGP, C-CAM), an adhesion molecule of the CEA family, in endometrial cancer. Am J Pathol 152:1401–1406
Bamberger AM, Sudahl S, Bamberger CM, Schulte HM, Löning T (1999) Expression pattern of the cell cycle inhibitor p27 and the cell-cycle promoter cyclin E in the human placenta throughout gestation: Implications for the control of proliferation. Placenta 20:401–406
Bamberger AM, Sudahl S, Löning T, Wagener C, Bamberger CM, Drakakis P, Coutifaris C, Makrigiannakis A (2000) The adhesion molecule CEACAM1 (CD66a, C-CAM, BGP) is specifically expressed by the extravillous intermediate trophoblast. Am J Pathol 156:1165–1170
Bewley CA, Gronenborn AM, Clore GM (1998) Minor groove-binding architectural proteins: structure, function and DNA recognition. Annu Rev Biopys Biomol Struct 27:105–131
Burrows TD, King A, Loke YW (1996) Trophoblast migration during human placental implantation. Hum Reprod Update 2:307–321
Chiappetta G, Bandiera A, Berlingieri MT (1995) The expression of the high mobility group HMGI(Y) proteins correlates with the malignan phenotype of human thyroid neoplasias. Oncogene 10:1307–1314
Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ (1994) Integrin switching regulates normal trophoblast invasion. Development 120:3657–3677
Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME (2001) The High Mobility Group Boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton organization in rat smooth muscle cells. J Cell Biol 152:1197–2006
Fages C, Nolo R, Huttunen HJ, Eskelinen E, Rauvala H (2000) Regulation of cell migration by amphoterin. J Cell Sci 113:611–620
Friedmann M, Holth LT, Zoghbi HY, Reeves R (1993) Organization, inducible-expression and chromosome localization of the human HMG-I(Y) nonhistone protein gene. Nucleic Acids Res 21:4259–4267
Huhtala P, Tuuttila A, Chow LT, Lohi J, Keski-Oja J, Tryggvason K (1991) Complete structure of the human gene for 92-kDa type IV collagenase: divergent regulation of expression for the 92- and 72- kilodalton enzyme genes in HT-1080 cells. J Biol Chem 266:16485–16490
Jochum W, Passegue E, Wagner EF (2001) AP-1 in mouse development and tumorigenesis. Oncogene 20:2401–2412
Johns EW (1982) History, definitions and problems. The HMG proteins. Academic Press, London, pp 1–7
Johnson KR, Lehn DA, Elton TS, Barr PJ, Reeves R (1988) Complete murine cDNA sequence, genomic structure, and tissue expression of the high mobility group protein HMGI(Y). J Biol Chem 263:18338–18342
Johnson KR, Lehn DA, Reeves R (1989) Alternative processing of mRNAs encoding mammalian chromosomal hig-mobility-group proteins HMG-I and HMG-Y. Mol Cell Biol 9:2114–2123
Kazmierczak B, Dal Cin P, Wanschura S, Borrmann L, Fusco A, Van den Berghe H, Bullerdiek J (1998) HMGIY is the target of 6p21.3 rearrangements in various benign mesenchymal tumors. Genes Chromosomes Cancer 23:279–285
Kuijpers TW, Hoogerwerf M, Van der Laan LJ, Nagel G, Van der Schoot CE, Grunert F, Roos D (1992) CD66 nonspecific cross-reacting antigens are involved in neutrophil adherence to cytokine-activated endothelial cells. J Cell Biol 118:457–466
Lewis H, Kaszubska W, DeLamarter JF, Whelan J (1994) Cooperativity between two NF-kappa B complexes, mediated by high-mobility-group protein I(Y), is essential for cytokine-induced expression of the E-selectin promoter. Mol Cell Biol 14:5701–5709
Liu F, Cahu K, Arlotta P, Ono SJ (2001) The HMG I Proteins: dynamic roles in gene activation, development and tumorigenesis. Immunol Res 23:35–51
Liu WM, Guerra-Vladusic FK, Kurakata S, Lupu R, Kohwi-Shigematsu T (1999) HMG-I(Y) recognizes base-unpairing regions of matrix attachment sequences and its increased expression is directly linked to metastatic breast cancer phenotype. Cancer Res 59:5695–5703
Lund T, Hotlund J, Fredriksen M, Laland SG (1983) On the presence of two new high mobility group-like proteins in HeLa cells. FEBS Lett 152:163–167
Müller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME (2001) The double life of HMGB1 chromatin protein: architectural factor and extacellular signal. EMBO J 20:4337–4340
Parkinen J, Rauvala H (1991) Interactions of plasminogen and tissue plasminogen activator (t-PA) with amphoterin. Enhancement of t-PA-catalyzed plasminogen activation by amphoterin. J Biol Chem 266:16730–16735
Polette M, Nawrocki B, Pintiaux A, Massenat C, Maquoi E, Volders L, Schaapa JP, Birembaut P, Foidart JM (1994) Expression of gelatinases A and B and their tissue inhibitors by cells of early and term human placenta and gestational endometrium. Lab Invest 71:838–846
Reeves R, Beckerbauer L (2001) HMGI/Y proteins: flexible regulators of transcription and chromatin structure. Biochem Biophys Acta 1519:13–29
Rogalla P, Drechsler K, Frey G, Henning Y, Helmke B, Bonk U, Bullerdiek J (1996) HMGI-C expression patterns in human tissues. Implications for the genesis of frequent mesenchymal tumors. Am J Pathol 149:775–779
Sato H, Seiki M (1994) Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene 8:395–405
Schoenmakers EF, Wanschura S, Mols R, Bullerdiek J, Van den Berghe H, Van de Ven WJ (1995) Recurrent rearrangements in the high mobility group protein gene, HMGI-C, in benign mesenchymal tumours. Nat Genet 10:436–444
Shih I-M, Kurman RJ (1997) New concepts in trophoblastic growth and differentiation with practical application for the diagnosis of gestational trophoblastic disease. Verh Dtsch Ges Pathol 81:266–272
Strauss F, Varhavsky A (1984) A protein binds to a satellite DA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell 37:889–901
Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM (2000) Blockage of RAGE-amphoterin signalling suppresses tumour growth and metastasis. Nature 405:345–360
Vallone D, Battista S, Pierantoni GM, Fedele M, Casalino L, Santoro M, Viglietto G, Fusco A, Verde P (1997) Neoplastic transformation of rat thyroid cells requires the junB and fra-1 gene induction which is dependent on the HMGI-C gene product. EMBO J 16:5310–5321
Whitley MZ, Thanos D, Read MA, Maniatis T, Collins T (1994) A striking similarity in the organization of the E-selectin and the beta-interferon gene promoters. Mol Cell Biol 14:6464–6475
Zhou X, Benson KF, Ashar HR, Chada K (1995) Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature 376:771–774
Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH (1997) Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest 99:2139–2151
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bamberger, AM., Makrigiannakis, A., Röser, K. et al. Expression of the high-mobility group protein HMGI(Y) in human trophoblast: potential role in trophoblast invasion of maternal tissue. Virchows Arch 443, 649–654 (2003). https://doi.org/10.1007/s00428-003-0892-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-003-0892-1