Abstract
To determine the cellular origin of plasmacytoid cells in salivary gland adenomas, immunohistochemistry was performed on sections from 12 pleomorphic adenomas rich in these cells. In normal salivary glands included in these sections, the myoepithelial cells (MECs) expressed α-smooth muscle actin (αSMA) and smooth muscle myosin heavy chain (SMMHC), whereas the duct luminal cells expressed keratins 19, 18 and 8. Some of the salivary duct basal cells expressed these keratins, and the acinar cells expressed keratins 18 and 8. The expression profile was similar in rat salivary glands not only after but also during development. The immature MECs never expressed the keratins nor did the immature duct cells express αSMA. In seven cases, up to 60% of the plasmacytoid cells expressed keratin 19. In three of these cases, about 10% of the plasmacytoid cells expressed keratin 18. No plasmacytoid cells expressed αSMA, SMMHC or keratin 8. These results indicate that plasmacytoid cells originate from luminal cells and not from MECs. Furthermore, in addition to the luminal tumor cells, the non-luminal cells could express keratins 19, 18 and 8. Therefore, it is necessary to re-evaluate the prevailing notion that non-luminal cells are modified MECs. Keratin 14, basic calponin, vimentin and p63 were bi-specific for the MECs and the duct cells. Therefore, expression of these proteins by significant numbers of the non-luminal tumor cells and the plasmacytoid cells never denied the above notion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myoepithelial cells (MECs) are cellular components of various kinds of salivary gland tumor. There is strong evidence that they exert ameliorative effects in these tumors [4], as demonstrated, for example, in a series of recent studies by Barsky et al. [36, 46, 47, 48]. They have demonstrated that MECs (1) promote the differentiation of tumor cells, (2) synthesize basement-membrane and non-basement-membrane components, (3) secrete high amount of tumor-suppressor maspin and various proteinase inhibitors some of which accumulate within MEC-derived extracellular matrix and (4) inhibit invasion and angiogenesis [36, 46, 47, 48]. Therefore, exact identification of MECs is necessary to predict the biological behavior of the tumor.
Myoepithelioma is believed to be a rare kind of pleomorphic adenoma that is almost entirely composed of MECs [20]. Contrary to our expectation, the biological behavior of myoepithelioma is unpredictable and even erratic. In fact, some authors consider that myoepithelioma is more aggressive than pleomorphic adenoma [44]. The cellular components of myoepithelioma are spindle, plasmacytoid (hyaline), epithelioid and clear cells. It is surprising to know that, except for the spindle cells and some of the epithelioid cells, the nature of MECs has not been confirmed in these cells yet [20]. Therefore, it is conceivable that they did not originate from MECs and, thus, are related to the aggressiveness of myoepithelioma.
Keratins belong to the family of intermediate filaments and are associated with cellular differentiation and cytoskeleton organization. They are expressed by epithelial cells in a cell-specific and differentiation-specific manner [30]. The MECs in salivary glands express keratins 5 and 14. These keratins, however, do not serve as specific markers for the MECs because they are also expressed by the basal cells of the ducts [18, 31, 37, 38]. On the other hand, the non-MECs express keratins 7, 8, 18 and 19. Keratins 8 and 18 are expressed by the acinar cells and duct cells, and keratins 7 and 19 by the duct cells [6, 18, 24, 28, 38]. Therefore, the presence of keratins 5 and 14 in combination with the absence of keratins 7, 8, 18 and 19 may serve as a reliable criterion for identification of salivary gland MECs.
The aim of the present study was to determine the cell of origin of plasmacytoid cells, the prototypic cells of unknown origin. They are oval to polygonal cells with an eccentrically placed nucleus and an eosinophilic hyaline cytoplasm. They are also the cellular constituents of pleomorphic adenoma [8, 20, 29]. Because previous studies have not observed the MEC character in plasmacytoid cells [13, 14, 21, 33, 38, 40], we decided to seek the non-MEC character in these cells. To this end, using antibodies specific for the non-MEC keratins, immunohistochemistry was performed on pleomorphic adenomas rich in plasmacytoid cells.
Materials and methods
All experiments were reviewed and approved by the Intramural Ethics Committee and Animal Use and Care Committee of Osaka University Graduate School of Dentistry prior to the study. A total of 12 cases of pleomorphic adenoma were selected from the files of the Clinical Laboratory at the Osaka University Dental Hospital (Table 1). They included normal salivary glands that were used as the positive control for immunohistochemistry. The tissues were obtained during surgery, fixed in 10% neutral buffered formalin and embedded in paraffin. Rat major salivary glands were removed from pre- and post-natal animals (from 17 days in utero to 3 months after birth; three animals per each age group), fixed in methacarn and embedded in paraffin according to the method described previously [37]. Sections (about 4-μm thick) were cut and mounted on silane-coated glass slides. One section from each tissue block was stained with hematoxylin and eosin to assess the histology, and the others were used for immunohistochemistry.
For human materials, except for α-smooth muscle actin (αSMA), antigens were retrieved by treating the sections in a microwave oven [38]. Immunohistochemistry was performed by indirect immunoperoxidase method described previously [38]. The primary monoclonal antibodies, their dilutions and their sources are listed in Table 2. After immunostaining, the sections were incubated with 3, 3′-diaminobenzidine terahydrochlorode-H2O2 solution and counterstained briefly with Mayer's hematoxylin. Negative controls for immunostaining were performed by substituting the primary antibodies with 0.01 M phosphate-buffered saline (pH 7.2) and normal mouse IgG.
Results
Some clinical features of the 12 patients are shown in Table 1. Seven were women and five were men. The ages of the patients ranged from 23 years to 66 years (mean, 46.5 years). The tumors preferentially arose in the palatine gland (nine cases). All the patients presented with the primary complaint of a painless mass. At surgery, one case (case 1) expressed a tumor-bearing surgical margin. At present (after 6 years and 11 months), however, the patient does not show any signs of recurrence. All the other patients showed neither recurrence nor malignant transformation during follow-up period from 3 months (case 2) to 10 years (case 9).
Normal salivary glands
Immunohistochemistry was performed on nine palatine glands, one labial gland, one submandibular gland and one parotid gland (Table 1). In these glands, the MECs expressed immunoreactivity for αSMA, smooth muscle myosin heavy chain (SMMHC), basic calponin, vimentin, keratin 14 and p63 (Fig. 1A, B, C). In the MECs, αSMA, SMMHC, basic calponin, vimentin and keratin 14 were seen in the cytoplasm, whereas p63 was seen in the nucleus. Alpha-SMA, SMMHC, basic calponin and keratin 14 were broadly distributed in the cytoplasm, whereas vimentin was concentrated around the nucleus. In contrast to αSMA and SMMHC, which were selective for the MECs, immunoreactivity for vimentin, basic calponin, keratin 14 and p63 was observed in the duct cells (Fig. 1B, D) [5, 18, 31, 37, 38, 51]. Keratin 8, keratin 18 and keratin 19 were selective for the duct cells and, thus, never seen in the MECs (Fig. 1E, F). Keratin 8 and keratin 18 were seen in the same cell types: the serous and mucous acinar cells, the duct luminal cells and some of the duct basal cells (Fig. 1E). The keratin 18 immunoreactivity was generally more intense than the keratin 8 immunoreactivity. Any differences were not detected in the staining between two antibodies against keratin 18. Keratin 19 immunoreactivity was seen in the duct luminal cells, sometimes in the duct basal cells and occasionally in the acinar cells (Fig. 1F). Apart from these parenchymal stainings, the stromal vascular walls were stained by the antibodies to αSMA, SMMHC and basic calponin (Fig. 1A, B). The anti-basic calponin antibody occasionally stained the nerve fibers (Fig. 1B).
Palatine (A, C–F) and parotid (B) glands. Alpha-smooth muscle actin is expressed by myoepithelial cells (MECs) around acini and intercalated ducts and smooth muscle cells in vascular walls (A). In addition to MECs and vascular walls, basic calponin is expressed by ducts (arrows) and nerve fibers (double arrows) (B). Vimentin is expressed by MECs around intercalated ducts (C) and acini (arrow in inset in C) and occasionally by duct cells (D). Ductal expression of vimentin is more frequent in inflamed glands. Keratin 18 (E) and keratin 19 (F) are expressed by duct cells. Keratin 18 (E) is also expressed by acinar cells. Note MECs around intercalated ducts are devoid of these keratins (arrows in E and F). Bars A, B 100 μm, ×200; C–F 50 μm, ×400
Except for keratin 8 and SMMHC, all the above proteins were detectable in rat tissues (Table 2). Cellular distribution of these proteins in adult rat (3-month-old) glands was basically the same as that in human glands (Fig. 2A, B, C). In the rat glands, however, keratin 19 was not seen in the intralobular striated ducts and its special form, the granular ducts in the submandibular glands (Fig. 2C). The duct cells and the nerve fibers were not stained by the anti-basic calponin antibody. To determine the expression of these proteins in the immature epithelial cells, immunohistochemistry was performed on the developing salivary glands.
Rat parotid (A, C, D), sublingual (B, E) and submandibular (F) glands. Mature glands (A–C) and immature glands: 1 day after birth (D, E), 18 days in utero (F) and 17 days in utero (inset in F). In mature glands, α-smooth muscle actin (αSMA) is expressed by myoepithelial cells (MECs) around intercalated ducts and smooth muscle cells in vascular walls (A). Acini of rat parotid glands are devoid of MECs. Besides interstitial mesenchymal cells, vimentin is expressed by MECs (arrow in inset in B) and occasionally by striated duct cells (arrowhead in B). Keratin 19 is expressed by intercalated duct cells and extralobular striated duct cells (inset in C). Intralobular striated duct cells and some duct basal cells (arrow in inset in C) do not express this keratin. In developing immature glands, αSMA is expressed only by MECs, whereas keratin 19 (D) only by duct cells. Nuclear staining of p63 is observed both in MECs and duct basal cells (E). Vimentin is expressed by terminal bud cells (inset in F), cellular cord cells, immature acinar cells (F) and immature duct cells (F). Bars 50 μm, ×400
After the early morphodifferentiation, each salivary gland primordium gives rise to an extensively branched system of cellular cords, each of which terminates in a spherical cellular mass—a terminal bud. Cellular cords develop into striated ducts and excretory ducts, and terminal buds develop into acini and intercalated ducts. The development of the striated duct/excretory duct precedes that of the acinus/intercalated duct, which starts at 18 days in utero in the submandibular and sublingual glands and immediately after birth in the parotid gland. The adult gland structures are established by 6 weeks after birth. The MECs first appear on the periphery of the terminal buds at the same time as the acinar development in the submandibular and sublingual glands, but slightly earlier than that in the parotid gland [39]. They showed immunoreactivity for αSMA, basic calponin, vimentin and p63 throughout the developmental period (Fig. 2E) [37]. In addition to the MECs, p63 was seen in the duct basal cells and vimentin in the terminal tubule and cellular cord cells and the nascent acinar and duct cells (Fig. 2E, F). Keratins were observed in the duct cells. Though keratin 14 was also observed in the MECs in the later developmental stage [37], the other keratins were never seen in the MECs (Fig. 2D). Keratin 19 appeared to occur in the duct cells earlier than keratin 18. At 18 days in utero when the submandibular and sublingual glands first expressed these keratins, keratin 19 is seen in more duct cells than is keratin 18. The immature serous acinar cells also expressed immunoreactivity for keratin 18, whereas the immature mucous cells in the sublingual glands were devoid of this protein.
Pleomorphic adenomas
Pleomorphic adenomas are composed of luminal tumor cells and non-luminal tumor cells. The former cells line neoplastic lumina and are surrounded by the latter cells. These cells make up solid proliferating structures and mesenchymal structures [20]. The luminal cells showed immunoreactivity for keratins 18 and 19 (Fig. 3A, B). They also sometimes expressed keratin 8, keratin 14 and basic calponin and occasionally vimentin (Fig. 3C) [38]. Many of the non-luminal cells expressed immunoreactivity for vimentin, keratin 14, basic calponin and p63 (Fig. 3C) [38]. They also sometimes expressed keratin 18, keratin 19 and αSMA and occasionally keratin 8 and SMMHC (Fig. 3A, B, D). The keratins 18-, 19- and 8-positive cells took both spindle (elongated) and non-spindle configurations, whereas the smooth muscle protein-positive cells were mostly spindle. The latter cells were usually observed on the periphery of the solid structure and occasionally in the mesenchymal structure (Fig. 3D).
Keratin 18 (A) and keratin 19 (B) are expressed by luminal tumor cells and sometimes by non-luminal tumor cells in pleomorphic adenomas. Vimentin is expressed by most non-luminal cells and occasional luminal cells (arrows in C). Alpha-smooth muscle actin is expressed by some non-luminal cells in solid epithelial sheets and by sporadic cells in mesenchymal structures (D). These cells usually have spindle appearance (inset in D). Bars A, C 100 μm, ×200; B, D 200 μm, ×100; inset in D 50 μm, ×400
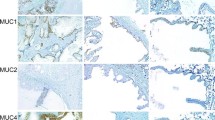
Immunoreactivity of the plasmacytoid cells for various proteins was summarized in Table 3. In all cases, more than 90% of the plasmacytoid cells strongly expressed immunoreactivity for vimentin (Fig. 4A). The vimentin immunoreaction was usually diffuse in the cytoplasm and sometimes accentuated the thin rim surrounding the central globular structure. Next to vimentin, basic calponin distributed broadly over the plasmacytoid cells in nine cases (75%). In seven cases (58%), up to 60% of the plasmacytoid cells expressed keratin 19 (Fig. 4B). In three of these cases, about 10% of the plasmacytoid cells weakly expressed keratin 18 (Fig. 4C). The keratin 19 immunoreaction was often concentrated to the thin rim. Keratin 14 was expressed in six cases and p63 in seven cases. We could not find any plasmacytoid cells that were definitively positive for αSMA, SMMHC or keratin 8 (Fig. 4D).
Plasmacytoid cells usually express vimentin (A), sometimes keratin 19 (B) and occasionally keratin 18 (C). Though elongated cells with somewhat eccentrically placed nuclei are positive for α-smooth muscle actin (αSMA) and may represent a transitional form between plasmacytoid cells and spindle-shaped neoplastic myoepithelial cells, authentic plasmacytoid cells never express αSMA (D). Bars 25 μm, ×800
Discussion
The present study has demonstrated that plasmacytoid cells are related to luminal cells but not MECs in pleomorphic adenomas. Small but definite number of the plasmacytoid cells expressed keratins 19 and 18, whereas none of them possessed αSMA and SMMHC. The number of the keratin 19-positive cells was larger than that of the keratin 18- and probably keratin 19-positive cells. Keratins 19 and 18 showed similar distribution in the normal salivary glands. They were expressed by the luminal cells and some of the basal cells in the ducts [18, 24, 26, 28, 38]. They also showed similar cellular distribution in the developing glands. The MECs never expressed these keratins either after or during development. Keratin 18, however, was constantly expressed by the acinar cells [18, 24, 26, 28]. During development, keratin 19 occurred in the duct cells earlier than keratin 18. These may indicate that keratin 19-positive tumor cells are more primitive than keratin 18-positive tumor cells.
A similar keratin profile has been found in pancreas, where keratin 18 is expressed by acinar and duct cells and keratin 19 by duct cells. During development, expression of keratin 18 by pancreatic cells is preceded by that of keratin 19. Pancreatic cancer cells with keratin 19 are thought to be less differentiated than those with keratins 18 and 19 [7]. Keratin 8 showed a cellular distribution similar to that of keratin 18 in the human salivary glands, suggesting that they make up a keratin-pair usually seen in simple epithelia [30]. Keratin 8, however, disappeared more frequently than keratin 18 after neoplastic transformation, and, thus, was not observed in the plasmacytoid cells.
Except for one study [45], previous immunohistochemistry could not demonstrate smooth muscle proteins in plasmacytoid cells [13, 14, 21, 33, 38, 40]. Many pleomorphic adenoma cells, including the plasmacytoid cells, however, were stained by an antibody to basic calponin, a putative regulatory protein in smooth muscle contraction [38, 40]. The anti-basic calponin antibody reacted not only with MECs and smooth muscle cells but also with duct cells, nerve fibers and keratinocytes [38]. Pleomorphic adenoma cells frequently possess tonofilaments and nerve proteins such as S100 protein and glial fibrillary acidic protein (GFAP) [1, 9, 10, 13, 14, 20, 34, 35, 52]. Therefore, these cells may have reacted with the antibody. S100 protein is not expressed by MECs but by duct cells [15, 34, 35]. MEC expression of GFAP is still a matter of controversy [1, 32, 34, 52].
Almost all the plasmacytoid cells expressed immunoreactivity for vimentin. It has been often difficult to identify vimentin immunostaining in MECs [18, 24, 33, 37], probably due to its restricted distribution in the cytoplasm [19, 23]. Despite this difficulty, the present and the previous [6, 24, 25] studies observed distinct vimentin staining in MECs. In this context, some authors have recommended this protein as a MEC marker [2, 3, 33]. The luminal tumor cells in the pleomorphic adenomas, however, occasionally expressed vimentin immunoreactivity. In the normal salivary glands, vimentin immunoreactivity was also observed in the duct cells [24]. Both the MECs and the duct cells expressed vimentin in the developing glands. Vimentin immunostaining in the terminal bud and cord cells strongly suggest that the precursors of these cells express this protein. Therefore, it can be concluded that vimentin expression does not necessarily mean the cell is derived from MEC. Vimentin expression is necessary for migration of epithelial cells in pathological and physiological processes [22]. Salivary cells migrate within and between each of the gland segments both during and after development [16, 39].
It has been hypothesized that MECs and duct basal cells make up a single cell lineage: MECs and duct basal cells are the proximal and the distal components, respectively, of a cellular continuum extending from acinus to distal most duct [11, 12]. However, the present observation of keratin 14 and p63 in both MECs and duct basal cells does not necessarily support the above hypothesis because keratin 14 was also expressed by the duct luminal cells, and keratins 8, 18 and 19 were observed in the duct basal cells [38]. Indeed, embryological studies of rodent salivary glands suggested that the MECs and the acinar/intercalated duct cells are derived from a common primordium, whereas the basal cells in the larger ducts and their luminal partners are derived from another primordium [39].
The characteristic hyaline cytoplasm of plasmacytoid cells is composed of haphazardly arranged filaments. Due to misinterpretation of these filaments as actin microfilaments, they were originally thought to be neoplastic MECs [27, 29, 42, 43, 50]. Though the filaments have been proved to be intermediate filaments, plasmacytoid cells are still considered neoplastic MECs because they are members of the non-luminal cells in pleomorphic adenomas [13]. According to these authors [9, 10], the luminal cells and the non-luminal cells in pleomorphic adenoma come from the luminal cells and the MECs in intercalated duct, respectively. During tumorigenesis, the MECs are modified to lose the smooth muscle nature and come to exhibit extensive morphological diversity (modified MECs). Our present and previous [38] studies have demonstrated that at least some of the modified MECs, irrespective of their cytomorphology, express the phenotype of the luminal cells. Though the notion that non-luminal cells are modified MECs has been adopted into many types of salivary gland tumors, it must be re-evaluated to predict their accurate biological behavior [38].
MECs, even after neoplastic transformation, possess anti-cancer properties and, consequently, MEC neoplasms have low-grade malignant nature [4, 46]. The atlas of salivary gland tumors published by the Armed Forces Institute of Pathology describes that myoepitheliomas are less likely to recur than pleomorphic adenomas [20], whereas the histological typing of salivary gland tumors by the World Health Organization says that myoepithelioma is characterized by more aggressive growth than pleomorphic adenoma and occasionally by transformation to malignancy [44]. By reviewing one Armed Forces Institute of Pathology case and 20 reported cases of malignant myoepitheliomas with follow-up information, Ellis and Auclair [20] referred to the high incidence of patients who died or were living with disease: six patients (29%) and seven patients (33%), respectively. The unpredictable and even erratic biological behavior of myoepitheliomas should relate to the fact that they are not composed of 100% neoplastic MECs. Indeed, luminal cells participate in many myoepitheliomas, as they often contain ductal structures [20]. In conclusion, we would like to propose that plasmacytoid cell tumors should not be considered a subtype of myoepithelioma but classified as plasmacytoid adenomas or adenocarcinomas [21]. In this context, it is noteworthy that malignant myoepitheliomas with predominant plasmacytoid cells exhibit more aggressive behavior than the other forms of these tumors [17, 49]. After the analysis of a large series of myoepithelial carcinomas, Savera et al. [41] concluded that the cell type and the prognosis did not show a statistically significant correlation. This conclusion, however, appears to be immature. There were only two cases of hyaline cell-rich myoepithelial carcinomas with available follow-up. One patient (case 12 of their study) died of the disease at 18 months after surgery. Another patient with cervical lymph node metastasis (case 1) is alive at 6 months, which is extremely shorter than the follow-up period of the other patients.
References
Achstätter T, Moll R, Anderson A, Kuhn C, Pitz S, Schwechheimer K, Franke WW (1986) Expression of glial filament protein (GFP) in nerve sheaths and non-neural cells re-examined using monoclonal antibodies, with special emphasis on the co-expression of GFP and cytokeratins in epithelial cells of human salivary gland and pleomorphic adenomas. Differentiation 31:206–227
Araújo VC, Araújo NS (1990) Vimentin as a marker of myoepithelial cells in salivary gland tumors. Eur Arch Otorhinolaryngol 247:252–255
Araújo VC, Carvalho YR, Araújo NS (1994) Actin versus vimentin in myoepithelial cells of salivary gland tumors. A comparative study. Oral Surg Oral Med Oral Pathol 77:387–391
Batsakis JG, El-Naggar AK (1999) Myoepithelium in salivary and mammary neoplasms is host-friendly. Adv Anat Pathol 6:218–226
Bilal H, Handra-Luca A, Bertrand JC, Fouret PJ (2003) p63 is expressed in basal and myoepithelial cells of human normal and tumor salivary gland tissues. J Histochem Cytochem 51:133–139
Born IA, Schwechheimer K, Maier H, Otto HF (1987) Cytokeratin expression in normal salivary glands and in cystadenolymphomas demonstrated by monoclonal antibodies against selective cytokeratin polypeptides. Virchows Arch 411:583–589
Bouwens L (1998) Cytokeratins and cell differentiation in the pancreas. J Pathol 184:234–239
Buchner A, David R, Hansen LS (1981) "Hyaline cells" in pleomorphic adenoma of salivary gland origin. Oral Surg Oral Med Oral Pathol 52:506–512
Dardick I, van Nostrand AWP, Jeans MTD, Rippstein P, Edwards V (1983) Pleomorphic adenoma, I: ultrastructural organization of "epithelial" regions. Hum Pathol 14:780–797
Dardick I, van Nostrand AWP, Jeans MTD, Rippstein P, Edwards V (1983) Pleomorphic adenoma, II: ultrastructural organization of "stromal" regions. Hum Pathol 14:798–809
Dardick I, Rippstein P, Skimming L, Boivin M, Parks WR, Dairkee SH (1987) Immunohistochemistry and ultrastructure of myoepithelium and modified myoepithelium of the ducts of human major salivary glands: histogenetic implications for salivary gland tumors. Oral Surg Oral Med Oral Pathol 64:703–715
Dardick I, Parks WR, Little J, Brown DL (1988) Characterization of cytoskeletal proteins in basal cells of human parotid salivary gland ducts. Virchows Arch 412:525–532
Dardick I, Thomas MJ, van Nostrand AWP (1989) Myoepithelioma-new concepts of histology and classification: a light and electron microscopic study. Ultrastruct Pathol 13:187–224
Dardick I, Cavell S, Boivin M, Hoppe D, Parks WR, Stinson J, Yamada S, Burns BF (1989) Salivary gland myoepithelioma variants. Histological, ultrastructural, and immunocytological features. Virchows Arch 416:25–42
Dardick I, Stratis M, Parks WR, DeNardi FG, Kahn HJ (1991) S-100 protein antibodies do not label normal salivary gland myoepithelium. Histogenetic implications for salivary gland tumors. Am J Pathol 138:619–628
Denny PC, Ball WD, Redman RS. (1997) Salivary glands: a paradigm for diversity of gland development. Crit Rev Oral Biol Med 8:51–75
Di Palma S, Guzzo M (1998) Myoepithelial carcinoma with predominance of plasmacytoid cells arising in a pleomorphic adenoma of the parotid gland. Histopathology 33:485
Draeger A, Nathrath WBJ, Lane EB, Sundström BE, Stigbrand TI (1991) Cytokeratins, smooth muscle actin and vimentin in human normal salivary gland and pleomorphic adenomas. Immunohistochemical studies with particular reference to myoepithelial and basal cells. APMIS 99:405–415
Eckert F, de Viragh PA, Schmid U (1994) Coexpression of cytokeratin and vimentin intermediate filaments in benign and malignant sweat gland tumors. J Cutan Pathol 21:140–150
Ellis GL, Auclair PL (1996) Tumors of the salivary glands. Atlas of tumor pathology, 3rd series, fascicle 17. Armed Forces Institute of Pathology, Washington, DC
Franquemont DW, Mills SE (1993) Plasmacytoid monomorphic adenoma of salivary glands. Absence of myogenous differentiation and comparison to spindle cell myoepithelioma. Am J Surg Pathol 17:146–153
Gilles C, Polette M, Zahm JM, Tournier JM, Volders L, Foidart JM, Birembaut P (1999) Vimentin contributes to human mammary epithelial cell migration. J Cell Sci 112:4615–4625
Gould VE, Koukoulis GK, Jansson DS, Nagle RB, Franke WW, Moll R (1990) Coexpression patterns of vimentin and glial filament protein with cytokeratins in the normal, hyperplastic, and neoplastic breast. Am J Pathol 137:1143–1155
Gustafsson H, Kjörell U, Eriksson A, Virtanen I, Thornell L-E (1988) Distribution of intermediate filament proteins in developing and adult salivary glands in man. Anat Embryol 178:243–251
Hirano T, Gluckman JL, deVries EJ (1990) The expression of a vascular smooth-muscle actin in salivary gland tumors. Arch Otolaryngol Head Neck Surg 116:692–696
Ihrler S, Zietz C, Sendelhofert A, Lang S, Blasenbreu-Vogt S, Löhrs U (2002) A morphogenetic concept of salivary duct regeneration and metaplasia. Virchows Arch 440:519–526
Kahn LB, Schoub L (1973) Myoepithelioma of the palate. Histochemical and ultrastructural observations. Arch Pathol 95:209–212
Leoncini P, Cintorino M, Vindigni C, Leoncini L, Armellini D, Bugnoli M, Skalli O, Gabbiani G (1988) Distribution of cytoskeletal and contractile proteins in normal and tumour bearing salivary and lacrimal glands. Virchows Arch 412:329–337
Lomax-Smith JD, Azzopardi JG (1978) The hyaline cell: a distinctive feature of "mixed" salivary tumours. Histopathology 2:77–92
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24
Moll R, Dhouailly D, Sun T-T (1989) Expression of keratin 5 as a distinctive feature of epithelial and biphasic mesotheliomas. An immunohistochemical study using monoclonal antibody AE14. Virchows Arch 58:129–145
Mori M, Ninomiya T, Okada Y, Tsukitani K (1989) Myoepitheliomas and myoepithelial adenomas of salivary gland origin. Immunohistochemical evaluation of filament proteins, S-100α and β, glial fibrillary acidic protein, neuron specific enolase, and lactoferrin. Pathol Res Pract 184:168–178
Morinaga S, Nakajima T, Shimosato Y (1987) Normal and neoplastic myoepithelial cells in salivary glands: an immunohistochemical study. Hum Pathol 18:1218–1226
Nakazato Y, Ishizeki J, Takahashi K, Yamaguchi H, Kamei T, Mori T (1982) Localization of S-100 protein and glial fibrillary acidic protein-related antigen in pleomorphic adenoma of the salivary glands. Lab Invest 46:621–626
Ninomiya T, Naito R, Okada Y, Kobayashi K, Mori M, Tsukitani K (1989) Immunohistochemical localization of the α and β subunits of S-100 protein in pleomorphic adenoma of the salivary glands. Virchows Arch 57:63–75
Nguyen M, Lee MC, Wang JL, Tomlinson JS, Shao Z-M, Alpaugh ML, Barsky SH (2000) The human myoepithelial cell displays a multifaceted anti-angiogenic phenotype. Oncogene 19:3449–3459
Ogawa Y, Yamauchi S, Ohnishi A, Ito R, Ijuhin N (1999) Immunohistochemistry of myoepithelial cells during development of the rat salivary glands. Anat Embryol 200:215–228
Ogawa Y, Toyosawa S, Ishida T, Ijuhin N (2000) Keratin 14 immunoreactive cells in pleomorphic adenomas and adenoid cystic carcinomas of salivary glands. Virchows Arch 437:58–68
Ogawa Y (2003) Immunocytochemistry of myoepithelial cells in the salivary glands. Prog Histochem Cytochem (in press)
Savera AT, Gown AM, Zarbo RJ (1997) Immunolocalization of three novel smooth muscle-specific proteins in salivary gland pleomorphic adenoma: assessment of the morphogenetic role of myoepithelium. Mod Pathol 10:1093–1100
Savera AT, Sloman A, Huvos AG, Klimstra DS (2000) Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol 24:761–774
Sciubba JJ, Goldstein BH (1976) Myoepithelioma. Review of the literature and report of a case with ultrastructural confirmation. Oral Surg Oral Med Oral Pathol 42:328–338
Sciubba JJ, Brannon RB (1982) Myoepithelioma of salivary glands: report of 23 cases. Cancer 49:562–572
Seifert G, Sobin LH (1991) Histological typing of salivary gland tumors. World Health Organization international classification of tumors, 2nd ed. Springer Verlag, Berlin Heidelberg New York
Simpson RH, Jones H, Beasley P (1995) Benign myoepithelioma of the salivary glands: a true entity? Histopathology 27:1–9
Sternlicht MD, Barsky SH (1997) The myoepithelial defense: a host defense against cancer. Med Hypotheses 48:37–46
Sternlicht MD, Safarians S, Rivera SP, Barsky SH (1996) Characterizations of the extracellular matrix and proteinase inhibitor content of human myoepithelial tumors. Lab Invest 74:781–796
Sternlicht MD, Kedeshian P, Shao Z-M, Safarians S, Barsky SH (1997) The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res 3:1949–1958
Suzuki H, Inoue K, Fujioka Y, Ishikura H, Furuta Y, Fukuda S (1998) Myoepithelial carcinoma with predominance of plasmacytoid cells arising in a pleomorphic adenoma of the parotid gland. Histopathology 32:86–87
Wayne Stromeyer F, Haggitt RC, Nelson JF, Hardman JM (1975) Myoepithelioma of minor salivary gland origin. Light and electron microscopical study. Arch Pathol 99:242–245
Weber A, Langhanki L, Schutz A, Gerstner, A, Bootz F, Wittekind C, Tannapfel A (2002) Expression profiles of p53, p63, and p73 in benign salivary gland tumors. Virchows Arch 441:428–436
Zarbo RJ, Regezi JA, Hatfield JS, Maisel H, Trojanowski JQ, Batsakis JG, Crissman JD (1988) Immunoreactive glial fibrillary acidic protein in normal and neoplastic salivary glands: a combined immunohistochemical and immunoblot study. Surg Pathol 1:55–63
Acknowledgement
The authors thank Dr. Ross T. Fernley for his critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogawa, Y., Kishino, M., Atsumi, Y. et al. Plasmacytoid cells in salivary-gland pleomorphic adenomas: evidence of luminal cell differentiation. Virchows Arch 443, 625–634 (2003). https://doi.org/10.1007/s00428-003-0890-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-003-0890-3