Abstract
We previously investigated sensory coupling of the sensed positions of cursor and hand in a cursor-control task and found differential characteristics of implicit and explicit measures of the bias of sensed hand position toward the position of the cursor. The present study further tested whether adaptation to a visuomotor rotation differentially affects these two measures. Participants made center-out reaching movements to remembered targets while looking at a rotated feedback cursor. After sets of practice trials with constant (adaptation condition) or random (control condition) visuomotor rotations, test trials served to assess sensory coupling. In these trials, participants judged the position of the hand at the end of the center-out movement, and the deviation of these judgments from the physical hand positions served as explicit measure of the bias of sensed hand position toward the position of the cursor, whereas the implicit measure was based on the direction of the return movement. The results showed that inter-individual variability of explicitly assessed biases of sensed hand position toward the cursor position was less in the adaptation condition than in the control condition. Conversely, no such changes were observed for the implicit measure of the bias of sensed hand position, revealing contrasting effects of adaptation on the explicit and implicit measures. These results suggest that biases of explicitly sensed hand position reflect sensory coupling of neural representations that are altered by visuomotor adaptation. In contrast, biases of implicitly sensed hand position reflect sensory coupling of neural representations that are unaffected by adaptation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When reaching movements are made, the position of the hand is monitored both visually and proprioceptively. The individual position estimates, based on each of the two modalities, are integrated to obtain a single estimate (Van Beers, Sittig, & Denier van der Gon, 1999). In general, multisensory integration can be modeled as a weighted average, with the weights of the individual estimates being matched to their relative reliabilities (Cheng, Shettleworth, Huttenlocher, & Rieser, 2007; Ernst & Bülthoff, 2004). Thereby the variance of the combined estimate, e.g., of the position of the hand, is minimized. Such optimal combinations are advantageous when the individual estimates refer to the same characteristic of the same object because the integrated perceptual estimate has a higher precision than each individual estimate. However, a less-than-perfect multisensory integration, called sensory coupling (cf. Bresciani, Dammeier, & Ernst, 2006; Ernst, 2006, 2012), has also been found in tool-use tasks where different sensory modalities refer to different objects, in particular in the task of controlling a cursor on a monitor by way of a computer mouse or a similar input device.
In a cursor-control task, the position of the effective part of the tool (the cursor) is specified by visual information, and the position of the hand is specified by proprioceptive information. These are positions of different objects that typically move in different planes, the cursor in the frontoparallel plane and the hand in the horizontal plane. In such a case, and even in the case where the cursor moves in the same plane (e.g., horizontal) as the hand, visual and proprioceptive information are no longer fully redundant in that they do not refer to the same object. But they are related to each other through the tool’s kinematic transformation. This systematic relation between the movements of the hand and the motions of the cursor is sufficient to prompt sensory coupling, which has been observed in different variants of cursor-control tasks (e.g., Debats, Ernst, & Heuer, 2017; Kirsch, Pfister, & Kunde, 2016; Ladwig, Sutter, & Müsseler, 2012, 2013; Rand & Heuer, 2013, 2016; Wendker, Sack, & Sutter, 2014). For example, when the direction of cursor motion is slightly rotated relative to the direction of hand movement, the sensed final hand position is biased toward the final position of the cursor, and the sensed final position of the cursor is biased toward the final position of the hand (Debats et al., 2017; Rand & Heuer, 2013, 2016). The magnitude of these biases reflects the strength of sensory coupling. However, sensory coupling and the resulting bias of sensed hand position toward the position of the cursor are not invariant across different methods of assessment. Here, we test whether two particular methods of assessment are affected differently by a preceding adaptation to a visuomotor rotation.
For the assessment of mutual biases of their sensed final positions, hand and cursor have to move in slightly different directions in each trial so that their final positions differ. These visuomotor rotations were randomized across trials and zero on average both in previous studies (Debats et al., 2017; Rand & Heuer, 2013, 2016, 2017, in press) and in the control condition of the present study. In a second (i.e., adaptation) condition of the present study, visuomotor rotations were constant across series of trials so that participants adapted to them (e.g., Abeele & Bock, 2001a, 2001b; Heuer, Hegele, & Rand, 2013; Krakauer & Mazzoni, 2011; Krakauer, Pine, Ghilardi, & Ghez, 2000; Morehead, Qasim, Crossley, & Ivry, 2015). We introduced the visuomotor rotation stepwise, a procedure that serves to reduce participants’ awareness of the rotation (Buch, Young, & Contreras-Vidal, 2003; Kagerer, Contreras-Vidal, & Stelmach, 1997). The adaptation and the control condition of the present experiment allowed us to compare the strength of sensory coupling for two different methods of assessment of the bias of sensed hand position toward the position of the cursor when participants were either adapted or unadapted to visuomotor rotations.
Explicit and implicit assessments of biases of sensed hand position
We tag the two methods of bias assessment used in this and previous studies (Rand & Heuer, 2013, 2016, 2017, in press) as explicit and implicit. Our explicit assessment of the bias of sensed hand position toward the position of the cursor is based on psychophysical judgments. In each test trial, participants perform a center-out reaching movement in a particular direction while looking at a visual feedback cursor moving in a slightly rotated direction and return to the starting position. Immediately afterwards, they give a psychophysical judgment of the perceived (and remembered) position of the hand at the end of the center-out movement. They do so by moving their hand on a circular path which runs through all possible end positions of reaching and stop at that position that matches the remembered hand position. In each single trial, the deviation of the judged hand position from the physical position of the hand at the end of reaching serves as an explicit measure of the bias of sensed hand position toward the position of the cursor. Across trials, the bias depends linearly on the difference between the physical positions of cursor and hand, that is, on the size of the visuomotor rotation (Debats et al., 2017; Rand & Heuer, 2013, 2016, 2017, in press). Therefore, we compute the slope of that linear regression for each participant and experimental condition and use it as an explicit measure of the (proportional) bias of sensed hand position. We use the term “proportional” because the bias is expressed as proportion of the visuomotor rotation.
Our implicit assessment of the bias of sensed hand position toward the position of the cursor is based on the movements of the participants without them being aware of judging the position of their hand. Specifically, we measure the angle between the reaching movement and the return movement back to the initial position of that reaching movement in each trial. The rationale of this implicit measure of the deviation of the sensed hand position from the physical position of the hand is based on the observation that discrepancies between sensed and physical locations of the hand give rise to systematic errors in subsequent movements (Bock & Eckmiller, 1986; Heuer & Sangals, 1998; Heuer & Sülzenbrück, 2012; Holmes, Crozier, & Spence, 2004; Holmes & Spence, 2005; Rossetti, Desmurget, & Prablanc, 1995; Vindras, Desmurget, Prablanc, & Viviani, 1998). The implicitly assessed bias of the sensed hand position in each trial is subjected to a linear regression on the visuomotor rotation for each participant. The slope of that linear regression serves as an implicit measure of the (proportional) bias of sensed hand position.
We use the terms “explicit” and “implicit” in a descriptive way for the procedures used to assess sensory coupling in a cursor-control task, specifically the bias of sensed hand position toward the position of the cursor. The explicit assessment is based on a psychophysical procedure where hand position is intentionally judged. The implicit assessment, in contrast, is based on the observed motor behavior without an intentional judgment by the participant. Similar to other fields such as the learning of sequences and other regularities in the environment, where the explicit–implicit distinction is firmly established (cf. Reber, 2013; Shanks & St. John, 1994), we ask whether the different types of assessment have different characteristics. Such differences would suggest that they are based on different neural representations of hand position. Previous studies have shown, for example, that implicitly and explicitly assessed biases are uncorrelated, that the explicitly assessed bias is about twice as strong as the implicitly assessed bias, and that the explicitly assessed bias increases at older adult age, whereas the implicitly assessed bias remains stable (Rand & Heuer, 2013). The explicitly assessed bias, but not the implicitly assessed bias, is reduced when the relative reliability of proprioceptive information on final hand position is increased (Rand & Heuer, 2016). These findings suggest that the different types of bias assessment indeed may be based on different neural representations of hand position. The present study extends our exploration of dissociations between explicitly and implicitly assessed biases of sensed hand position in a cursor-control task to the effects of adaptation to visuomotor rotations. More specifically, we investigate whether the two types of bias differentially change when the bias due to sensory coupling is combined with a bias due to adaptation to a visuomotor rotation (i.e., proprioceptive recalibration, see more details in the next section).
Sensory coupling and proprioceptive recalibration
The distinction between sensory coupling and proprioceptive recalibration is essential for the purpose of the present study. The first critical difference is the test condition. Sensory coupling is tested in a condition in which both proprioceptive and visual information is presented—a bimodal condition. For the assessment of biases, a certain discrepancy between both sources is introduced, for example by means of a prism or by a visuomotor rotation in a cursor-control task. Proprioceptive recalibration, conversely, is tested in a condition in which only proprioceptive information on hand position is provided—a unimodal condition. The typical finding in an adaptation experiment is a shift of the judged position of the hand toward the physical position of the cursor in the preceding adaptation trials (Cressman & Henriques, 2009, 2010; Simani, McGuire, & Sabes, 2007; Synofzik, Lindner, & Thier, 2008; Wilke, Synofzik, & Lindner, 2013; Zbib, Henriques, & Cressman, 2016). Note that the distinction between a bimodal and a unimodal test condition refers to the stimulus to be judged, but not to the procedure used to obtain the judgments.
The second critical difference is the time course. The biases due to sensory coupling are immediate, that is, they depend on the intermodal discrepancy in the current trial and vary across trials when the discrepancy is varied. In the classic literature on prism adaptation (cf. Welch, 1978), the immediate bias of sensed hand direction toward the direction of the cursor—a result of sensory coupling—is also known as visual capture (Hay, Pick, & Ikeda, 1965). Proprioceptive recalibration, in contrast, develops in the course of a number of adaptation trials with a certain discrepancy between proprioceptive and visual information. It depends on a consistent discrepancy across trials, whereas sensory coupling can be observed when the discrepancy varies from trial to trial and is zero on average.
In the present study, we use bimodal test conditions after a preceding adaptation period. How does the observed bias—the deviation of the sensed position of the hand from its physical position—change when the bias due to sensory coupling (i.e., the coupling bias) is combined with the bias due to adaptation (i.e., the proprioceptive recalibration)? A simple formal analysis suggests the following hypothesis that is not evident intuitively.
Let h′ be the expected value of the sensed hand position under unimodal test conditions, that is, judgments of the final position of the hand after a movement being made without visual feedback. Similarly, let c′ be the expected value of the sensed cursor position under unimodal test conditions, that is, judgments of the final position of the cursor after cursor motion without accompanying hand movement. Then under bimodal test conditions where judgments are made after a movement with visual feedback, the expected value of the sensed hand position, h′′, is
with wc as the coupling weight which represents the shift of the sensed hand position towards the sensed position of the cursor (cf. Debats et al., 2017; Eq. 1). The variables of this equation are illustrated in Fig. 1a, where positions are marked by polar coordinates with constant amplitudes, so that only directions matter. This is also the case in the experiment, where we focus on the deviations of the sensed positions from the physical positions of cursor, c, and hand, h: Δh′ = h′ − h, Δc′ = c′ − c, and Δh′′ = h′′ − h. In Fig. 1a, Δh′′ is counterclockwise and thus positive. Replacing the sensed positions in Eq. (1) by their deviations from physical positions and re-arranging terms results in
a Illustrations of different positions of cursor and hand in the control and adaptation conditions; h and c are physical positions; h′ and c′ are judged positions of cursor and hand in unimodal test trials; h′′ and c′′ are judged positions of cursor and hand in bimodal test trials. b Proportional bias Δh′′/(c − h) as a function of the coupling weight wc, where Δh′′, the deviation of h′′ from h, is expressed as proportion of the visuomotor rotation, the discrepancy between c and h. The dashed and dotted lines refer to the control and adaptation condition, respectively. The coupling weight wc and the proportional bias are identical in the control condition, whereas the slope of the proportional bias as a function of the coupling weight is reduced in the adaptation condition
In this equation, all deviations of judgments from physical positions of cursor and hand are expressed as proportions of the discrepancy between cursor and hand position, that is, as proportions of the visuomotor rotation so that they vary between 0 and 1 (or between − 1 and 0).
For the control condition of the present experiment with randomly varying visuomotor rotations (Fig. 1a, control), the expected values of the unimodal judgments h′ and c′ roughly correspond to the physical positions, so that the proportional deviations Δh′/(c − h) and Δc′/(c − h) are approximately zero (cf. Debats et al., 2017). In this case, the proportional deviation Δh′′/(c − h), as assessed under bimodal test conditions, is an estimate of the coupling weight wc (cf. Eq. 2). This is illustrated by the dashed line in Fig. 1b (control), which shows Δh′′/(c − h) as a function of wc, and the coefficient of wc in Eq. (2) is one. In the adaptation condition (Fig. 1a, adaptation), the expected values of the unimodal judgments h′ and c′ should be shifted away from the respective physical positions, so that Δh′ becomes larger (more positive) and Δc′ smaller (more negative). The increase of Δh′ represents the well-established proprioceptive recalibration, and the change of Δc′ represents the less well-established visual recalibration (cf. Van Beers, Wolpert, & Haggard, 2002; Hatada, Miall, & Rossetti, 2006). The consequence of these adaptive changes, the one being negative and the other one being positive, is that the coefficient of wc in Eq. (2) becomes less than one. This is illustrated by the dotted line in Fig. 1b (adaptation), where the slope of Δh′′/(c − h) as a function of wc is reduced in the adaptation condition as compared with the control condition.
In the following, we consider individuals who differ with respect to the coupling weight and the adaptation effects (proprioceptive and visual recalibration). Note that observed biases Δh′′/(c − h) are measured individually in the present study as the proportional biases of sensed hand position toward the cursor position (i.e., the slopes of linear regressions, see the previous section). Some individuals will have smaller coupling weights (wc), meaning that their observed biases of sensed hand position toward the cursor position, Δh′′/(c − h), in the control condition are small and closer to 0 (cf. Fig. 1b, control). Some individuals will have larger coupling weights, meaning that their observed biases of sensed hand position toward the cursor position in the control condition are large and closer to 1. Other individuals with moderate coupling weights lie in-between. In the adaptation condition, however, the observed individual biases Δh′′/(c − h) are affected by both the proprioceptive and visual recalibration (Fig. 1b, adaptation). If there were only proprioceptive recalibration, the observed biases in the adaptation condition should be consistently larger than in the control condition. If there were only visual recalibration, they should be consistently smaller. But when there are both types of recalibration (as shown in Fig. 1b, adaptation), smaller biases in the control condition (small coupling weights wc) should become larger after adaptation due to the proprioceptive recalibration (Δh′/(c − h)), and larger biases in the control condition (large coupling weights wc) should become smaller due to the visual recalibration (Δc′/(c − h)). Consequently, adaptation should result in smaller or larger observed biases Δh′′/(c − h) in the adaptation condition depending on the relative sizes of the proprioceptive and the visual recalibration. Thus, there are no clear expectations for the means of observed biases across individuals—they could be larger or smaller after adaptation depending on the relative sizes of proprioceptive and visual recalibration. Independent of a difference in the means, however, the inter-individual variability of observed biases Δh′′/(c − h) in the adaptation condition should be smaller than in the control condition, as evidenced from Fig. 1b.
This hypothesis implies that sensory coupling is based on the sensed positions of hand and cursor as they are changed by adaptation. The implication is likely to hold for the explicit assessment of the bias of the sensed position of the hand under bimodal test conditions. The reason is, first, that proprioceptive recalibration has mainly been shown by psychophysical procedures, that is, by explicit assessments under unimodal test conditions (Cressman & Henriques, 2009, 2010; Izawa, Criscimagna-Hemminger, & Shadmehr, 2012; Simani et al., 2007; Synofzik et al., 2008; Wilke et al., 2013; Zbib et al., 2016). The second reason is that explicitly assessed biases in bimodal tests have been shown to depend on the explicit judgments of hand and cursor positions in unimodal tests (Debats et al., 2017). However, the implication may not hold for the implicit assessment of sensory coupling, which might rely on representations of hand and cursor positions that are unaffected by adaptation. After all, neural representations of proprioceptive information reside in various areas of the human brain, both cortically and subcortically (e.g., Goble et al., 2012), and different methods of assessing the sensed position of the hand might tap different representations. Therefore, we felt it worthwhile to inquire whether adaptation to visuomotor rotations has same or different effects on explicit and implicit methods of assessing the bias of sensed hand position toward the position of the cursor.
Materials and methods
Participants
Twenty-nine healthy right-handed young adults (mean ± SD: 22.6 ± 2.5 years; 14 males and 15 females) signed informed consent and participated in the study. Initially, 28 participants were tested. However, one participant was identified as having outliers for the explicitly assessed bias of cursor direction and was replaced by another participant. The study was conducted in accordance with the Declaration of Helsinki and with general approval by the ethics committee of the Leibniz Research Centre for Working Environment and Human Factors.
Apparatus
The experimental setting was similar to the one used in our previous studies (Rand & Heuer, 2013, 2016, 2017, in press). In brief, seated participants held a stylus with their right hand and made three-stroke movements from a starting position (SP) to a first target (T1) and then to a second target (T2), and back to T1 on a digitizer (Wacom Intuos 4 XL, 133 Hz sampling rate). They faced a monitor which was covered by a large black circular screen with a semi-circular window (32 cm in diameter) in its center. T1 (1.4 cm in diameter) was located in that center, and the SP (1.2 cm in diameter) was located 3 cm below T1. T2 (1 cm in diameter) was presented at pseudo random locations, ranging continuously from − 60° to + 60° relative to the central location (12 o’clock position), on an invisible circle with a radius of 15 cm around T1. Corresponding to this circle, the workspace on the digitizer was bordered by a semi-circular plastic ring, the “stopper ring”, with a radius of 15 cm around T1. An opaque board placed above the participants’ arm blocked their direct view of the hand.
Design and procedure
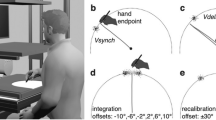
Each participant was tested both in the control condition and the adaptation condition. These conditions differed in the serial order of the different types of trial, but within each trial the sequence of events was the same. At the beginning of a trial, participants were guided to the SP by arrows shown on the monitor. One second after the stylus was in the SP, T2 appeared for 1 s (Fig. 2a, first panel). Subsequently, T1 appeared (Fig. 2a, second panel). After a delay of 0.5 s, an auditory go-signal was presented. The participants then made three-stroke movements from the SP to T1 (first stroke), to T2 (second stroke), and back to T1 (third stroke) at a comfortable speed. When the first stroke was made to T1, this target disappeared. This stroke was introduced because the participants would naturally look at T1 during the movement (Neggers & Bekkering, 2000; Rand & Stelmach, 2010), which prevented them from keeping their gaze on T2 to remember those locations. Because T2 was no longer visible after the initial presentation, the participants next made the second stroke to the remembered T2 (Fig. 2a, third panel) until the movement was stopped by the stopper ring. Afterwards, they made a return movement (third stroke) back to the remembered T1 location (Fig. 2a, fourth panel).
Behavioral task of a three-stroke movement and judgments of hand and cursor positions (a). SP, T1 and T2 refer to a starting position, a first target, and a second target, respectively. The visual feedback of the second stroke (from T1 to the remembered T2) is rotated and displayed simultaneously with hand movements. After the second stroke, the participants make a return movement back to T1 without the visual feedback as the third stroke, and subsequently make an explicit judgment regarding the hand or cursor position. Arrows with solid line refer to motions of visual feedback (third panel) and a position marker (fifth panel, top). Arrows with dashed line refer to hand movements (not the visual feedback). b Implicit measure of hand position. The directional deviation α′ of the sensed hand position (dotted outline circle) from the physical one (black circle) at the end of the second stroke is estimated from the directional deviation α of the hand position at the end of the third stroke (solid outline circle) from its remembered target (T1, gray circle)
The participants made the first and second strokes with concurrent visual feedback provided by a cursor on the monitor, but the third strokes without visual feedback. Only during the second stroke, the direction of cursor motion was rotated relative to the direction of hand movement. In the control condition, the rotation angle in each trial was randomly chosen out of six rotations [clockwise (CW) direction: − 25°, − 15°, − 5°; counter-clockwise (CCW) direction: 5°, 15°, 25°]. In the adaptation condition, the rotation angle in each trial was chosen out of ten rotations (− 25°, − 20°, − 15°, − 10°, − 5°, 5°, 10°, 15°, 20°, 25°) in specific orders as described below.
Note that the second stroke was performed with the presence of both proprioceptive and visual information, as described above. This bimodal procedure was used for both practice and test trials (see below), which was essential for studying sensory coupling and makes a clear distinction from studies of sensory recalibration (see the section: Sensory coupling and proprioceptive recalibration in “Introduction”).
The participants were instructed to make three distinct reaching movements to the targets without lifting the stylus from the digitizer surface and make a brief stop at the end of each movement. Regarding the second stroke, the participants were not informed of the nature of the feedback distortion, but they were told that the motions of visual feedback of the second stroke may be or may not be different from their hand movements and that they must adjust hand movements during that stroke so that the feedback cursor reaches to the remembered T2. They were instructed to make accurate movements at a comfortable speed. There were no reaction time or movement time constraints.
There were two types of trials, practice trials and test trials. Only the test trials included an explicit judgment after the three-stroke movement. One second after completing the third stroke (Fig. 2a, fourth panel), participants were asked to judge the position of either the hand or the cursor at the end of the second stroke. More specifically, to indicate the type of judgment to be performed, either the word “Hand” (for a judgment of hand position) or “Cursor” (for a judgment of cursor position) appeared briefly in the center of the monitor together with an arrow pointing to either the right (for a judgment using a CCW motion) or the left (for a judgment using a CW motion). Thence, the specified judgment followed.
For the judgment of cursor position (Fig. 2a, fifth panel, top), a short (2 cm) straight line served as a spatial direction marker and was moved on the monitor screen at constant speed (like a clock arm) CCW or CW on a circular path, beginning at a starting position 102° to the right or left of the vertical. It marked the peripheral end of a radial line from T1 to the circumference of the invisible ring of 15 cm diameter centered at T1. The participant instructed the examiner to stop and finely adjust (back and/or forth) the line to the position that matched the judged position of the cursor at the end of the second stroke. As needed, the position of the line was further adjusted (back and/or forth) by participants verbally instructing the experimenter to start and subsequently stop the movement of the line until they were satisfied with the position.
For the judgment of hand position (Fig. 2a, fifth panel, bottom), the participant first moved the pen (held by the right hand) to the right (or left) from the end point of the third stroke until the pen contacted the stopper ring. From that right (or left) lower corner of the stopper ring, the participant continued moving the pen CCW (or CW) along the ring, and stopped where he/she judged the hand position to match the position of the hand at the end of the second stroke. This hand judgment was performed without any visual feedback. Judged position (hand or cursor position) and direction of line or hand movement during the judgment (CW or CCW) were randomized across trials, with the constraint of equal frequencies.
Note that both the motion of the visual marker and the movement of the hand during the judgments differed from cursor motion and hand movement, respectively, during the second stroke. Therefore, only positions could be matched, but not movement directions and/or amplitudes.
The participants underwent 3-day experiments, 1 day for the control condition and 2 days for the adaptation condition. The arrangements of practice and tests trials and the rotation angles in the two conditions are shown in Table 1. In the adaptation condition, visuomotor rotations during the second stroke were gradually introduced in 5° steps in the order of 5°, 10°, 15°, 20°, 25°, 20°, 15°, 10°, and 5° on the 1 day and in the order of − 5°, − 10°, − 15°, − 20°, − 25°, − 20°, − 15°, − 10°, and − 5° on the other day. The order of positive and negative rotation days was counterbalanced across participants. For each rotation angle, participants performed 40 practice trials. After completion of the practice trials for each of the six rotation angles − 25°, − 15°, − 5°, 5°, 15°, and 25°, a set of test trials with the same rotation angle as in the preceding practice trials was performed. The control condition also consisted of practice and test trials. However, visuomotor rotations in both the practice and the test trials were randomly chosen from the set (− 25°, − 15°, − 5°, 5°, 15°, 25°), with the constraint of equal frequencies of the six angles and thus an average angle of 0°. Thereby adaptation to a visuomotor rotation was prevented. The number of test trials for each visuomotor rotation was the same for the adaptation and the control condition, with the only difference being the sequence of visuomotor rotations. The number of practice trials was somewhat reduced in the control condition to reduce the total duration of the experiment (from 4 to 3 days) but still maintain the same total number of trials on each day (432 trials). Given that the control condition did not involve any adaptation, the reduction of the number of practice trials did not affect the main distinction (i.e., with or without adaptation) between the adaptation and control conditions. The order of the control and adaptation conditions was counterbalanced across participants to avoid any practice effect that would systematically confound the differences between conditions.
On the first day of the experiment, there were ten familiarization trials without the judgment that included the procedure with/without the visual-feedback rotation and four warm-up trials that included all the procedure of the test trials (two trials each for hand and cursor judgments). The same four warm-up trials were also performed on the second and third day of the experiment. Within each set of test trials of each condition (Table 1), trials with explicit-hand and explicit-cursor judgment were randomized, again with the constraint of equal frequencies. There were short breaks after the second, fifth, eighth, and eleventh trial sets on each day of the experiment. In total, we recorded 288 practice and 144 test trials in the control condition and 720 practice and 144 test trials in the adaptation condition for each participant. The 144 test trials for each condition, 24 trials for each of the six angular rotations, were analyzed.
Data analysis
Data analyses were similar to those of our previous studies (Rand & Heuer, 2013, 2016, 2017, in press). The angular deviation of the judged hand or cursor position from the actual hand or cursor position at the end of the second stroke was measured in each test trial (the CCW direction had a positive sign) and served as the explicit measure of the bias in that trial. Individual means were computed for judged positions of cursor and hand for each condition (control and adaptation) and each visual-feedback rotation. The overall influence of the rotated visual feedback on the angular deviations of the judged from the actual hand or cursor positions was assessed by the slope of the linear regression of the angular deviation (dependent variable) on the visual-feedback rotation (independent variable), which was computed for each participant, condition, and type of judgment (cursor or hand). These slope parameters specify the strength of sensory coupling in terms of the proportional biases of the judgments of hand and cursor positions in degree per degree of the visual-feedback rotation.
As implicit measure of the bias of sensed hand position toward the position of the cursor in each trial, we computed the angular deviation of the direction of the third stroke from the direction of the second stroke, namely, the angle α (Fig. 2b) between the line connecting T1 with the end of the second stroke (i.e., line A) and the line connecting the end of the second stroke with the end of the third stroke (i.e., line B) was measured. The angle α’, which is α′ = α, was used as the implicit measure of the bias in that trial. This measure exploits the existence of error propagation in successive reaching movements (Bock & Eckmiller, 1986; Heuer & Sangals, 1998; Heuer & Sülzenbrück, 2012), in particular the propagation of errors that originate from visually induced deviations between the physical and the sensed position of the hand (Holmes et al., 2004; Holmes & Spence, 2005; Rossetti et al., 1995). In our case, the visually induced angular deviations of the sensed position of the hand from the actual one at the end of the second stroke (angle α′ in Fig. 2b) result from the rotated visual feedback, and they are estimated from the angular error of the return movement (angle α in Fig. 2b). When the sensed position deviated in the CCW or CW direction from the actual position of the hand, the angular deviation (α′) had a positive or negative sign, respectively. Individual means and standard deviations of the angular deviations were computed for each condition (control and adaptation) and each visual-feedback rotation across trials including both types of judgments (cursor and hand) because the third strokes were made before the direction to be judged was instructed. The angular deviations α′ were subjected to the same linear regressions as the angular deviations of the explicit judgments of hand direction from the actual direction to obtain a measure of the implicitly assessed bias as proportion of the visual-feedback rotation (i.e., proportional bias).
To identify changes of movement characteristics of the second stroke due to adaptation, we measured the deviation of the initial movement direction from the target direction, and the target error for each test trial. For these measures, we computed again individual slopes of the linear regressions on the visual-feedback rotation. These analyses served to check that adaptation indeed occurred (initial movement direction) and to test whether movement end points in the two experimental conditions (target error) were different.
The deviation of the initial movement direction from the target direction (i.e., initial direction error) was the deviation between two vectors. The first vector was defined by the starting position of the hand and its position after 10 mm distance. The second vector, which served as the reference, was defined by the starting position of the hand and the center of T2. The deviation of the initial movement direction from the target direction was positive (or negative) when a hand-path was directed CCW (or CW) compared to the reference line. In the control condition, the initial direction should be consistently toward the remembered visual target, that is, the deviation from the target direction should be close to zero on average and independent of the visuomotor rotation. In the adaptation condition, in contrast, the deviation of the initial movement direction from the target direction should depend on the visuomotor rotation: the slope of the linear regression of the deviation on the visuomotor rotation should be negative, indicating the adaptive change of the initial movement direction which is proportional to the visual-feedback rotation, but in the opposite direction.
The target error was the angular deviation of the cursor position at the end of the second stroke from the position of T2 (This deviation is identical to the angular deviation of the hand from its correct final position). The target error was positive (or negative) when the cursor position was shifted CCW (or CW) compared to the position of T2. If the cursor reached the remembered position of the target T2 consistently both in the control and the adaptation condition, the target error should be close to zero and not depend on the visuomotor rotation. In that case, the slope of its linear regression on the visual-feedback rotation should be zero as well.
The data were screened for outliers both among trials and among participants. Based on the linear regressions applied separately for each type of bias assessment (explicit cursor, explicit hand, implicit hand), each condition (control, adaptation), and each participant, trials with angular deviations outside the range of predicted deviations ± 3 standard deviations of the residuals were eliminated as outliers among trials. In total, 0.57% of test trials were removed from all analyses. Subsequently, the proportional bias parameters for each type of assessment and each condition were screened for outliers among participants. Means and standard deviations across all participants were calculated for the three types of measurement and the two conditions, and bias parameters outside the range of mean ± 3 standard deviations were defined as outliers. These computations were repeated until no further outliers were found. As the result, one participant was identified as having outliers for the explicitly assessed bias of cursor direction and was excluded from all analyses. This participant was replaced by a new one.
The individual regression parameters (slopes and intercepts) of the explicit and implicit bias measures of hand direction were subjected to a 2 (condition: control vs adaptation) × 2 (type of measure: hand-explicit vs hand-implicit) repeated-measures ANOVA. For analyzing the inter-individual variability of the different bias measures (the explicit and implicit measures of hand direction and the explicit measure of cursor direction), group’s mean regression coefficient (slope) was first calculated across participants in each condition and each measure, and next absolute deviations of individual values from the group’s mean value were calculated (cf. Levene, 1960). The larger these are, the larger is the inter-individual variability. These values were subjected to non-parametric tests to compare differences between the conditions and between the measures. Furthermore, individual regression parameters for the explicit bias measure of cursor direction, initial movement direction, and target error were compared between the control and adaptation conditions by using t tests.
Results
Our main interest is in the effects of adaptation on explicitly and implicitly assessed proportional biases of sensed hand position toward the position of the cursor. These findings are reported first, followed by a simulation based on the assumptions about the combination of adaptive changes and sensory coupling outlined in the Introduction. Next we report the findings for the explicit measure of sensed cursor position, and finally present the results for the initial directions and target errors of the reaching movements (second stroke).
Explicit and implicit assessments of the proportional bias of sensed hand position
In both the control and the adaptation condition, the mean angular deviation of the judged hand direction from the physical direction showed a steep positive slope as a function of the visual-feedback rotation (Fig. 3, squares). This indicates a strong explicitly assessed (proportional) bias toward the direction of the cursor. Turning to the implicit measure of the bias of hand direction, there were again positive slopes as a function of the visual-feedback rotation in both conditions (Fig. 3, triangles). However, these slopes were less steep than those observed for the explicit measure, indicating a weaker implicitly assessed than explicitly assessed (proportional) bias toward the direction of the cursor.
Mean explicit and implicit measures (i.e., angular deviation of the judged hand or cursor position from the actual hand or cursor position) as a function of the rotation of visual feedback. The mean values across all participants are plotted for explicit measures of cursor position (circles) and hand position (squares) and implicit measures of hand position (triangles). Open and filled symbols refer to the control and adaptation conditions, respectively. The error bars represent the SE
The means (and standard errors) of the individually estimated biases (proportional biases as measured by the slopes of the linear regressions) for the explicit measure of sensed hand direction toward the direction of the cursor were 0.595 ± 0.055 for the control and 0.646 ± 0.032 for the adaptation condition. The comparable means for the implicitly assessed biases of sensed hand direction were 0.351 ± 0.014 and 0.332 ± 0.011, respectively. A 2 (condition: control vs adaptation) × 2 (type of measure: hand-explicit vs hand-implicit) ANOVA revealed a significant main effect of type of measure (F(1,27) = 47.51, p < 0.001): the explicitly assessed bias was significantly stronger than the implicitly assessed bias. There was no condition effect (F(1,27) = 0.57, p > 0.05), and the interaction between condition and type of measure just fell short of statistical significance (F(1,27) = 3.68, p = 0.06). The explicitly assessed bias was numerically larger in the adaptation than in the control condition, though not significantly so (t(27) = 1.39, p = 0.18), whereas the implicitly assessed bias was numerically smaller in the adaptation condition (t(27) = 1.49, p = 0.15).
Figure 4 shows histograms of the individual (proportional) bias assessments in the control and the adaptation condition. For the explicitly assessed bias of sensed hand position, there was a conspicuous reduction of the variability in the adaptation condition, resulting from the reduction of the frequency of small biases (< 0.30) from 4 to 0 and of large biases (> 0.85) from 6 to 2 (see Fig. 4b as compared to Fig. 4a). The means (and standard errors) of the absolute deviations of the individual biases from their condition means were 0.230 ± 0.032 for the control and 0.143 ± 0.016 for the adaptation condition; the associated medians were 0.207 and 0.124, respectively (To make sure that these differences were not simply due to the three individual biases close to zero in the control condition, we repeated the analysis with a reduced sample: the mean absolute deviations were 0.185 ± 0.022 for the control and 0.127 ± 0.008 for the adaptation condition). The comparable means for the implicitly assessed biases of sensed hand direction were 0.061 ± 0.008 and 0.051 ± 0.005 for the control (Fig. 4c) and adaptation (Fig. 4d) condition, respectively; the associated medians were 0.052 and 0.053.
Individual (proportional) biases (i.e., the slope of the linear regression). Histograms of directional biases for explicit measures of hand positions (a, b), implicit measures of hand positions (c, d), and explicit measures of cursor positions (e, f) are shown for the control condition (upper panels) and the adaptation condition (lower panels). The binning size of each plot is 0.05°
Separate Wilcoxon signed-rank tests indicated a significant difference between conditions for the explicitly assessed bias (z = 2.80, p < 0.01; in the reduced sample: z = 2.22, N = 25, p < 0.05) but not for the implicitly assessed one (z = 0.96, p = 0.34). We also compared the change from the control to the adaptation condition between the two types of assessment by means of a Wilcoxon signed-rank test, which was significant as well (z = 2.28, p < 0.05). Thus for the explicit, but not for the implicit assessment, the inter-individual variability was reduced in the adaptation condition, and the change from control to adaptation condition was significantly different for the two measures. In addition, the inter-individual variability of the explicitly assessed biases was significantly larger than for the implicitly assessed ones both in the control condition (z = 4.19, p < 0.01) and the adaptation condition (z = 4.12, p < 0.01).
In addition to the slopes of the individual linear regressions for explicit and implicit proportional bias measures of sensed hand position, we examined whether the intercepts of the same regressions differed between the control and adaptation conditions. For the explicitly assessed bias of sensed hand position (Fig. 3, squares), the means (and standard errors) of the individual intercepts were 2.125 ± 0.539 in the control condition and 2.271 ± 0.576 in the adaptation condition, both of which were significantly greater than zero (control: t(27) = 3.94; adaptation: t(27) = 3.94, p < 0.01 for both). The comparable mean intercepts for the implicitly assessed bias (Fig. 3, triangles) were 0.978 ± 0.239 and 1.278 ± 0.358, respectively. Both means were significantly greater than zero (control: t(27) = 4.09; adaptation: t(27) = 3.57, p < 0.01 for both). A 2 (condition: control vs adaptation) × 2 (type of measure: hand-explicit vs hand-implicit) ANOVA revealed that the condition effect (F(1,27) = 0.97) and the interaction effect (F(1,27) = 0.075) were non-significant (p > 0.05 for both). The intercepts of the explicit measure tended to be greater than those of the implicit measure (F(1,27) = 3.45, p = 0.074). These findings indicate that both implicit and explicit measures of sensed hand position were slightly, but systematically shifted counterclockwise relative to the hand’s physical position, independently of the visuomotor rotation and to a similar extent in control and adaptation conditions.
Simulations of explicit and implicit assessments of the bias of sensed hand position
We hypothesized that the inter-individual variability of explicitly assessed (proportional) biases of sensed hand position, but not of implicitly assessed biases, would be reduced after adaptation. Our experimental data (Fig. 4a–d) confirmed our hypothesis. The rationale of this expectation is outlined in the “Introduction” (the section: “Sensory coupling and proprioceptive recalibration” in "Introduction", Fig. 1). In Fig. 5, we relate this rationale to the experimental data by way of simulations based on Eq. (2).
Proportional biases of sensed hand positions toward the cursor directions in the adaptation condition are plotted as a function of those in the control condition for the implicit (a) and explicit (b) assessments. Note that the proportional biases in the control condition serve as estimates of the coupling weight wc. Black dots refer to the experimentally observed individual biases and contour plots refer to the simulated biases. The contour lines mark the boundaries of the central areas which cover 67, 95, and 99% of all cases. Straight lines are identity lines
For the simulations, we used a large sample size of 107 to obtain robust estimates of the distributions of the expected individual biases of send hand position toward the position of the cursor. For the individual coupling weights wc, we assumed normally distributed random variables in the control-condition session and the adaptation-condition session, which are correlated between the sessions. Means and standard deviations of the coupling weights were set to the observed means and standard deviations of the observed proportional biases in the control condition, 0.351 and 0.075 for the implicit assessments and 0.595 and 0.289 for the explicit assessments. The correlations between the individual coupling weights in the different sessions were estimated by the correlations between biases computed separately from odd-numbered and even-numbered trials in the control condition (split-half reliability), being 0.74 and 0.87 for the implicit and explicit assessments, respectively. For the adaptation condition, means and standard deviations of the adaptive changes of sensed positions of hand and cursor (proprioceptive recalibration, Δh′/(c − h), and visual recalibration, Δc′/(c − h), Fig. 1b) were set to 0.336 and 0.140 for the hand and to 0.145 and 0.043 for the cursor. These values were chosen for the simulated biases of the explicit measure in the adaptation condition to have the same mean (0.645), the same standard deviation (0.168), and the same correlation with the biases in the control condition (0.78) as the observed biases have. Adaptive changes were zero for the control condition.
The bivariate distributions of the simulated biases in the control and adaptation condition were smoothed and are shown as contour plots in Fig. 5. The contour lines of the figure mark the boundaries of the central areas which cover 67, 95, and 99% of all cases. The simulated distributions (and the observed data) are close to the identity line for the implicit assessments (Fig. 5a), whereas they clearly deviate from it for the explicit assessments (Fig. 5b). Note that for the explicit assessments, most of the smaller biases in the control condition have their counterparts in the adaptation condition mostly above the identity line, reflecting the effect of the proprioceptive recalibration. In contrast, most of the larger biases in the control condition have their adaptation-condition counterparts below the identity line, reflecting the effect of visual recalibration.
Explicitly assessed bias of sensed cursor position
The slope of the mean angular deviation of the judged cursor position from the physical position as a function of the visual-feedback rotation was negative and small in absolute terms for both the control and the adaptation condition (Fig. 3, circles). This indicates a weak bias toward the position of the hand. The mean proportional biases (and standard errors) of cursor judgments toward the position of the hand were − 0.053 ± 0.009 for the control condition and − 0.039 ± 0.007 for the adaptation condition. These means were not significantly different (t(27) = 1.22, p = 0.23). The inter-individual variability of the proportional biases was also similar between the two conditions (Fig. 4e, f). The means (and standard errors) of the absolute deviations of the individually estimated biases from their condition means were 0.038 ± 0.006 for the control and 0.031 ± 0.004 for the adaptation condition; the associated medians were 0.029 and 0.028, respectively. These means were not significantly different (Wilcoxon signed-rank test, z = 0.46, p = 0.65). Additionally, means (and standard error) of the individual intercepts of the linear regressions for the explicit bias measures (Fig. 3, circles) were − 0.348 ± 0.169 in the control condition, which was significantly smaller than zero (t(27) = − 2.06, p < 0.05), and − 0.106 ± 0.208 in the adaptation condition, which did not differ from zero (t(27) = 0.511, p < 0.05). The condition difference was not significant (t(27) = 1.68, p > 0.05).
Target error and initial movement direction
Mean target errors in the control condition were generally small, but they increased slightly from about − 0.3° for the − 25° rotation to about 1.7° for the 25° rotation (Fig. 6a, open circles). The mean (and standard error) of the slopes of the individual linear regressions of the target error on the rotation angle was 0.046 ± 0.010. In the adaptation condition, the mean slope was significantly reduced (Fig. 6a, filled circles) to 0.015 ± 0.006 (t(27) = 35.90, p < 0.001). Thus, in the control condition, the visuomotor rotation was slightly less compensated at the end of reaching than in the adaptation condition.
The mean initial movement directions relative to the direction of the target T2 were about 3° in the control condition and independent of the visuomotor rotation (Fig. 6b, open circles). The mean (and standard error) of the slopes of the individual regressions of initial direction on visual-feedback rotation was 0.003 ± 0.008 and not significantly different from zero (t(27) = 0.32, p = 0.75). In the adaptation condition, conversely, the mean initial movement direction relative to the direction of the target was about 18° for the − 25° rotation and about − 14° for the 25° rotation (Fig. 6b, filled circles), and the mean of the individual slopes was − 0.672 ± 0.016. Thus, the initial movement directions were adapted to compensate for about 67% of the visual-feedback rotation. The difference between the mean slopes of the two conditions was significant (t(27) = 35.90, p < 0.001). This considerable difference in initial directions together with the very small difference in target errors indicates that the reaching movements were more curved in the control condition than in the adaptation condition, in particular at the larger visuomotor rotations.
Discussion
In previous studies of sensory coupling in cursor-control tasks, we had found a number of differences and dissociations between implicit and explicit assessments of the bias of the sensed position of the hand toward the position of the cursor (Rand & Heuer, 2013, 2016, 2017, in press). Here we extend these findings by showing different effects of adaptation to visuomotor rotations: explicitly assessed proportional biases have a reduced inter-individual variability after adaptation as compared with the control condition, whereas there is no such difference for the implicitly assessed proportional biases. We shall first elaborate on the different effects of adaptation on our implicit and explicit assessments. Thereafter, we discuss the implications of the current findings together with those of differences and dissociations observed previously. Finally, we turn to discuss additional findings that are not at the focus of this study.
Contrasting effects of adaption on explicit and implicit bias assessments
This study was motivated by the possibility that sensory coupling might be based on different representations of hand position depending on the type of measurement used. More specifically, explicit assessments might be based on representations of hand and cursor positions that are affected by visuomotor adaptation (e.g., Cressman & Henriques, 2009, 2010; Izawa et al., 2012; Simani et al., 2007; Synofzik et al., 2008; Wilke et al., 2013; Zbib et al., 2016). Implicit assessments, in contrast, might be based on representations of hand and cursor positions that remain unaffected. If this were the case, the observed biases of sensed hand position toward the position of the cursor for the implicit assessments should be the same for the control and the adaptation condition. This is what we found. For explicit assessments, the effect of adaptation should be less straightforward and essentially result in a reduced inter-individual variability. Again this is what we found. We outlined the rationale of this expectation (the section: “Sensory coupling and proprioceptive recalibration” in "Introduction”, Fig. 1) and further illustrated it by way of simulation (Fig. 5). The essence of the rationale for the explicit assessments is that adaptation induces proprioceptive and visual recalibration, which reduces the discrepancy between the sensed hand and cursor positions compared with that of the respective physical positions. As the CNS takes into account that reduced discrepancy, the range of the component of the explicitly assessed biases of hand position toward the cursor position that results from sensory coupling also reduces. This in turn reduces the observed inter-individual variability of the explicit measure in the adaptation condition compared with the control condition.
In the present study, the presence of proprioceptive and visual recalibration can be only inferred from the observed changes of inter-individual variability of the proportional biases of explicitly assessed hand positions toward the position of the cursor. In future studies, they can be assessed separately in unimodal test trials, which we have not done because of the already long duration of the experiment. It should become possible, however, when time is saved, e.g., by only one visuomotor rotation for adaptation (instead of the 6 rotations of the present study). With such a design, adaptation effects are constant for the different visuomotor rotations used in bimodal test trials, and hence, the expected findings are quite different from the findings with the present design. Namely, the individual intercepts of the regressions of the observed biases of sensed hand position toward the position of the cursor should be affected by proprioceptive and/or visual recalibration, rather than the individual slopes. How they are affected by these recalibrations should be predictable from separate assessments of the effects of adaptation in unimodal test trials.
A striking parallel to our main finding has recently been reported with a quite different paradigm. The temporal integration of auditory and visual stimuli is known to adapt to systematic stimulus onset asynchronies, so that asynchronous stimuli come to be perceived as synchronous (e.g., Harrar & Harris, 2008). Judgments of synchronicity of bimodal stimuli are psychophysical judgments and explicit. Synchronous visual and auditory stimuli do also result in particularly fast reaction times, faster than expected by statistical facilitation (or by responding to the signal that in a particular trial is processed more rapidly). This speed-up of reaction time is a behavioral measure of sensory integration and thus implicit. It turned out not to be affected by adaptation to asynchronous auditory and visual stimuli (Harrar, Harris, & Spence, 2017). Thus, again implicit and explicit measures of sensory integration respond differently to adaptation (though here it is a comparison between different experiments), even though the measures and the type of adaptation are grossly different from those of the present study.
Do explicit and implicit measures of sensory coupling depend on different representations of sensory information?
Sensory coupling in cursor-control tasks can be formalized as a weighted average of individual estimates of hand and cursor positions (Debats et al., 2017). Depending on how sensory coupling is assessed, the individual estimates that are averaged may differ. The implicit and explicit assessments that we used in the present and previous (Rand & Heuer, 2013, 2016, 2017, in press) studies are primarily different ways of measurement. However, the differences and dissociations that we found can be taken to suggest that they tap different representations of hand position. In particular, we found selective effects of visuomotor adaptation (present study), of enhanced proprioceptive information after the end of the forward movement (Rand & Heuer, 2016), and of aging (Rand & Heuer, 2013) on explicitly assessed biases of sensed hand position toward the position of the cursor, but not on implicit assessments. Regarding differences, we found weaker implicitly assessed biases with smaller intra- and inter-individual variability than explicitly assessed biases. Finally, the individual explicit and implicit biases are uncorrelated (This was also the case in the present study, r = 0.27, p = 0.16, in the control condition and r = 0.04, p = 0.84, in the adaptation condition).
Can these differences and dissociations be understood in terms of a single coupled estimate of hand position that is used for different purposes, psychophysical judgments (explicit assessments) and motor control (implicit assessments)? Certainly some of the differences such as those in the means and individual standard deviations could be explained in this way. But overall the various differences and dissociations of explicit and implicit bias assessments are consistent with the view that the two types of measure tap different ones of the several neural representations of proprioceptive information (e.g., Brown, Rosenbaum, & Sainburg, 2003a, 2003b; Goble et al., 2012; Proske & Gandevia, 2012; van Dam & Ernst, 2013). A possible distinction of the functional roles of these different representations is in terms of motor control and conscious awareness. This distinction is quite prominent for the visual modality (Milner & Goodale, 1995, 2008), though not undisputed (e.g., Schenk, Franz, & Bruno, 2011), and less prominent for the proprioceptive modality (Dijkerman & de Haan, 2007; Paillard, 1991).
From the available findings, we can tentatively characterize the two representations of hand position that are probably tapped by the implicit and explicit assessment procedures. The implicit representation is a rather low-level representation close to the information provided by the sensors and thus to the physical position of the hand, whereas the explicit one is a rather high-level representation which is more influenced by concurrent stimuli (cf. Kavounoudias et al., 2008; Reichenbach, Thielscher, Peer, Bülthoff, & Bresciani, 2014) and capable to feed consciousness. Thus, sensory coupling of high-level representations is stronger than sensory coupling of low-level representations, and it is more subject to the age-related difficulty in keeping visual and proprioceptive information on cursor and hand position apart (Rand, Wang, Müsseler, & Heuer, 2013; Rand & Heuer, 2013). Sensory coupling of the low-level representation is a rapid process that occurs already during movement, whereas sensory coupling of the high-level representation is a later process that is affected by proprioceptive information after the end of the movement (Rand & Heuer, 2016). Only the high-level representations are affected by proprioceptive recalibration (this study).
Implications of additional findings
The main findings of the present experiment regarding sensed hand positions were accompanied by no effect of adaptation on the explicitly assessed bias of the sensed position of the cursor toward the position of the hand. This bias was much smaller than the explicitly assessed bias of the sensed hand position. Perhaps small variations of such small biases are difficult or impossible to detect in the face of noisy data. The asymmetry of the explicitly assessed biases of the sensed hand position toward the position of the cursor and the sensed cursor position toward the position of the hand is a rather consistent finding (Debats et al., 2017; Rand & Heuer, 2013, 2016, 2017, in press). Nevertheless, the asymmetry can be modulated by the reliability of the individual estimates of hand and cursor positions (Debats et al., 2017), being consistent with the principle of reliability-based weighting that is typically observed for sensory coupling.
The examination of movement characteristics revealed an adaptation in terms of motor performance: about 67% of the visuomotor rotation was compensated by the planned movement direction, as assessed by the direction of the movement during the first centimeter of amplitude. Thus, our experimental manipulation to induce visuomotor adaptation was successful. Although the initial movement directions differed considerably between the adaptation and the control condition, the target error at the end of the movement was only slightly different. The compensatory adjustments during the movements were stronger in the control condition than in the adaptation condition, so that the final hand positions that were judged were almost identical in the control and adaptation conditions.
One could expect that the sensorimotor adaptation to the visuomotor rotations is correlated with the proprioceptive recalibration. However, the magnitudes of proprioceptive recalibration and sensorimotor adaptation have been shown to be uncorrelated in healthy adults (Cressman & Henriques, 2009, 2010; Izawa et al., 2012). Furthermore, sensorimotor adaptation and proprioceptive recalibration have different time courses (Zbib et al., 2016). Consistent with these findings, we found no correlation between the individual changes of the initial movement directions (expressed as proportion of the visuomotor rotation) from the control to the adaptation condition and the corresponding changes of the explicitly assessed proportional biases (r = − 0.14, p = 0.48).
References
Abeele, S., & Bock, O. (2001a). Mechanisms for sensorimotor adaptation to rotated visual input. Experimental Brain Research, 139, 248–253.
Abeele, S., & Bock, O. (2001b). Sensorimotor adaptation to rotated visual input: Different mechanisms for small versus large rotations. Experimental Brain Research, 140, 407–410.
Bock, O., & Eckmiller, R. (1986). Goal-directed arm movements in absence of visual guidance: Evidence for amplitude rather than position control. Experimental Brain Research, 62, 451–458.
Bresciani, J.-P., Dammeier, F., & Ernst, M. O. (2006). Vision and touch are automatically integrated for the perception of sequences of events. Journal of Vision, 6, 554–564.
Brown, L. E., Rosenbaum, D. A., & Sainburg, R. L. (2003a). Limb position drift: Implications for control of posture and movement. Journal of Neurophysiology, 90, 3105–3118.
Brown, L. E., Rosenbaum, D. A., & Sainburg, R. L. (2003b). Movement speed effects on limb position drift. Experimental Brain Research, 153, 266–274.
Buch, E. R., Young, S., & Contreras-Vidal, J. L. (2003). Visuomotor adaptation in normal aging. Learning & Memory, 10, 55–63.
Cheng, K., Shettleworth, S. J., Huttenlocher, J., & Rieser, J. J. (2007). Bayesian integration of spatial information. Psychological Bulletin, 133, 625–637.
Cressman, E. K., & Henriques, D. Y. P. (2009). Sensory recalibration of hand position following visuomotor adaptation. Journal of Neurophysiology, 102, 3505–3518.
Cressman, E. K., & Henriques, D. Y. P. (2010). Reach adaptation and proprioceptive recalibration following exposure to misaligned sensory input. Journal of Neurophysiology, 103, 1888–1895.
Debats, N. B., Ernst, M. O., & Heuer, H. (2017). Perceptual attraction in tool-use: Evidence for a reliability-based weighting mechanism. Journal of Neurophysiology, 117, 1569–1580.
Dijkerman, H. C., & de Haan, E. H. F. (2007). Somatosensory processes subserving perception and action. Behavioral and Brain Sciences, 30, 189–239.
Ernst, M. O. (2006). A Bayesian view on multimodal cue integration. In G. Knoblich, I. M. Thornton, M. Grosjean, & M. Shiffrar (Eds.), Human body perception from the inside out (pp. 105–131). Oxford: Oxford University Press.
Ernst, M. O. (2012). Optimal multisensory integration: Assumptions and limits. In B. E. Stein (Ed.), The New Handbook of Multisensory Processes (pp. 1084–1124). Cambridge: MIT Press.
Ernst, M. O., & Bülthoff, H. H. (2004). Merging the senses into a robust percept. Trends in Cognitive Sciences, 8, 162–169.
Goble, D. J., Coxon, J. P., Van Impe, A., Geurts, M., Van Hecke, W., Sunaert, S., … Swinnen, S. P. (2012). The neural basis of central proprioceptive processing in older versus younger adults: An important sensory role for right putamen. Human Brain Mapping, 33, 895–908.
Harrar, V., & Harris, L. R. (2008). The effect of exposure to asynchronous audio, visual, and tactile stimulus combinations on the perception of simultaneity. Experimental Brain Research, 186, 517–524.
Harrar, V., Harris, L. R., & Spence, C. (2017). Multisensory integration is independent of perceived simultaneity. Experimental Brain Research, 235, 763–775.
Hatada, Y., Miall, R. C., & Rossetti, Y. (2006). Long-lasting aftereffect of a single prism adaptation: shifts in vision and proprioception are independent. Experimental Brain Research, 173, 415–424.
Hay, J. C., Pick, H. L., & Ikeda, K. (1965). Visual capture produced by prism spectacles. Psychonomic Science, 2, 215–216.
Heuer, H., Hegele, M., & Rand, M. K. (2013). Age-related variations in the control of electronic tools. In C. M. Schlick, E. Frieling, & J. Wegge (Eds.), Age-Differentiated Work Systems (pp. 369–390). Heidelberg: Springer.
Heuer, H., & Sangals, J. (1998). Task-dependent mixtures of coordinate systems in visuomotor transformations. Experimental Brain Research, 119, 224–236.
Heuer, H., & Sülzenbrück, S. (2012). The influence of the dynamic transformation of a sliding lever on aiming errors. Neuroscience, 207, 137–147.
Holmes, N. P., Crozier, G., & Spence, C. (2004). When mirrors lie: “Visual capture” of arm position impairs reaching performance. Cognitive, Affective & Behavioral Neuroscience, 4, 193–200.
Holmes, N. P., & Spence, C. (2005). Visual bias of unseen hand position with a mirror: Spatial and temporal factors. Experimental Brain Research, 166, 489–497.
Izawa, J., Criscimagna-Hemminger, S. E., & Shadmehr, R. (2012). Cerebellar contributions to reach adaptation and learning sensory consequences of action. Journal of Neuroscience, 32, 4230–4239.
Kagerer, F. A., Contreras-Vidal, J. L., & Stelmach, G. E. (1997). Adaptation to gradual as compared with sudden visuo-motor distortions. Experimental Brain Research, 115, 557–561.
Kavounoudias, A., Roll, J. P., Anton, J. L., Nazarian, B., Roth, M., & Roll, R. (2008). Proprio-tactile integration for kinesthetic perception: An fMRI study. Neuropsychologia, 46, 567–575.
Kirsch, W., Pfister, R., & Kunde, W. (2016). Spatial action–effect binding. Attention, Perception & Psychophysics, 78, 133–142.
Krakauer, J. W., & Mazzoni, P. (2011). Human sensorimotor learning: Adaptation, skill, and beyond. Current Opinion in Neurobiology, 21, 1–9.
Krakauer, J. W., Pine, Z. M., Ghilardi, M. F., & Ghez, C. (2000). Learning of visuomotor transformations for vectorial planning of reaching trajectories. Journal of Neuroscience, 20, 8916–8924.
Ladwig, S., Sutter, C., & Müsseler, J. (2012). Crosstalk between proximal and distal action effects during tool use. Zeitschrift für Psychologie, 220, 10–15.
Ladwig, S., Sutter, C., & Müsseler, J. (2013). Intra- and intermodal integration of discrepant visual and proprioceptive action effects. Experimental Brain Research, 231, 457–468.
Levene, H. (1960). Robust tests for equality of variances. In I. Olkin, S. G. Ghurye, W. Hoeffding, W. G. Madow, & H. B. Mann (Eds.), Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling (pp. 278–292). Stanford: Stanford University Press.
Milner, A. D., & Goodale, M. A. (1995). The Visual Brain in Action. Oxford: Oxford University Press.
Milner, A. D., & Goodale, M. A. (2008). Two visual systems re-viewed. Neuropsychologia, 46, 774–785.
Morehead, J. R., Qasim, S. E., Crossley, M. J., & Ivry, R. (2015). Savings upon re-aiming in visuomotor adaptation. Journal of Neuroscience, 35, 14386–14396.
Neggers, S. F. W., & Bekkering, H. (2000). Ocular gaze is anchored to the target of an ongoing pointing movement. Journal of Neurophysiology, 83, 639–651.
Paillard, J. (1991). Motor and representational framing of space. In J. Paillard (Ed.), Brain and Space (pp. 163–182). Oxford: Oxford University Press.
Proske, U., & Gandevia, S. C. (2012). The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiological Reviews, 92, 1651–1697.
Rand, M. K., & Heuer, H. (2013). Implicit and explicit representations of hand position in tool use. PLoS ONE, 8, e68471.
Rand, M. K., & Heuer, H. (2016). Effects of reliability and global context on explicit and implicit measures of sensed hand position in cursor-control tasks. Frontiers in Psychology, 6, 2056.
Rand, M. K., & Heuer, H. (in press). Dissociating explicit and implicit measures of sensed hand position in tool use: effect of relative frequency of judging different objects. Attention, Perception, & Psychophysics.
Rand, M. K., & Stelmach, G. E. (2010). Effects of hand termination and accuracy constraint on eye-hand coordination during sequential two-segment movements. Experimental Brain Research, 207, 197–211.
Rand, M. K., Wang, L., Müsseler, J., & Heuer, H. (2013). Vision and proprioception in action monitoring by young and older adults. Neurobiology of Aging, 34, 1864–1872.
Reber, P. J. (2013). The neural basis of implicit learning and memory: A review of neuropsychological and neuroimaging research. Neuropsychologia, 51, 2026–2042.
Reichenbach, A., Thielscher, A., Peer, A., Bülthoff, H. H., & Bresciani, J.-P. (2014). A key region in the human parietal cortex for processing proprioceptive hand feedback during reaching movements. NeuroImage, 84, 615–625.
Rossetti, Y., Desmurget, M., & Prablanc, C. (1995). Vector coding of movement: Vision, proprioception, or both? Journal of Neurophysiology, 74, 457–463.
Schenk, T., Franz, V., & Bruno, N. (2011). Vision-for-perception and vision-for-action: Which model is compatible with the available psychophysical and neuropsychological data? Vision Research, 51, 812–818.
Shanks, D. R., & St. John, M. F. (1994). Characteristics of dissociable human learning systems. The Behavioral and Brain Sciences, 17, 367–447.
Simani, M. C., McGuire, L. M., & Sabes, P. N. (2007). Visual-shift adaptation is composed of separable sensory and task-dependent effects. Journal of Neurophysiology, 98, 2827–2841.
Synofzik, M., Lindner, A., & Thier, P. (2008). The cerebellum updates predictions about the visual consequences of one’s behavior. Current Biology, 18, 814–818.
Van Beers, R. J., Sittig, A. C., & Denier van der Gon, J. J. (1999). Integration of proprioceptive and visual position-information: An experimentally supported model. Journal of Neurophysiology, 81, 1355–1364.
Van Beers, R. J., Wolpert, D. M., & Haggard, P. (2002). When feeling is more important than seeing in sensorimotor adaptation. Current Biology, 12, 834–837.
Van Dam, L. C. J., & Ernst, M. O. (2013). Knowing each random error of our ways, but hardly correcting for it: An instance of optimal performance. PLoS ONE, 8, e78757.
Vindras, P., Desmurget, M., Prablanc, C., & Viviani, P. (1998). Pointing errors reflect biases in the perception of the initial hand position. Journal of Neurophysiology, 79, 3290–3294.
Welch, R. B. (1978). Perceptual modification. Adapting to altered sensory environments. New York: Academic Press.
Wendker, N., Sack, O. S., & Sutter, C. (2014). Visual target distance, but not visual cursor path length produces shifts in motor behavior. Frontiers in Psychology, 5, 225.
Wilke, C., Synofzik, M., & Lindner, A. (2013). Sensorimotor recalibration depends on attribution of sensory prediction errors to internal causes. PLoS ONE, 8, e54925.
Zbib, B., Henriques, D. Y. P., & Cressman, E. K. (2016). Proprioceptive recalibration arises slowly compared to reach adaptation. Experimental Brain Research, 234, 2201–2213.
Acknowledgements
This research was supported by the German Research Foundation (DFG) (Grant number Ra 2183/1-3). We thank Maleen Greine and Franziska Schywalski for their support in data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the German Research Foundation (DFG) (Grant number Ra 2183/1-3).
Conflict of interest
Miya Rand declares that she has no conflict of interest. Herbert Heuer declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Rand, M.K., Heuer, H. Contrasting effects of adaptation to a visuomotor rotation on explicit and implicit measures of sensory coupling. Psychological Research 83, 935–950 (2019). https://doi.org/10.1007/s00426-017-0931-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00426-017-0931-1