Abstract
Strigolactones (SLs) are carotenoid-derived molecules, which regulate various developmental and adaptation processes in plants. These are engaged in different aspects of growth such as development of root, leaf senescence, shoot branching, etc. Plants grown under nutrient-deficient conditions enhance SL production that facilitates root architecture and symbiosis of arbuscular mycorrhizal fungi, as a result increases nutrient uptake. The crosstalk of SLs with other phytohormones such as auxin, abscisic acid, cytokinin and gibberellins, in response to abiotic stresses indicates that SLs actively contribute to the regulatory systems of plant stress adaptation. In response to different environmental circumstances such as salinity, drought, heat, cold, heavy metals and nutrient deprivation, these SLs get accumulated in plant tissues. Strigolactones regulate multiple hormonal responsive pathways, which aids plants to surmount stressful environmental constraints as well as reduce negative impact on overall productivity of crops. The external application of SL analog GR24 for its higher bioaccumulation can be one of the possible approaches for establishing various abiotic stress tolerances in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
SLs promote AMF symbiosis that fulfills nutrient requirement of plants.
-
SLs interact with other phytohormones and affect the physiology of plant.
-
SLs counteract the ill effects of abiotic stresses.
-
SLs-related mutant plant possesses a number of impaired developmental processes.
Introduction
Plants encounter multiple environmental stresses, time-to-time, in their entire lifecycle. These environmental stresses (such as biotic and abiotic) are culpable for crop deterioration and have become a hazard for sustainable farming (Abouqamar et al. 2017). To shield crops from various biotic stresses (such as diseases, pests, weeds, etc.), chemical solutions are known to be a well-established segment, while, only limited measures are available to counteract abiotic stresses (like drought, salt, heavy metal, nutrient deficiency, temperature, etc.) at various stages of growth and development. Plants have evolved various responses as a survival stratagem towards unfavorable circumstances by eliciting a series of signals for reprogramming of metabolic and genetic pathways (Banerjee et al. 2017). Phytohormones are inherent signaling molecules which harmonize with various stresses and boost-up the growth as well as development of plants by producing complex responses under such environmental circumstances (Choudhary et al. 2012). They act centrally and govern stress reactions even at minute concentrations such as 10–5–10–6 mol L−1 (Banerjee and Roychoudhury 2018). The standard plant growth regulators (PGRs) are composed mainly of abscisic acid (ABA), auxins, gibberellins (GAs), cytokinins (CKs), brassinosteroids (BRs), and ethylene. In addition, certain metabolites such as nitric oxide (NO), jasmonic acid (JA), salicylic acid (SA), and polyamines (PAs) are considered as advanced PGRs. To this categorical list, strigolactones (SLs) seem to be the most recent ones (Banerjee et al. 2017).
Strigolactones were discovered in the root exudates of Gossypium hirsutum in 1966 as a crystalline germination stimulant of the parasitic plant Striga lutea (Cook et al. 1966). The central location of SL biosynthesis is the root; additionally, lower portion of the shoot has also been proposed as one of the important sites. In the year 2005, Akiyama et al. identified a hyphal branching factor from Lotus japonicus root exudates cultivated in low-phosphate circumstances. This has been identified as a novel SL ( +)-5-deoxystrigol (5-DS), showing another feature of this class of molecules. It was observed that SL is an important host-derived signaling molecule that regulates hyphal branching to set up a positive relationship between plants and arbuscular mycorrhizal fungi (AMF). Plant root liberates SL to attract AMF and, to build a symbiotic association that supplies essential minerals and water to plants, and in return plants provide carbohydrates to their fungal partners. This symbiotic association enables approximately 80% of land-plants to benefit from the consumption of nutrients and minerals for optimal growth, particularly under nutrient-deficient condition (Smith and Read 2010).
In the recent years, SLs have attracted a great deal of attention because of their essential roles in regulating numerous physiological and molecular pathways throughout the plant response to abiotic stresses (Banerjee et al. 2017). These govern various aspects of plant growth like impeding shoot branching, shaping root structure, facilitating leaf senescence, and regulating secondary growth (Brewer et al. 2013). To date, different SLs have been determined as germination stimulants from various species such as strigol from Gossypium hirsutum, Sorghum bicolor, Zea mays and Panicum miliaceum, sorgolactone from Sorghum bicolor, alectrol from Vigna unguiculata and, orobanchol from Trifolium pratense (Yoneyama et al. 2007b).
Strigolactones act as growth and development regulators in response to various cues, impacting plant architecture both endogenously as phytohormones and exogenously in the rhizosphere. Looking to their twin roles, SLs can be intended to generate crops which are resistant to environmental stresses. In this review, we will be dealing with the SLs biosynthesis, activity and functions, crosstalk with different plant hormones, and their performance during development and growth of plant with special prominence on stress adaption and tolerance mechanism.
Biosyntheses of SLs
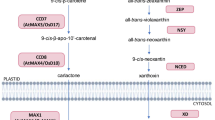
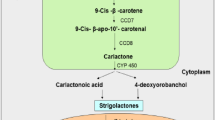
In the recent years, more than 20 SLs and SL-like compounds have been identified from the root exudates of several plants. Strigolactones are carotenoid-derivatives containing conserved tricyclic lactone ring (termed as ABC ring) in their structure, which is bound by an enol-ether bond to the inevitable α,β-unsaturated furanone ring known as D ring. For the production of SL carotenoids, both the plastidial and cytosolic enzymes act sequentially (Alder et al. 2012). In certain cases, carotenoid cleaving enzymes [usually belongs to the universal class of Carotenoid Cleavage Oxygenases (CCOs)] are involved in the formation of apocarotenoid which breaks C = C bonds and produces two carbonyl products. Many of the CCOs accelerate secondary cleavage of apocarotenoids rather than carotenoids (Walter and Strack 2011). The CCOs enzymes are usually known to be Carotenoid Cleavage Dioxygenases (CCDs) in plants, although many of them are yet to be proved experimentally. There are five main sub-families of CCD in plants; CCD1, CCD4, CCD7, CCD8, and 9-Cisepoxycarotenoid Dioxygenases (NCEDs) (Walter and Strack 2011). The 5-DS is considered as the precursor of SLs, due to its simple configuration and extensive abundance in both monocots and dicots. It was extracted from the root exudates of Lotus japonicus that lack any alternative on the A and B rings (Akiyama et al. 2005). Strigolactones are derived by acetylation, ketolation, hydroxylation, decarboxylation, oxidation, dehydration, and epoxidation reactions of 5-DS (Al-Babili and Bouwmeester 2015).
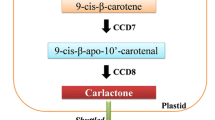
Plastid is the major site for the biosyntheses of apocarotenoids. The plastidial enzymes D27, CCD7, and CCD8 sequentially convert all-trans-β-carotene into carlactone (CL). Initially, carotenoid isomerase D27 transforms all-trans-β-carotene into the 9-cis-β-carotene, which is further transformed into the 9-cis-β-apo-10′-carotene by CCD7. Finally, CCD8 act on this compound and convert it into the CL. At this stage, formation of D ring is completed. The in vitro study has proved that the enzymes like all-trans-/9-cis-β-carotene isomerase Dwarf 27 (D27) and CCD7 and 8 are involved in the production of CL, which is a precursor of SLs (Alder et al. 2012). The More Axillary Growth 1 (MAX1), the cytochrome P450 monooxygenase enzyme (in Arabidopsis thaliana), and their associated homologs oxidizes CLs to various SLs through unspecified infrequent steps, which are catalyzed by novel enzymes (Mishra et al. 2017). Far along, it was reported that the two relevant CCDs; CCD7 and CCD8 [denoted as D17/ High Tillering Dwarf1 (HTD1) and D10 in Oryza sativa, Ramosus 5 (RMS5) and RMS1 in Pisum sativum, Decreased Apical Dominance3 (DAD3) and DAD1 in Petunia atkinsiana, MAX3 and MAX4 in Arabidopsis thaliana, respectively] functions sequentially in the SLs syntheses pathway (Arite et al. 2007). Cloning and sequencing experiments demonstrated that the RMS5/MAX3/D17 (HTD1) and RMS1/MAX4/D10/DAD1 encodes CCD7 and CCD8, respectively, and cytochrome P450; CYP711A1 is encoded by MAX1 (Schwartz et al. 2004). Most evidently, the knock-out or knock-down of CCD7 enzyme block or decrease the production of SL which later results in multiple shoot branching in plants (Vogel et al. 2010).
SLs transporter
The transporter of SLs has been characterized firstly in Petunia hybrida, termed as Pleiotropic Drug Resistance1 (PDR1). This transporter belongs to the ABC transporter family and is mentioned as an active transporter of SLs. In addition, one more ABC transporter; ABCG59 has been reported in Medicago truncatula for SLs transportation (Banasiak et al. 2020). In rhizosphere, secretion of SL takes place via PDR1, which is expressed near axillary bud that indicates it could be involved in the xylem loading in shoots. Furthermore, it has also been shown that SLs are not noticed in xylem saps of Oryza sativa, Lycopersicon esculentum and Arabidopsis thaliana, demonstrating that transportation of endogenous and exogenous SLs does not occur via xylem, but from the root to shoot (Kretzschmar et al. 2012).
Perception and signaling cascade of SLs
The F-box protein (MAX2/D3/RMS4) and α/β-hydrolase (D14/AtD14/DAD2) proteins may play a significant role throughout the perception of SLs in plant (Mishra et al. 2017). Strigolactones are sensed by the α/β-fold hydrolase that further delivers the signal to a leucine (Leu)-rich-repeat F-box protein (MAX2 in Arabidopsis thaliana; D3 in Oryza sativa), which acts like a recognition subunit in an SKP1-CUL1-F-box-protein (SCF)-type ubiquitin ligase complex. The SCF-type ubiquitin ligase further activates the 26S proteasome to degrade transcription repressor, such as Suppressor of MAX2 1-Like (SMXLs) in Arabidopsis thaliana and D53 in Oryza sativa (Wang et al. 2015). The D14 contain an exclusively conserved catalytic triad (Ser-His-Asp) that is essential for hydrolysis and signaling of SL. In higher plants, F-box protein MAX2 and α/β-hydrolase (D14/DAD2) are the core elements of SL perception complex (Hamiaux et al. 2012).
The process of signal transduction begins with the binding of SL to the “open state” pocket of D14/AtD14, and α/β-hydrolase receptor (Arite et al. 2007). Core elements of SL-signaling pathway comprises of activators such as D14 and D3 in Oryza sativa, correlated with AtD14 and MAX2 in Arabidopsis thaliana, as well as repressors such as D53 (Oryza sativa) and, SMXL 6, 7 and 8 (Arabidopsis thaliana) (Zhou et al. 2013). After binding with SL, D14/AtD14 induces deletion of ABC ring, enabling D ring to remain tightly and permanently attached to the D14/AtD14 catalytic cavity, while the conformational change in D14/AtD14 results in a “closed state” that triggers its interaction with the D3/MAX2-based SCF complex (Yao et al. 2016). Once the SLs are formed in the roots, they are acropetally relocated from the rhizosphere to shoot or exude through the transporter.
SLs application methods
Strigolactones are a class of chemical compounds produced by plants. But, certain environmental circumstances result into its deprivation in plants, thereby affecting their growth and development. In the recent years, lots of information about the structure and bio-properties of naturally occurring SLs have been gained. Scientists have discovered various methods for the synthesis of SL analogs with superior bioactivity. These analogs are implemented in plants via different application methods including foliar spray, growth medium, solution, etc. (Table 1).
Physiological roles of SLs in plants
Strigolactones are reported to be associated with a wide range of developmental processes like root growth, development of flower, leaf senescence, and photomorphogenesis, excluding their significant roles in shoot architecture (Zhang et al. 2018). However, it has also been demonstrated that SLs inhibit lateral root (LR) formation, but enhance the height of plant, senescence, secondary growth, and root hairs (Brewer et al. 2013). Indeed, detailed analyses of mutants of many species have revealed that the SLs control numerous facets of plant growth and development (Fig. 1).
Root development
Strigolactone affects various features of root development to govern the architecture of overall root system. Ruyter-Spira et al. (2011) revealed the biosyntheses or responses of SLs in max mutant of Arabidopsis thaliana, in which more LRs, and shorter primary roots with fewer meristem cells were formed than those appearing in wild types (WTs), demonstrating the role of SLs during root system development. It has been noticed that the exogenous application of GR24 affects LR formation, lateral root primordia development, and length of primary root in Arabidopsis thaliana. Involvement of GR24-mediated rise in the size of the transition zone into overall increase in primary root length was comparatively higher. Likewise, Benkova et al. (2003) examined that the primary root elongation is mostly interceded by GR24 which positively monitors numerous meristematic cells and transition zones. However, short root phenotype of SL-deficient mutant was unaffected by GR24 application but not in max2 mutant.
Auxin and its transport have a very important function in the identification of root system architecture, and auxin fluxes are controlled by SLs; the extent of this new plant hormone has been examined for root development processes (Fig. 2). The meristem and transition zone sizes are dependent on the auxin concentration gradient in radicles (Benkova et al. 2003). In Arabidopsis thaliana, the CCD8 (the SL-biosynthetic gene) is primarily activated in root cortical as well as epidermal cells of the transition-elongation zone in response to auxin exposure (Bainbridge et al. 2005).
Possible interaction of SLs with different phytohormones: SLs interact with different phytohormones and regulates various aspects of plants. A SLs enhance the PR growth and suppress LR formation as well as bud outgrowth by inhibiting PIN proteins, (auxin transporters). B The accumulation of SLs also inhibits CK to release BRC1 from suppression. As a result, bud outgrowth is prevented. The leaf senescence and mesocotyl length are also suppressed by CK. Strigolactones increase SAG expression and triggers leaf senescence. C Condition-dependent SL-ABA interaction was observed. Stress stimuli-induced simultaneous up-regulation of both the phytohormones in a mutually co-operative manner. However, during seed germination, SLs triggered CYP707A1 (the ABA catabolic gene), which reduced ABA level and stimulated seed germination. But under stress environment like drought and salinity, SLs increase gene expression of ABA, which causes stomatal closure. D Both SLs and GA are involved in shoot branching as well as seed germination. Strigolactones promote proteasomal degradation of DELLA (GA signaling inhibitor), as a result seed germination occurred. Green arrows denote activation of processes, while red lines represent phytohormone repression to various plant features. Dotted lines represent catabolism of ABA. PR, Primary roots; LR, Lateral roots; GA, Gibberellic acid; CK, Cytokinins; ABA, Abscisic acid; SL, Strigolactone; SAG, senescence associated gene
Another impact of SL is on length of root hairs. Under optimal growth condition, SLs promote root hair elongation, while inhibiting the formation of LRs (Fig. 1; Ruyter-Spira et al. 2011). Enhanced elongation of root hair in WT, and SL-deficient mutants (max3 and max4 but not in SL-response mutant max2) of Arabidopsis thaliana was observed when various synthetic analogs of SL were exogenously applied; proposing that the role of SL on root hair elongation is influenced by MAX2. Hence, SLs have an encouraging effect on root hair length and is facilitated via MAX2 (Kapulnik et al. 2011).
Shoot branching
Branching inhibiting signal (BIS), key player in the process of apical dominance, and SLs are similar or closely related as both are the derivatives of carotenoids (Ruyter-Spira et al. 2011). A group of shoot-branching mutants that have defective genes like d in Oryza sativa, max in Arabidopsis thaliana, dad in Petunia hybrida, and rms in Pisum sativum are involved in unraveling the SL biosynthesis, perception and transportation (Table 2; Kretzschmar et al. 2012). It has been reported that the genes like MAX3 (AtCCD7), MAX4 (AtCCD8), and cytochrome P450 MAX1 (AtCyp711A1) are associated with BIS biosynthesis in Arabidopsis thaliana, while, F-box and Leu-rich repeats containing MAX2 are likely to act in the signal perception/transduction. Increased numbers of shoot branching have been observed in plants mutated with any of the MAX genes. Exposure of a synthetic analog of SL; GR24, can rescue this mutant phenotype in a MAX2-dependent manner (Ruyter-Spira et al. 2011). Similarly, in case of ccd8 mutants of Pisum sativum (rms1) and Oryza sativa (d10), the SL was not produced in measurable amount, though their increased-branching phenotypes might be retrieved by the use of GR24. In another study it has been observed that the addition of GR24 negatively regulated increased branching in SL-deficient mutants. In a similar manner, enhanced branching phenotype was also detected in SL-response mutant, but this was not averted by GR24, supporting that the affected factors may possibly be involved in perception of SLs (Umehara et al. 2008).
Leaf senescence
Numerous abiotic factors including temperature and drought can influence leaf senescence in plants. Throughout the initial phase of senescence, cellular metabolic processes and gene expression patterns drastically alter. The most significant change is the degradation of chloroplasts followed by chlorophyll (Chl) destruction as well as continuous denaturation of chloroplast proteins like RuBisCo and Chl-a/b binding protein (CAB), which changes leaf color from green to yellow (Yamada and Umehara 2015). Phytohormones such as ABA, JA and ethylene promote senescence; while, CK is known as an inhibitor of this process (Fig. 2). Additionally, SLs tend to be a class of phytohormones that control senescence of leaf, because a delay in this process was observed in SL-deficient, and SL-insensitive mutants (Table 2; Yamada et al. 2014). However, in SL-deficient mutants; d27, d17 and d10 (Oryza sativa) as well as max1, max3 and max4 (Arabidopsis thaliana), the rate of leaf senescence greatly increased when GR24 was exogenously applied, but did not impose any effect on SL-insensitive mutants d3 and d14 of Oryza sativa and, max2 and atd14 of Arabidopsis thaliana (Yamada and Umehara 2015). Yamada et al. (2014) demonstrated that the exogenous application of GR24 recovered normal course of senescence in terms of leaf color, Chl content, and electrolyte leakage in SL-deficient Oryza sativa mutants, but not in SL-insensitive mutants. The SL-related mutants expressed delayed senescence phenotype, while GR24 increased senescence, suggesting that the SLs promote process of leaf senescence.
Stomatal closure
Plants have established a variety of stratagems to deal with various abiotic stresses like decreasing loss of water through stomatal closure that enhances tolerance against drought condition. Strigolactones have been shown to improve the syntheses of hydrogen peroxide (H2O2) and NO, and triggering Slow Anion Channel-Associated1 (SLAC1) anion channel, which stimulates stomatal closure, subsequently supporting plant adaptability towards environmental stresses (Zhang et al. 2018). The plasma membrane protein SLAC1 is very important for closure of stomata in response to CO2, calcium ions, ABA, change in humidity, ozone, light/dark transitions, H2O2 and NO (Vahisalu et al. 2008). Lv et al. (2017) indicated that for enhanced SL response, both H2O2 and NO are required in guard cell. It has been observed that stomatal closure induced by SL got damaged in the slac1 mutant, reflecting that the SLAC1 is essential for the SL-induced stomatal closure. As a result, destruction of the SLAC1 anion channel lead to SL and ABA insensitivity in stomata, signifying that the two signaling pathways may overlap at the SLAC1 anion channel by modifying the osmotic pressure that triggers stomatal movement. The interaction between SLs and ABA has been shown to play a key role in the integration of stress signals to regulate the development and functioning of the stomata (Fig. 2; Ha et al. 2014). In addition, a greater stomatal opening has been observed in SL-biosynthetic (max1-1, max3-9, and max4-1), and SL-signaling (atd14-5 and max2-1) Arabidopsis thaliana mutants as compared to WTs, suggesting that the SLs positively regulate stomatal closure (Lv et al. 2017). Liu et al. (2015a, b) noticed enhanced stomatal conductance and more widely opened stomata in SL mutants of Lotus japonicus (Table 2).
Recently, Visentin et al. (2020) found that SLs improved the ability of stomatal movement as a systemic stress signal component in Solanum lycopersicum. It has also been noted that the exogenous SLs enhanced the mature miR156 levels in the leaves of Solanum lycopersicum, both in absence and presence of drought stress. These results make SLs the first known molecular unit leading to induction of miR156 and its stress-related effects in the drought-triggered pathway.
Chlorophyll synthesis
To perform the process of photosynthesis, Chl is considered as one of the chief elements that reflects the photosynthetic capacity of plants. It has been observed that the abiotic stresses viz.; salt and drought stress mainly restricts photosynthesis and diffusion of CO2 by reducing conductivity inside the stomatal and mesophyll cells. However, very limited information is yet available regarding the impacts of SL treatment on photosynthesis, oxidative stress, and transcriptomics of plants under salinity or drought condition. Reductions in carotenoids and Chl-b content with increasing salinity/ alkalinity stress has previously been noticed in Leymus chinensis, which might be due to the enhanced activity of Chl degrading enzyme or inhibition in Chl synthesis. Additionally, supply of CO2 to RuBisCo may also have been declined due to the decreased stomatal conductivity, leading to inhibited CO2 assimilation (Lin et al. 2017). Similarly, Ma et al. (2017) observed a reduction in Chl content with an increasing concentration of salt in Brassica napus. However, exogenous application of GR24 revealed a significant improvement in the Chl content under salinity stress. In one more study, Min et al. (2018) noted a reduced Chl content in Vitis vinifera under drought stress, while exogenous application of GR24 restored the amount of Chl.
Moreover, several other studies revealed that the light-harvesting complexes of plants are positively regulated by SLs. These include multi-subunit membrane-protein complexes and, the components of the PSI and PSII (key converters of the sunlight into the chemical energy). A reduced level of Chl was determined in the SL-deficient Solanum lycopersicum mutant (Sl-ORT1) due to the decreased expressions of genes of RuBisCo as well as CAB protein precursor. In contrast, external application of GR24 induced expressions of these genes, suggesting the optimistic role of SLs in regulation of light-harvesting components (Mayzlish-Gati et al. 2010).
There are many evidences implying the imperative role of SLs in light-harvesting complex; even though, more studies are required to find the connection points between SLs and light-harvesting components. Apart from that, since light is the main component for carotenoid biosynthesis and SLs are also assumed to be derived from this pathway, it could be hypothesized that the interaction between SLs and light-associated pathways follows the feedback loop needed for coordinated growth and optimal development of plant.
Crosstalk between SLs and different phytohormones
A single phytohormone regulates several elements of plant growth and development. Consequently, most of the developmental processes like hypocotyl elongation, LR development, secondary growth, etc., are affected by various hormones that act together to improve growth and reproduction (Fig. 2; de Saint Germain et al. 2013). Phytohormones are the primary transporters of signaling indicators. The molecular regulation of whole cascade was done by phytohormones that prompt stress reactions. Being a novel hormone, SLs also support the above perceptions.
Interactions between SLs and ABA
Abscisic acid is known as a universal stress phytohormone because of its cooperation and regulation in all kinds of abiotic stresses. It regulates stomatal closure and activates a long distant signaling during environmental stresses such as drought, salinity, pathogen attack and injury. Besides stomatal closure for coping with stress, alteration in ABA levels also induces transcription of protein-encoding genes, including dehydrins, osmoprotectants, salinity and drought-related genes that trigger stress tolerance in plants. In stressed leaves and roots, the de novo synthesis of ABA has also been reported (Fujita et al. 2011).
Both SLs and ABA are derivatives of carotenoid and are considered as stress hormones. The regulatory role of ABA in biosynthesis of SL has previously been suggested as a correlation between ABA levels and SLs. The NCEDs enzyme (family of CCD enzymes) catalyzes the 9-cis-epoxycarotenoids to xanthoxin, i.e. very initial step in ABA biosynthetic pathway. Reduced concentration of SL was registered in the Solanum lycopersicum, when treated with specific NCEDs inhibitors that block the ABA biosynthesis. This decreased concentration of SLs in NCED mutants seems to suggest that the NCED is not associated directly with SL biosynthesis, rather its biosynthetic product ABA may be involved in regulating biosynthesis of SL (Lopez-Raez et al. 2010). Ha et al. (2014) revealed that the SL positively regulates the stress and/or ABA-responsive genes expression associated with plant development and abiotic stress responses. A large proportion of stress-inducible and/ or ABA-inducible genes along with the expression of ABA importer genes (ABCG22 and ABCG40) were seen to be negatively regulated in the max2 mutant of Arabidopsis thaliana during water stress.
It has been popularly reported that a wide range of physiological mechanisms are controlled via ABA and SLs (Ren et al. 2018). Abscisic acid also plays a crucial role in AM symbiosis. In addition to its role in the protection of stress in the mycorrhizal roots, studies have also revealed that ABA is essential for the symbiosis development, completion of arbuscular formation, and promotion of sustainable plant root colonization. Elevated level of ABA in mycorrhiza-associated stressed plants would therefore help to foster stress tolerance, while at the same time enhancing and sustaining AM symbiosis (Lopez-Raez 2016). Additionally, SLs and ABA are essential for the salt stress response regulation as well as for establishment of symbiotic relationships among host plant and AMF. Under stress conditions, exogenous application of ABA may increase the accumulation of SLs (Ren et al. 2018).
Interactions between SLs and auxin
Auxins are key growth promoters, and are also reported to induce expressions of SL associated genes such as CCD7 and CCD8 in Arabidopsis thaliana, Oryza sativa, Pisum sativum and Chrysanthemum morifolium (Lopez-Raez 2016). The shoot branching is reported to be regulated by SLs and auxin (Fig. 2). The increased-branching phenotype of rms mutants of Pisum sativum, dad mutants of Petunia hybrida, and max mutants of Arabidopsis thaliana has been observed. Strigolactones can inhibit shoot branching via their regulation through auxin transport (Fig. 2). These findings demonstrate that increased shoot branching of max mutant is due to the increased auxin transport capability. This indicates that the MAX pathway can function as a second messenger of auxin to regulate bud growth. Auxin triggers the expression of MAX-dependent genes, which transfer their signals into the buds and directly inhibit their development, hence preventing shoot branching in Arabidopsis thaliana (Bennett et al. 2006). Xu et al. (2015) also detected an interaction between auxin and SLs which regulate shoot branching in Oryza sativa.
The PIN protein, an auxin transport protein, has been shown to mediate the amount and route of polar auxin transport (Bennett et al. 2006). The max mutant of Arabidopsis thaliana leads to increased stem conductivity for auxin, and enhanced expression of several auxin transporters of PIN family (PIN1 and PIN3). The higher content of auxin in max mutant facilitates positive feedback control of MAX pathway and up-regulate gene expressions of MAX3 and MAX4. As a result, the low SL would increase auxin content that further triggers biosynthesis of SL. This indicates that the crucial function of the MAX pathway is to alter the expression of PIN in the stems (Hayward et al. 2009). In addition, the OsPIN expression levels, especially OsPIN9, also decreased significantly in response to GR24 treatment in the nodes of Oryza sativa, suggesting that the GR24 reduces auxin transportation capacity (Xu et al. 2015). Zhang et al. (2020b) revealed that both endogenous and exogenous SLs interact with canalization-dependent developmental processes, namely auxin feedback on PIN polarity and clathrin-mediated endocytosis of PIN proteins in Pisum sativum and Arabidopsis thaliana. This study revealed a non-transcriptional mechanism by which SL regulates vascular tissue formation and regeneration by uncoupling auxin feedback on PIN polarity and trafficking.
Interactions between SLs and CK
The three hormones namely auxin, CK, and SLs are associated with bud outgrowth control; the auxin and SLs suppress bud outgrowth, while it is promoted by CK (Fig. 2). Cytokinins are a group of phytohormones that positively regulate the initiation and outgrowth of axillary buds (Dun et al. 2012). Manandhar et al. (2018) observed that the increasing amount of endogenous SLs in less-branched varieties may have repressed the concentration of endogenous CKs leading to inhibition of axillary buds in Zantedeschia aethiopica. Similar results have also been registered in Oryza sativa, where bud growth was triggered by CK, while it was inhibited by exogenously applied GR24 (Xu et al. 2015). The AXR1 pathway incites SL biosynthesis followed by simultaneous suppression of CK synthesis. In plants, CKs might be affected by SLs; the SL increased-branching mutants of Pisum sativum has low CK levels in their xylem sap compared to WT ones which is due to the long-distance feedback regulation of SL branching pathway. It has been also suggested that Branched 1 (BRC1) is a transcription factor and is regulated by SL and CK to govern bud outgrowth (Fig. 2). Therefore, reduced PsBRC1 expression was noted in SL mutants, which was further enhanced by the exogenous application of GR24 (Dun et al. 2012). In another study, increased-branching phenotype Psbrc1 mutant of Pisum sativum was observed (Braun et al. 2012). In SL-deficient and response mutants of Pisum sativum and other species, reduced and normal levels of CK were determined in root and shoot tissues respectively. Adenosine phosphate-isopentenyltransferase-1 (PsIPT1) is the gene involved in the CK biosynthesis. In SL-related mutants of Pisum sativum, expression of PsIPT1 gene was found to be increased significantly which resulted in elevated biosynthesis of CK (Braun et al. 2012; Dun et al. 2012).
The length of mesocotyl highly elongates when seedlings are grown in the dark, while gets reduced under light conditions (Hu et al. 2010). It has been perceived that the SLs negatively regulate mesocotyl development via suppressing cell division in Oryza sativa under dark condition. In contrast, increased elongation in mesocotyl was reported in SL-related d mutants due to increased cell division, which raised the possibility that CK would be linked with the augmentation of mesocotyls cell division in d mutant (Hu et al. 2014). Likewise, a relationship among SL and CK has also been studied in the mesocotyl of Arabidopsis thaliana, and it was found that they have an antagonistic relationship in the SL-deficient d10-1 mutant, under dark conditions, whereas; in SL-insensitive mutant d14-1, this adverse phenomenon was not observed (Ha et al. 2014).
Interaction between SLs and GA
Gibberellic acid is among the primary factors contributing to plant height, as proposed by various techniques aimed at reducing the height of a number of crops. Similar to the GA signaling pathway, SL uses an F-box protein (RMS4/D3/MAX2) and α/β-fold hydrolase (D14/DAD2). Shorter stature phenotypes of SL-deficient and SL-response mutants of different species such as Oryza sativa, Arabidopsis thaliana, Solanum lycopersicum, Pisum sativum, Petunia hybrida, and Lotus japonicus have been observed, than of their WT ones (deSaint Germain et al. 2013).
Several researches have been done to uncover the potential correlations between SLs and GAs during shoot-branching control, and how they regulate each other (Marzec 2017). The actions of SLs were monitored by evaluating the possible interactions between these two hormones, i.e. the consequence of SLs on GA1 level (basic bioactive GA in Pisum sativum), and their significant effects on GA signaling, specifically on DELLA protein (Fig. 2; de Saint Germain et al. 2013). It was observed that the application of bioactive forms of GA; GA1, GA3, and GA4 negatively regulated SL biosynthesis in Oryza sativa and Lotus japonicus. Evidences showed that GA controls biosynthesis of SL independently from SL signaling, as GA3 treatment reduced the level of endogenous SLs in SL-insensitive mutants d3-1 and d14-1 of Oryza sativa. Furthermore, it was analyzed that GA-biosynthesis mutant was insensitive to GR24 treatment, while this treatment inhibited the second tiller bud growth in the 2-week old seedlings of WT. This suggested that the shoot branching is likely to be controlled by SLs in coordination with GAs (Ito et al. 2017).
Both biosynthetic and signaling pathways of GAs are well known. Earlier results suggested that the perception and signaling of SLs are close to that of GAs; hence it was assumed that the knowledge of GAs could also be relevant in SL biology (Marzec 2017). Similar to SL mutants, more shoot branched phenotypes have been noticed in semi dwarf mutants of Gibberellin Oxidase5, 6, and 9 (OsGAox5, OsGAox6, and OsGAox9) in Oryza sativa, wherein GA biosynthesis is disrupted. The association between SL receptor D14 and Slender1 (SLR1) has also been shown to be induced by SL. The SLR1 is a representative of DELLA protein that inhibits GA signaling (Nakamura et al. 2013). This suggests that SLs and GAs interact as SLR1 is degraded in an SL-dependent manner, similar to how binding of the GA receptor Gibberellic-Acid Insensitive1 (GID1) to a GA molecule enhances the interaction of GID1 and DELLA proteins in the GA signaling pathway. The resulting association of GID1-DELLA with the SCF complex protein leads to polyubiquitination of DELLA followed by 26S proteasomal degradation (Marzec 2017). However, further studies are required for the confirmation of crosstalk between SL and GA by analyzing the hormone status of different genetic backgrounds, and interaction between SL receptor and single DELLA protein.
Roles of SLs in abiotic stresses
Once the plant experiences certain environmental difficulties, the SL levels increase to optimize and perk up the growth strategies to fit in such circumstances. Abiotic stresses like salinity, drought, extreme temperatures, heavy metals, lack of availability of nutrients, etc., are cumulatively a serious threat to crop plants and thereby, global food security. Strigolactones are capable of conferring tolerance to various abiotic stresses in plants (Fig. 3).
SLs during nutrient starvation
Phosphorus (P) is an essential macronutrient that plants require since it is a structural component of cell macromolecules and plays a critical role in main metabolic functions (Mayzlish-Gati et al. 2012). To deal with P-starvation, plants have developed several strategies to achieve localized P-sources. The structural changes that occur in P deficiency include modifications in root architecture and root hair density in plants. Strigolactones control over LR formation has been seen to be affected by the concentration of inorganic phosphate (Pi). During sufficient availability of Pi, SLs repress the LR formation, while it was induced under low Pi level (Kapulnik et al. 2011; Ruyter-Spira et al. 2011). Hence, SLs have been suggested to be plant architecture regulators, especially in response to Pi. The Pi starvation-induced (PSI) genes are significantly linked with plants in response to low Pi conditions and are widely seen as markers under such circumstances. These genes are transcribed during Pi stressed condition but in SL mutant of Arabidopsis thaliana, the PSI expression could not be controlled by Pi, which might be due to the regulation of these genes by SL (Mayzlish-Gati et al. 2012). Reduced primary root growth and increased density of LR were observed in Arabidopsis thaliana under P-deficient condition (Rasmussen et al. 2013). Similarly, one more factor, the seminal root elongation can also be affected by Pi deficiency. Under P-deficient conditions, SL producing (d10 and d27) or SL-insensitive (d3) mutants of Oryza sativa showed a reduced seminal root (Sun et al. 2014). Santoro et al. (2020) studied in Solanum lycopersicum that exogenous SLs increased fresh weight, number of primary and lateral roots in low P compared to high P seedlings. In addition, SLs imposes a change in the root anatomy which was essentially required for development of roots under P shortage. This finding implies that the effects of rac-GR24 treatment on root architecture are influenced by P levels.
Roots are the main sites to produce SLs, and under normal growth conditions their production is much higher than those in above ground tissues. Unlike roots, in P deficiency (Lotus japonicus) or both P and Nitrogen (N)-deficiencies (Sorghum bicolor), there were few or no impacts on the SL content in their shoots respectively. This information shows that the SL levels primarily increase in the roots under nutritional deficiencies rather than in over ground organs, at least in the context of Lotus japonicus and Sorghum bicolor (Yoneyama et al. 2015). In Sorghum bicolor, the concomitant deprivation of P and N has a lesser impact compared to individual N or P deficiency on root SL exudation (Yoneyama et al. 2007a). Similar observation was noted in roots of Oryza sativa that the deterioration of both N and P triggered a lower exudation of SLs than the P deficiency alone (Jamil et al. 2011). Strigolactones can act as signaling molecules all through nutrient stress as Pi and N deficiencies induce their deposition in root tissues. As a result, SLs elevate Pi mobility and take-up during severe deficiency. Either P or N deficiency enhanced the production of SL by stimulating the expression of D10, D17, and D27, while suppressing D3, D14 and D53 expression in WT Oryza sativa (Sun et al. 2014). In roots of Arabidopsis thaliana, the N deficiency also increased transcription of MAX3 and MAX4, which may indicate that the SLs accumulated in root tissues due to N starvation (Ito et al. 2016). Under P-deficient conditions, the expression of SL transporter-encoding gene PDR1 (in the roots) had been enhanced, which was necessary for root-to-shoot translocation of SLs in the Petunia hybrida and Lotus japonicus (Kretzschmar et al. 2012; Liu et al. 2015a, b).
The establishment of AMF symbiosis requires a high level of collaboration between two partners on the basis of a finely regulated molecular interaction. The host plants produce signaling molecule and exudate it into rhizosphere to initiate this symbiotic interaction (in nutrient-deficient circumstances), which induces AMF followed by hyphae growth (Aroca et al. 2013). Phosphorus is known to be the least available essential nutrient in the soil. Plants quickly build a P-depletion zone across their roots due to the low mobility of P in soil solution. In Arabidopsis thaliana, that low-phosphate condition induced the SL biosynthesis and its secretion by roots, thereby promoting the interaction of plant with AMF as well as triggering the AMF branching (Toh et al. 2012). The absorption of P occurs through fine external hyphae of AMF by extending beyond the depletion zone. It was demonstrated that the Trifolium pratense produced high levels of SL in low P conditions (Yoneyama et al. 2007b). In addition, Pan et al. (2016) also observed in Zea mays that low P induces ZmCCD7, the SL-biosynthetic gene. Leguminous plants and rhizobia make a symbiotic association and obtain N from the root nodules; in this case only P scarcity improves exudation of SL, that attracts AM symbionts for the availability of P. But, in non-leguminous plants like Sorghum bicolor, both N and P deficiencies facilitate SL exudation, indicating that such plants rely on AM symbionts for the demand of both N and P (Yoneyama et al. 2007a).
SLs during drought stress
Among the abiotic stresses that plants experience, one of the most common types of environmental stresses is water deficiency, which interferes with development and has antagonistic effect on their survival and productivity (Ruiz-Lozano et al. 2016). When plants are subjected to drought stress, the electron transport chain gets disrupted, leading to oxidative stress and reactive oxygen species (ROS) accumulation, which in turn causes damage to certain organelles, including chloroplasts, mitochondria and peroxisomes (Min et al. 2018). Plants have evolved various defense mechanisms to optimize water uptake like morphological adaption, osmotic alteration, closure of stomata and improvement in antioxidant system. Ruiz-Lozano et al. (2016) noticed reduction in SL level under drought stress which is seen to be positively related with the down regulation of SL-biosynthetic gene SlCCD7 in Solanum lycopersicum. In Vitis vinifera, it has been observed that the polyethylene glycol-induced drought stress increased the production of H2O2 and malondialdehyde (MDA), whereas; reduced Chl content and altered antioxidant defense system. In contrast, GR24 treatment displayed greater tolerance to drought stress by regulation of Chl components and photosynthetic rate (Min et al. 2018). One more study revealed that the application of GR24 decreased the H2O2 and MDA content, suggesting that SL may act as ROS scavenger and reduce lipid peroxidation in Triticum aestivum under drought condition (Sedaghat et al. 2017). It has been investigated that max mutant (max2, max3 and max4) of Arabidopsis thaliana releases more water as compared to WT ones, which could be attributed to the change in rate of transpiration. The exogenous treatment of SL recovered the drought-sensitive phenotypes of SL-deficient max3 and max4 mutants at the same level of WT, while in SL-response max2 mutant, no significant effect was observed (Ha et al. 2014). These findings also provide support that SLs act as common regulators to induce stomatal closure. Strigolactones also possess indirect pathway to trigger stomatal closure i.e. regulation of ABA biosynthesis (Fig. 2). A comparative analysis of SL mutants (d3, d10, d17 and d27) of Oryza sativa revealed that d27 produced low level of ABA as compared to other d mutants, indicating that D27 could be associated in the regulation of ABA biosynthesis. In comparison to the elevated ABA level in SL biosynthetic/signaling mutants, the reduced ABA content in d27 demonstrated the potential role of OsD27 in the formation of ABA. These findings revealed that in these mutant lines the ABA content is positively linked with the expression level of D27 gene. Therefore, it can be said that the regulation of SL and ABA homeostasis is connected via D27, the β-carotene isomerase encoding gene, which increases drought tolerance in plants (Haider et al. 2018). In one of the mutants of Hordeum vulgare, hvd14.d gene was reported as hypersensitive to drought stress, and has been found to lower relative water content, disorganise chloroplast structure, impair photosynthesis, alter stomatal density, and slows stomatal closure as compared to those observed in the WT ones. Further findings suggested that a disruption in ABA metabolism and/or signaling pathways could be the cause of the drought-sensitive phenotype of SL mutant. These results revealed a correlation between SL signaling and drought stress response that is ABA-dependent (Marzec et al. 2020). In another study it was analyzed that smxl6, 7, 8 triple mutant of Arabidopsis thaliana was more drought tolerant than WT. During drought, the triple mutant showed increased synthesis of anthocyanin, improved detoxification ability, and enhanced sensitivity to ABA. They also found higher transcript levels of LEA/ABR, LEA18, and LEA76, but lower expressions of PDH1, PDH2, WRKY46, and QQS genes in mutant than that determined in the WT (Li et al. 2020).
In addition to fundamental defensive systems towards environmental stresses, plants may develop symbiotic relations with wide range of microorganisms residing in rhizosphere, which can relieve the stress symptoms. The AM symbiosis reduces stress-induced negative impacts in the majority of cases studied, which helps to host plants more tolerant against drought (Aroca et al. 2013). Due to the lack of AM symbiosis, reduction in SLs level with increased ABA concentration was observed in Solanum lycopersicum and Lactuca sativa plants grown under drought condition (Ruiz-Lozano et al. 2016).
SLs during salinity stress
In recent years, salinization is one of the most significant agricultural and environmental problems in a voluminous area of the world. It has been observed that currently salt containing soil occupies more than 7% of land area, and by the mid of the twenty-first century it is expected to increase up to 50% (Ruiz-Lozano et al. 2012). However, the inhibiting effects of salinity stress can be alleviated by the exogenous treatment of GR24, as demonstrated by a rise in the photosynthetic capacity in Brassica napus (Ma et al. 2017). Ha et al. (2014) revealed that the SL-deficient (max3 and max4) and SL-signaling (max2) mutants of Arabidopsis thaliana were hypersensitive to salinity at the germination/vegetative stages of growth as compared to their WT ones. In another experiment, two Triticum aestivum cultivars namely S-24 and PARI-73 were selected to analyze the role of GR24 against salt stress. The growth characteristics were significantly reduced, while GR24 treated seed population showed a differential growth rate (Kausar and Shahbaz 2017). In salt-stressed Oryza sativa, Ling et al. (2020) observed higher contents of MDA and ROS that leads to cellular damage, whereas seedlings treated with GR24 greatly reduced the levels of both, maintaining the standard growth of plant. Likewise, in Salvia nemorosa, elevated MDA and H2O2 contents have been noted under salinity. Additionally, the concentration of essential oil was also found to be reduced. The exogenous application of GR24 diminished the MDA accumulation and H2O2, while positively influenced the yield of essential oil (Sharifi and Bidabadi 2020). Recently, Sarwar and Shahbaz (2020) investigated the effects of salt stress in Helianthus annuus and noted that various growth parameters including photosynthetic pigments, net CO2 assimilation, and stomatal conductance were greatly reduced, while pre-sowing treatment of GR24 significantly reversed these traits. This treatment also increased the levels of Na+, K+, and Ca2+ in shoots and roots. Similarly, significant reductions in biomass of callus, soluble proteins, water potential and K+ and Ca2+, while increases in the MDA, Na+ and H2O2 were determined in Helianthus annuus during salt stress, which got further reversed by the application of GR24 (Zulfiqar et al. 2020).
Both SL and ABA play pivotal roles to regulate salt stress responses and establishment of AMF symbiosis. Ren et al. (2018) revealed that the external application of ABA, NaCl, and H2O2 up-regulated the expressions of SL-biosynthetic genes including CCD7, CCD8 (in root) as well as MAX2 (in shoot) of Sesbania cannabina. It has also been detected that tungstate and dimethylthiourea (DMTU; a scavenger of H2O2), repressed the expressions of CCD7 and CCD8 in AMF associated Sesbania cannabina under salinity stress. Additionally, the ABA-induced production of SL was also blocked by the pre-treatment of DMTU and TIS108 (SL biosynthesis inhibitor). Therefore, it could be said that ABA is essential for AMF-induced SL synthesis under salt stress. Likewise, the SL synthesis in AMF inoculated Lactuca sativa roots under salinity stress, was found to be positively connected. Moreover, ABA biosynthetic gene also showed a positive correlation with SLs syntheses under this stress. The mycorrhizal Lactuca sativa developed better under salinity in contrast to non-mycorrhizal ones. This indicates that under symbiotic conditions, association of these two phytohormones enhances activities of plants towards salt tolerance (Aroca et al. 2013).
SLs during light stress
Unlike any other stress, light is a prevalent environmental factor that cannot be avoided. Level of the SL in plants is also influenced by this factor, indirectly justifying the need of SLs in light signal cascades. Pleiotropic Photosignaling (PPS) encodes MAX2/ORE9 F-box proteins which facilitates the process of photomorphogenesis, and suppresses hypocotyl elongation. The pps mutant of Arabidopsis thaliana displayed hyposensitive response to blue, red and far red light (Shen et al. 2007). Strigolactone inhibits hypocotyl growth in a light-dependent manner, and this inhibition requires functional Elongated Hypocotyl5 (HY5). The HY5 negatively regulates hypocotyl growth, and SLs act positively to regulate abundance of HY5. Elongated hypocotyl phenotypes of hy1 and hy2 mutants have been recovered by GR24, but not hy5, which might be due to the SLs insensitive hy5 mutant. On the other hand, Constitutive Photomorphogenic1 (COP1) is another factor which promotes hypocotyl elongation under dark condition. The exogenous treatment of GR24-mediated nuclear exclusion of COP1; as a result stabilization of HY5 occurred which decreased the hypocotyl elongation. Both light and SL inhibit COP1 function, which indicates that the SL act as a supplement of light under dark condition (Tsuchiya et al. 2010). Xie et al. (2020) noted that in Arabidopsis thaliana, FHY3 and FAR1; phytochrome A mediated light signaling transcription factors directly up-regulates the expressions of SMXL6 and SMXL7 (SLs repressors) genes to promote shoot branching. These two factors also interact with Squamosa Promoter Binding Protein-Like9 (SPL) and SPL15, which suppresses the BRC1 thereby promoting shoot branching.
In general, phytochromes are involved in absorption of light by plants, and inhibit mesocotyl growth. Any damage in SL biosynthesis or signaling triggers extra elongation of mesocotyl under dark environment. It has previously been mentioned how CK and SL interact to regulate mesocotyl growth in SL-related d mutants (d10-1, d17-1, d27-1, d14 and d3) of Oryza sativa grown in dark condition. The application of GR24 was seen to inhibit mesocotyl growth in d10-1, d17-1 and d27-1 mutants, but not in the SL-insensitive d14 and d3 mutants (Hu et al. 2010). In one more experiment, in the mesocotyls of d10-1 and d14-1 of Oryza sativa, the CK-responsive type-A Response Regulator (RR) genes (OsRR4, OsRR5, OsRR7, OsRR9/10 and OsRR11) were found to be highly expressed, whereas the expression of OsTCP5 (Teosinte Branched1/Cycloidea/PCFs5), that inhibits growth and proliferation, was repressed in the mesocotyl of these mutants. An application of GR24 triggered the expression of OsTCP5 in the d10-1 (SL biosynthesis mutant), while it did not affect the OsTCP5 expression in the d14-1 (SL-signaling mutant) (Hu et al. 2014). Similar result was also registered by Kameoka and Kyozuka (2015) in the d3 and d14 mutants of Oryza sativa, where both were insensitive to GR24. The low light stress resulted into numerous metabolic changes in Solanum lycopersicum by inhibiting photosynthetic characteristics and disordering the assimilative metabolisms, which were restored by the application of GR24 (Lu et al. 2019).
SLs during heat stress
Heat stress is the key determinant restricting the root development of the cold-season plants, as the growth of roots requires relatively low temperature, therefore being very sensitive to rising environmental temperatures. The beneficial effects of GR24 on elongation of crown roots, and number of root cells has been noted by observing alterations in expression patterns of genes associated with cell division, and cell cycle in root tips, such as Proliferating Cell Nuclear Antigen (PCNA), Cyclin-D2 (CycD2) and Cyclin-Dependent Kinase B (CDKB) in heat stressed Festuca arundinacea (Hu et al. 2018). The SL-mediated regulation of germination has been demonstrated all through the heat stress in SL-defective Arabidopsis thaliana mutants (Table 2; Tsuchiya et al. 2010). Omoarelojie et al. (2020) have observed that the GR24 treatment alleviated the inhibitory effects of heat stress on seed germination of Lupinus polyphyllus. The GR24 enhances antioxidants activities which scavenges ROS during germination phase (Fig. 3). The temperature-induced dormancy of Pasteuria ramosa seeds throughout the warm stratification was found to be inverted by the application of SLs. The exogenous application of GR24 increased the expression of ABA catabolic gene (CYP707A1), and as a result, breakdown of ABA occurred and seeds get germinated even under heat stress (Lechat et al. 2015). It has been reported by Toh et al. (2012) that the seed germination response of max mutant of Arabidopsis thaliana was highly sensitive as compared to WT under heat stress. The exogenous treatment of GR24 recovered the max1-1 mutant phenotypes but not max2-1. Reduction in the rate of seed germination at high temperature was noticed in Lactuca sativa which was further stimulated by the application of SLs, by decreasing ABA/GA ratio (Gonai et al. 2004). The above observations suggest that the SLs might have an indirect communication with GA to monitor seed germination (Fig. 2).
SLs during chilling stress
Low temperature negatively affects crop production in several places around the world, triggering economic loss (Thakur et al. 2010). It has been noted that chilling temperature greatly reduced pace of photosynthesis and leaf area in Arabidopsis thaliana max (max2-1 and max4-1) and Pisum sativum rms4 mutants as compared to their WTs. As mentioned earlier that the max and rms4 are SLs-related genes, therefore, it could be assumed that the SL plays efficient role in low temperature tolerance in these plants (Fig. 3; Cooper et al. 2018). Zhang et al. (2020a) reported alteration in growth parameters during chilling stress in Brassica rapa; while, GR24 application substantially increased photosynthesis, cell viability, soluble protein and proline content, antioxidant enzymes and lowered the relative conductivity. They further noticed that GR24 significantly enhanced the expression of cold-related genes such as antioxidant enzymes, NADPH oxidase, MAPK, etc. Another study revealed that chilling treatment greatly decreases quantum efficiencies of photosystem II, and stimulates dehydration, leading to disruption of water status in Vigna radiata. The GR24 treatment notably reduced lipid peroxidation level and increased proline content, soluble sugar, activities of lipoxygenase and phenylpropanoid pathway enzymes, thereby alleviating chilling stress (Omoarelojie et al. 2021).
The gene expression analysis of Oryza sativa revealed similar levels of D27 and D17 in roots of non-mycorrhizal plants during both lower and normal temperature, whereas; the expressions of D10 and Os01g0701500 genes were seen to be down regulated during low temperature. However, significant up-regulation of all the above genes was reported in mycorrhizal Oryza sativa roots at low temperature (Liu et al. 2015a, b). It has previously been observed that the AMF increases accumulation of SLs by stimulating expression of their biosynthetic genes in host plants. Hence, it can be assumed that the SL was possibly involved in the regulation of chilling stress in plants. Till date, limited information is available regarding the role of SLs under chilling stress. In coming future, more analysis will certainly be required to find out the accurate mechanism of SLs towards chilling stress alleviation in plants.
SLs during heavy metal stress
All around the globe, heavy metals are considered to be the second most devastating environmental threat based on their degree of danger to the human population. In plants, toxicity of heavy metal is exhibited retardation in their growth and development resulting due to accumulation in their organs, which might be controlled by applying various phytohormones (Sytar et al. 2018). The toxicity of heavy metals varies with the type of metal elements. Tai et al. (2017) analyzed the effects of Cadmium (Cd) and its uptake in Panicum virgatum. Suppression in growth attributes, photosynthetic rate, Chl content and antioxidants activities, while enhanced levels of ROS were noticed. In contrast, improved growth, Chl content and antioxidants activities with lower accrual of Cd were discernible in the GR24 added Cd grown tissues. Recently, Mostofa et al. (2021) comparatively analyzed the role of SL in WT and, d10 and d17 mutants of Oryza sativa under Arsenic (As) stress. The transcript level of antioxidant enzymes including OsCuZnSOD1, OsCuZnSOD2, OsAPX1, OsAPX2 and OsCATA were noted to be relatively higher in WT roots than SLs mutant roots. Whereas, the expression of transporter genes (OsPT1, OsPT2, OsPT4 and OsPT8) was seen to be up-regulated in mutant plants as compared to WTs.
The membrane transporters play a crucial role in the uptake of bio-available metal ions from the soil, and co-transportation of different metal (loid) cations occur through the plasma membranes of roots. These transporters are essential to maintain physiological concentration of metal ion at the cellular level, and they also contribute to metal-induced stress responses (DalCorso et al. 2013). Arsenic (V) is structurally analogous to Pi and easily emerges via plant roots by means of Pi transporters. A number of categorized high affinity Pi transporters have been defined in the Pi transporter1 family (Pht1; TC 2.A.1.9, the PHS family), some of which are also engaged in the transport of As (V) in plants. As a result, As-toxicity causes Pi deficiency in plants (Li et al. 2016). Earlier, in this review, we have already stated that the SLs play a crucial role in plant mechanisms towards nutrient deficiencies and this deficiency stimulates expressions of SL biosynthesis genes (e.g. D10, D17, D27, OsMAX1, etc.) in roots. Hence, it could be assumed that the SLs may possibly be involved in mitigation of heavy metal stress in plants. However, further research is needed to determine the molecular pathways by which SLs acts on heavy metal stressed plants to alleviate their harmful effects.
Conclusions and future perspectives
Strigolactones are carotenoid-derived plant secondary metabolite molecules. They act on different aspects of plants to regulate their growth mechanisms, including seed germination, shoot branching, leaf senescence, root development, etc. Stress may have an impact on SL biosynthesis, signaling, and crosstalk with other plant hormones. In the recent years, SLs have raised tremendous attention because of their significant roles in adaptation of plants to several abiotic stresses like drought, salinity, nutrient deficiency, heat, chilling and heavy metals, as well as in control of a number of physiological and molecular processes. Collectively, all these areas signify the enormous impact of SLs in modern agriculture. Hence, application of SLs may offer an immense promise for the creation of new approaches and technologies that are consistent with evolving principles of sustainable agriculture.
Author contribution statement
SK conceptualized the topic, and finalized the manuscript draft. AB, BY and JC gathered the information and drafted the manuscript. All the authors read and approved the final manuscript.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Abouqamar S, Moustafa K, Tran LS (2017) Mechanisms and strategies of plant defense against Botrytis cinerea. Crit Rev Biotechnol 37:262–274. https://doi.org/10.1080/07388551.2016.1271767
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827. https://doi.org/10.1038/nature03608
Al-Babili S, Bouwmeester HJ (2015) Strigolactones, a novel carotenoid-derived plant hormone. Annu Rev Plant Biol 66:161–186. https://doi.org/10.1146/annurev-arplant-043014-114759
Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S (2012) The path from b-carotene to carlactone, a strigolactone-like plant hormone. Science 335:1348–1351. https://doi.org/10.1126/science.1218094
Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51:1019–1029. https://doi.org/10.1111/j.1365-313X.2007.03210.x
Aroca R, Ruiz-Lozano JM, Zamarreno AM, Paz JA, Garcia-Mina JM, Pozo MJ, Lopez-Raez JA (2013) Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J Plant Physiol 170:47–55. https://doi.org/10.1016/j.jplph.2012.08.020
Bainbridge K, Sorefan K, Ward S, Leyser O (2005) Hormonally controlled expression of the Arabidopsis MAX4 shoot branching regulatory gene. Plant J 44:569–580. https://doi.org/10.1111/j.1365-313X.2005.02548.x
Banasiak J, Borghi L, Stec N, Martinoia E, Jasinski M (2020) The full-size ABCG transporter of Medicago truncatula is involved in strigolactone secretion, affecting arbuscular mycorrhiza. Front Plant Sci 11:18. https://doi.org/10.3389/fpls.2020.00018
Banerjee A, Roychoudhury A (2018) Strigolactones: multi-level regulation of biosynthesis and diverse responses in plant abiotic stresses. Acta Physiol Plant 40:86. https://doi.org/10.1007/s11738-018-2660-5
Banerjee A, Wani SH, Roychoudhury A (2017) Epigenetic control of plant cold responses. Front Plant Sci 8:1643. https://doi.org/10.3389/fpls.2017.01643
Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602. https://doi.org/10.1016/S0092-8674(03)00924-3
Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis thaliana MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16:553–563. https://doi.org/10.1016/j.cub.2006.01.058
Bidabadi SS, Sharifi P (2021) Strigolactone and methyl jasmonate-induced antioxidant defense and the composition alterations of different active compounds in Dracocephalum kotschyi Boiss under drought stress. J Plant Growth Regul 40:878–889. https://doi.org/10.1007/s00344-020-10157-6
Braun N, deSaint GA, Pillot JP, Boutet-Mercey S, Dalmais M, Antoniadi I, Li X, Maia-Grondard A, Signor CL, Bouteiller N, Luo D, Bendahmane A, Turnbull C, Rameau C (2012) The Pisum sativum TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol 158:225–238. https://doi.org/10.1104/pp.111.182725
Brewer PB, Koltai H, Beveridge CA (2013) Diverse roles of strigolactones in plant development. Mol Plant 6:18–28. https://doi.org/10.1093/mp/sss130
Choudhary SP, Yu JQ, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS (2012) Benefits of brassinosteroid crosstalk. Trends Plant Sci 17:594–605. https://doi.org/10.1016/j.tplants.2012.05.012
Cook CE, Whichard LP, Turner B, Wall ME, Egley GH (1966) Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science 154:1189–1190. https://doi.org/10.1126/science.154.3753.1189
Cooper JW, Hu Y, Beyyoudh L, Yildiz Dasgan H, Kunert K, Beveridge CA, Foyer CH (2018) Strigolactones positively regulate chilling tolerance in Pisum sativum and in Arabidopsis thaliana. Plant Cell Environ 41:1298–1310. https://doi.org/10.1111/pce.13147
DalCorso G, Manara A, Furini A (2013) An overview of heavy metal challenge in plants: from roots to shoots. Metallomics 5:1117–1132. https://doi.org/10.1039/C3MT00038A
de Saint Germain A, Ligerot Y, Dun EA, Pillot JP, Ross JJ, Beveridge CA, Rameau C (2013) Strigolactones stimulate internode elongation independently of gibberellins. Plant Physiol 163:1012–1025. https://doi.org/10.1104/pp.113.220541
Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2012) Antagonistic action of strigolactone and cytokinin in bud out growth control. Plant Physiol 158:487–498. https://doi.org/10.1104/pp.111.186783
Foo E, Yoneyama K, Hugill CJ, Quittenden LJ, Reid JB (2013) Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol Plant 6:76–87. https://doi.org/10.1093/mp/sss115
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525. https://doi.org/10.1007/s10265-011-0412-3
Gonai T, Kawahara S, Tougou M, Satoh S, Hashiba T, Hirai N, Kawaide H, Kamiya Y, Yoshioka T (2004) Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. J Exp Bot 55:111–118. https://doi.org/10.1093/jxb/erh023
Ha CV, Leyva-Gonzalez MA, Osakabe Y, Tran UT, Nishiyama R, Watanabe Y, Tanaka M, Seki M, Yamaguchi S, Dong NV, Shinozaki KY, Shinozaki K, Estrella LH, Tran LSP (2014) Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc Natl Acad Sci USA 111:851–856. https://doi.org/10.1073/pnas.1322135111
Haider I, Jimenez BA, Bruno M, Bimbo A, Flokova K, Abuauf H, Ntui VO, Guo X, Charnikhova T, Al-Babili S, Bouwmeester HJ, Ruyter-Spira C (2018) The interaction of strigolactones with abscisic acid during the drought response in Oryza sativa. J Exp Bot 69:2403–2414. https://doi.org/10.1093/jxb/ery089
Hamiaux C, Drummond RS, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC (2012) DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr Biol 22:2032–2036. https://doi.org/10.1016/j.cub.2012.08.007
Hayward A, Stirnberg P, Beveridge C, Leyser O (2009) Interactions between auxin and strigolactone in shoot branching control. Plant Physiol 151:400–412. https://doi.org/10.1104/pp.109.137646
Hu Z, Yan H, Yang J, Yamaguchi S, Maekawa M, Takamure I, Tsutsumi N, Kyozuka J, Nakazono M (2010) Strigolactones negatively regulate mesocotyl elongation in Oryza sativa during germination and growth in darkness. Plant Cell Physiol 51:1136–1142. https://doi.org/10.1093/pcp/pcq075
Hu Z, Yamauchi T, Yang J, Jikumaru Y, Mayama TT, Ichikawa H, Takamure I, Nagamura Y, Tsutsumi N, Yamaguchi S, Kyozuka J, Nakazono M (2014) Strigolactone and cytokinin act antagonistically in regulating rice mesocotyl elongation in darkness. Plant Cell Physiol 55:30–41. https://doi.org/10.1093/pcp/pct150
Hu Q, Zhang S, Huang B (2018) Strigolactones and interaction with auxin regulating root elongation in tall fescue under different temperature regimes. Plant Sci 271:34–39. https://doi.org/10.1016/j.plantsci.2018.03.008
Ito S, Ito K, Abeta N, Takahashi R, Sasaki Y, Yajima S (2016) Effects of strigolactone signaling on Arabidopsis thaliana growth under nitrogen deficient stress condition. Plant Signal Behav 11:e1126031. https://doi.org/10.1080/15592324.2015.1126031
Ito S, Yamagami D, Umehara M, Hanada A, Yoshida S, Sasaki Y, Yajima S, Kyozuka J, Tanaka MU, Matsuoka M, Shirasu K, Yamaguchi S, Asami T (2017) Regulation of strigolactone biosynthesis by gibberellin signaling. Plant Physiol 174:1250–1259. https://doi.org/10.1104/pp.17.00301
Jamil M, Charnikhova T, Cardoso C, Jamil T, Ueno K, Verstappen F, Asami T, Bouwmeester HJ (2011) Quantification of the relationship between strigolactones and Striga hermonthica infection in Oryza sativa under varying levels of nitrogen and phosphorus. Weed Res 51:373–385. https://doi.org/10.1111/j.1365-3180.2011.00847.x
Kameoka H, Kyozuka J (2015) Down regulation of Oryza sativa DWARF 14 LIKE suppress mesocotyl elongation via a strigolactone independent pathway in the dark. J Genet Genomics 42:119e124
Kapulnik Y, Delaux PM, Resnick N, Mayzlish-Gati E, Wininger S, Bhattacharya C, Delmas NS, Combier JP, Becard G, Belausov E, Beeckman T, Dor E, Hershenhorn J, Koltai H (2011) Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis thaliana. Planta 233:209–216. https://doi.org/10.1007/s00425-010-1310-y
Kausar F, Shahbaz M (2017) Influence of strigolactone (GR24) as a seed treatment on growth, gas exchange and chlorophyll fluorescence of wheat under saline conditions. Int J Agric Biol 19:321–327
Kretzschmar T, Kohlen W, Sasse J, Borghi L, Schlegel M, Bachelier JB, Reinhardt D, Bours R, Bouwmeester HJ, Martinoia E (2012) A Petunia hybrida ABC protein controls strigolactone-dependent symbiotic signaling and branching. Nature 483:341–344. https://doi.org/10.1038/nature10873
Lechat MM, Brun G, Montiel G, Veronesi C, Simier P, Thoiron S, Pouvreau JB, Delavault P (2015) Seed response to strigolactone is controlled by abscisic acid-independent DNA methylation in obligate root parasitic plant, Phelipanche ramosa L Pomel. J Exp Bot 66:3129–3140. https://doi.org/10.1093/jxb/erv119
Li N, Wang J, Song WY (2016) Arsenic uptake and translocation in plants. Plant Cell Physiol 57:4–13. https://doi.org/10.1093/pcp/pcv143
Li W, Nguyen KH, Tran CD, Watanabe Y, Tian C, Yin X, Li K, Yang Y, Guo J, Miao Y, Yamaguchi S, Tran LSP (2020) Negative roles of strigolactone-related SMXL6, 7 and 8 proteins in drought resistance in Arabidopsis. Biomolecules 10:607. https://doi.org/10.3390/biom10040607
Lin J, Wang Y, Sun S, Mu C, Yan X (2017) Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci Total Environ 576:234–241. https://doi.org/10.1016/j.scitotenv.2016.10.091
Ling F, Su Q, Jiang H, Cui J, He X, Wu Z, Zhang Z, Liu J, Zhao Y (2020) Effects of strigolactone on photosynthetic and physiological characteristics in salt-stressed rice seedlings. Sci Rep 10:6183. https://doi.org/10.1038/s41598-020-63352-6
Liu W, Kohlen W, Lillo A, den Camp RO, Ivanov S, Hartog M, Limpens E, Jamil M, Smaczniak C, Kaufmann K, Yang WC, Hooiveld GJEJ, Charnikhova T, Bouwmeester HJ, Bisseling T, Geurts R (2011) Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 23:3853–3865. https://doi.org/10.1105/tpc.111.089771
Liu Z, Li Y, Wang J, He X, Tian C (2015b) Different respiration metabolism between mycorrhizal and non-mycorrhizal rice under low-temperature stress: a cry for help from the host. J Agric Sci 153:602–614. https://doi.org/10.1017/S0021859614000434
Liu J, He H, Vitali M, Visentin I, Charnikhova T, Haider I, Schubert A, Ruyter-Spira C, Bouwmeester HJ, Lovisolo C, Cardinale F (2015a) Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: exploring the interaction between strigolactones and ABA under abiotic stress. Planta 241:1435–1451. https://doi.org/10.1007/s00425-015-2266-8
Lopez-Raez JA (2016) How drought and salinity affect arbuscular mycorrhizal symbiosis and strigolactone biosynthesis? Planta 243:1375–1385. https://doi.org/10.1007/s00425-015-2435-9
Lopez-Raez JA, Kohlen W, Charnikhova T, Mulder P, Undas AK, Sergeant MJ, Verstappen F, Bugg TDH, Thompson AJ, Ruyter-Spira C, Bouwmeester H (2010) Does abscisic acid affect strigolactone biosynthesis? New Phytol 187:343–354. https://doi.org/10.1111/j.1469-8137.2010.03291.x
Lu T, Yu H, Li Q, Chai L, Jiang W (2019) Improving plant growth and alleviating photosynthetic inhibition and oxidative stress from low-light stress with exogenous GR24 in tomato (Solanum lycopersicum L.) seedlings. Front Plant Sci 10:490. https://doi.org/10.3389/fpls.2019.00490
Lv S, Zhang Y, Li C, Liu Z, Yang N, Pan L, Wu J, Wang J, Yang J, Lv Y, Zhang Y, Jiang W, She X, Wang G (2017) Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytol 217:290–304. https://doi.org/10.1111/nph.14813
Ma N, Hu C, Wan L, Hu Q, Xiong J, Zhang C (2017) Strigolactones improve plant growth, photosynthesis, and alleviate oxidative stress under salinity in rapeseed (Brassica napus) by regulating gene expression. Front Plant Sci 8:1671. https://doi.org/10.3389/fpls.2017.01671
Manandhar S, Funnell KA, Woolley DJ, Cooney JM (2018) Interaction between strigolactone and cytokinin on axillary and adventitious bud development in Zantedeschia. J Plant Physiol Pathol 6:1. https://doi.org/10.4172/2329-955X.1000172
Marzec M (2017) Strigolactones and gibberellins: a new couple in the phytohormone world? Trends Plant Sci 22:813–815. https://doi.org/10.1016/j.tplants.2017.08.001
Marzec M, Daszkowska-Golec A, Collin A, Melzer M, Eggert K, Szarejko I (2020) Barley strigolactone signaling mutant hvd14.d reveals the role of strigolactones in ABA-dependent response to drought. Plant Cell Environ 43:2239–2253. https://doi.org/10.1111/pce.13815
Mayzlish-Gati E, LekKala SP, Resnick N, Wininger S, Bhattacharya C, Lemcoff JH, Kapulnik Y, Koltai H (2010) Strigolactones are positive regulators of light-harvesting genes in tomato. J Exp Bot 61:3129–3136. https://doi.org/10.1093/jxb/erq138
Mayzlish-Gati E, De-Cuype C, Goormachtig S, Beeckman T, Vuylsteke M, Brewer PB, Beveridge CA, Yermiyahu U, Kaplan Y, Enzer Y, Wininger S, Resnick N, Cohen M, Kapulnik Y, Koltai H (2012) Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol 160:1329–1341. https://doi.org/10.1104/pp.112.202358
Min Z, Li R, Chen L, Zhang Y, Li Z, Liu M, Ju Y, Fang Y (2018) Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol Biochem 135:99–110. https://doi.org/10.1016/j.plaphy.2018.11.037
Mishra S, Upadhyay S, Shukla RK (2017) The role of strigolactones and their potential cross-talk under hostile ecological conditions in plants. Front Physiol 7:691. https://doi.org/10.3389/fphys.2016.00691
Mostofa MG, Rahman MM, Nguyen KH, Li W, Watanabe Y, Tran CD, Zhang M, Itouga M, Fujita M, Tran LP (2021) Strigolactones regulate arsenate uptake, vacuolar sequestration and antioxidant defense responses to resist arsenic toxicity in rice roots. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.125589
Nakamura H, Xue YL, Miyakawa T, Hou F, Qin HM, Fukui K, Shi X, Ito E, Ito S, Park SH, Miyauchi Y, Asano A, Totsuka N, Ueda T, Tanokura M, Asami T (2013) Molecular mechanism of strigolactone perception by DWARF14. Nat Commun 4:2613. https://doi.org/10.1038/ncomms3613
Omoarelojie LO, Kulkarni MG, Finnie JF, Pospisil T, Strnad M, Staden JV (2020) Synthetic strigolactone (rac-GR24) alleviates the adverse effects of heat stress on seed germination and photosystem II function in lupine seedlings. Plant Physiol Biochem 155:965–979. https://doi.org/10.1016/j.plaphy.2020.07.043
Omoarelojie LO, Kulkarni MG, Finnie JF, Staden JV (2021) Strigolactone analog (rac-GR24) enhances chilling tolerance in mung bean seedlings. S Afr J Bot 140:173–181. https://doi.org/10.1016/j.sajb.2021.03.044
Pan X, Zheng H, Zhao J, Xu Y, Li X (2016) ZmCCD7/ZpCCD7 encodes a carotenoid cleavage dioxygenase mediating shoot branching. Planta 243:1407–1418. https://doi.org/10.1007/s00425016-2479-5
Rasmussen A, Mason MG, De Cuyper C, Brewer PB, Herold S, Agusti J, Geelen D, Greb T, Goormachtig S, Beeckman T, Beveridge CA (2013) Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol 158:1976–1987. https://doi.org/10.1104/pp.111.187104
Ren CG, Kong CC, Xie ZH (2018) Role of abscisic acid in strigolactone induced salt stress tolerance in arbuscular mycorrhizal Sesbania cannabina seedlings. BMC Plant Biol 18:74. https://doi.org/10.1186/s12870-018-1292-7
Ruiz-Lozano JM, Porcel R, Azcon C, Aroca R (2012) Regulation of arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J Exp Bot 11:4033–4044. https://doi.org/10.1093/jxb/ers126
Ruiz-Lozano JM, Aroca R, Zamarreno AM, Molina S, Andreo-Jimenez B, Porcel R, Garcia-Mina JM, Ruyter-Spira C, Lopez Raez JA (2016) Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ 39:441–452. https://doi.org/10.1111/pce.12631
Ruyter-Spira C, Kohlen W, Charnikhova T, van Zeijl A, van Bezouwen L, de Ruijter N, Cardoso C, Lopez-Raez JA, Matusova R, Bours R, Verstappen F, Bouwmeester H (2011) Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiol 155:721–734. https://doi.org/10.1104/pp.110.166645
Santoro V, Schiavon M, Gresta F, Ertani A, Cardinale F, Sturrock CJ, Celi L, Schubert A (2020) Strigolactones control root system architecture and tip anatomy in Solanum lycopersicum L. plants under P starvation. Plants 9:612. https://doi.org/10.3390/plants9050612
Sarwar Y, Shahbaz M (2020) Modulation in growth, photosynthetic pigments, gas exchange attributes and inorganic ions in sunflower (Helianthus annuus L.) by strigolactones (GR24) achene priming under saline conditions. Pak J Bot 52:23–31. https://doi.org/10.30848/PJB2020-1(4)
Schwartz SH, Qin X, Loewen MC (2004) The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J Biol Chem 279:46940–46945. https://doi.org/10.1074/jbc.M409004200
Sedaghat M, Sarvestani ZT, Emam Y, Bidgoli AM (2017) Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol Biochem 119:59–69. https://doi.org/10.1016/j.plaphy.2017.08.015
Sedaghat M, Sarvestania ZT, Emamb Y, Bidgolia AM, Sorooshzadeh A (2020) Foliar-applied GR24 and salicylic acid enhanced wheat drought tolerance. Russ J Plant Physl 67:733–739. https://doi.org/10.1134/S1021443720040159
Sharifi P, Bidabadi SS (2020) Strigolactone could enhances gas-exchange through augmented antioxidant defense system in Salvia nemorosa L. plants subjected to saline conditions stress. Ind Crop Prod 151:112460. https://doi.org/10.1016/j.indcrop.2020.112460
Shen H, Luong P, Huq E (2007) The F-Box protein MAX2 functions as a positive regulator of photomorphogenesis in Arabidopsis. Plant Physiol 145:1471–1483. https://doi.org/10.1104/pp.107.107227
Smith S, Read DJ (2010) Mycorrhizal symbiosis, 3rd edn. Academic Press, New York
Sun H, Tao J, Liu S, Huang S, Chen S, Xie X, Yoneyama K, Zhang Y, Xu G (2014) Strigolactones are involved in phosphate and nitrate-deficiency induced root development and auxin transport in rice. J Exp Bot 65:6735–6746. https://doi.org/10.1093/jxb/eru029
Sytar O, Kumari P, Yadav S, Brestic M, Rastogi A (2018) Phytohormone priming: regulator for heavy metal stress in plants. J Plant Growth Regul 38:739–752. https://doi.org/10.1007/s00344-018-9886-8
Tai Z, Yin X, Fang Z, Shi G, Lou L, Cai Q (2017) Exogenous GR24 alleviates cadmium toxicity by reducing cadmium uptake in switch grass (Panicum virgatum) seedlings. Int J Environ Res Public Health 14:852. https://doi.org/10.3390/ijerph14080852
Thakur P, Sanjeev K, Malik JA, Berger JD, Nayyar H (2010) Chilling stress effects on reproductive development in grain crops: an overview. Environ Exp Bot 67:429–443. https://doi.org/10.1016/j.envexpbot.2009.09.004
Toh S, Kamiya Y, Kawakami N, Nambara E, McCourt P, Tsuchiya Y (2012) Thermoinhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant Cell Physiol 53:107–117. https://doi.org/10.1093/pcp/pcr176
Tsuchiya Y, Vidaurre D, Toh S, Hanada A, Nambara E, Kamiya Y, Yamaguchi S, McCourt P (2010) A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat Chem Biol 6:741–749. https://doi.org/10.1038/nchembio.435
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200. https://doi.org/10.1038/nature07272
Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmaki A, Brosche M, Moldau H, Desikan R, Schroeder JI, Kangasjarvi J (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signaling. Nature 452:487–491. https://doi.org/10.1038/nature06608
Visentin I, Pagliarani C, Deva E, Caracci A, Tureckova V, Novak O, Lovisolo C, Schubert A, Cardinale V (2020) A novel strigolactone-miR156 module controls stomatal behavior during drought recovery. Plant Cell Environ 43:1613–1624. https://doi.org/10.1111/pce.13758
Vogel JT, Walter MH, Giavalisco P et al (2010) SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J 61:300–311. https://doi.org/10.1111/j.1365-313X.2009.04056.x
Walter MH, Strack D (2011) Carotenoids and their cleavage products: biosynthesis and functions. Nat Prod Rep 28:663–692. https://doi.org/10.1039/c0np00036a
Wang L, Wang B, Jiang L, Liu X, Li X, Lu Z, Meng X, Wang Y, Smith SM, Li J (2015) Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-Like SMXL repressor proteins for ubiquitination and degradation. Plant Cell 27:3128–3142. https://doi.org/10.1105/tpc.15.00605
Xie Y, Liu Y, Ma M, Zhou Q, Zhao Y, Zhao B, Wang B, Wei H, Wang H (2020) Arabidopsis FHY3 and FAR1 integrate light and strigolactone signaling to regulate branching. Nat Commun 11:1955. https://doi.org/10.1038/s41467-020-15893-7
Xu J, Zha M, Li Y, Ding Y, Chen L, Ding C, Wang S (2015) The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in Oryza sativa L. Plant Cell Rep 34:1647–1662. https://doi.org/10.1007/s00299-015-1815-8
Yamada Y, Umehara M (2015) Possible roles of strigolactones during leaf senescence. Plants 4:664–677
Yamada Y, Furusawa S, Nagasaka S, Shimomura K, Yamaguchi S, Umehara M (2014) Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 240:399–408. https://doi.org/10.1007/s00425-014-2096-0
Yang YY, Ren YR, Zheng PF, Zhao LL, You CX, Wang XF, Hao YJ (2020) Cloning and functional identification of a strigolactone receptor gene MdD14 in apple. Plant Cell Tiss Org 140:197–208. https://doi.org/10.1007/s11240-019-01722-3
Yao RF, Ming ZH, Yan LM, Li SH, Wang F, Ma S, Yu C, Yang M, Chen L, Chen L, Li Y, Yan C, Miao D, Sun Z, Yan J, Sun Y, Wang L, Chu J, Fan S, He W, Deng H, Nan F, Li J, Rao Z, Lou Z, Xie D (2016) DWARF14 is a non canonical hormone receptor for strigolactone. Nature 536:469–473. https://doi.org/10.1038/nature19073
Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H (2007b) Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 225:1031–1038. https://doi.org/10.1007/s00425-006-0410-1
Yoneyama K, Xie X, Kusumoto D, Sekimoto H, Sugimoto Y, Takeuchi Y, Yoneyama K (2007a) Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 227:125–132. https://doi.org/10.1007/s00425-007-0600-5
Yoneyama K, Kisugi T, Xie X, Arakawa R, Ezawa T, Nomura T, Yoneyama K (2015) Shoot derived signals other than auxin are involved in systemic regulation of strigolactone production in roots. Planta 241:687–698. https://doi.org/10.1007/s00425-014-2208-x
Zhang Y, Lv S, Wang G (2018) Strigolactones are common regulators in induction of stomatal closure in planta. Plant Signal Behav 13:e144432. https://doi.org/10.1080/15592324.2018.1444322
Zhang J, Mazur E, Balla J, Gallei M, Kalousek P, Medvedova Z, Li Y, Wang Y, Prat T, Vasileva M, Reinohl V, Prochazka S, Halouzka R, Tarkowski P, Luschnig C, Brewer PB, Friml J (2020b) Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization. Nat Commun 11:3508. https://doi.org/10.1038/s41467-020-17252-y
Zhang X, Zhang L, Sun Y, Zheng S, Wang J, Zhang T (2020a) Hydrogen peroxide is involved in strigolactone induced low temperature stress tolerance in rape seedlings (Brassica rapa L.). Plant Physiol Biochem 157:402–415. https://doi.org/10.1016/j.plaphy.2020.11.006
Zheng X, Li Y, Xi X, Ma C, Sun Z, Yang X, Li X, Tian Y, Wang C (2021) Exogenous Strigolactones alleviate KCl stress by regulating photosynthesis, ROS migration and ion transport in Malus hupehensis Rehd. Plant Physiol Biochem 159:113–122. https://doi.org/10.1016/j.plaphy.2020.12.015
Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, Wu F, Mao H, Dong W, Gan L, Ma W, Gao H, Chen J, Yang C, Wang D, Tan J, Zhang X, Guo X, Wang J, Jiang L, Liu X, Chen W, Chu J, Yan C, Ueno K, Ito S, Asami T, Cheng Z, Wang J, Lei C, Zhai H, Wu C, Wang H, Zheng N, Wan J (2013) D14-SCF (D3)-dependent degradation of D53 regulates strigolactone signaling. Nature 504:406–410. https://doi.org/10.1038/nature12878
Zulfiqar H, Shahbaz M, Ahsan M, Nafees M, Nadeem H, Akram M, Maqsood A, Ahmar S, Kamran M, Alamri S, Siddiqui MH, Saud S, Fahad S (2020) Strigolactone (GR24) induced salinity tolerance in sunflower (Helianthus annuus L.) by ameliorating morpho-physiological and biochemical attributes under in vitro conditions. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10256-4
Acknowledgements
The authors would like to thank University Grants Commission, New Delhi, and Council of Scientific and Industrial Research, New Delhi, for awarding fellowship to Anita Bhoi under Research Fellowship [F.No. 16-6(DEC. 2018)/2019(NET/CSIR), dated July 24, 2019]. The authors would also like to thank Pt. Ravishankar Shukla University, Raipur, and University Grants Commission, New Delhi, for awarding fellowship to Jipsi Chandra under Research Fellowship (No. 79/8/Fin.Sch/2014, dated 16.04.14) and National Fellowship for students of Other Backward Classes (F./2016-17/NFO-2015-17-OBC-CHH-27902) respectively.
Funding
The authors would like to thank University Grants Commission, New Delhi, and Council of Scientific and Industrial Research, New Delhi, for awarding fellowship to Anita Bhoi under Research Fellowship [F.No. 16–6(DEC. 2018)/2019(NET/CSIR), dated July 24, 2019]. The authors would also like to thank Pt. Ravishankar Shukla University, Raipur, and University Grants Commission, New Delhi, for awarding fellowship to Jipsi Chandra under Research Fellowship (No. 79/8/Fin.Sch/2014, dated 16.04.14) and National Fellowship for students of Other Backward Classes (F./2016–17/NFO-2015–17-OBC-CHH-27902) respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors that they have no conflict of interest.
Additional information
Communicated by Gerhard Leubner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhoi, A., Yadu, B., Chandra, J. et al. Contribution of strigolactone in plant physiology, hormonal interaction and abiotic stresses. Planta 254, 28 (2021). https://doi.org/10.1007/s00425-021-03678-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03678-1