Abstract
Main conclusion
The recombinant caffeic acid 3-O-methyltransferase gene has been cloned and characterized from Neem. The gene is involved in ferulic acid biosynthesis, a key intermediate component of lignin biosynthesis.
Abstract
Azadirachta indica (Neem) is a highly reputed traditional medicinal plant and is phytochemically well-known for its limonoids. Besides limonoids, phenolics are also distinctively present, which add more medicinal attributes to Neem. Caffeic acid is one of such phenolic compound and it can be converted enzymatically into another bioactive phytomolecule, ferulic acid. This conversion requires transfer of a methyl group from a donor to caffeic acid under the catalytic action of an appropriate methyltransferase. In this study, caffeic acid 3-O-methyltransferase gene from Neem (NCOMT) fruits has been isolated and heterologously expressed in E. coli. The recombinant NCOMT enzyme was purified, which exhibited efficient catalytic conversion of caffeic acid into ferulic acid, a highly potential pharmaceutical compound. The purified recombinant enzyme was physico-kinetically characterized for its catalysis. The analysis of tissue-wide expression of NCOMT gene revealed interesting pattern of transcript abundance reflecting its role in the development of fruit tissues. Further, NCOMT was heterologously overexpressed in Withania somnifera and Ocimum species, to analyze its role in ferulic acid biosynthesis in planta. Thus, the study provides insight for the endogenous role of NCOMT in ferulic acid biosynthesis en route to lignin, an important structural component. To the best of our knowledge, NCOMT pertains to be the first enzyme of the secondary metabolism that has been purified and kinetically characterized from Neem. This study may also have important prospects of applications as the observation on heterologous expression of NCOMT showed its involvement in the maintenance of the in vivo pool of ferulic acid in the plants. Thus, the study involving NCOMT opens up new dimensions of metabolic engineering approaches for the biosynthesis of potential therapeutically important phytomolecules in heterologous systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
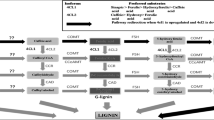
Caffeic acid 3-O-methyltransferase (COMT) belongs to the family of S-adenosyl-L-methionine (SAM) dependent O-methyltransferase and catalyzes the conversion of caffeic acid into ferulic acid, an important step in the biosynthesis of lignin. Lignin is a heteropolymer, composed of three main units p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S). The composition and structure of lignin polymer differs considerably within and among plants due to variation in monomeric ratio especially in S and G units. Ferulic acid is a major metabolic intermediate in the route to lignin biosynthesis in plants (Ou and Kwok 2004; Kumar and Pruthi 2014; Li et al. 2015). COMTs are considered as multifunctional enzymes, which can perform methylation of several substrates such as caffeic acid, coniferyl alcohols, free acids and aldehydes as well as methoxylation of other derivatives of syringyl subunits (Li et al. 2015).
There are limited reports available in respect to isolation and characterization of COMT cDNA from plant sources such as Ligusticum chuanxiong, Populus tremula, Iris hollandica, Arabidopsis thaliana, Medicago sativa etc. (Kumar and Pruthi 2014; Li et al. 2015). Arabidopsis thaliana COMT (AtCOMT) has been reported to exhibit significant N-acetylserotonin methyltransferase activity also, catalyzing the conversion of N-acetyl serotonin into its methylated product (Byeon et al. 2014). In most of the reports, the involvement of COMT enzymatic activity was shown towards the lignin biosynthesis. COMT is also known as an enzyme of commercial significance for its applications for the production of ferulic acid, a potential therapeutic and nutraceutical compound (Ou and Kwok 2004; Kumar and Pruthi 2014).
Ferulic acid is one of the most abundant phenolic acid in plants and is used as an ingredient of many drugs, in functional foods and nutraceuticals (Kumar and Pruthi 2014). As a potential therapeutic agent, ferulic acid is in the experimental stage for its potential action in the treatment of serious diseases like diabetes, cancer, neuro-degenerative, cardiovascular etc. (Ou and Kwok 2004; Kumar and Pruthi 2014). In plants, ferulic acid is rarely present in free form, as usually it is encountered in the cross linked form like cross-linked with polysaccharides, and proteins etc. Therefore, its cross-linking property is used in the preparation of complex gels in food applications (Ou and Kwok 2004; Kumar and Pruthi 2014). Ferulic acid has been approved in several countries as a food additive to prevent lipid peroxidation due to its effective scavenging activity towards free radicals (Srinivasan et al. 2007). Commercial significance of ferulic acid in pharmaceutical and food industry necessitates exploration of multiple opportunities of usage of alternative plant resources and approaches for its production.
The Azadirachta indica (Neem) is a medicinal tree well known for its characteristic triterpenoids called limonoids (tetranortriterpenoids). Besides, the plant is also reported to have notable quantities of various phenolics such as gallic acid, tannic acid, caffeic acid, ferulic acid, chlorogenic acid, quinones, quercetin, kaempferol, epicatechin and 4-caffeoryl quinic acid, hydroquinone, protocatechuic acid etc. Many of the phenolics have been shown to possess diverse biological activities such as anti-fungal, anti-feedant, anti-bacterial, anti-oxidant, anti-mutagenic and anti-carcinogenic (Singh et al. 2005; Krishnan et al. 2011; Narnoliya et al. 2014). Therefore, Neem could be a potential bioresource for the production of phenolics such as ferulic acid.
Despite diverse applications of Neem, till date there is no detailed report available on molecular attributes of any enzyme from the plant in the perspectives of biochemical/catalytic characteristics as a purified native or recombinant preparation. Recently, genome, transcriptome and ESTs data resources of Neem become available in the public domain, which could be used for the identification and elucidation of biosynthetic pathways related to secondary metabolites such as limonoids and phenolics (Krishnan et al. 2011, 2012; Narnoliya et al. 2014; Rajakani et al. 2013, 2014). Transcriptomics and genomics analysis provide information about genes and trasncripts related to specific pathways present in that particular organism which could be further used for the improvement of quality and quantity of related metabolites. Such genomics and transcriptomics data from several medicinal plants including Ocimum species, Withania somnifera (known as Ashwagandha) and Neem have become available, which provides valuable information about their important biosynthetic pathways (Narnoliya et al. 2014, 2017; Sangwan et al. 2018; Chandra et al. 2020). Our earlier studies have shown the elevated terpenoids accumulation in synchronization with the enhanced expression of genes of their metabolic pathways, using W. somnifera and Ocimum as heterologous experimental system as a proof of concept (Bansal et al. 2018).
In case of Neem, however, none of the enzymes related to either primary or secondary metabolism appears to have been kinetically characterized for their catalytic reaction till date. Therefore, the present study was aimed to isolate and characterize NCOMT from Neem as a beginning for the enzymological study of the plant and examine the impact of metabolic engineering of heterologous plant systems with Neem NCOMT. Accordingly, an important NCOMT gene was identified and cloned from A. indica fruit tissue. The recombinant NCOMT was purified from E. coli, genetically transformed to express NCOMT, was physico-catalytically characterized. Further, the investigations on the impact of transient over-expression of the gene in heterologous plants- Withania somnifera and Ocimum species (O. sanctum, O. basilicum, and O. kilimandscharicum) along with a stable transformation in O. gratissimum reflected its preeminent role in ferulic acid biosynthesis. Therefore, NCOMT may hold the potential to serve as a tool/target for the metabolic engineering for the improvement in the production of ferulic acid.
Materials and methods
Plant material and chemicals
Azadirachta indica, Withania somnifera and Ocimum species (O. sanctum, O. basilicum, O. kilimandscharicum and O. gratissimum) were grown and maintained at the experimental farm of CSIR-Central Institute of Medicinal and Aromatic Plant, Lucknow, India. Samples were immediately flash-frozen in liquid nitrogen and stored at − 80 °C until use. All the kits and chemicals were purchased from Thermo Scientific (Revert Aid cDNA synthesis kit), Applied Biosystems (SYBR green ROX master mix), Sigma (CTAB, IPTG, caffeic acid, ferulic acid, methyl jasmonate, salicylic acid), Merck (trifluoroacetic acid) and HiMedia (indole-3-acetic acid, abscisic acid, tris buffer, MgCl2).
Total RNA isolation and cDNA synthesis
Total RNA was extracted from Neem fruit tissue by the modified CTAB method (Rajakani et al. 2013), and was quantitatively as well as qualitatively analyzed by Nano Drop-1000 spectrophotometer. Thereafter, DNase treated total RNA was used for cDNA synthesis by Revert Aid cDNA synthesis kit (Thermo Scientific) according to the manufacturer’s instructions.
Isolation of full- length NCOMT cDNA from Neem fruit
Degenerate primers were used for the amplification of core fragment which was made full length using 3′ and 5′ RACE approach (Bansal et al. 2018). The full-length cDNA of NCOMT was amplified by using full-length primers, designed from the assembled NCOMT sequence and confirmed by sequencing.
Sequence analysis and 3D structure prediction
NCBI Blastx tool (http://blast.ncbi.nlm.nih.gov/Blast/) was used for searching similarities between NCOMT cDNA and other plants OMT(s) sequences present in the NCBI database. ClustalW2 tool (http://www.ebi.ac.uk/Tools/msa/clustalw2) was used for sequence alignment. The ORF (open reading frame) finder graphical analysis tool of NCBI (www.ncbi.nlm.nih.gov/projects/gorf) was used to predict the coding region of the NCOMT gene. Molecular mass and isoelectric point (pI) was determined by Compute pI/Mw tool hosted at Expasy web browser (http://cn.expasy.org/tools/protparam.html/). A 3D homology-based model was generated by Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2) using caffeic acid/5-hydroxyferulic2 acid 3/5-o-methyltransferase (PDB: c1kyzC) of Medicago sativa as a template. The generated NCOMT model was superimposed with its template using UCSF Chimera package. Enzyme binding site was predicted by 3D ligand site (http://www.sbg.bio.ic.ac.uk/~3dligandsite/). Evolutionary relationships were assessed between NCOMT and OMT(s) from other plants through constructing a phylogenetic tree by the maximum likelihood method using MEGA 5.01 software (http://www.megasoftware.net).
Heterologous expression and purification of NCOMT in E. coli
The full-length NCOMT gene was used for genetic transformation of E. coli BL21 (DE3) cells and overexpression of the gene was induced by 0.8 mM IPTG at 16 °C for an overnight duration, according to manufacturer’s instructions. Crude protein was extracted from induced culture and the recombinant protein was purified through Ni–NTA affinity column chromatography (Qiagen, Hilden, Germany). All the eluted fractions were screened for the presence of protein (A280) as well as for its catalytic activity. Concentration of purified protein preparation was determined by Bradford’s method (Bradford 1976). The purified recombinant NCOMT protein was verified by Western blotting. In brief, eluted proteins samples were subjected to SDS-PAGE and transferred the protein bands to PVDF membrane. The membrane was washed with PBS solution and transferred to the primary antibody (Anti-His antibodies, 0.5 μg ml−1) solution and later on, after washing with PBC-Tris buffer, into the solution of the secondary antibody (alkaline phosphatase enzyme-linked anti-rabbit IgG antibodies, 0.2 μg ml−1). Blotted NCOMT protein was screened for binding with anti-His antibodies which was further detected by BCIP/NBT chromogenic reaction on the membrane.
Enzymatic assay and reaction product estimation
The NCOMT standard reaction mixture consisted of 2 mM caffeic acid, 0.5 mM SAM (S-adenosyl-L-methionine) and 1 µg purified recombinant NCOMT in 100 mM Tris buffer (pH 7.5) containing 2 mM MgCl2 and 5 mM DTT (Lee et al. 2014). The reaction mixture was incubated at 37 °C for 60 min and the reaction product was extracted by ethyl acetate and analyzed by reverse-phase HPLC with a RP-C18 column (Nova-Pak, 4 mm, 3.9 × 150 mm, Waters). The reaction product was resolved using the HPLC operational parameters of methanol (A) and water (B) in the presence of 0.3% trifluoroacetic acid (TFA) as mobile phase in gradient mode with a flow rate of 0.8 ml min−1 and detection wavelength was 320 nm. The reaction products were also validated according to the method developed by Janicsak et al. (2013). Reaction mixtures lacking substrate or enzyme served as control. To study the catalytic kinetics of this enzyme, the standard assay reaction composition and conditions were altered as the specific requirement. The effect of pH on NCOMT activity was studied by using assay buffers of varied pH, and for thermostability, varied temperature range was used. The varying concentration of caffeic acid and SAM were used in the enzymatic assay to deduce Km and Vmax values for both the substrates.
Expression analysis of NCOMT transcripts in Neem tissues and under elicitor treatment
Gene-specific primers were designed for NCOMT using Beacon Designer 8.0 for the quantitative real-time PCR (qRT-PCR) analysis. Total cDNA was synthesized using 5 µg of DNase treated RNA, extracted from different tissues. Neem tissues were treated with different concentrations of various elicitors such as methyl jasmonate (MJ), salicylic acid (SA), indole-3-acetic acid (IAA), abscisic acid (ABA). Wounding (30 min, 1, 2, 4, 8, 12 and 24 h) and UV (30 min, 1, 2, and 5 h) were also used for expression analysis. Reaction mixture for qRT-PCR was prepared in a 20 µl of total reaction volume, consisting of ~ 100 ng cDNA as template, 10 µl of SYBR green ROX master mix (ABI Biosystems) and 5 pM of each gene-specific primer. Reactions for qRT-PCR were set in triplicate using cycling conditions (95 °C for 10 s for one cycle, 95 °C for 15 s and 50 °C for 1 min for 40 cycles and for melting curve analysis 95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s). The reaction was performed in a Step One™ real-time PCR system (Applied Biosystems). The expression of the gene was normalized against β-actin gene as an endogenous control. The relative gene expression levels were indicated by relative quantification (RQ) values, which were calculated following the 2−ΔΔCT method (Pfaffl 2004).
Construction of NCOMT over-expression construct in pBI121
The NCOMT was cloned in pJET1.2 cloning vector (Thermo Scientific) and then digested with BamHI and SacI restriction enzymes and ligated into pBI121 vector, predigested with BamHI and SacI restriction enzymes. The ligated pBI121-NCOMT construct was transformed into Agrobacterium tumefaciens strain EHA101 by using the freeze-thaw method and positive colonies were confirmed by PCR and sequencing. Thereafter, positive clones were used for plant transformation study as earlier (Bansal et al. 2018).
Genetic transformation of NCOMT in W. somnifera and Ocimum sp.
NCOMT was transiently over-expressed in W. somnifera and Ocimum species (O. sanctum, O. basilicum, O. kilimandscharicum and O. gratissimum) using young and healthy leaves. Stable transgenic lines were also generated in O. gratissimum using NCOMT. The genetic transformation of W. somnifera and Ocimum sp. was carried out as per the protocol of Mishra et al. (2012, 2015) and Bansal et al. (2018), respectively. Transformed tissues were used for molecular and biochemical analysis. The transgenic nature of the lines was confirmed by PCR analysis using genomic DNA as template and primer set of nptII gene. Expression of NCOMT was checked in transformed leaf tissues by real-time PCR as described above. Untransformed and empty vector transformed leaf tissues were used as controls and three independent biological experiments with triplicates were performed. For metabolite analysis, ferulic acid was extracted from transformed leaf tissues by the extraction process reported by Janicsak et al. (2013) and analyzed by HPLC as described earlier.
Statistical analysis
All the experimental data obtained are the mean of at least three independent biological replicates (enzyme assay and real-time experiments) and the results are presented with standard deviation. t-test was used to analyze significance, at P < 0.05 for (*), P ≤ 0.01 for (**) and P ≤ 0.001 for (***) with the help of GraphPaid Prism 7.03 software.
Results
Cloning of NCOMT and sequence analysis
A partial amplicon of ~ 600 bp was obtained by PCR using cDNA library of A. indica fruit tissue as a template and degenerate primers. Further, 3′ RACE and 5′ RACE primers resulted in amplification of ~ 500 bp amplicons for both sides which were assembled with partial amplicon (internal fragment) to obtain a full-length NCOMT. The full-length NCOMT contained 1284 bp with 35 bp 5′ UTR, 148 bp 3′ UTR region and 1098 bp coding region encoding a polypeptide of 366 amino acid residues. The full-length NCOMT gene sequence exhibited significant homology with O-methyl transferases (OMTs) from other plants such as Citrus aurantium (85%), Citrus sinensis (85%), Jatropha curcas (85%), Gossypium arboreum (85%), Ricinus communis (84%) and Prunus mume (84%).
The theoretical pI and molecular weight of the NCOMT protein were computed to be 6.21 and 39.93 kDa, respectively. Generally, plant OMTs contain three signature motifs referred to as S-adenosyl-l-methionine (SAM) binding, catalytic and phenolic substrate binding motifs. Multiple sequence alignment of NCOMT amino acid sequence with other plant OMTs showed the presence of all the three signature motifs (Fig. S1). The SAM binding motif [VDVGGGXG] was observed to be located in approximately middle of the protein (amino acid residues from 206 to 213), phenolic substrate binding residues in the enzyme were W (267), IMLAHN (320 to 325) and the catalytic site residues were His (270), Glu (298) and Glu (330), respectively.
Molecular modeling and evolutionary analysis
A 3D homology-based protein model was generated using caffeic acid/5-hydroxyferulic2 acid 3/5-O-methyltransferase (PDB: c1kyzC) as a template (Fig. 1a). A total of 355 (97%) residues of NCOMT could be modeled with 100% confidence level by the single highest scoring template. The superimposition of NCOMT model on the template (PDB: c1kyzC) showed an almost perfect match and their spatial differences indicated its sequence variability (Fig. 1b). Binding site prediction studies revealed the involvement of at least 21 amino acid residues in the binding of a substrate (Fig. 1c). The phylogenetic tree generated through the maximum likelihood method based on the Jones-Taylor-Thornton model indicated that NCOMT clustered together with Citrus aurantium OMT (Fig. 2). Both, the genus Citrus and Azadirachta, fall under the same order Sapindales.
Homology based 3D modeling of NCOMT. a 3D structure of NCOMT predicted by Phyre2. Caffeic acid/5-hydroxyferulic2 acid 3/5-O-methyltransferase (PDB: c1kyzC) was used as a template. b Superimposition of NCOMT on the template. c Ligand binding pocket (light green) predicted by 3DLigandSite, residues involved as active site are depicted in blue. Visualizations of modeled structures were performed by UCSF Chimera package
Phylogenic analysis of Azadirachta indica caffeic acid 3-O-methyltransferase with other plants OMT proteins. Phylogeny of NCOMTs was inferred through the maximum likelihood method using MEGA 5 software. A total of 27 protein sequences used for evolutionary analysis from following plants viz Azadirachta indica (NCOMT), Citrus aurantium (ADK97702), Jatropha curcas (ACT87981), Gossypium arboretum (KHG13289), Gossypium hirsutum (ACT32029), Boehmeria nivea (ABG27066), Eucalyptus camaldulensis (ACY66932), Betula platyphylla (AGG91492), Punica granatum (AID68566), Rosa chinensis (BAC78827), Malus domestica (ABI54119), Theobroma cacao (EOY23716), Fragaria x ananassa (AAF28353), Pyrus x bretschneideri (AGS44640), Populus tomentosa (AFZ78575), Caragana korshinskii (AEV93478), Glycine max (AEI54336), Medicago truncatula (AES72647), Glycine soja (KHN23296), Medicago sativa (ACY06328), Linum usitatissimum (AGO50639), Leucaena leucocephala (ABS57468), Arabidopsis lyrata (EFH40568), Arabidopsis thaliana (NP_200227), Camellia sinensis (ADN27527), Ipomoea nil (BAE94403) and Salvia miltiorrhiza (AEO14870)
Recombinant NCOMT protein purification and catalytic activity
The recombinant pET28a-NCOMT construct was expressed heterologously in E. coli BL21 (DE3) cells as an N-terminal polyhistidine-tagged protein. The recombinant NCOMT protein was extracted from IPTG induced cultures and confirmed on 12% SDS-PAGE. Further, Ni–NTA eluted recombinant enzyme preparation was observed to be homogeneous by its state of purity as revealed by a single polypeptide (~ 40 kDa). The results from Western blotting using anti His-antibody also confirmed the enzyme as a monomeric protein (Fig. S2). The recombinant NCOMT protein was assayed for its catalytic attributes with respect to the acceptance of caffeic acid as an acceptor substrate for methyl group transfer from SAM. Ferulic acid as a product of the catalytic reaction was identified through HPLC (Fig. 3) as well as TLC (Fig. S3).
Kinetic attributes of NCOMT
The catalytic activity of NCOMT was analyzed at various pH and temperatures using caffeic acid as substrates (Fig. 4a, b). The highest activity was observed at pH 7.5 with 100 mM Tris–Cl as assay buffer (Fig. 4a). NCOMT showed maximum activity at 37 °C and only 40% activity was remained at 50 oC. Although 30% catalytic activity was retained at 60 °C and at 70 °C, activity was completely lost. For determining, the thermo-stability, temperature-based assays were performed by incubating the protein at various temperatures for 30 min. The catalytic activity was substantially retained till incubation at 60 °C whilst it was completely lost at 70 °C (Fig. 4b).
Kinetic parameters of recombinant NCOMT enzyme using caffeic acid as substrate and SAM as co-substrate. a Effect of pH on NCOMT activity. b Effect of temperature on NCOMT activity. c Double reciprocal plots of substrate saturation kinetics for substrate. d Double reciprocal plots of substrate saturation kinetics for cofactor. [t-test was used to analyze significance, at P < 0.05 for (*), P ≤ 0.01 for (**) and P ≤ 0.001 for (***) with the help of GraphPaid Prism 7.03 software.]
The substrate saturation curve of the enzyme was hyperbolic for caffeic acid but with a slight lag of activity response at an initial lower concentration. Yet the substrate saturation curve of the enzyme for SAM was normal hyperbolic. Thus, the enzyme followed almost normal Michaelis–Menten kinetics. The Km, Vmax and kcat values of NCOMT enzyme with caffeic acid and SAM were determined via double reciprocal plot (Fig. 4c, d). The Km values of NCOMT for caffeic acid and SAM were observed to be 7.14 mM and 208 µM, respectively, while the kcat values for the two substrates were computed to be 256.32 and 44.32 s−1, respectively. The Vmax values for caffeic acid and SAM were 7.69 and 1.42 µM min−1 mg−1, respectively. The kinetic parameters of the NCOMT catalyzed reaction gave an estimate of catalytic efficiency (kcat/Km) of the recombinant enzyme as 3.59 × 104 and 2.13 × 105 M−1 s−1 toward caffeic acid and SAM, respectively.
NCOMT expression analysis
The pattern of NCOMT gene expression was analyzed by qRT-PCR in Neem tissues, different developmental stages of fruit and different fruit parts (Fig. 5a–c). Results revealed that NCOMT was constitutively expressed at high level in fruit tissues followed by stem and leaf tissues. The expression of NCOMT was much higher in fruit tissues than in buds. Stem, leaf and flower tissues have 28, 4.5 and 2-folds higher transcript abundance than bud tissue (Fig. 5a). Expression of NCOMT was continuously increased from immature green to mature ripen yellow stage of fruit development. NCOMT transcript abundance was almost 2, 3 and 10 fold higher in mature green, half ripen yellow green and fully ripen yellow fruit in comparison to immature green fruits (Fig. 5b). Elevated expression levels of NCOMT were observed in tegmen tissue, followed by endocarp and cotyledons. Interestingly, fruit mesocarp, epicarp and embryo showed a similar pattern having lower transcript abundance (Fig. 5c).
Real-time expression profile representing the transcript abundance of NCOMT. a Expression profile in different tissues. b Expression profile in different developmental stages of fruit. c Expression profile in different fruit parts. BU bud; FL flower; SE fruit; LE leaf; ST Stem; NF1 immature green fruit; NF2 mid mature green fruit; NF3 mature green fruit; NF4 half ripen yellow green fruit; NF5 fully ripen yellow fruit; EP epicarp; ME mesocarp; TE tegmen; EN endocarp; CO cotyledons and EM embryo. [t-test was used to analyze significance, at P < 0.05 for (*), P ≤ 0.01 for (**) and P ≤ 0.001 for (***) with the help of GraphPaid Prism 7.03 software.]
Effect of elicitors on NCOMT transcript profiles
The exposure of fruit to different concentrations of MJ and SA led to up regulation of NCOMT transcripts (Fig. 6a, b). The exposure to wounding was unable to alter the transcript levels of NCOMT (Fig. 6c). Under UV stress conditions, the levels of NCOMT transcript was increased up to threefold after 30 min and subsequently reached up to a maximum level of 5.5-folds after 1 h of exposure. Extended UV exposure for 2 h exhibited a slight decline in NCOMT transcript levels (4.5-fold) and after 5 h of exposure it remained about 4-fold (Fig. 6d). The abundance of NCOMT transcript was up regulated after IAA treatment. The expression was increased up to 20-fold at 100 μM IAA and at 200 μM IAA, the expression level was raised up 50-fold. The maximum transcript abundance (300-fold) was observed at 500 μM concentration of IAA (Fig. 6e). However, the NCOMT transcript was unaffected after ABA treatment (Fig. 6f).
Real-time expression profile representing the transcript abundance of NCOMT in elicitor treated fruits. a Methyl jasmonate (MJ). b Salicylic acid (SA). c Wounding. d UV treatment. e Auxin (IAA) treatment. f Abscisic acid (ABA) treatment. All experiments were performed in triplicate with three biological repeats
Over-expression of NCOMT
NCOMT was cloned in pBI121 plant expression vector and the gene construct was used for transient transformation by using leaf tissues of W. somnifera as well as different species of Ocimum (O. sanctum, O. basilicum, O. kilimandscharicum and O. gratissimum). Stable transgenic lines of O. gratissimum, containing NCOMT gene were generated and confirmed by the presence of the nptII gene through PCR analysis. In the present study, three independent lines of the transiently transformed leaf tissues and five lines of stable transformed leaf tissues were examined. Overexpression of NCOMT gene in W. somnifera was confirmed by real-time PCR, suggesting 2- to 2.5-fold higher expression, and transformed Ocimum sp. also have ~ 4–14-folds enhanced transcript levels compared to controls (Fig. 7a, b). The metabolite analysis was performed through estimation of the ferulic acid content in transgenic W. somnifera leaves as well as in Ocimum sp. leaves (O. sanctum, O. basilicum, O. kilimandscharicum and O. gratissimum). About up to ~ 1.5–2.5 fold increase was noted for ferulic acid content in transformed leaf tissues of W. somnifera, O. sanctum, O. basilicum, O. kilimandscharicum and O. gratissimum as compared to un-transformed leaf tissues (Fig. 7c, d).
Analysis of transient and stable transformed NCOMT in Ocimum species and W. somnifera. a Expression analysis of gus gene in stable lines of O. gratissimum. b Expression analysis of gus gene in transiently transformed tissues from different Ocimum species and W. somnifera. c Estimation of ferulic acid in stable transgenic lines of O. gratissimum. d Estimation of ferulic acid in transiently transformed tissues from different Ocimum species and W. somnifera. C untransformed control; V empty vector transformed control; G NCOMT gene transformed tissue; S1–S5 Stable lines of O. gratissimum; OB O. basilicum; OS O. sanctum; OK O. kilimandscharicum; OG O. gratissimum; WS Withania somnifera. [t-test was used to analyze significance, at P < 0.05 for (*), P ≤ 0.01 for (**) and P ≤ 0.001 for (***) with the help of GraphPaid Prism 7.03 software.]
Discussion
COMT enzyme converts caffeic acid into ferulic acid, a key component of lignin. Ferulic acid is also used in pharmaceuticals, nutraceuticals and food industries. It has been reported to exhibit a notable range of biomedical activities such as antioxidant, antidiabetic, anticarcinogenic, antiallergic, antimicrobial, antiviral, antiaging, vasodilator, hepatoprotective, anti-inflammatory, antithrombotic etc. (Srinivasan et al. 2007; Kumar and Pruthi 2014). It is also used as a preclusion agent for food discoloration, a growth enhancer as well as a precursor of vanillin, the high demand flavoring agent (Kumar and Pruthi 2014). Natural sources of ferulic acid are limited, therefore it is desired to explore and examine other sources/approaches of its production. In this study, an O-methyl transferase named NCOMT was isolated and characterized from Neem, which exhibited significant potential for the biogeneration of ferulic acid from caffeic acid. The NCOMT gene, isolated from cDNA library of A. indica fruit, contains 1098 bp open reading frame encoding polypeptide of 366 amino acids. Other plants OMT such as L. chuanxiong, Vanda mimi Palmer, Iris hollandica have 362, 367, 365 amino-acids and 5.94, 5.74, 5.54 isoelectric points, respectively, which is quite close to the NCOMT isoelectric point (Yoshihara et al. 2008; Aiman et al. 2015; Li et al. 2015). A significant sequence similarity (> 80%) of NCOMT confirms it as caffeic acid O-methyltransferase gene (COMT). Generally, plant O-methyltransferases possess three signature motifs as SAM-binding, catalytic and phenolic substrate binding motifs (Kim et al. 2006; Byeon et al. 2015). Multiple sequence alignment of NCOMT with other plants OMT sequences indicates the presence of all three-signature motifs in appropriate manner. The SAM binding motif situated at the middle position and phenolic substrate binding and catalytic site binding motifs are present at the C terminal of NCOMT sequence. All these motifs are identical to other plants COMT enzymes, and strongly suggest this as caffeic acid O-methyltransferase enzyme. Previous reports indicate that COMT(s) lack any signal sequence for subcellular localization and are localized in the cytosol such as rice COMT, Vanda Mimi Palmer OMT, Arabidopsis OMT (Lee et al. 2014; Aiman et al. 2015; Byeon et al. 2015).
Homology modeling suggests that NCOMT model is perfectly matched with caffeic acid/5-hydroxyferulic2 acid 3/5-O-methyltransferase model of Medicago sativa (PDB: c1kyzC) whose crystal structure is already available in PDB database. These results strongly indicated that the NCOMT protein may also be functional in accordance with the Medicago sativa COMT protein. Binding site prediction analysis proposes participation of almost 21 residues as a functional substrate binding site. It was previously reported in Medicago sativa that Lys-265, Asp-206, Asp-231 and Asp-251 are the key residues, which are involved in SAM binding (Zubieta et al. 2002). Here, in the present investigation, we also observed a similar structural composition in predicted substrate binding site as Lys-266, Asp-207, Asp-232 and Asp-252, which strongly supports NCOMT enzyme as kinetically active. Evolutionary relationship of NCOMT with other COMT protein sequences clustered it with Citrus aurantium OMT, which falls under the same order Sapindales.
Homogeneity of the purified recombinant NCOMT protein was confirmed by SDS-PAGE and Western-blot analysis. Purified NCOMT protein was used for enzyme assay to confirm the reaction product as ferulic acid. NCOMT enzyme follows the Michaelis–Menten kinetics and displays a hyperbolic substrate saturation curve for its substrate as well as cofactor. The Km, Vmax and kcat values of the NCOMT enzyme with caffeic acid and SAM are quite comparable with other plants COMT enzymes. Previous studies on OMTs reported an elevated Km with significant catalytic efficiency like OMT-II; 1.1 and OMT-II; 2.2 enzyme from Thalictrum tuberosum, catalyzing caffeic acid at Km of 0.6 and 1.1 mM, respectively (Frick and Kutchan 1999). Several other studies have revealed that COMT(s) were also able to methylate N-acetylserotonin (Byeon et al. 2014). Thus, COMT may catalyze multiple reactions with significant catalytic efficiency as shown in Arabidopsis: it catalyzes methylation of caffeic acid and N-acetylserotonin with Km values of 103 and 233 µM, respectively (Byeon et al. 2014, 2015). In another report, it was shown that A. thaliana caffeic acid O-methyltransferase (AtCOMT) enzyme converted N-acetylserotonin into 5-methoxytryptamine with low catalytic activity having a Km of 3.996 mM (Lee et al. 2014). Therefore, COMT is a multifunctional enzyme and able to catalyze methylation of an array of substrates including phenolics, flavonoids, and aryl alkylamines (Byeon et al. 2014).
The COMT enzyme exhibited a wide range of catalytic efficiency (kcat/Km) ranging from mM−1 s−1 to nM−1 s−1. The COMT enzyme of sweet basil exhibited 0.27 nM−1 s−1 catalytic efficiency (Gang et al. 2002), VanOMT3 isolated from Vanilla planifolia 0.6 mM−1 s−1 (Li et al. 2006), COMT of Clarkia breweri 9.93 nM−1 s−1 (Wang and Pichersky 1998), and a much higher efficiency was shown for Rauvolfia serpentina OMTs (Wiens and Luca 2016). The higher catalytic efficiency made NCOMT to be a catalytically efficient enzyme.
Optimum NCOMT activity is recorded at pH 7.5, which is equivalent to OMT(s) from other plants such as sweet basil and Iris hollandica OMT; both have maximal enzyme activity at pH 7.5–8.0 (Gang et al. 2002; Yoshihara et al. 2008). Recently, two OMT(s) characterized from R. serpentina roots showed significant variation in pH optima. RsOMT1 showed maximum enzymatic activity between pH 7.5–10 while RsOMT3 exhibited between 6.5–8.0 pH (Wiens and Luca 2016). Thermo-stability results of NCOMT exhibited 37 °C as its optimum temperature. At higher temperature, its enzyme activity gradually decreases and at 70 °C, activity is completely lost. Previous studies of COMT suggest that it remains active at 60 °C, at higher temperatures integrity of enzyme is destroyed and unable to perform catalytic conversion. The OMT(s) characterized by R. serpentina lost their catalytic activity above 60 °C (Wiens and Luca 2016). Reports of LcCOMT enzyme isolated from Ligusticum chuanxiong had its optimum pH at 7 and temperature at 37 ℃ (Li et al. 2015).
Abundance of NCOMT transcripts was determined in tissues, fruit ontogeny, fruit parts and elicitor treated fruits. Fruit ontogeny specific expression pattern of NCOMT reveals that continuous increment in expression from immature to fully mature (ripen) fruits. When expression was analyzed within fruit parts, it showed interesting results. The tegmen part of fruit had a maximal level of expression followed by endocarp and cotyledons, but, interestingly, mesocarp, epicarp and embryo exhibited very low expression. This indicates that NCOMT has a tissue-specific role in Neem plants.
The signaling molecules, MJ and SA, are known for modulating the expression of genes involved in defense responses including secondary metabolites. Treatment of MJ and SA modulators resulted in the induction of NCOMT gene expression. These observations are in agreement with earlier reports of the application of MJ treatment for increasing phenolics production. Elevated level of COMT expression was observed in Hibiscus cannabinus after exposure of MJ and SA (Kim et al. 2013).
In general, wounding induced genes which were implicated in lignin biosynthesis but the caffeoyl- coenzyme A 3-O-methyltransferase gene from switchgrass displayed similar transcript abundance in leaf and stem tissue after exposing to wounding stress (Liu et al. 2016). Here, wounding exposure was unable to alter the expression level of NCOMT. UV stress slightly increased the level of NCOMT transcripts but IAA up-regulated notable transcript levels. ABA treatment had similar results as wounding and was not able to enhance remarkable abundance. Ligusticum chuanxiong OMT showed enhanced expression under different stress conditions (Li et al. 2015).
The most common function of COMT enzyme is biosynthesis of ferulic acid, an intermediate component of lignin biosynthesis. Although it exhibits maximal enzyme activity toward caffeic acid substrate, it can significantly convert N-acetylserotonin into melatonin. This is also confirmed by in vitro experiments in Arabidopsis and rice (Lee et al. 2014; Byeon et al. 2014, 2015). Several reports are available exhibiting the significance of the COMT enzyme in the regulation of lignin content. Down regulation of Brachypodium distachyon COMT reduced total lignin quantity, thus enhanced ethanol yield observed from plant biomass (Trabucco et al. 2013). Knocking out of Arabidopsis COMT results in a lower production of melatonin compared to wild type, suggesting its involvement in melatonin pathway as well (Byeon et al. 2014).
Ferulic acid profiling was performed in different tissues of Neem. Several reports are available showing production of ferulic acid in varying range in plant tissues (Kumar and Pruthi 2014). Ferulic acid was not detected in flowers of Neem, yet a substantial level (0.38–1.16 µg g−1 fresh weight) was observed in fruit parts, raw fruit (green fruit) epicarp, mesocarp and seed (Singh et al. 2005). These results were quite comparable with the results on NCOMT transcripts abundance, which reveals minimal expression in epicarp followed by mesocarp (pulp) and maximal in seed parts (tegmen, endocarp, cotyledons and embryo) of green fruits. Singh et al. (2005) reported maximal yield of ferulic acid in ripen fruit (18.51 µg g−1 fresh weight), which is approximately 8.8-fold higher than in raw green fruit (2.09 µg g−1 fresh weight). The expression of NCOMT is about ten fold higher in ripen yellow fruits in comparison to unripe green fruits. Thus, NCOMT expression profile clearly indicates that the maturation of flowers is accompanied by a higher requirement of ferulic acid content as observed by Singh et al. (2005) in Neem tissues, probably for cellular multi-functionality, especially for lignin biosynthesis.
NCOMT was transiently overexpressed in W. somnifera leaf tissue and its expression pattern was observed to be almost similar with ferulic acid content. Over-expressed W. somnifera leaf tissue contained a higher levels of NCOMT expression as well as ferulic acid as compared to untransformed controls. To probe the functional role of NCOMT in ferulic acid biosynthesis, it was transiently transformed in Withania somnifera and Ocimum species (O. sanctum, O. basilicum, O. kilimandscharicum and O. gratissimum) and stably transformed in O. gratissimum, to evaluate its over-expression impact on ferulic acid content. In this study, ferulic acid could be enhanced up to ~ 1.5–2.5 fold in transgenic lines of heterologous tissues. It is in agreement with previous reports exhibiting enhanced levels of ferulic acid or lignin in transgenic plants, transformed by COMT gene (Oraby and Ramadan 2015). Silencing of COMT genes of Brassica napus resulted in the reduction of the lignin content without affecting seed oil composition (Oraby and Ramadan 2015). Other COMTs were also involved in catalytic conversion of important molecules as shown earlier (Byeon et al. 2014, 2015). Here we report the first gene from the reputed medicinal plant Neem, NCOMT, which was cloned and physico-kinetically characterized. It was shown that NCOMT was involved in ferulic acid biosynthesis from caffeic acid, a key step in lignin biosynthesis. It could also serve as a new source for the biosynthesis of ferulic acid, a therapeutic agent (Srinivasan et al. 2007). In the present study, we demonstrated that metabolic engineering can be used to improve the production of ferulic acid. We discussed two approaches for ferulic acid production from caffeic acid using the COMT enzyme: first through isolation of the recombinant enzyme from engineered microbes (E. coli), followed by enzymatic assay in an in-vitro system, and second through the genetic transformation of the NCOMT gene in suitable plants (Withania somnifera and Ocimum sp.). This study provides insights into the ferulic acid production which plays a major role in lignin biosynthesis.
Conclusions
In this study, a NCOMT gene of 1098 bp was identified, isolated and cloned from A. indica fruit tissues. NCOMT was heterologously expressed in E. coli and the purified recombinant protein catalyzed the conversion of caffeic acid into ferulic acid. In-silico structural analysis of NCOMT suggested that the 3D structure of NCOMT is closely similar to OMT from Medicago sativa. Further, the NCOMT enzyme was kinetically characterized. It exhibited a significant catalytic efficiency (kcat/Km). The observed optimum pH was 7.5 and temperature was 37 ℃ for NCOMT with caffeic acid as substrate. The transcript abundance of NCOMT in different Neem tissues revealed maximal expression in fruit tissues, followed by fully mature fruit and tegmen tissues, respectively. The elevated level of NCOMT expression was observed in most of the elicitor treated fruit tissues. For in-planta functional characterization of NCOMT, it was transiently over-expressed in W. somnifera and Ocimum species (O. sanctum, O. basilicum, O. kilimandscharicum and O. gratissimum), and stable transforments of O. gratissimum were generated. The transformed tissues were able to accumulate an enhanced ferulic acid content with enhanced levels of NCOMT expression as compared to untransformed tissues.
Author contributions statement
RSS and NSS planned the experiments and prepared the manuscript. LKN, JSJ and SB were involved in performing the experiments and in the compilation of data. All the authors critically read the paper and approved.
Abbreviations
- COMT:
-
Caffeic acid 3-O-methyltransferase
- SAM:
-
S-adenosyl-l-methionine
- IPTG:
-
Isopropyl β-d-1 thiogalactopyranoside
- MJ:
-
Methyl jasmonate
- SA:
-
Salicylic acid
References
Aiman BM, Mohd-Hairul AR, Hian TS (2015) Sequence analysis of new isolated O-methyltransferase transcript (VMPOMT) from Vanda Mimi Palmer. Scholars Acad J Biosci 3:239–247. https://doi.org/10.1186/1472-6750-13-61
Bansal S, Narnoliya LK, Mishra B, Chandra M, Yadav RK, Sangwan NS (2018) HMG-CoA reductase from Camphor Tulsi (Ocimum kilimandscharicum) regulated MVA dependent biosynthesis of diverse terpenoids in homologous and heterologous plant systems. Sci Rep 8:3547. https://doi.org/10.1038/s41598-017-17153-z
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Byeon Y, Lee HY, Lee K, Back K (2014) Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J Pineal Res 57:219–227. https://doi.org/10.1111/jpi.12160
Byeon Y, Choi GH, Lee HY, Back K (2015) Melatonin biosynthesis requires N-acetylserotonin methyltransferase activity of caffeic acid O-methyltransferase in rice. J Exp Bot 66(21):6917–6925. https://doi.org/10.1093/jxb/erv396
Chandra M, Maurya S, Sangwan NS (2020) Comparative transcriptome analysis to identify putative genes related to trichome development in Ocimum species. Mol Biol Rep 47(9):6587–6598. https://doi.org/10.1007/s11033-020-05710-1
Frick S, Kutchan TM (1999) Molecular cloning and functional expression of O-methyltransferases common to isoquinoline alkaloid and phenylpropanoid biosynthesis. Plant J 17:329–339. https://doi.org/10.1046/j.1365-313X.1999.00379x
Gang DR, Lavid N, Zubieta C, Chen F, Beuerle T, Lewinsohn E, Noel JP, Pichersky E (2002) Characterization of phenyl-propene O-methyltransferases from sweet basil: facile change of substrate specificity and convergent evolution within a plant O-methyltransferase family. Plant Cell 14:505–519. https://doi.org/10.1105/tpc.010327
Janicsak G, Háznagy-Radnai E, Engel R, Blunden G, Máthé I (2013) TLC-densitometry of rosmarinic and caffeic acids in the evaluation of Lamiaceae species growing in central Europe. JPC- J Planar Chromatogr Modern TLC 26:132–136. https://doi.org/10.1556/JPC.26.2013.2.5
Kim BG, Lee Y, Hur HG, Lim Y, Ahn JH (2006) Flavonoid 3’ -O-methyltransferase from rice: cDNA cloning, characterization and functional expression. Phytochemistry 67:387–394. https://doi.org/10.1016/j.phytochem.2005.11.022
Kim J, Choi B, Cho BK, Lim HS, Kim JB, Natarajan S, Kwak E, Bae H (2013) Molecular cloning, characterization and expression of the caffeic acid O-methyltransferase (COMT) ortholog from kenaf (Hibiscus cannabinus). Plant Omics 6:246
Krishnan NM, Swetansu P, Deepak SA, Hariharan AK, Gaur P, Chaudhary R, Jain P, Vaidyanathan S, Krishna PB, Panda B (2011) De novo sequencing and assembly of Azadirachta indica fruit transcriptome. Curr Sci 101:1–12
Krishnan NM, Pattnaik S, Jain P, Gaur P, Choudhary R, Vaidyanathan S, Deepak S, Hariharan AK, Krishna PB, Nair J, Varghese L, Valivarthi NK, Dhas K, Ramaswamy K, Panda B (2012) A draft of the genome and four transcriptomes of a medicinal and pesticidal angiosperm Azadirachta indica. BMC Genomics 13:464–472. https://doi.org/10.1186/1471-2164-13-464
Kumar N, Pruthi V (2014) Potential applications of ferulic acid from natural sources. Biotechnol Rep 4:86–93. https://doi.org/10.1016/j.btre.2014.09.002
Lee HY, Byeon Y, Lee K, Lee HJ, Back K (2014) Cloning of Arabidopsis serotonin N-acetyltransferase and its role with caffeic acid O-methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localizations. J Pineal Res 57:418–426. https://doi.org/10.1111/jpi.12181
Li HM, Rotter D, Hartman TG, Pak FE, Havkin-Frenkel D, Belanger FC (2006) Evolution of novel O-methyltransferases from the Vanilla planifolia caffeic acid O-methyltransferase. Plant Mol Biol 61:537–552. https://doi.org/10.1007/s11103-006-0029-4
Li JJ, Zhang G, Yu JH, Li YY, Huang XH, Wang WJ, Liao H (2015) Molecular cloning and characterization of caffeic acid 3-O-methyltransferase from the rhizome of Ligusticum chuanxiong. Biotech Lett 37:2295–2302. https://doi.org/10.1007/s10529-015-1917-y
Liu SJ, Huang YH, He CJ, Cheng FANG, Zhang YW (2016) Cloning, bioinformatics and transcriptional analysis of caffeoyl-coenzyme A 3-O-methyltransferase in switchgrass under abiotic stress. J Integr Agr 15:636–649. https://doi.org/10.1016/S2095-3119(16)61363-1
Mishra S, Sangwan RS, Bansal S, Sangwan NS (2012) Efficient transgenic plant production of Withania coagulans (Stocks) Dunal mediated by Agrobacterium tumefaciens from leaf explants of in vitro multiple shoot culture. Protoplasma 250:451–458. https://doi.org/10.1007/s00709-012-0428-0
Mishra S, Bansal S, Sangwan RS, Sangwan NS (2015) Genotype independent and efficient Agrobacterium-mediated genetic transformation of the medicinal plant Withania somnifera Dunal. J Plant Biochem Biotechnol 25:191–198. https://doi.org/10.1007/s13562-015-0324-8
Narnoliya LK, Rajakani R, Sangwan NS, Gupta V, Sangwan RS (2014) Comparative transcripts profiling of fruit mesocarp and endocarp relevant to secondary metabolism by suppression subtractive hybridization in Azadirachta indica (neem). Mol Biol Rep 41:3147–3162. https://doi.org/10.1007/s11033-014-3174-x
Narnoliya LK, Kaushal G, Singh SP, Sangwan RS (2017) De novo transcriptome analysis of rose-scented geranium provides insights into the metabolic specificity of terpene and tartaric acid biosynthesis. BMC Genomics 18:74. https://doi.org/10.1186/s12864-016-3437-0
Oraby HF, Ramadan MF (2015) Impact of suppressing the caffeic acid O-methyltransferase (COMT) gene on lignin, fiber, and seed oil composition in Brassica napus transgenic plant. Eur Food Res Technol 240:931–938. https://doi.org/10.1007/s00217-014-2397-3
Ou S, Kwok KC (2004) Ferulic acid: pharmaceutical functions, preparation and applications in foods. J Sci Food Agric 84:1261–1269. https://doi.org/10.1002/jsfa.1873
Pfaffl MW (2004) Quantification strategies in real-time PCR. In: Bustin SA (ed) The real-time PCR encyclopedia A-Z of quantitative PCR. International University Line, La Jolla, CA, USA, pp 87–112
Rajakani R, Narnoliya L, Sangwan NS, Sangwan RS, Gupta V (2013) Activated charcoal-mediated RNA extraction method for Azadirachta indica and plants highly rich in polyphenolics, polysaccharides and other complex secondary compounds. BMC Res Notes 6:125. https://doi.org/10.1186/1756-0500-6-125
Rajakani R, Narnoliya LK, Sangwan NS, Sangwan RS, Gupta V (2014) Subtractive transcriptomes of fruit and leaf reveal differential representation of transcripts in Azadirachta indica. Tree Genet Genomes 10:1331–1351. https://doi.org/10.1007/s11295-014-0764-7
Sangwan NS, Jadaun J, Tripathi S, Mishra B, Narnoliya LK, Sangwan RS (2018) Plant metabolic engineering. In Omics technology and bioengineering. Towards improving quality of life, Vol 2. Academic Press, ScienceDirect, USA. ISBN:978-0-12-815870-8. pp 143–175. https://doi.org/10.1016/B978-0-12-815870-8.00009-7
Singh UP, Maurya S, Singh DP (2005) Phenolic acids in neem (Azadirachta indica) a major pre-existing secondary metabolites. J Herb Pharmacother 5:35–43. https://doi.org/10.1080/J157v05n01_05
Srinivasan M, Sudheer AR, Menon VP (2007) Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 40(2):92–100
Trabucco GM, Matos DA, Lee SJ, Saathoff AJ, Priest HD, Mockler TC, Sarath G, Hazen SP (2013) Functional characterization of cinnamyl alcohol dehydrogenase and caffeic acid O-methyltransferase in Brachypodium distachyon. BMC Biotechnol 13:61. https://doi.org/10.1186/1472-6750-13-61
Wang J, Pichersky E (1998) Characterization of s-adenosyl-l-methionine:(iso) eugenolo-methyltransferase involved in floral scent production in Clarkia breweri. Arch Biochem Biophys 349(1):153–160
Wiens B, De Luca V (2016) Molecular and biochemical characterization of a benzenoid/phenylpropanoid meta/para-O-methyltransferase from Rauwolfia serpentina roots. Phytochemisty 132:5–15. https://doi.org/10.1016/j.phytochem.2016.10.004
Yoshihara N, Fukuchi-Mizutani M, Okuhara H, Tanaka Y, Yabuya T (2008) Molecular cloning and characterization of O-methyltransferases from the flower buds of Iris hollandica. J Plant Physiol 165:415–422. https://doi.org/10.1016/j.jplph.2006.12.002
Zubieta C, Kota P, Ferrer JL, Dixon RA, Noel JP (2002) Structural basis for the modulation of lignin monomer methylation by caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase. Plant Cell 14:1265–1277. https://doi.org/10.1105/tpc.001412
Acknowledgements
RSS and NSS are thankful to HCP-0010, MLP-5 and BSC-203 CSIR network project for providing financial assistance. LKN, JSJ and SB are thankful to University Grants Commission and CSIR for senior research fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Soheil S Mahmoud.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2020_3514_MOESM1_ESM.tif
Supplementary file1 Fig. S1 Multiple sequence alignment of the deduced amino acid sequences of Azadirachta indica COMT and other plants OMT. Citrus aurantium (ADK97702), Jatropha curcas (ACT87981), Eucalyptus camaldulensis (ACY66932), Rosa chinensis (BAC78827), Gossypium hirsutum (ACT32029), Malus domestica (ABI54119), Theobroma cacao (EOY23716), Fragaria x ananassa (AAF28353), Populus tomentosa (AFZ78575), Medicago truncatula (AES72647), Arabidopsis thaliana (NP_200227), Camellia sinensis (ADN27527) and Salvia miltiorrhiza (AEO14870). Sequence in black box represents the S-adenosyl-L-methionine (SAM) binding motif and red box represents phenolic substrate binding residues (TIF 1431 KB)
425_2020_3514_MOESM2_ESM.tiff
Supplementary file 2 Fig. S2 Western-blot analysis of Ni-NTA purified NCOMT protein. Lane M Pre-stained protein molecular marker; W1 Ni NTA purified fraction 1; W2 Ni NTA purified fraction 2 (TIFF 348 KB)
425_2020_3514_MOESM3_ESM.tif
Supplementary file3 Fig. S3 NCOMT enzyme assay validation through TLC. CA caffeic acid; FA ferulic acid; C1 control assay without substrate; C2 control assay without protein, E1 and E2 enzyme assay (TIF 245 KB)
Rights and permissions
About this article
Cite this article
Narnoliya, L.K., Sangwan, N., Jadaun, J.S. et al. Defining the role of a caffeic acid 3-O-methyltransferase from Azadirachta indica fruits in the biosynthesis of ferulic acid through heterologous over-expression in Ocimum species and Withania somnifera. Planta 253, 20 (2021). https://doi.org/10.1007/s00425-020-03514-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03514-y