Abstract
Main conclusion
Overexpression ofPeVQ28in Arabidopsis regulated the expression of salt/ABA-responsive genes and indicated thatPeVQ28may affect the ABA synthesis induced by stress in plants by regulating salt tolerance.
Abstract
Plant-specific VQ proteins, which contain a conserved short FxxhVQxhTG amino acid sequence motif, play an important role in abiotic stress responses, but their functions have not been previously studied in Moso bamboo (Phyllostachys edulis). In this study, real-time quantitative PCR analysis indicated that expression of PeVQ28 was induced by salt and abscisic acid stresses. A subcellular localization experiment showed that PeVQ28 was localized in the nuclei of tobacco leaf cells. Yeast two-hybrid and bimolecular fluorescence complementation analyses indicated that PeVQ28 and WRKY83 interactions occurred in the nucleus. The PeVQ28-overexpressing Arabidopsis lines showed increased resistance to salt stress and enhanced sensitivity to ABA. Compared with wild-type plants under salt stress, PeVQ28-transgenic plants had lower malondialdehyde and higher proline contents, which might enhance stress tolerance. Overexpression of PeVQ28 in Arabidopsis enhanced expression of salt- and ABA-responsive genes. These results suggest that PeVQ28 functions in the positive regulation of salt tolerance mediated by an ABA-dependent signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The environment has an important impact on plant growth and development. Numerous environmental factors have negative, and even harmful, effects on plants. Such factors include biotic stresses, for example, diseases, insect pests, and weeds, and abiotic stresses, such as drought, salinity, and high and low temperatures (Ingram and Bartels 1996; Katiyaragarwal. 2006; Yamaguchi-Shinozaki and Shinozaki 2006). Among the abiotic stresses, drought, salinity, and low temperature have the greatest influence on plants (Xiong et al. 2002; Jakab et al. 2005). Plants have evolved numerous mechanisms to enable adaption to detrimental environmental changes. During these adaption periods, plants regulate their response to stress through the regulation of the osmotic balance, synthesis of stress-associated proteins and antioxidants, and expression of characteristic genes (Shinozaki and Yamaguchi-Shinozaki 1997; Zhu 2002). In particular, valine–glutamine (VQ) motif-containing proteins are indicated to be involved in plant responses to abiotic stresses (Perruc et al. 2004; Lai et al. 2011; Hu et al. 2013b; Kim et al. 2013; Song et al. 2016; Wang et al. 2017).
The plant-specific VQ proteins are of increasing interest owing to their interactions with WRKY transcription factors (Lai et al. 2011). At present, 34, 40, 61, 18, and 74 VQ members have been identified in Arabidopsis, rice (Oryza sativa), maize (Zea mays), grape (Vitis vinifera), and soybean (Glycine max), respectively (Cheng and Chen 2012; Li et al. 2014; Wang et al. 2014, 2015b; Song et al. 2016). The VQ proteins are structurally characterized by five highly conserved amino acids in the core sequence FxxxVQxLTG, where x represents any amino acid and VQ are the highly conserved amino acid residues (Pecher et al. 2014). Recently, it was observed that the last three amino acids in the conserved domain may be of other types besides LTG, such as FTG, ITG, LTA, and VTG (Kim et al. 2013).
As a transcriptional regulator, VQ proteins can interact with a variety of proteins to regulate several physiological and biochemical processes in plants. The WRKY transcription factors are the most important interactors with VQ proteins (Wu et al. 2017). For example, AtVQ15 interacts with WRKY25 and WRKY51 to regulate plant tolerance to salt and osmotic stress (Perruc et al. 2004). In addition, transient expression in protoplasms of Arabidopsis leaves revealed that most VQ members are capable of interacting with each other (Wang et al. 2015a).
The transcriptional expression of VQ genes is induced or inhibited by salt, drought, low nitrogen, temperature stress, and ABA, indicating that the VQ protein family plays an important role in the regulation of plant responses to abiotic stress (Hu et al. 2013b; Wang et al. 2014, 2015b). In an Arabidopsis study (Hu et al. 2013b), AtVQ9 (AtMVQ10) mutants showed a higher germination rate and superior seedling growth under NaCl treatment, whereas the reverse was true in overexpression plants (Perruc et al. 2004). These results indicate that AtVQ9 is involved in the negative regulation of Arabidopsis resistance to NaCl stress. Perruc et al. (2004) observed that AtVQ15 (AtCAMBP25) overexpression lines were highly sensitive to NaCl and osmotic stress during seed germination and seedling growth, whereas the mutant showed enhanced resistance. Thus, AtVQ15 might negatively regulate the tolerance of Arabidopsis to NaCl and osmotic stresses. In maize and rice, ZmVQ19 and ZmVQ54, and OsVQ2, OsVQ16, and OsVQ20, respectively, were highly expressed during drought induction (Wang et al. 2015b). In soybean, GmVQ6 and GmVQ53 were highly expressed in roots and stems under low-nitrogen conditions, which indicated that these genes affected growth and development (Wang et al. 2014). Collectively, these results suggest that VQ proteins play important roles in plant growth and responses to environmental stresses.
The majority of functional studies of VQ genes have been conducted on Arabidopsis. In our previous study, 29 VQ proteins were identified in Moso bamboo (Phyllostachys edulis) and were classified in seven subfamilies on the basis of phylogenetic relationships (Wang et al. 2017). As an extension of our previous research, in the present study we characterized the function of PeVQ28. The PeVQ28 cDNA was isolated from Moso bamboo and transformed into Arabidopsis by Agrobacterium-mediated methods to obtain PeVQ28-overexpressing Arabidopsis plants. The present results will aid in elucidating the molecular mechanism of PeVQ28 and provide a theoretical reference for breeding of stress resistance in Moso bamboo.
Materials and methods
Experimental materials, growth conditions, and stress treatment
Seedlings of Moso bamboo (Phyllostachys edulis (Carrière) J.Houz.) were cultivated in a greenhouse [24 °C, 16 h/8 h (light/dark) photoperiod, and 80% relative humidity]. The Moso bamboo seeds were collected from the Tianmu Mountain National Nature Reserve in Zhejiang Province, China. The growing medium was a mixture of black soil and vermiculite (3:1, v/v) (Wu et al. 2015; Wang et al. 2016; Chen et al. 2017; Gao et al. 2017; Liu et al. 2017). For application of stress treatments, the soil mixture was irrigated with either 100 μM ABA (Kim et al. 2013) or 200 mM NaCl solutions (Zhao et al. 2012). Leaves were harvested at 0, 1, 3, 6, 12, and 24 h after treatment, respectively. In addition, untreated root (R), stem (St), young leaf (YL), leaf (L), shoot (S), and rhizome (Rh) samples were immediately frozen in liquid nitrogen and stored at − 80 °C for RNA extraction, and roots were used as comparators.
Seeds of tobacco (Nicotiana tabacum L.), wild-type (WT) Arabidopsis [Colombia (Col-0) ecotype], and three T3 transgenic lines (L1, L3, and L10) were incubated in Murashige and Skoog (MS) medium at 4 °C for 3 d to undergo vernalization, then transferred to a greenhouse [24 °C, 16 h/8 h (light/dark), 80% relative humidity] for 10 d, and transplanted into square pots (12 cm diameter) containing a mixture of black soil and vermiculite (1:3, v/v). Two- to three-week-old seedlings were used for the experiments.
Isolation of PeVQ28 and generation of Arabidopsis transgenic lines
The 513 bp coding sequence of PeVQ28 was cloned using the gene-specific primers 5′-ATGGGGGAGTACCACAGAATGA-3′ (forward) and 5′-TTAAGATGCGTACATTTCACCAACC-3′ (reverse).
The SmaI and SalI restriction sites were inserted upstream and downstream, respectively, of the coding sequence of PeVQ28. The constructs were transformed into Arabidopsis Col-0 plants by Agrobacterium-mediated transformation using a previously described method (Wu et al. 2017).
RNA isolation and RT-qPCR analysis of gene expression
Total RNA was extracted as described previously (Wang et al. 2017). The PeVQ28 cDNA was synthesized using PrimeScript™ RT Master Mix (Takara, Tokyo, Japan) in accordance with the manufacturer's instructions. The PeVQ28 gene-specific primers and ABA-associated gene primers were designed using Primer Express 5.0 (Table S1). Real-time quantitative PCR (RT-qPCR) was performed on an ABI 7300 real-time system in accordance with a previously reported procedure (Wang et al. 2017). Data were processed following the methods of Livak and Schmittgen (Livak and Schmittgen 2001; Zhao et al. 2012). The tonoplast intrinsic protein 41 (TIP41) was used as an internal control (Fan et al. 2013).
Subcellular localization analysis
The coding sequence of PeVQ28, lacking the stop codon, was amplified by PCR using the following primers: 5′-TGCTCTAGAATGGGGGAGTACCACA-3′ (forward, XbaI site) and 5′-CGCGGATCCAGATGCGTACATTTCA-3′ (reverse, BamHI site). The amplified fragment was inserted into the pCAMBIAI1305 vector (Clontech, Beijing, China) containing the CaMV35S promoter and the green fluorescent protein (GFP) gene to generate the p1305-CaMV35S-PeVQ28-GFP fusion expression vector. The control vector was p1305-CaMV35S-GFP. The suspension was infiltrated into tobacco leaves using an injection method and the GFP fluorescence was observed using a confocal microscope (LSM710, Carl Zeiss, Jena, Germany) (Dai et al. 2007; Cao et al. 2016).
Yeast two-hybrid and bimolecular fluorescence complementation (BiFC) analyses
The coding regions of PeVQ28 and PeWRKY83 (Table S2), which contained the EcoRI and BamHI restriction enzyme sites, were amplified by PCR. The pGBKT7 and pGADT7 vectors, and the target fragments were digested with the EcoRI and BamHI restriction enzymes, recovered, and ligated to generate the recombinant plasmid vectors pGBKT7-PeWRKY83 and pGADT7-PeVQ28. The recombinant plasmids of the experimental group (pGBKT7-PeWRKY83 + pGADT7-PeVQ28), the positive control (pGBKT7-T53 + pGADT7-T), and the negative controls (pGADT7-PeVQ28 + pGBKT7 and pGBKT7-PeWRKY83 + pGADT7) were transformed into yeast (Saccharomyces cerevisiae) strain AH109. The transformed yeast cells were plated on deficient media (SD/-Leu/-Trp or SD/-Ade/-Leu/-Trp/-His) for 3–5 days to determine protein–protein interactions.
The BiFC assay was performed as previously reported (Walter et al. 2004). Briefly, PeWRKY83 and PeVQ28 containing the XbaI and BamHI restriction enzyme sites were enzymatically digested and inserted into the pUC-SPYNE and pUC-SPYCE vectors, recovered, and ligated to obtain the recombinant plasmid vectors PeVQ28-nYFP and PeWRKY83-cYFP.
Stress resistance of transgenic Arabidopsis
First, a seed germination experiment was performed. Plants of the Arabidopsis WT and transgenic lines were cultured under identical conditions [24 °C, 16 h/8 h (light/dark), and 80% relative humidity] and seeds were collected. The seeds were germinated on plates containing MS medium supplemented with ABA (0, 0.1, 0.5, or 1 µM) or NaCl (0, 100, or 150 mM). The germination frequency was calculated after 3–5 days.
Second, the main root length of seedlings was measured. Seeds were germinated on standard MS medium following the aforementioned method, and then seedlings were transferred to MS plates containing ABA (0, 10, 30, or 50 µM) or NaCl (0, 100, 150, or 200 mM). After growth for 7 days, the main root length of each seedling was recorded.
Finally, 3-week-old T3 transgenic lines (L1, L3, and L10) and WT Arabidopsis seedlings planted in the same pot were treated with salt solution (50, 100, 200, or 300 mM NaCl), and the control seedlings were irrigated with water. The pots were randomly arranged in a greenhouse and grown at 24 °C, in which the lighting conditions and humidity did not change. After 10–15 d of continuous treatment, the pots were irrigated with water, and plants allowed to recover for 7 days.
Measurement of growth and stress-related parameters
The percentage survival, relative water content (RWC) (Bates et al. 1973; Zhao et al. 2014), malondialdehyde (MDA) content (Heath and Packer 1968), activities of catalase (CAT) (Rorth and Jensen 1967; Goldstein. 1968; Del Rio et al. 1977), peroxidase (POD) (Sequeira and Mineo 1966; Kochba et al. 1977), and superoxide dismutase (SOD) (Liu et al. 2012, Zhou and Prognon 2005), and proline (PRO) content (Bates et al. 1973) were determined. All enzyme activity analysis was performed using Arabidopsis leaves.
Statistical analysis
Data analyses were performed using Excel and SPSS v10.0 (SPSS, Inc., Chicago, IL, USA), and Student’s t test was used to determine the significance of differences between means. The mean values and standard deviations (SDs) were obtained from three biological and three technical replicates, and significant differences relative to controls are indicated at **P < 0.05 and *P < 0.01.
Results
Identification and expression pattern analysis of PeVQ28
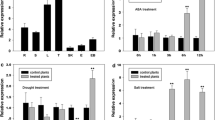
In our previous research on the expression patterns of the VQ genes of Moso bamboo, we noted that PeVQ28 was induced by salt and ABA (Wang et al. 2017). Therefore, PeVQ28 was selected for further functional analyses. Gene-specific primers were designed on the basis of the gene sequence, and total RNA extracted from the leaves was reverse-transcribed to isolate the full-length cDNA of bamboo PeVQ28. Sequencing demonstrated that the PCR-amplified PeVQ28 gene fragment (accession no. PH01007611G0010) contained a 513 bp open reading frame encoding a 170-amino acid protein with a conserved FxxxVQxLTG domain (amino acids 47–57). The cDNA sequence of PeVQ28 showed 100% sequence similarity to the sequence in the bamboo database (https://www.bamboogdb.org/). A putative nuclear localization signal (NLS) sequence was predicted to be located in the region of amino acids 79–110 (Fig. 1a). The VQ genes are widely involved in abiotic stress responses in plants. To explore whether PeVQ28 is responsive to stress, the expression levels under ABA and NaCl stresses were observed using qRT-PCR (Fig. 1b). The expression of PeVQ28 was significantly up-regulated by salt treatment and was high at a relatively early stage (after 3 h treatment). Under ABA stress, the expression of PeVQ28 was induced at 1 h and 24 h. Thus, PeVQ28 was induced by salt and ABA, which indicated that the gene may play key roles in response to abiotic stress and the ABA-signaling pathway.
Sequence analysis and expression pattern of PeVQ28.aPeVQ28 nucleotide and deduced amino acid sequences. The VQ motif was indicated by black solid lines, and two putative NLS sequences are enclosed by red lines. bPeVQ28 expression analysis (RT-qPCR) in Moso bamboo young leaf subjected to ABA and NaCl treatments, respectively. c Expression patterns of PeVQ28 in various tissues: L leaf, R root, Rh rhizome, S shoot, St stem, YL young leaf
In addition, six tissues were selected to examine the tissue-specific expression of PeVQ28 (Fig. 1c). PeVQ28 was expressed in all of the tested tissues and organs, including root, stem, young leaf, leaf, shoot, and rhizome. The highest expression level detected was in the leaves.
Subcellular localization of PeVQ28
According to the application method of the cNLS Mapper website (Conti et al. 1998; Ossareh-Nazari et al. 2001; Harel and Forbes 2004; Kosugi et al. 2008a, b, 2009), analyzing whether a particular sequence contains an NLS sequence produces three different results: (1) score of 7–9, the gene is only located in the nucleus; (2) score of 3–6, the gene is located in the nucleus and cytoplasm; and (3) score of 1 or 2, the gene is located only in the cytoplasm. We analyzed the protein sequence of PeVQ28 using cNLS Mapper and obtained a score of 7, which indicated that an NLS sequence was present and that PeVQ28 was localized in the nucleus (Fig. 1a). To confirm this prediction, the PeVQ28-GFP fusion construct (35S::PeVQ28-GFP) was used to examine the subcellular localization of PeVQ28. The GFP signal was detected in the nuclei of tobacco cells. In the control (35S::GFP), GFP signal was observed throughout the cell (Fig. 2). Thus, PeVQ28 is a nuclear-localized protein (Figs. 1a, 2).
Analysis of the interaction between PeVQ28 and PeWRKY83
In Arabidopsis, the majority of AtVQ proteins interact with WRKY transcription factors. To verify whether PeVQ28 can interact with the WRKY transcription factor PeWRKY83, we performed yeast two-hybrid and BiFC experiments.
The yeast cells harboring the recombinant plasmids (pGBKT7-PeWRKY83 + pGADT7-PeVQ28, pGBKT7-T53 + pGADT7-T, pGADT7-PeVQ28 + pGBKT7, and pGBKT7-PeWRKY83 + pGADT7) grew normally on SD/-Leu/-Trp medium (Fig. 3a). However, on SD/-Ade/-Leu/-Trp/-His medium, the yeast cells of the positive control and the experimental group grew normally, whereas those of the negative control group did not grow normally. Thus, PeVQ28 interacted with the PeWRKY83 transcription factor.
Interaction of PeVQ28 with PeWRKY83. a Interaction of PeVQ28 with PeWRKY83 in yeast. The bait construct (pGBKT7-PeVQ28) and the prey constructs (pGADT7-PeWRKY83) were co-transformed yeast strain AH109, then examined on SD/-Leu/-Trp and SD/-Ade/-His/-Leu/-Trp plates. Positive control, pGBKT7-53 + pGADT7-T; negative control, pGBKT-53 + pGADT7-Lam. b BiFC assays of PeVQ28 interaction with PeWRKY83 in vivo. Yellow fluorescent protein (YFP) images were detected at an approximate frequency of 8.46% (1100 of 1300 tobacco leaf epidermal cells analyzed exhibited BiFC events)
Tobacco transformed with PeVQ28-nYFP + PeWRKY83-cYFP was observed to show YFP signal concentrated in the nucleus, whereas the control groups (PeVQ28-nYFP + cYFP and PeWRKY83-cYFP + nYFP) did not show a fluorescent signal (Fig. 3b). Thus, the BiFC assay also indicated that PeVQ28 and the PeWRKY83 transcription factor were capable of interacting.
Molecular analysis of PeVQ28-containing transgenic Arabidopsis
To verify the effects of PeVQ28 on plant stress tolerance, PeVQ28 was transformed into the WT Arabidopsis (Col-0) under the control of the CaMV35S promoter (Fig. S1a). A total of 30 independent transgenic plants was obtained, and the expression level of PeVQ28 was determined by β-glucuronidase assay (GUS) and RT-qPCR in eight randomly selected lines (Fig. S1a, c). PeVQ28 was not expressed in WT plants, but was expressed in transgenic lines. According to the RT-qPCR analysis, the expression levels were greater in the L1, L3, L8, and L10 lines, but those of the first three lines were more similar (Fig. S1d), so they were used in the subsequent stress-resistance experiments.
Resistance of PeVQ28-overexpressing Arabidopsis plants to salt stress
We first performed a seed germination experiment on MS plates supplemented with different concentrations of NaCl. On the 0 mM NaCl MS plate, no differences were observed among the germination frequencies of the three transgenic lines (L1, L3, and L8) and that of WT seeds. In contrast, under high-salt-stress conditions induced by 100 or 150 mM NaCl treatment, the germination frequency of the WT and transgenic lines was significantly inhibited, but that of the WT was significantly lower than the latter (Fig. 4a, b and Table S3).
Germination and phenotypes of PeVQ28 in transgenic Arabidopsis under salt tolerance. a Germination performance of PeVQ28-overexpression and WT seeds on MS medium containing 0, 100, or 150 mM NaCl. b Calculation of the germination rates of transgenic and WT seeds. c Effect of salt stress on root length of transgenic and WT plants. d Calculation of the root length of transgenic and WT plants. Values are means ± SE (n = 10). *P < 0.05, t test; **P < 0.01, t test
Next, main root length assays were conducted on MS plates in the presence of 0 or 150 mM NaCl. On the 0 mM NaCl MS plate, no significant differences were observed among the growth rates of the three transgenic strains (L1, L3, and L8) and that of the WT. Under the 150 mM NaCl treatment, the transgenic plants exhibit less severe growth inhibition than that of the WT (Fig. 4c), and the main root lengths of the overexpression lines (3.9–4.1 cm) showed less inhibition than that of the WT (2.1 cm) (Fig. 4d).
The resistance to salt stress of overexpression plants grown in soil was examined. The phenotype of plants in the 50 mM NaCl treatment was not significantly different (Fig. 5a). The stress-induced phenotype was evident after treatment with 100, 200, and 300 mM NaCl. On the basis of the phenotype assessment, we analyzed the growth and selected stress-related parameters of WT and transgenic plants in response to treatment with 200 mM NaCl. After 15 d of treatment with 200 mM NaCl, the majority of WT plants had withered, whereas the transgenic lines grew well (Fig. 5a). The percentage survival of the transgenic plants (43.58–47.66%) was notably higher than that of the WT plants (33.89%) (Fig. 5b). In response to treatment with 200 mM NaCl, the RWC, PRO content, and activities of SOD, POD, and CAT in the WT plants were lower than those in the PeVQ28-overexpressing plants (Fig. 5c–g). No significant differences in MDA content between the transgenic and WT plants were observed before stress; but after salt stress treatment, the MDA content in transgenic plants was significantly lower than that in WT plants (Fig. 5h). In summary, under high-salinity conditions, PeVQ28-overexpressing plants showed stronger salt-stress resistance than that of WT plants.
Salt stress of PeVQ28 in transgenic Arabidopsis plants. a Performance of WT and transgenic plants before and after salt treatment with 200 mM NaCl. b Survival rates of WT and transgenic plants after recovery for 7 d following salt stress. PRO (c), RWC (d), SOD (e), POD (f), CAT (g) and MDA content (h) were measured in WT and transgenic plants after salt treatment. Values are means ± SE (n = 3). *P < 0.05, t test; **P < 0.01, t test
Sensitivity of PeVQ28-overexpressing Arabidopsis plants to ABA
The enhanced tolerance of PeVQ28-overexpressing plants to salt stress motivated us to evaluate whether PeVQ28 had an effect on ABA signaling. We first examined the ABA sensitivity of PeVQ28-transgenic plants in a seed germination experiment. Under the control condition, no significant difference was observed in the germination frequencies of WT and transgenic seeds (Fig. 6a). In response to supplementation with 0.5 or 1.0 µM ABA, the germination frequencies of WT and transgenic seeds decreased, but the germination frequency of transgenic seeds was less strongly reduced than that of the WT seeds (Fig. 6b).
ABA sensitivity of PeVQ28 transgenic Arabidopsis. a Germination performance of PeVQ28-overexpression and WT seeds on MS medium containing 0, 0.5, or 1.0 μM ABA. b Calculation of the germination rates of transgenic and WT seeds. c Effect of salt stress on root length of transgenic and WT plants. d Calculation of the root length of transgenic and WT plants. Values are means ± SE (n = 10). *P < 0.05, t test; **P < 0.01, t test
The root growth of WT and PeVQ28-overexpressing seedlings was determined under treatment with 0 or 30 µM ABA. No significant difference in main root length between WT and PeVQ28-overexpressing seedlings was observed under the control condition; however, in response to 30-µM ABA treatment, the growth of PeVQ28-transgenic seedlings was restrained more severely than that of WT seedlings (Fig. 6c, d). Thus, overexpression of PeVQ28 increased the ABA sensitivity of Arabidopsis seedlings.
We also determined the ABA content of WT and PeVQ28-overexpressing plants. The content of ABA in PeVQ28-overexpressing plants was higher than that in WT plants (Fig. 6e).
Expression of ABA-related genes in PeVQ28-overexpressing Arabidopsis plants
To investigate the molecular mechanism of the PeVQ28-mediated response to stress conditions, we analyzed the expression levels of nine marker genes (AtAAO3, AtNCED2, AtRD29A, AtRD29B, AtABF1, AtNCED3, AtABI1, AtP5CS1, and AtPP2CA) in PeVQ28-overexpressing Arabidopsis lines. In response to 5 d of salt treatment, ABA biosynthesis genes were activated in WT and PeVQ28-overexpressing lines, whereas the expression levels of AtAAO3, AtNCED2, AtRD29A, AtRD29B, AtABF1, and AtNCED3 in transgenic plants were significantly higher than those in WT plants (Fig. 7). In addition, AtP5CS1, AtABI1, and AtPP2CA showed higher expression levels in transgenic plants than in WT plants (Fig. 7). These results suggested that PeVQ28 overexpression also induced ABA synthesis in transgenic plants under salt-stress conditions, thereby leading to increased expression levels of ABA-signaling and ABA-related genes.
Relative expression levels of stress/ABA-responsive genes in transgenic Arabidopsis plants under normal salt stress. Leaves of WT and transgenic Arabidopsis plants were sampled after salt stress. Y-axis: relative expression levels; X-axis: the time course of stress treatments. Mean values ± SE (n = 6)
Discussion
The VQ proteins are plant-specific proteins. In our previous research, we identified 29 VQ genes in the Moso bamboo genomic database and classified them into seven subfamilies (I–VII) on the basis of evolutionary relationships (Wang et al. 2017). The genome-wide bioinformatics and stress-induced expression patterns of the VQ gene family have been analyzed in a number of species, whereas functional studies have focused on Arabidopsis VQ genes, such as AtVQ9 (AtMVQ10) and AtVQ15 (AtCAMBP25) (Perruc et al. 2004; Hu et al. 2013b). No functional analyses have been reported for PeVQ genes. Based on the results of previous bioinformatics research in combination with the literature on the VQ gene family, we selected the potential abiotic stress-related gene PeVQ28 for functional analysis. The expression level of PeVQ28 in response to different stress treatments was analyzed (Fig. 1b). PeVQ28 was up-regulated under high-salt and ABA stress conditions. The expression level was 80-fold higher under high-salt conditions, indicating that the gene was likely induced by an adversity-triggered stress-response mechanism. We extracted six tissue samples (R, St, YL, L, S and Rh) of Moso bamboo for RT-PCR experiments. The results showed that PeVQ28 was highest expressed in L, followed by YL. It shows that PeVQ28 was highly expressed in L, so the materials used in subsequent experiments were all L.
First, the localization of the PeVQ28 protein was analyzed by transiently transforming the constructed p1305-CaMV35S-PeVQ28-GFP fusion expression vector into tobacco leaves, and was shown to localize in the nucleus (Fig. 2). Second, the VQ protein family can interact with WRKY transcription factors in Arabidopsis (Hu et al. 2013a). To verify the interaction between a VQ protein and a WRKY transcription factor in Moso bamboo, yeast two-hybrid and BiFC assays were performed (Fig. 3). The PeVQ28 protein, both in vivo and in vitro, could interact with WRKY transcription factors and form a complex localized in the nucleus. The VQ-assisted WRKY transcription factor is involved in the regulation of plant growth and development, and response to abiotic and biotic stresses, and provided a pointer for exploration of the function of PeVQ28 (Perruc et al. 2004; Wang et al. 2015a; Wu et al. 2017).
Overexpression of a gene is a direct and effective method to examine the biological function of the gene (Hao et al. 2011; He et al. 2011; Ge et al. 2017). As expected, transgenic Arabidopsis plants were significantly more tolerant to salt stress (Fig. 4). In addition, the sensitivity of PeVQ28-overexpressing plants to exogenous ABA was significantly increased during the germination and seedling stages (Fig. 6). Thus, PeVQ28 may play an important role in ABA-dependent signaling and promote different resistance levels to different stresses. The present research showed that PeVQ28 might act as a positive regulator of ABA-dependent signaling pathways to improve plant resistance to salt stress.
Plants have evolved diverse mechanisms to adapt to changes in the environment. It is well known that plants suffering from abiotic stress often accumulate reactive oxygen species leading to lipid peroxidation and oxidative stress (Xiong et al. 2002; Mittler et al. 2004). The content of MDA is a parameter often used as an indicator of reactive oxygen species stress in plant cells. Under salt stress, the MDA content of WT plants was higher than that of PeVQ28-transgenic plants, which showed that overexpression of PeVQ28 may lead to increased resistance to oxidative stress induced by salt stress (Fig. 5h). Many studies have shown that accumulation of free PRO is an important mechanism in response to abiotic stress, because it can stabilize subcellular structures by regulating intracellular osmotic potential to prevent damage (Liu and Zhu 1997; Armengaud et al. 2004; Xiang et al. 2007). The present data indicated that PeVQ28-overexpressing plants accumulate significantly higher quantities of PRO than that of WT under stress conditions (Fig. 5c). The increase in PRO content may explain the higher osmotic pressure, which leads to lower water potential and the more efficient conservation of water by plants (Song et al. 2011). Consistent with this, leaves of PeVQ28-overexpressing plants showed a higher RWC than that of WT plants (Fig. 5d), which indicated that the transformants showed increased ability to conserve water under stress conditions. Given that PRO, CAT, SOD, and POD can be used, to a certain extent, as antioxidants to decrease oxidative damage under stress, increased PRO content and CAT, SOD, and POD activities in transgenic plants may result in lower MDA contents under stress conditions (Fig. 5c–h) (May 2008). As a result, the enhanced PRO content of PeVQ28-overexpressing plants may be a primary mechanism to improve salt-stress resistance.
Abscisic acid can inhibit seed germination, seedling growth, and plant development. Under a variety of stresses, ABA-dependent signaling pathways lead to ABA accumulation (Iuchi et al. 2001; Tan et al. 2003; Koiwai et al. 2004; Nambara and Marion-Poll 2005). In plants, the overexpression of certain genes induces the expression of stress-responsive genes, which in turn result in increased resistance to diverse stresses. Under non-stress conditions, AtAAO3, AtNCED2, AtRD29A, AtRD29B, AtABF1, and AtNCED3 showed similarly low expression levels in PeVQ28-overexpressing and WT plants. However, under salt stress, the expression levels of these six genes in the overexpression plants were higher than those in WT plants (Fig. 7), indicating that PeVQ28 plays a role in stress-induced ABA biosynthesis. In addition, the expression levels of the ABA-signaling genes ABI1, AtP5CS1, and AtPP2CA were examined in overexpression and WT plants under non-stress and salt-stress conditions (Fig. 7) (Hirayama and Shinozaki 2007). AtABI1 and AtPP2CA encode protein phosphatase 2C, which plays a major role in ABA-mediated signaling networks associated with stress responses. Under salt stress, the expression levels of ABI1 and AtPP2CA in PeVQ28-overexpressing plants were higher than those in WT plants. In addition, the induced expression of AtP5CS1 may lead to the accumulation of PRO in transgenic plants, thus contributing to the enhanced tolerance of PeVQ28-transgenic plants to stress conditions. Thus, the increased resistance of PeVQ28-overexpressing plants to salt stress may result, in part, from the enhanced expression of these genes.
Protein–protein interactions are the basis for all metabolic activities in cells. VQ proteins participate in interactions with other proteins (Hu et al. 2013a, b; Wu et al. 2017). For example, AtVQ14 interacts with AtWRKY10 to form a complex protein that affects seed size (Wang et al. 2010), and AtVQ22 interacts with AtWRKY28 to negatively regulate jasmonic acid-mediated disease-related signaling pathways (Hu et al. 2013a). In the present research, we tested the interaction between PeVQ28 and PeWRKY83 using a yeast two-hybrid assay (Fig. 3a), which revealed that PeVQ28 is able to interact with PeWRKY83. This result was verified using a BiFC analysis (Fig. 3b). Thus, based on this research and previous surveys, we conclude that proteins encoded by the VQ family in Moso bamboo participate in protein–protein interactions.
Author contribution statement
XRC and YJW conceived the study, performed the main experimental analyses, and drafted the manuscript. RX carried out the software analyses and generated the figures and tables. YMG processed the experimental data and drafted the manuscript. HWY reviewed the project and edited the manuscript. YX conceived and guided the experiments, coordinated the project, and drafted the manuscript. All authors read and approved the final manuscript.
Availability of data and materials
The genome sequences of Moso bamboo and Arabidopsis were downloaded from the Bamboo Genome Database (https://www.bamboogdb.org/) and The Arabidopsis Information Resource (https://www.arabidopsis.org), respectively.
Abbreviations
- BiFC:
-
Bimolecular fluorescence complementation;
- CAT:
-
Catalase
- MDA:
-
Malondialdehyde
- POD:
-
Peroxidase
- POR:
-
Proline
- SOD:
-
Superoxide dismutase
- RWC:
-
Plant relative water content
- VQ:
-
Valine–glutamine motif-containing protein
References
Armengaud P, Thiery L, Buhot N, Grenier-De March G, Savoure A (2004) Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Plant Physiol 120:442–450
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Cao Y, Han Y, Li D, Yi L, Cai Y (2016) MYB transcription factors in Chinese pear (Pyrus bretschneideri Rehd.): Genome-wide identification, classification, and expression profiling during fruit development. Front Plant Sci 7:557
Chen D, Chen Z, Wu M, Wang Y, Wang Y, Yan H, Xiang Y (2017) Genome-wide identification and expression analysis of the HD-Zip gene family in Moso bamboo (Phyllostachys edulis). J Plant Growth Reg 36:323–337
Cheng Y, Chen Z (2012) Structural and functional analysis of VQ motif-containing proteins in Arabidopsis as interacting proteins of WRKY transcription factors. Plant Physiol 159:810–825
Conti E, Uy M, Leighton L, Blobel G, Kuriyan J (1998) Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin alpha. Cell 94:193–204
Dai X, Xu Y, Ma Q, Xu W, Wang T, Xue Y, Chong K (2007) Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol 143:1739–1751
Del Rio LA, Ortega MG, Lopez AL, Gorgo JL (1977) A more sensitive modification of the catalase assay with the Clark oxygen electrode: application to the kinetic study of the pea leaf enzyme. Anal Biol 80:409–415
Fan C, Ma J, Guo Q, Li X, Wang H, Lu M (2013) Selection of reference genes for quantitative real-time PCR in bamboo (Phyllostachys edulis). PLoS One 8:e56573
Gao Y, Liu H, Wang Y, Li F, Xiang Y (2017) Genome-wide identification of PHD-finger genes and expression pattern analysis under various treatments in moso bamboo (Phyllostachys edulis). Plant Physiol Biochem 123:378–391
Ge W, Zhang Y, Cheng Z, Hou D, Li X, Gao J (2017) Main regulatory pathways, key genes and microRNAs involved in flower formation and development of moso bamboo (Phyllostachys edulis). Plant Biotchenol J 15:82–96
Goldstein DB (1968) A method for assay of catalase with the oxygen cathode. Anal Biol 24:431–437
Hao YJ, Wei W, Song QX, Chen HW, Zhang YQ, Wang F, Zou HF, Lei G, Tian AG, Zhang WK (2011) Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J 68:302–313
Harel A, Forbes DJ (2004) Importin beta: conducting a much larger cellular symphony. Mol Cell 16:319–330
He X, Hou X, Shen Y, Huang Z (2011) TaSRG, a wheat transcription factor, significantly affects salt tolerance in transgenic rice and Arabidopsis. FEBS Lett 585:1231–1237
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hirayama T, Shinozaki K (2007) Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends Plant Sci 12:343–351
Hu P, Zhou W, Cheng Z, Fan M, Wang L, Xie D (2013a) JAV1 controls jasmonate-regulated plant defense. Mol Cell 50:504–515
Hu Y, Chen L, Wang H, Zhang L, Wang F, Yu D (2013b) Arabidopsis transcription factor WRKY8 functions antagonistically with its interacting partner VQ9 to modulate salinity stress tolerance. Plant J 74:730–745
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:377–403
Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27:325–333
Jakab G, Zimmerli L, Métraux JP, Mauch-Mani B (2005) Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol 139:267–274
Katiyaragarwal S (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45:523–539
Kim DY, Kwon SI, Choi C, Lee H, Ahn I, Park SR, Bae SC, Lee SC, Hwang DJ (2013) Expression analysis of rice VQ genes in response to biotic and abiotic stresses. Gene 529:208–214
Kochba J, Lavee S, Spiegelroy P (1977) Differences in peroxidase activity and isoenzymes in embryogenic ane non-embryogenic ‘Shamouti’ orange ovular callus lines. Plant Cell Physiol 18:463–467
Koiwai H, Seo M, Koshiba T (2004) Tissue-specific localization of an abscisic acid biosynthetic enzyme, AAO3, in Arabidopsis. Plant Physiol 134:1697–1707
Kosugi S, Hasebe M, Entani T, Takayama S, Tomita M, Yanagawa H (2008a) Design of peptide inhibitors for the importin α/β nuclear import pathway by activity-based profiling. Chem Biol 15:940–949
Kosugi S, Hasebe M, Tomita M, Yanagawa H (2008b) Nuclear export signal consensus sequences defined using a localization-based yeast selection system. Traffic 9:2053–2062
Kosugi S, Hasebe M, Tomita M, Yanagawa H, Hunt T (2009) Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc Natl Acad Sci USA 106:10171–10176
Lai Z, Li Y, Wang F, Cheng Y, Fan B, Yu JQ, Chen Z (2011) Arabidopsis sigma factor binding proteins are activators of the WRKY33 transcription factor in plant defense. Plant Cell 23:3824–3841
Li N, Li X, Xiao J, Wang S (2014) Comprehensive analysis of VQ motif-containing gene expression in rice defense responses to three pathogens. Plant Cell Rep 33:1493–1505
Liu J, Zhu JK (1997) Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol 114:591–596
Liu SP, Pang XL, Cao JY, Xue H, Chen ZL (2012) Measurement of SOD in fresh Jujube fruit and analysis of its content. Hunan Agric Sci 15:14
Liu H, Min W, Zhu D, Feng P, Wang Y, Yue W, Yan X (2017) Genome-wide analysis of the AAAP gene family in moso bamboo (Phyllostachys edulis). BMC Plant Biol 17:29
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25:402–408
May G (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53:11–28
Mittler R, Vanderauwera S, Gollery M, BreusegemF V (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Ossareh-Nazari B, Gwizdek C, Dargemont C (2001) Protein export from the nucleus. Traffic 2:684
Pecher P, Eschen-Lippold L, Herklotz S, Kuhle K, Naumann K, Bethke G, Uhrig J, Weyhe M, Scheel D, Lee J (2014) The Arabidopsis thaliana mitogen-activated protein kinases MPK3 and MPK6 target a subclass of ‘VQ-motif’-containing proteins to regulate immune responses. New Phytol 203:592–606
Perruc E, Charpenteau M, Ramirez BC, Jauneau A, Galaud JP, Ranjeva R, Ranty B (2004) A novel calmodulin-binding protein functions as a negative regulator of osmotic stress tolerance in Arabidopsis thaliana seedlings. Plant J 38:410–420
Rorth M, Jensen PK (1967) Determination of catalase activity by means of the Clark oxygen electrode. Biochim Biophys Acta 139:171–173
Sequeira L, Mineo L (1966) Partial purification and kinetics of indoleacetic acid oxidase from tobacco roots. Plant Physiol 41:1200–1288
Shinozaki K, Yamaguchi-Shinozaki K (1997) Gene expression and signal transduction in water-stress response. Plant Physiol 115:327–334
Song S, Chen Y, Zhao M, Zhang WH (2011) A novel Medicago truncatula HD-Zip gene, MtHB2, is involved in abiotic stress responses. Envir Exp Bot 69:1–9
Song W, Zhao H, Zhang X, Lei L, Lai J (2016) Genome-wide identification of VQ motif-containing proteins and their expression profiles under abiotic stresses in maize. Front Plant Sci 6:1177
Tan BC, Joseph LM, Deng WT, Liu LJ, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35:44–56
Walter M, Chaban C, SchTze K, Batistic O, Weckermann K, Nake C, Blazevic D, Grefen C, Schumacher K, Oecking C (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40:428–438
Wang A, Garcia D, Zhang H, Feng K, Chaudhury A, Berger F, Peacock WJ, Dennis ES, Luo M (2010) The VQ motif protein IKU1 regulates endosperm growth and seed size in Arabidopsis. Plant J 63:670–679
Wang X, Zhang H, Sun G, Jin Y, Qiu L (2014) Identification of active VQ motif-containing genes and the expression patterns under low nitrogen treatment in soybean. Gene 543:237–243
Wang H, Hu Y, Pan J, Yu D (2015a) Arabidopsis VQ motif-containing proteins VQ12 and VQ29 negatively modulate basal defense against Botrytis cinerea. Sci Rep 5:14185
Wang M, Vannozzi A, Wang G, Zhong Y, Corso M, Cavallini E, Cheng ZM (2015b) A comprehensive survey of the grapevine VQ gene family and its transcriptional correlation with WRKY proteins. Front Plant Sci 6:417
Wang T, Yue JJ, Wang XJ, Xu L, Li LB, Gu XP (2016) Genome-wide identification and characterization of the Dof gene family in moso bamboo (Phyllostachys heterocycla var. pubescens). Genes Genom 38:733–745
Wang Y, Liu H, Zhu D, Gao Y, Yan H, Yan X (2017) Genome-wide analysis of VQ motif-containing proteins in Moso bamboo (Phyllostachys edulis). Planta 246:165–181
Wu H, Lv H, Li L, Liu J, Mu S, Li X, Gao J (2015) Genome-wide analysis of the AP2/ERF transcription factors family and the expression patterns of DREB genes in Moso bamboo (Phyllostachys edulis). PLoS One 10:e0126657
Wu M, Liu H, Han G, Cai R, Pan F, Xiang Y (2017) A moso bamboo WRKY gene PeWRKY83 confers salinity tolerance in transgenic Arabidopsis plants. Sci Rep 7:11721
Xiang Y, Huang Y, Xiong L (2007) Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol 144:1416–1428
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14:S165–S183
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Zhao H, Ma H, Yu L, Wang X, Zhao J (2012) Genome-wide survey and expression analysis of amino acid transporter gene family in rice (Oryza sativa L.). PLoS One 7:e49210
Zhao Y, Ma Q, Jin X, Peng X, Liu J, Deng L, Yan H, Sheng L, Jiang H, Cheng B (2014) A novel maize homeodomain-leucine zipper (HD-Zip) I gene, Zmhdz10, positively regulates drought and ssalt tolerance in both rice and Arabidopsis. Plant Cell Physiol 55:1142–1156
Zhou JY, Prognon P (2005) Raw material enzymatic activity determination: a specific case for validation and comparison of analytical methods—the example of superoxide dismutase (SOD). J Pharm Biomed 40:1143–1148
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Acknowledgements
We thank the Laboratory of Modern Biotechnology, National Engineering Laboratory of Crop Stress Resistance Breeding, and Key Laboratory of Crop Biology of Anhui Province members for assistance with this study. We thank Lesley Benyon, Ph.D., from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This study was supported by the National Science Foundation of China (Grant no. 31670672) and the National Science and Technology Support Program (Grant no. 2015BAD04B0302).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, X., Wang, Y., Xiong, R. et al. A Moso bamboo gene VQ28 confers salt tolerance to transgenic Arabidopsis plants. Planta 251, 99 (2020). https://doi.org/10.1007/s00425-020-03391-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03391-5