Abstract
In response to the rapid expansion of the worldwide population, there is an urgent need for the development of new, more environmentally-conscious, food sources. In this context, microalgae, such as Euglena, are of interest due to their capacity for large-scale cultivation and flexible bioproduct output. Media recycling techniques have been proposed as waste- and cost-reducing measures for large-scale algal cultivation. Culture dynamics and phytohormone production during novel fermentation conditions can enhance growth and biomass production; yet, little is known about the impacts on growth regulating phytohormones such as cytokinins (CKs) and abscisic acid (ABA) under these conditions. Using Euglena gracilis, an organic culture medium was recycled at 25, 50 and 75% inclusion rates through multiple growth cycles to evaluate impacts on biomass production and phytohormone profiles. A 25% recycled inclusion rate resulted in biomass accumulation similar to the control over one growth cycle. Subsequent recycled rates and growth cycling reduced E. gracilis biomass accumulation relative to the control, even with cell counts continuing to increase throughout, highlighting that the recycled medium inclusion and the number of repeated culture cycles impacts the biomass output of E. gracilis. Phytohormone analysis indicated that E. gracilis synthesized CKs via the tRNA-degradation pathway with increased hormone levels corresponding to higher recycle rates; ABA increased in the supernatant at higher recycle rates but was not detected in any pellet fractions. This research expands upon the roles of CKs and ABA as signaling molecules and adds guidance for building a suite of conditions specific for industrial product development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae are a popular ingredient source for food, animal feed, biofertilizer and biofuel industries (Fernández et al. 2021). Industrial interest in this polyphyletic group is due to the capacity of microalgae to produce a range of micronutrients, lipids, and proteins thus paving the way for novel development of functional food and bioproducts (Hlavova et al. 2015; Vigani et al. 2015; Mathieu-Rivet et al. 2020; Fernández et al. 2021). Ease of large-scale cultivation, sustainability and effectiveness for target compound production or bioproduct development are key metrics needed to support industrial applications. Euglenoids, specifically Euglena gracilis Klebs (strain Z) (Euglenophyceae), have garnered a growing commercial and academic interest, enough to spur the formation of the Euglena International Network (Ebenezer et al. 2022) a venture including academics and industry experts seeking to understand the biology and versatility of Euglenoids.
Euglena gracilis is a photosynthetic flagellate microalga. In the free-swimming stage it reproduces rapidly through binary fission; however, in unfavourable environments, cells become non-motile and encyst (Leedale 1964; Cook 1968). Euglena gracilis is capable of heterotrophic, autotrophic and mixotrophic growth and can utilize exogenous organic materials and a variety of input sources (Kim et al. 2021) while also being amenable to large scale cultivation and growth through a variety of cultivation methods (for example: fed-batch or continuous; Chen 1996; Perez-Garcia et al. 2011; Gissibl et al. 2019). The unique evolutionary position of E. gracilis is reflected by a dynamic genome and complex metabolism making this species a candidate for obtaining nutritional ingredients of interest for industrial platforms and production (O'Neill et al. 2015; Gissibl et al. 2019).
Algal production industries continue to face challenges related to efficient biomass production at low cost (Acién et al. 2012). In response to this, reuse of spent growth media with leftover nutrient/components for subsequent algal cultivation—referred to as nutrient recycling—is an economic approach that can significantly reduce the waste stream volume (Rodolfi et al. 2003; Rösch et al. 2012; Farooq et al. 2014; Farooq 2021). The residual nutrients, as a complex mixture, include varying concentrations of organic carbon (sugars and carbohydrates), nitrogen (inorganic and complex organic nitrogen), essential minerals and remaining extracellular metabolites and undefined chemical ingredients (Discart et al. 2014; Liu et al. 2016; Farooq 2021). The viability of nutrient recycling depends on a clear understanding of operating scale, the composition of the medium, the proportion of recycling volume, fortification type, system sterility (ex. filter or autoclave) and the characteristics of the selected microalgae species (Ruiz et al. 2016). Species-dependent variability in growth rates and yield occurs when a medium recycling technique is applied to heterotrophic cultivation (Hnain et al. 2011; Loftus and Johnson 2017). Medium recirculation resulted in reduced biomass production and growth rates for Nannochloropsis sp., Arthrospira platensis and Chlorella zofingiensis (Fret et al. 2017), whereas Tetraselmis sp. and Chlorella vulgaris were cultivated with medium recycling without a significant impact on growth (Lowrey et al. 2016). Thus, the selection of medium and microbe is critical to growth output.
Addition of growth enhancing compounds such as phytohormones (plant hormones) has been used to trigger increased growth and biomass production in various systems (Yu et al. 2015; Zhu et al. 2019; Xu and Xiong 2023). Phytohormones are present in many organisms including bacteria, fungi, protists, algae and mammals (Stirk and van Staden 2010; Spíchal 2012; Noble et al. 2014; Morrison et al. 2017; Seegobin et al. 2018; Aoki et al. 2019a). Due to the evolutionarily close relationship between plants and algae, phytohormones can be correlated with regulatory processes, such as reproduction, defence responses, differentiation, development, and cell division in algae (Stirk et al. 2003; Santner et al. 2009). Cytokinin (CK), the crucial growth phytohormone responsible for cytokinesis (cell division), can be found in all algal groups (Schwender et al. 2001; Stirk et al. 2003) and therefore understanding their balance is crucial for evaluating growth and development. Since plant hormones are thought to have evolved from pre-existing elements of the primary metabolism in algae (Pils and Heyl 2009), they are valuable to investigate outside of the plant kingdom. To date there are no published CK profiles of heterotrophically grown E. gracilis cultivated in recycled media to reduce input costs. Thus, it is worth understanding the mechanisms involved in E. gracilis CK production and its relationship with biomass production. Another phytohormone, abscisic acid (ABA) is also present in some algae (Hartung 2010) with levels altered when exposed to stressful conditions like salinity, nitrogen deficiency, heat, drought, acidity, oxidation, light, and alkaline environments (Cowan and Rose 1991; Tominaga et al. 1993; Hartung 2010; Noble et al. 2014; Nguyen et al. 2023). Abscisic acid has contradictory responses in different aspects of cellular functions acting as either a stimulatory or inhibitory agent for permeability of solutes (Ord et al. 1977; Hirsch et al. 1989), increasing ion uptake or having both positive and negative responses for respiration, differentiation, and cell growth. Thus, it is important to recognize the relationships of ABA with growth and yield profiles since the effect of ABA is species-dependent (Hirsch et al. 1989). Specifically, in E gracilis, ABA and CK appeared to act synergistically together to improve culture growth (Noble et al. 2014) and ABA supplementation increased CK production, and mitigated stress responses through cross-talk with CK (Nguyen et al. 2023).
The current study addressed the practical application of using recycled medium for culturing heterotrophically grown E. gracilis. On another level, we investigated the mechanistic reasons for growth responses by examining phytohormone (CK and ABA) production over growth cycles and linking this to E. gracilis phytohormone biosynthesis and impacts on biomass metrics.
Materials and methods
Organism and culture medium
Euglena gracilis was purchased from UTEX Culture Collection of Algae (UTEX 753; University of Texas, Houston, TX, USA). Axenic liquid cultures of E. gracilis were maintained heterotrophically in sterile Noble Organic Medium (NOM): molasses (3 g L−1), yeast extract (5 g L−1), ammonium phosphate monobasic (2 g L−1), vegetable oil (2 g L−1), and ethanol (2 g L−1); that was previously developed by Noblegen Inc, Peterborough, ON, Canada. Euglena gracilis was cultured in 2 L flasks with ajar caps, in an incubator set at 100 rpm and 29.0 °C (Adolf Kuhner AG, Model ISF-4-V). These preliminary cultures were harvested as a cell concentrate (over 5 mL) via centrifugation; 2400 × g, 5 min) to inoculate subsequent cultures. Each cycle round was inoculated with cells maintained in NOM at a 2 × 106 cells mL−1 inoculum. Recycled supernatant was recirculated from the initial culture in prepared dilutions (75%, 50%, 25%, 0% recycled to fresh media) and compared to the control; a batch technique was utilized for culturing.
Experimental design
Experimental parameters, conditions for heterotrophic and batch culturing techniques for microalgae were determined with input from an industry partner, Noblegen Inc. Euglena gracilis cultures were grown for 3 days per cycle as described below, in 500 mL NOM using 1 L baffled bottom culture flasks (Fisher Scientific, Canada) with vented PTFF closure caps (0.22 µm) at 100 rpm and 29.0 °C (Adolf Kuhner AG, Model, ISF-4-V).
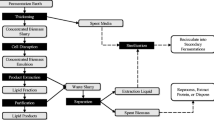
Four recycled rates were selected (0%, 25%, 50%, and 75%) representing the percent of spent medium in the culture flask. An initial culture round (cycle 0), conducted for 3 days, was started with 2 × 106 cells mL−1 inoculum (2 L flasks containing 1 L medium); this spent supernatant was collected and used to begin the subsequent cycles. Growth consisted of four cycle rounds with four flasks and three replicates for each treatment group. Cycles were represented as follows: cycle 1 (day 1 – day 3), cycle 2 (day 1 – day 3), cycle 3 (day 1 – day 3) and cycle 4 (day 1 – day 3), days are represented as day(s) post inoculation (DPI). For simplicity in discussions each recycle rate is termed as a percentage of spent medium included in the total medium volume: 0% or control (0% spent medium: 100% fresh growth medium), 25% (25% spent medium: 75% fresh growth medium), 50% (50% spent medium: 50% fresh growth medium) and 75% (75% spent medium: 25% fresh growth medium). To fully explain the cycling procedure, as an example, the 25% recycle rate was prepared as follows: following three days of growth, one flask (cycle 0) was harvested (4696 × g; 5 min) and the supernatant (spent medium) was used to generate the culture medium (25% spent medium: 75% fresh growth medium) this was used to inoculate four flasks in cycle 1. Cultures were grown for three days and then harvested and the pooled spent media from flasks in cycle 1 was used to supply spent medium for cycle 2. Subsequent cycles were set up in the same way (Fig. S1). Samples (23 mL) were collected daily for phytohormone analysis (10 mL), and 10 mL was centrifuged to assess biomass accumulation, 2 mL was used for glucose content and 1 mL for morphological assessment and pH testing.
Growth parameters
Macro-characteristics of culture health were assessed throughout the experiment including: cell morphology, cell count, biomass accumulation, pH, and glucose content. Cell morphology was monitored using a microscope (Life Technologies EVOS FL Auto AMAFD1000) according to Noble et al. (2014) (10 µL, 20 × objective with a 200 µm field of view) for all replicates and all four recycle rates.
Cell density was measured using a Cell Counter (Life Technologies, Countess II FL, Model AMOAF1000) and a Countess II FL double-well slide. Consistent cell count parameters were maintained throughout the experiment: Auto Final Count threshold size of 6 µm to 60 µm; Auto Lighting 0 – 147 brightness units, and a maximum threshold of shape – oval to circular per 10 μL sample. Cell counts were performed in duplicate as the number of cells per mL.
Biomass accumulation was measured by collecting 10 mL culture in pre-weighed 15 mL tubes, samples were centrifuged at 2,400 × g, 5 min. Following centrifugation the sample was separated into supernatant and pellet. The resultant pellet was washed twice with 5 mL deionized water and centrifuged (2,400 × g, 5 min), water was removed and the remaining pellet was weighed and freeze-dried (Labconco, Model 7,806,021) for 24 h. Wet and dry mass were recorded.
pH was measured in 2 mL aliquots of culture medium (containing cells) twice per cycle at the start of cultivation: day(s) post inoculation (DPI) 1 and at the completion of the cycle, DPI 3, for all cycles (Sevenmulti Mettler Toledo AG, Model 8603 pH meter).
Glucose content was measured at the beginning and end of each cycle in 2.5 mL culture using a Biochemical Analyzer (YSI 2700 Select). Before sampling, the biochemical analyzer was calibrated using YSI 1531 D-Glucose Standard 9.00 g L−1 and re-calibrated after five samples or after a 15-min standby period. A YSI 2357 Buffer Concentrate Kit and YSI 2776 D-Glucose Standard 2.50 g L−1, L-lactate 0.50 g L−1 concentrations were used to measure against each sample. Linearity checks with a 9.0 g L−1 linearity check solution (2.25 g glucose/250 mL water), were examined alongside the standard and fell between ± 5% of labelled glucose. Triplicate samples were analyzed for 1 min. Samples were diluted eightfold so that the substrate concentration was within the linear range of the glucose curve (between 8.55 g L−1 and 9.45 g L−1).
Phytohormone extraction and detection
A modified protocol described by Kisiala et al. (2013) and Noble et al. (2014) was used for CK and ABA extraction of E. gracilis. Collected samples of E. gracilis pellet and supernatant were freeze-dried and stored at -80 °C until phytohormone extraction. Upon extraction, samples were re-suspended in extraction buffer Bieleski #2 (Methanol:Water:Formic Acid; CH4O:H2O:CH2O2 [15:4:1, v/v/v]), spiked with 144 ng of [2H4] abscisic acid (ABA; PBI, Canada) and 10 ng of each of the deuterated internal CK standards, (OlChemIm Ltd., Czech Republic; Table 1), and homogenized using a ball mill grinder (RetschMM300; 5 min, 25 Hz) at 4°C with zirconium oxide grinding beads (Comeau Technique Ltd., Canada). The samples were allowed to extract passively overnight (approximately 12 h) at -20°C. Following overnight extraction, samples were centrifuged (10 min at 11,200 × g) and supernatant was collected. Remaining sample pellets were re-extracted with 1 mL Bieleski #2 extraction buffer at -20°C for 30 min. Samples were dried in a speed vacuum concentrator at 35°C (UVS400, Thermo Fisher Scientific, USA).
Extraction residues were reconstituted in 1 mL 1 M formic acid (pH 1.4) to ensure complete protonation of all CKs. Each extract was purified on a mixed-mode, reverse-phase, cation-exchange cartridge (IRIS MCX 6 mL; 25–35 µ 200 mg; Canadian Life Sciences, Canada). Extraction followed methods listed in Kisiala et al. (2013) with the elution of ABA, CKs: nucleotides (NTs); followed by free bases (FBs), ribosides (RBs), glucosides (GLUCs) and methylthiolated CKs (METs). All samples were evaporated to dryness in a speed vacuum concentrator at 35 °C and stored at -20 °C.
Nucleotides were dephosphorylated using three units of bacterial alkaline phosphatase in 1 mL 0.1 M ethanolamine-hydrochloric acid (pH 10.4) for 12 h at 37 °C (Emery et al. 2000). The resulting RBs were brought to dryness in a speed vacuum concentrator at 35 °C and represent a pooled contribution of mono/di/tri phosphate NTs. Dephosphorylated samples were reconstituted in 1.5 mL double distilled water for further purification on a reversed-phase C18 column (C18/14%, 6 mL, 500 mg; Canadian Life Sciences, Canada) following the method described in Kisiala et al. (2013). Prior to HPLC–MS/MS analysis, all dried samples were reconstituted in 1.5 mL of starting conditions (CH3COOH:CH3CN:ddH2O [0.08:5.0:94.92, v/v/v]).
Hormones were identified and quantified by HPLC–MS/MS analysis. A 25 μL sample volume was injected into a Dionex Ultimate 3000 HPLC coupled to a QExactive Orbitrap mass spectrometer (Kisiala et al. 2019; Thermo Scientific). Compounds were resolved using a reversed-phase C18 column (Kinetex 2.6μ C18 100 A, 2.1 × 50 mm; Phenomenex, USA). All hormone fractions were eluted with a multistep gradient of component A: water (H2O) with 0.08% acetic acid (CH3COOH) mixed with component B: acetonitrile (CH3CN) with 0.08% CH3CO2H at a flow rate of 0.3 mL min−1 for ABA and 0.4 mL min−1 for CKs. The initial conditions were 5% B increasing linearly to 10% B over two minutes followed by an increase to 95% B over 6.5 min; 95% B was held constant for 1.5 min before returning to starting conditions for 5 min of recalibration.
Cytokinin and ABA samples were analyzed using a Thermo Fisher Scientific QExactive Orbitrap (USA) equipped with heated electrospray ionization (HESI) source in positive ion mode, all conditions followed those in Kisiala et al. (2019). Raw data files collected during HPLC–MS/MS analysis were processed using the Thermo Fisher Scientific Xcalibur software (v. 3.0.63; USA) as described in Kisiala et al. (2019). Quantification was achieved through isotope dilution analysis based on the recovery of 2H-labelled internal standards (Table 1).
Recognizing that all organic media contains background CK levels (Aoki et al. 2021) endogenous CK analysis was normalized by subtracting the average background levels of existing endogenous hormones in the Noble Organic Media (NOM; media blank; Table S1) and CK concentrations were calculated as per mL of culture.
Statistical analysis
Statistical analyses were conducted using Statistica 8.0 software on phytohormone data from supernatant and pellet fractions. Phytohormone levels were analyzed using Statistica 8.0 software to determine if data approximated normal distribution. Data was compared within each recycle rate and cycle with a two-way ANOVA followed by Duncan's multiple range test for post hoc analysis (P ≤ 0.5) to determine significant effects for cycle DPI per recycling rate and effects of cycle per recycling rate. Data points and error bars for metrics and phytohormone data reflect means ± standard error for 3 biological replicates obtained from separate culture flasks.
Results
Growth analytics
Control cells using 0% recycling showed "classical" E. gracilis growth as defined by Noble et al. (2014) (Fig. S2a). The cells were cylindrical or spindle-shaped with a slightly attenuated and rounded anterior body and between 40–80 μm long and 18–25 μm wide. As recycle rate increased from 25–75%, cells became irregular in shape and at the 75% rate, they were circular (Fig. S2d); visible cellular motility also decreased.
Cell counts increased in values throughout (with the exception of 75% for cycle 1; Fig. S3). These cell number increases indicated that, regardless of cycle and recycle rate, cells were able to divide and increase in number similar to the 0% control (Fig. S3).
Biomass accumulation
In general, biomass accumulation was indicative of culture growth, whereby an increasing trend of biomass occurred in every cycle for the control (0%), recycling rate 25%, 50% rate, and 75% rate, with the exception of 75% rate in cycle 1 (~ 12% decrease in dry biomass relative to the starting biomass, which correlated with decreased cell count; Figs. 1, S3). Recycling at a 25% rate and single cycle round increased biomass accumulation by 24% relative to the 0% control, indicating that an initial exposure to recycled media increased biomass accumulation (Fig. 1). Subsequent cycles 2–4 at a 25% recycle rate resulted in a biomass reduction of 33- ~ 51% relative to the control. The 50% recycle rate resulted in a biomass reduction of 67–77% across cycles 1–3 and a reduction of only ~ 14% in cycle 4 relative to the control growth. The 75% recycle rate, with the exception of cycle 1, had a biomass reduction of ~ 74–85% relative to the control. This suggested that the highest recycle rate of 75% resulted in a lower biomass accumulation relative to the control for all cycles.
Cumulative recycling of supernatant reduced biomass accumulation in all recycled rates relative to the control, and the percentage reduction was stable across cycles 2–4, indicating that percent recycled media and, to a lesser degree the cumulative recycling of media, had an impact on growth (Fig. 1).
Glucose content and pH
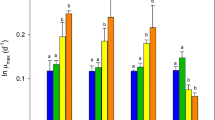
Glucose consumption levels for cycle 1 and 4 for each recycle rate, are represented as a value relative to the control (0% recycle rate; Fig. 2). Glucose reduction in the media was proportional to glucose utilization by the culture. Glucose utilization relative to the control was impacted by the recycle rate (Fig. 2) with higher recycle rates (75%) resulting in a reduction in glucose use (30–40%).
Percentage glucose consumption relative to control measured from Euglena gracilis cultures grown in recycled media rates of 0, 25, 50 and 75%. Glucose measurements were collected at day(s) post inoculation (DPI) 1 and 3 for cycles 1 and 4. Values presented as a percentage of the control calculated using the formula: (([treatment glucose] start-[treatment glucose] end)/ (([control glucose] start-[control glucose] end))*100. Analysis was performed using a Biochemical Analyzer YSI 2700 Select using YSI 1531with D- Glucose Standard 9.00 g L−1 , n = 3
There was a trend towards increased pH from DPI 1 to DPI 3 in all recycled rates and cycles, except for the 0% recycled rate which remained pH stable at ~ 3.2 (Fig. 3). Higher recycling rates and increased cycle number resulted in greater pH increases. For cycle 4, 25% had a ~ threefold relative pH increase compared to the control, 50% a ~ eightfold relative increase and 75% had a ~ 17 fold relative increase compared to the control.
Phytohormone profiles
CK levels varied between the extracellular supernatant (excreted) and cell pellet (retained) as well as between recycle rates and cycle periods. In general, the CK profiles in supernatants were dominated by RB and GLUC CKs (Fig. 4; Table S2), whereas pellets were dominated by NT followed by FB/RB CKs (Fig. 5; Table S3). The dominant CK type present in the freebase-riboside-nucleotide (FBRNT) forms for the supernatant and pellet was iP followed by cisZ (Tables S2 & S3). Measurements of CK levels were categorized into CK forms: freebase (FB) considered active; riboside (RB) considered moderately active; nucleotides (NTs) which are considered inactive precursors; glucosides (GLUCs) generally considered inactivated conjugates; and methylthiolated (MET) CKs for which functions are unknown. The CKs included in these categories are as shown in Table 1 (Sakakibara 2006). Analyses also looked at CK types which were divided into the following groups: dihydrozeatin (DHZ), trans-zeatin (transZ), isopentenyl adenine (iP), and cis-zeatin (cisZ) and their respective conjugates. ABA was analyzed as a common interactive agent with CK and known to be produced under varying conditions in Euglena and other algae (Kiseleva et al. 2012; Lu et al. 2014; Noble et al. 2014).
Total CK (pmol mL−1) detected in the supernatant of Euglena gracilis cultures grown in recycled media rates of 0, 25, 50 and 75%. Analysis was conducted on supernatant collected at day(s) post inoculation (DPI) 1 and 3 for cycles 1 and 4. Total CKs are subdivided into GLUC, MET, NT, RB and FB (Table 1) to show the representation of each form. Error bars represent standard error (SE) for Total CKs, n = 3. Cytokinins (CKs) were analyzed using a Dionex Ultimate 3000 HPLC coupled to a QExactive Orbitrap mass spectrometer (high-performance liquid chromatography-tandem mass spectrometry; HPLC–MS/MS)
Total CK (pmol mL−1) detected in the pelleted biomass from Euglena gracilis cultures grown in recycled media rates of 0, 25, 50 and 75%. Analysis was conducted on pellet harvested at day(s) post inoculation (DPI) 1 and 3 for cycles 1 and 4. Total CKs are subdivided into GLUC, MET, NT, RB and FB (Table 1) to show the representation of each form. Error bars represent standard error (SE) for Total CKs, n = 3. Cytokinins (CKs) were analyzed using a Dionex Ultimate 3000 HPLC coupled to a QExactive Orbitrap mass spectrometer (high-performance liquid chromatography-tandem mass spectrometry; HPLC–MS/MS)
Supernatant ABA levels
ABA levels were only detected within the supernatant fraction and levels generally increased with higher recycling rates (Fig. 6). Starting levels of ABA in cycle 1, DPI 1, showed increased ABA relative to control levels for the recycling rates with 25% having a 2.4 fold increase, 50% a 5.4 fold and 75% a 7.3 fold increase relative to the control (Fig. 6). At the end of cycle 1, ABA levels had decreased for all recycle rates. In cycle 4, both 50 and 75% had detectable levels of ABA, with levels decreasing over the cycle.
Abscisic acid (ABA; pmol mL−1) detected in the supernatant from Euglena gracilis cultures grown in recycled media rates of 0, 25, 50 and 75%. Analysis was conducted on supernatant collected at day(s) post inoculation (DPI) 1 and 3 for cycles 1 and 4. Error bars represent standard error (SE), n = 3. Abscisic Acid (ABA) was analyzed using Dionex Ultimate 3000 HPLC coupled to a QExactive Orbitrap mass spectrometer (high-performance liquid chromatography-tandem mass spectrometry; HPLC–MS/MS)
Total CK: Supernatant
In general, total CK levels increased with increased recycling rates (~ 6000 pmol mL−1 0% to greater than 20,000 pmol mL−1 50%: cycle 1 DPI 1; Fig. 4 and Table S2). In cycle 1, CK levels decreased for recycling rates 0%, 25% and 50% but increased for recycling rate 75% over time (from DPI 1 to DPI 3).
The cytokinin profile changed through repeated recycling. For DPI 1 recycling rates 0%, 25% and 50% were within 10,000 pmol mL−1 to 13,000 pmol mL−1 range, whereas 75% recycling was 2.9-fold higher relative to the control. CK levels were reduced at the end of cycle 4 for recycling rates 0%, 25% and 75%. In most cases, throughout the cycles there was an overall decrease in total CKs with the exception of 75% (cycle 1) and 50% (cycle 4).
CK forms: Supernatant
Within the supernatant fraction in general, RBs and GLUCs were the predominant CK form (categories as in Table 1). At the beginning of cycle 1, RBs were the predominant form for most recycle rates representing 64, 54, 23 and 44% of the total CKs for 0%, 25%, 50% and 75% recycled rates respectively. In contrast, at the end of cycle 1, RBs had decreased, and an increased proportion of GLUCs were present due to a reduction in DHZR and iPR and an increase in transZOG and cisZOG (Fig. 4, Table S2). NTs were also elevated in the 50% and 75% recycled rate at DPI 1 for cycle 1 and reduced in levels during the cycle, but they still represented a higher proportion of overall CKs in 50% and 75% recycled rate at 18% and 17%, respectively, compared to the 0% and 25% recycled rate at 4% each. A difference in form dominance occurred between different recycle rates over time (Fig. 4, Table S2).
During cycle 4, the overall proportion of CK forms represented were RB and GLUCs for most recycle rates. For recycle rate 75%, there was a decrease in overall GLUC % from 33 to 14% between DPI 1 and 3 respectively. NTs were also higher in the 75% recycled rate representing greater than 30% of the total CK whereas other rates had levels ranging from 2% (0% at DPI 1) to 11% (50% at DPI 3; Fig. 4). In general, supernatant profiles showed dominance of RB and GLUC, with changing proportions relative to time and recycle rate.
CK types: Supernatant
CKs can be divided into types including iP, cisZ, transZ and DHZ types. Among the four CK types within the FBRNT grouping, iP was the most dominant representing greater than 80% (cycle 1 DPI 1) of the FBRNT total CKs for each recycle rate followed by cisZ types which increased from DPI 1 to 3 by greater than 10% in all recycle rates (Table S2). Higher levels of cisZ FBRNT representation occurred at DPI 3 in cycle 1 and continued for cycle 4 with the exception of 75% which had 8% cisZ type FBRNT CKs (DPI 3, cycle 4; Table S2).
The methylthiolated CK fraction was dominated by zeatin types representing greater than 74% of the total MET forms throughout the recycle rates and cycles. Glucoside forms showed a dominance of DHZ type across recycle rates and time with an accumulation of cisZ type GLUCs between the DPI 1 and 3 for cycle 1.
Overall, the main CKs detected in the supernatant were iP and cisZ CK types.
Total CK: Pellet
Total CK concentrations in cell pellets remained relatively steady through cycle 1 at DPI 1 and 3 and for cycle 4 at DPI 1 (Fig. 5). The initial concentration measured in cycle 1 for recycling rates 0%, 25% and 50% were comparable (770—835 pmol mL−1) whereas the 75% recycling rate was marginally higher (greater than 1000 pmol mL−1). For cycle 4, (Fig. 5), levels among recycling rates were between 817 and 1055 pmol mL−1 (DPI 1). By DPI 3 the higher recycling rates (50 and 75%) had increased total CK concentrations to greater than 1550 pmol mL−1 (Fig. 5).
CK forms: Pellet
The dominant CK forms identified in the pellet were different from those of the supernatant. The pellet was dominated by NTs representing, in general, greater than 60% of the total CK levels with the exception of 0% and 75% for DPI 1 cycle 1 (Fig. 5), followed by FBs. Higher levels of METs (greater than 250 pmol mL−1) occurred at the higher recycling rates of 50% and 75%, and this was due to the later cycle abundance of 2MeSiP (cycle 4; Table S3). Among the pellet fractions, all forms were detected and led to similar profiles with a few exceptions. For instance, by DPI 3 of cycle 4 the relative profile abundance of METs was higher than FB or RB respectively (with the exception of 0% recycled rate), this trend was not seen in the supernatant profiles. Of the MET CKs, 2MeSiP was reduced between the beginning and end of cycle 1, whereas it increased between the beginning and end of cycle 4 for 25, 50 and 75% recycled rates (Table S3).
CK types: Pellet
The iP types from the FBRNT group highly dominated the pellet fraction across all cycles and recycle rates being present at levels greater than 74% and upwards of 92% (50%, 75% respectively, DPI 3 cycle 4) in the FBRNT grouping (Table S3). iP levels were due to iPNT where levels increased between DPI 1 and 3 for all samples with the exception of the 0% recycle rate in cycle 4 (Table S3). cisZ types from the FBRNT group were the next most abundant CK type representing ~ 5% or greater of the FBRNT grouping. For the methylthiolated CK forms the most abundant type was 2MeSiP representing greater than 61% of the methylthiolated CK forms in all recycle rates and cycles. For glucosides, the dominant form was cisZ type representing ~ 65% or greater levels in all recycle rates and cycles (Table S3), which is in contrast to the dominance of DHZ-GLUCs found in the supernatant. The trend towards iP and cisZ CK types suggested a dominance of the tRNA degradation pathway in this system.
Discussion
Understanding the interplay that occurs within a culture system is crucial to optimizing growth parameters and product output. The variety of culturing parameters and input sources for E. gracilis provides an opportunity to examine the dynamics of cellular production and phytohormone signaling that occurs within different fermentation strategies, specifically in recycling of spent media.
Performance of E. gracilis cultures under media recycling
Cell numbers increased for each recycle rate throughout the cycles at comparable levels (Fig. S3), whereas dry biomass was reduced at higher recycle rates (Fig. 1) and reduced glucose consumption relative to the control as recycle rate increased (Fig. 2). While biomass and glucose utilization were reduced relative to the control, cell number remained similar. The biomass differences between treatments may be due to changes in cellular composition. Cultivation conditions for E. gracilis significantly impact the culture profile (Wang et al. 2018; Gissibl et al. 2019; Yoshioka et al. 2020). For example, compositional shifts between protein and paramylon (a storage polysaccharide in E. gracilis) occur when pH changes during E. gracilis stationary growth. Under these conditions paramylon increased with a substantial decrease in protein content at a pH above 8.0 (Hayashi et al. 1994). This highlights the versatility in growth parameters that E. gracilis can adapt to, as well as how cellular composition can change with variation in external parameters. In the current study, pH shifted due to recycled media across cycles (but remained less than pH 5) and may have resulted in changes to cellular composition. While determining the overall culture compositional profile was not a goal for this paper, it could provide further insight into the impacts of recycled organic media on biomass output and composition. The use of recycled organic media—made using sterile handling without the need for filtration or between cycle sterilization—does result in adequate E. gracilis growth, providing another growth condition in which this versatile microalga can proliferate.
CK biosynthesis
Recent CK studies on Euglena have been mostly limited to photosynthetic cultivation (Noble et al. 2014; Nguyen et al. 2023). The growth conditions greatly impact the nature of CK profiles. In the current study, supernatant levels were upwards of 29,000 pmol mL−1 at the 75% cycle 4, DPI 1. This was higher than in other studies where media source, phototrophic vs. heterotrophic growth as well as culture fraction influenced cytokinin levels. For example, toxic metals including nickel, lead and cadmium modified the CK profiles of E. gracilis where the control treatment had nucleotides and methylthiolated CKs and some aromatic CKs at levels much lower (less than 272 pmol gDW−1 for total CKs; Nguyen et al. 2023) than what were detected in the current study (minimum level of ~ 760 pmol moL−1: 0% cycle 4, DPI 1 to greater than 1600 pmol mL−1 for total pellet CKs: 50% cycle 4, DPI 3). Euglena growth under non-axenic phototrophic conditions in defined media had total CK levels less than 3 pmol gFW−1 in pellets and less than 4 pmol mL−1 for supernatant (Noble et al. 2014).
In the current study, a variety of CKs were detected that may have originated from the organic media in which E. gracilis was grown as well as the culturing conditions to which it was subjected. Culturing conditions have a distinct impact on E. gracilis metabolite production. For example, E. gracilis can be triggered to produce several cyclic lipopeptides containing asparagine and non-proteinogenic amino acids referred to as euglenatides, that are not found under normal culturing conditions. Euglena gracilis growth under nitrogen limiting, phototrophic, synthetic media with the addition of a key amino acid, glutamic acid, resulted in the production of these euglenatides some of which resemble nemamides and have antiproliferative properties (Aldholmi et al. 2022). Euglena can produce a variety of compounds under different growth conditions which is likely why varying CK profiles are found between this study and other studies where E. gracilis was cultured under phototrophic conditions (Noble et al. 2014; Nguyen et al. 2023).
CK types can vary based on environmental conditions, tissue type or developmental stage (Schäfer et al. 2015). The dominance of a particular CK type can also indicate the activation of a particular biosynthetic pathway. For example, systems are typically more active in the de novo pathway when dominated by transZ CK types whereas those dominated by the tRNA degradation pathway often have more cisZ CKs (Sakakibara 2006). In this study, cisZ CK types and related conjugates were most abundant, followed by iP and DHZ. Methylthiolated CKs were detected and these are also proposed to be synthesized by the tRNA degradation pathway (Morrison et al. 2015; Žižková et al. 2017; Gibb et al. 2020). Though the tRNA degradation pathway is the only widely accepted source of cisZ, transZ derivatives have also been detected in the tRNA extracts of some algae including Scendedesmus obliquus (Žižková et al. 2017). CKs found in E. gracilis to date are typically dominated by iP, cisZ and methylthiolated CK types (Swaminathan and Bock 1977; Noble et al. 2014; Nguyen et al. 2023). CKs isolated from the tRNA fraction of phototrophically grown E. gracilis were identified as cisZR, 2MeScisZR, and iPR as the dominant forms. Subsequent analysis leads to suggested localization of these CKs in E. gracilis by comparing light and dark grown wildtype cultures as well as chloroplast bleached mutants. It was found that iPR was located mainly in the cytoplasmic and chloroplastic tRNA, cisZR was found mainly in the cytoplasmic tRNA and 2MeScisZR was localized in the chloroplast tRNA (Swaminathan et al. 1977). This prevalence of cisZ over transZ CK types suggested by the hormone data corresponds well with findings within other microalgae which have higher reported cisZ CK levels (Stirk et al. 2003; Jirásková et al. 2009; Stirk and Staden 2010; Kiseleva et al. 2012; Noble et al. 2014; Žižková et al. 2017).
cisZ and iP-CK type
The concentration, forms, and CK types present in cells are controlled by biosynthesis pathways. In the case of E. gracilis, this involves tRNA degradation (Swaminathan and Bock 1977; Swaminathan et al. 1977; Noble et al. 2014; Nguyen et al. 2021). With dominant production of iP FBRNTs and cisZ type CKs, this study highlighted that these CK types fluctuate and their levels may be connected to increased culturing time and media recycling. The balance of cisZ and iP CK types between supernatant and pellet environments in E. gracilis aligns with the results of other microalgae, which monitored CK levels. Those studies indicated that the microbes have the capacity to synthesize and excrete or retain only certain types of CKs into the surrounding environment to control growth (Ördög et al. 2004; Jirásková et al. 2009; Xinyi et al. 2016). Similarly, proportions of isomers differ greatly during the lifespan of Arabidopsis, maize, annual ryegrass and chickpea associated with growth-limiting conditions resulting from internal and/or external cues (Gajdosová et al. 2011). This suggests that cisZ CKs may be responsible for the maintenance of CK activity necessary for survival and recovery from restrictive conditions. Accordingly, the balance of cisZ with other CK types like iP-CKs, is likely important in response to stress conditions.
CKs in supernatant and pellet fractions
In general, the supernatant fraction had higher CK RBs and GLUCs and pellets had higher levels of NT and FB CK forms. These data highlight the difference in CK retention and excretion.
The accumulation of GLUCs in the supernatant indicated that E. gracilis can deactivate CKs into GLUC forms. As RBs are thought to be an intermediate form acting as precursors to the most active FBs (Nguyen et al. 2021; Romanov and Schmülling 2022), the higher abundance in the supernatant at the start of growth coincides with the potential for converting RBs to FBs under favourable growth conditions (Stirk et al. 2013). The high amounts of GLUCs within the supernatant fraction of E. gracilis could be due to many reasons. First, as GLUCs are the largest form of CKs found, the presence of these molecules outside of the cell indicates a potential for a secretory pathway via a vesicle exocytosis pathway – which may selectively and actively secrete the glucoside conjugates (Satiat-Jeunemaitre and Hawes 1993; Bajguz and Piotrowska 2009). Analysis of pellets of a variety of cyanobacteria and algae detected relatively small amounts of CK-O and N glucosides. Although, in some samples glucosides were detected endogenously, the lack of GLUC detection may be because the supernatant fraction was not examined (Žižková et al. 2017) whereas in the current study, both the pellet and supernatant were analyzed resulting in higher GLUC detection in the supernatant.
CKs in the pellet fraction were different from those released to the supernatant in the present study. As NTs are abundant and first produced in the pellet (where biosynthesis occurs), extremely low detected levels of NTs in the supernatant correspond to retention of precursors in the pellet. This finding is in line with other studies where NTs were selectively retained in mammalian HeLa cells (Aoki et al. 2019b). Also, with the increase of NTs and decrease in other forms, it suggests that NTs are selectively retained (unable to be transported) by CK transporters like purine permeases (Bürkle et al. 2003), equilibrative nucleoside transporters for nucleosides (Hirose et al. 2005) or ATP-binding cassette transporters (ABCG14; Ko et al. 2014) with selectivity for transporting smaller, less charged CKs in E. gracilis. Within the pellet fraction, METs increased late in the cycles and were elevated with higher recycling rates. This later incorporation of METs may indicate that these forms of CK are useful to E. gracilis, however, the function of METs in E. gracilis or any other organisms remains unknown (Gibb et al. 2020). While documented in prokaryotes in high levels along with iP derivatives, this system accumulated METs within the pellet, a result that was similar to a study of Rhizobium bacteria (Kisiala et al. 2013).
As nutrition and CK content are closely related (Sakakibara 2021), perhaps under recycled conditions, E. gracilis regulates a switch of CK forms to best adapt to the changing stressful nutrient conditions via a complex network of hormone signaling analogous to quorum sensing in bacteria.
Higher concentrations of phytohormones correspond with greater media recycling rates
This study showed that using higher recycling of media increased both CK and ABA supernatant concentrations– in the 50% and particularly in the 75% recycling trials.
Another possibility is that secreted active CKs under recycled conditions, may have an intercellular communication purpose analogous to quorum sensing – a series of chemical signals coordinated as a response to population density to regulate hormonal balance under specific conditions (Zhou et al. 2016). In the microbial world, quorum sensing is a common means of cell-to-cell communication. Much like CKs, quorum sensing is mediated by low molecular weight molecules that can move across membranes and accumulate in targeted locations. CKs often create a nutrient sink to perform their action, where quorum sensing molecules amass in a dense environment where they are produced to regulate growth at high densities once a threshold is met, thereby causing a coordinated response (Proust et al. 2011). Cross-kingdom quorum sensing signaling between bacteria and host confers indirect biological functions like slowed growth and other physiological functions, production of secondary metabolites and can be involved in defense responses (Gambello and Iglewski 1991; Chi et al. 2017). Perhaps then, ABA and CK levels are altered within the supernatant with higher recycling rates as a signal of substandard growth conditions.
Impact of ABA
ABA is often present in plant cells when nutrient availability is low (Nambara and Marion-Poll 2005) in a manner opposite to CKs; however, ABA can also be present when cells are actively dividing (Takezawa et al. 2011; Noble et al. 2014). In this study, ABA concentrations were only detected in the supernatant fraction indicating that they were produced and quickly secreted. Thus, ABA likely played an inhibitory role in E. gracilis biomass as the levels increased with increasing recycling rates. CKs and ABA did not show an opposite trend as is typically seen in higher plants. Instead, both hormones increased with increasing recycling rates, suggesting that E. gracilis may differ from hormonal communication in higher order plants (Hirsch et al. 1989; Yokoya et al. 2010). This may suggest that Euglena can secrete ABA preferentially into the surrounding environment to respond to nutrient stress (Lu and Xu 2015; Romanenko et al. 2015; Chi et al. 2017; Han et al. 2018). Overall, growth parameter changes reflected the dynamic work of hormone regulation.
Conclusions
This study provided novel insights into the dynamics of endogenous and exogenous CKs and ABA and growth characteristics of heterotrophic E. gracilis cultured in recycled media. Under four recycled conditions using an organic medium, the results showed that recycling culture medium is viable for use as a growth medium but a recycling rate above 25% can result in reduced dry weight biomass. To further understand biochemical mitigation of any stress the media recycling caused, ABA and CK phytohormones were profiled. This study is the first to report detected levels of approximately 22 different CKs in addition to ABA, in E. gracilis cells and supernatants. Euglena gracilis synthesizes CKs via the tRNA-degradation pathway. Accordingly, the cells increased in forms and types of CKs (cisZCKs, iPCKs) under media recycling conditions. Both CKs and ABA showed corresponding increased levels with increasing recycling rates. E. gracilis synthesizes ABA and exports it to the surrounding medium but does not retain it in the cells. Extracellular applications of combinations of CK and ABA synergistically acted to increase culture growth (Noble et al. 2014) and ABA can increase endogenous CKs to help E. gracilis overcome some levels of heavy metal stress (Nguyen et al. 2023). Thus, more research is required to determine if CK or ABA could be used to override negative impacts of industrial-scale Euglena fermentation with recycled media. Based on the data, industry could, for example, adjust parameters to enhance iP and cisZ CK types during the growth process.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Acién FG, Fernández JM, Magán JJ, Molina E (2012) Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol Adv 30:1344–1353
Aldholmi M, Ahmad R, Carretero-Molina D, Pérez-Victoria I, Martín J, Reyes F, Genilloud O, Gourbeyre L, T. Gefflaut T, Carlsson H, Maklakov A, O’Neill E, Field RA, Wilkinson B, O’Connell M, Ganesan A, (2022) Euglenatides, potent antiproliferative cyclic peptides isolated from the freshwater photosynthetic microalga Euglena gracilis. Angew Chem Int 61:e202203175
Aoki MM, Kisiala AB, Rahman T, Morrison EN, Emery RJN (2021) Cytokinins are pervasive among common in vitro culture media: An analysis of their forms, concentrations and potential sources. J Biotechnol 334:43–46
Aoki MM, Kisiala AB, Li S, Stock NL, Brunetti CR, Huber RJ, Emery RJN (2019a) Cytokinin Detection during the Dictyostelium discoideum life cycle: Profiles are dynamic and affect cell growth and spore germination. Biomolecules 9:702
Aoki MM, Seegobin M, Kisiala A, Noble A, Brunetti C, Emery RJN (2019) Phytohormone metabolism in human cells: Cytokinins are taken up and interconverted in HeLa cell culture. FASEB Bioadv 1:320–331
Bajguz A, Piotrowska A (2009) Conjugates of auxin and cytokinin. Phytochemistry 70:957–969
Bürkle L, Cedzich A, Döpke C, Stransky H, Okumoto S, Gillissen B, Kühn C, Frommer WB (2003) Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. Plant J 34:13–26
Chen F (1996) High cell density culture of microalgae in heterotrophic growth. Trends Biotech 14:421–426
Chi W, Zheng L, He C, Han B, Zheng M, Gao W, Sun C, Zhou G, Gao X (2017) Quorum sensing of microalgae associated marine Ponticoccus sp. PD-2 and its algicidal function regulation. AMB Express 7:59
Cook J R (1968) The cultivation and growth of Euglena. In: Buetow DE (ed.), The Biology of Euglena. I: General Biology and Ultrastructure. Academic Press, New York, pp 243–314
Cowan AK, Rose PD (1991) Abscisic acid metabolism in salt-stressed cells of Dunaliella salina: Possible interrelationship with β-carotene accumulation. Plant Physiol 97:798–803
Discart V, Bilad MR, Marbelia L, Vankelecom IFJ (2014) Bioresource Technology Impact of changes in broth composition on Chlorella vulgaris cultivation in a membrane photobioreactor (MPBR) with permeate recycle. Bioresour Technol 152:321–328
Ebenezer TE, Low RS, O'Neill EC, Huang I, DeSimone A, Farrow SC, Field RA, Ginger ML, Guerrero SA, Hammond M, Hampl V, Horst G, Ishikawa T, Karnkowska A, Linton EW, Myler P, Nakazawa M, Cardol P, Sánchez-Thomas R, Saville BJ, Shah MR, Simpson AGB, Sur A, Suzuki K, Tyler KM, Zimba PV, Hall N, Field MC (2022) Euglena International Network (EIN): Driving euglenoid biotechnology for the benefit of a challenged world. Biol Open 11:bio059561
Emery RJN, Ma Q, Atkins CA (2000) The forms and sources of cytokinins in developing white lupine seeds and fruits. Plant Physiol 123:1593–1604
Farooq W, Suh WI, Park MS, Yang J (2014) Water use and its recycling in microalgae cultivation for biofuel application. Bioresour Technol 184:73–81
Farooq W (2021) Sustainable production of microalgae biomass for biofuel and chemicals through recycling of water and nutrient within the biorefinery context: A review. GCB Bioenergy 13:914–940
Fernández FGA, Reis A, Wijffels RH, Barbosa M, Verdelho V, Llamas B (2021) The role of microalgae in the bioeconomy. New Biotech 61:99–107
Fret J, Roef L, Blust R, Diels L, Tavernier S, Vyverman W, Michiels M (2017) Reuse of rejuvenated media during laboratory and pilot-scale cultivation of Nannochloropsis sp. Algal Res 27:265–273
Gambello MJ, Iglewski BH (1991) Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol 173:3000–3009
Gajdosová S, Spíchal L, Kamínek M, Hoyerová K, Novák O, Dobrev PI, Galuszka P, Klíma P, Gaudinová A, Zizková E, Hanus J, Dancák M, Trávnícek B, Pesek B, Krupicka M, Vanková R, Strnad M, Motyka V (2011) Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J Exp Bot 62:2827–2840
Gibb M, Kisiala AB, Morrison EN, Emery RJN (2020) The origins and roles of methylthiolated cytokinins: Evidence from among life Kingdoms. Front Cell Dev Biol 8:605672
Gissibl A, Sun A, Care A, Nevalainen H, Sunna A (2019) Bioproducts from Euglena gracilis: Synthesis and applications. Front Bioeng Biotechnol 7:108
Han X, Zeng H, Bartocci P, Fantozzi F, Yan Y (2018) Phytohormones and effects on growth and metabolites of microalgae: A review. Fermentation 4:25
Hartung W (2010) The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Funct Plant Biol 37:806–812
Hayashi M, Toda K, Ishiko H, Komatsu R, Kitaoka S (1994) Effects of shifting pH in the stationary phase of growth on the chemical composition of Euglena gracilis. Biosci Biotech Biochem 58:1964–1967
Hirose N, Makita N, Yamaya T, Sakakibara H (2005) Functional characterization and expression analysis of a gene, OsENT2, encoding an equilibrative nucleoside trans-porter in rice suggest a function in cytokinin transport. Plant Physiol 138:196–206
Hirsch R, Hartung W, Gimmler H (1989) Abscisic acid content of algae under stress. Bot Acta 102:326–334
Hlavova M, Turoczy Z, Bisova K (2015) Improving microalgae for biotechnology — From genetics to synthetic biology. Biotechnol Adv 33:1194–1203
Hnain AK, Cockburn LM, Lefebvre DD (2011) Microbiological processes for waste conversion to bioenergy products: Approaches and directions. Environ Rev 19:214–237
Jirásková D, Poulíčková A, Novák O, Sedláková K, Hradecká V, Strnad M (2009) High-throughput screening technology for monitoring phytohormone production in microalgae. J Phycol 45:108–118
Kim S, Wirasnita R, Lee D, Yu J, Lee T (2021) Enhancement of growth and paramylon production of Euglena gracilis by upcycling of spent tomato byproduct as an alternative medium. Appl Sci 11:8182
Kiseleva AA, Tarachovskaya ER, Shishova MF (2012) Biosynthesis of phytohormones in algae. Russ J Plant Physiol 59:595–610
Kisiala A, Kambhampati S, Stock NL, Aoki M, Emery RJN (2019) Quantification of cytokinins using high-resolution accurate-mass orbitrap mass spectrometry and parallel reaction monitoring (PRM). Anal Chem 91:15049–15056
Kisiala A, Laffont C, Emery RJ, Frugier F (2013) Bioactive cytokinins are selectively secreted by Sinorhizobium meliloti nodulating and nonnodulating strains. Mol Plant Microbe Interact 26:1225–1231
Ko D, Kang J, Kiba T, Park J, Kojima M, Do J, Kim KY, Kwon M, Endler A, Song WY, Martinoia E, Sakakibara H, Lee Y (2014) Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc Natl Acad Sci U S A 111:7150–7155
Leedale GF (1964) Pellicle structure in Euglena. Brit Phycol Bull 2:291–306
Liu L, Pohnert G, Wei D (2016) Extracellular metabolites from industrial microalgae and their biotechnological potential. Mar Drugs 14:191
Loftus SE, Johnson ZI (2017) Cross-study analysis of factors affecting algae cultivation in recycled medium for biofuel production. Algal Res 24:154–166
Lowrey J, Armenta RE, Brooks MS (2016) Nutrient and media recycling in heterotrophic microalgae cultures. Appl Microbiol Biotechnol 100:1061–1075
Lu Y, Tarkowská D, Turečková V, Luo T, Xin Y, Li J, Wang Q, Jiao N, Strnad M, Xu J (2014) Antagonistic roles of abscisic acid and cytokinin during response to nitrogen depletion in oleaginous microalga Nannochloropsis oceanica expand the evolutionary breadth of phytohormone function. Plant J 80:52–68
Lu Y, Xu J (2015) Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends Plant Sci 20:273–282
Mathieu-Rivet E, Mati-Baouche N, Walet-Balieu ML, Lerouge P, Bardor M (2020) N- and O-glycosylation pathways in the microalgae polyphyletic group. Front Plant Sci 11:609993
Morrison EN, Emery RJ, Saville BJ (2015) Phytohormone involvement in the Ustilago maydis- Zea mays Pathosystem: Relationships between abscisic acid and cytokinin levels and strain virulence in infected cob tissue. PLoS One 10:e0130945
Morrison EN, Emery RJN, Saville BJ (2017) Fungal derived cytokinins are necessary for normal Ustilago maydis infection of maize. Plant Pathol 66:726–742
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Nguyen HN, Nguyen TQ, Kisiala AB, Emery RJN (2021) Beyond transport: cytokinin ribosides are translocated and active in regulating the development and environmental responses of plants. Planta 254:45
Nguyen NH, Nguyen QT, Dang DH, Emery RJN (2023) Phytohormones enhance heavy metal responses in Euglena gracilis: Evidence from uptake of Ni, Pb and Cd and linkages to hormonomic and metabolomic dynamics. Environ Pollut 320:121094
Noble A, Kisiala A, Galer A, Clysdale D, Emery RJN (2014) Euglena gracilis (Euglenophyceae) produces abscisic acid and cytokinins and responds to their exogenous application singly and in combination with other growth regulators. Eur J Phycol 49:244–254
O’Neill EC, Trick M, Hill L, Rejzek M, Dusi RG, Hamilton CJ, Zimba PV, Henrissat B, Field RA (2015) The transcriptome of Euglena gracilis reveals unexpected metabolic capabilities for carbohydrate and natural product biochemistry. Mol Biosyst 11:2808–2820
Ord G, Cameron I, Fensom D (1977) The effect of pH and ABA on the hydraulic conductivity of Nitella membranes. Can J Bot 55:1–4
Ördög V, Stirk WA, Lenobel R, Bancifova M, Strnad M, van Staden J, Szigeti J, Nemeth L (2004) Screening microalgae for some potentially useful agricultural and pharmaceutical secondary metabolites. J Appl Phycol 16:309–314
Perez-Garcia O, Escalante FME, De-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res 45:11–36
Pils B, Heyl A (2009) Unraveling the evolution of cytokinin signaling. Plant Physiol 151:782–791
Proust H, Hoffmann B, Xie X, Yoneyama K, Schaefer DG, Yoneyama K, Nogué F, Rameau C (2011) Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 138:1531–1539
Rodolfi L, Zittelli GC, Barsanti L, Rosati G, Tredici MR (2003) Growth medium recycling in Nannochloropsis sp. mass cultivation. Biomol Eng 20:243–248
Romanenko EA, Kosakovskaya IV, Romanenko PA (2015) Phytohormones of microalgae: Biological role and involvement in the regulation of physiological processes. Pt I. Auxins, abscisic acid, ethylene. Int J Algae 17:275–289
Romanov GA, Schmülling T (2022) On the biological activity of cytokinin free bases and their ribosides. Planta 255:27
Rösch C, Skarka J, Wegerer N (2012) Materials flow modeling of nutrient recycling in biodiesel production from microalgae. Bioresour Technol 107:191–199
Ruiz J, Olivieri G, de Vree J, Bosma R, Willems P, Reith JH, Eppink MHM, Kleinegris DMM, Wijffels RH, Barbosa MJ (2016) Towards industrial products from microalgae. Energy Environ Sci 9:3036–3043
Sakakibara H (2006) Cytokinins: Activity, biosynthesis, and translocation. Annu Rev Plant Biol 57:431–439
Sakakibara H (2021) Cytokinin biosynthesis and transport for systemic nitrogen signaling. Plant J 105:421–430
Santner A, Calderon-Villalobos LI, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5:301–307
Satiat-Jeunemaitre B, Hawes C (1993) Insights into the secretory pathway and vesicular transport in plant cells. Biol Cell 79:7–15
Schäfer M, Brütting C, Meza-Canales ID, Großkinsky DK, Vankova R, Baldwin IT, Meldau S (2015) The role of cis-zeatin-type cytokinins in plant growth regulation and mediating responses to environmental interactions. J Exp Bot 66:4873–4884
Schwender J, Gemünden C, Lichtenthaler HK (2001) Chlorophyta exclusively use the 1-deoxyxylulose 5-phosphate/2-C-methylerythritol 4-phosphate pathway for the biosynthesis of isoprenoids. Planta 212:416–423
Seegobin M, Kisiala A, Noble A, Kaplan D, Brunetti C, Emery RJN (2018) Canis familiaris tissues are characterized by different profiles of cytokinins typical of the tRNA degradation pathway. FASEB J 32:6575–6581
Spíchal L (2012) Cytokinins - Recent news and views of evolutionally old molecules. Funct Plant Biol 39:267–284
Stirk W, Novák O, Strnad M, van Staden J (2003) Cytokinins in macroalgae. Plant Growth Regul 41:13–24
Stirk WA, van Staden J (2010) Flow of cytokinins through the environment. Plant Growth Regul 62:101–116
Stirk WA, Ördög V, Novák O, Rolčík J, Strnad M, Bálint P, van Staden J (2013) Auxin and cytokinin relationships in 24 microalgal strains. J Phycol 49:459–467
Swaminathan S, Bock RM (1977) Isolation and identification of cytokinins from Euglena gracilis transfer ribonucleic acid. Biochemistry 16:1355–1360
Swaminathan S, Bock RM, Skoog F (1977) Subcellular localization of cytokinins in transfer ribonucleic acid. Plant Physiol 59:558–563
Takezawa D, Komatsu K, Sakata Y (2011) ABA in bryophytes: how a universal growth regulator in life became a plant hormone? J Plant Res 124:437–453
Tominaga N, Takahata M, Tominaga H (1993) Effects of NaCl and KNO3 concentrations on the abscisic acid content of Dunaliella sp. (Chlorophyta). Hydrobiologia 267:163–168
Vigani M, Parisi C, Rodriguez-Cerezo E, Barbosa MJ, Sijtsma L, Ploeg M, Enzing C (2015) Food and feed products from micro-algae: Market opportunities and challenges for the EU. Trends Food Sci Technol 42:81–92
Wang Y, Seppänen-Laakso T, Rischer H, Wiebe MG (2018) Euglena gracilis growth and cell composition under different temperature, light and trophic conditions. PLoS One 13:e0195329
Xu JM, Xiong JQ (2023) Boosting the yields of microalgal biomass and high-value added products by phytohormones: A mechanistic insight using transcriptomics. J Cleaner Production 393:136175
Xinyi E, Crofcheck C, Crocker M (2016) Application of recycled media and algae-based anaerobic digestate in Scenedesmus cultivation. J Renew Sustain Energy 8:013116
Yokoya NA, Stirk WA, van Staden J, Novak O, Tureckova V, Pencik A, Strnad M (2010) Endogenous cytokinins, auxins, and abscisic acid in red algae from Brazil. J Phycol 46:1198–1205
Yoshioka K, Suzuki K, Osanai T (2020) Effect of pH on metabolite excretion and cell morphology of Euglena gracilis under dark, anaerobic conditions. Algal Res 51:102084
Yu X, Chen L, Zhang W (2015) Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front Microbiol 6:56
Zhou J, Lyu Y, Richlen M, Anderson DM, Cai Z (2016) Quorum sensing is a language of chemical signals and plays an ecological role in algal-bacterial interactions. CRC Crit Rev Plant Sci 35:81–105
Zhu J, Hong DD, Wakisaka M (2019) Phytic acid extracted from rice bran as a growth promoter for Euglena gracilis. Open Chem 17:57–63
Žižková E, Kubeš M, Dobrev PI, Přibyl P, Šimura J, Zahajská L, Záveská Drábková L, Novák O, Motyka V (2017) Control of cytokinin and auxin homeostasis in cyanobacteria and algae. Ann Bot 119:151–166
Acknowledgements
The authors wish to acknowledge the financial support from the Natural Sciences and Engineering Council of Canada Discovery Grant (NSERC RGPIN-05436) and NSERC College-University Idea to Innovation Grant Program (CUI2I 470778-14). The authors thank Canadian Foundation for Innovation: Leaders Opportunity Fund and Ontario Research Fund: Research Infrastructure for funding instrumentation, including the Thermo Fisher QExactive Orbitrap, in the Water Quality Centre at Trent University.
Funding
Financial support from the Natural Sciences and Engineering Council of Canada Discovery Grant (NSERC RGPIN-05436) to RJNE, ENM and TS and NSERC College-University Idea to Innovation Grant Program (CUI2I 470778–14) to RJNE, ABK and AMK is gratefully acknowledged. Partial funding to AMK was provided by Noblegen Inc. The authors thank Canadian Foundation for Innovation: Leaders Opportunity Fund and Ontario Research Fund: Research Infrastructure for funding instrumentation, including the Thermo Fisher QExactive Orbitrap, in the Water Quality Centre at Trent University.
Author information
Authors and Affiliations
Contributions
AMK: Methodology, Formal analysis, Investigation, Writing Original Draft; ENM: Data analysis, Figure and Table creation, Writing-review and Editing; TS: Writing Draft; ABK: Formal analysis; KHR: Investigation; AN: Funding acquisition; RJN: Conceptualization, Project administration, Supervision, Funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Noblegen Inc. is a commercial, for-profit organization that is using E. gracilis in future food products and biosolutions. Partial funding for this research was provided by Noblegen Inc. KHR is an employee of Noblegen Inc. AN is the CEO and founder of Noblegen Inc. and is a shareholder.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuhne, A.M., Morrison, E.N., Sultana, T. et al. Cultivation of heterotrophic Euglena gracilis: The effects of recycled media on culture growth and associations with growth regulating phytohormone profiles. J Appl Phycol 35, 2161–2175 (2023). https://doi.org/10.1007/s10811-023-03062-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03062-4