Abstract
Main conclusion
Recent publications have increased our knowledge of how pectin composition and the degree of homogalacturonan methylesterification impact the biochemical and biomechanical properties of plant cell walls, plant development, and plants’ interactions with their abiotic and biotic environments. Experimental observations have shown that the relationships between the DM, the pattern of de-methylesterificaton, its effect on cell wall elasticity, other biomechanical parameters, and growth are not straightforward. Working towards a detailed understanding of these relationships at single cell resolution is one of the big tasks of pectin research.
Pectins are highly complex polysaccharides abundant in plant primary cell walls. New analytical and microscopy techniques are revealing the composition and mechanical properties of the cell wall and increasing our knowledge on the topic. Progress in plant physiological research supports a link between cell wall pectin modifications and plant development and interactions with the environment. Homogalacturonan pectins, which are major components of the primary cell wall, have a potential for modifications such as methylesterification, as well as an ability to form cross-linked structures with divalent cations. This contributes to changing the mechanical properties of the cell wall. This review aims to give a comprehensive overview of the pectin component homogalacturonan, including its synthesis, modification, regulation and role in the plant cell wall.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pectin as biochemical components of the plant cell wall
Cell walls play diverse functions including protection and interaction with the environment. The cell wall is also responsible for the plant cell’s capacity to develop turgor pressure, which is necessary for cell growth and ultimately for supporting the entire plant body structure.
Plant cell walls
The plant cell wall is a highly dynamic structure, continually being modified throughout development and in reaction to diverse environmental conditions. The genesis of each cell wall is the division process: during cell division, the primary cell wall grows from the cell plate to the nearest wall (Bordenave and Goldberg 1993). Subsequently, the surface area of this primary cell wall greatly increases at a special interface, the middle lamella, between the two newly formed, adjacent, primary cell walls. Finally, once growth has ceased, secondary cell walls are deposited onto the primary cell walls from the plasma membrane side. The secondary cell wall composition depends on the tissue and function of the cell, but their pectin content is lower than that of primary cell walls (Caffall and Mohnen 2009). Current models describe the primary cell wall as a complex highly cross-linked polymer framework (Fry 2004; Somerville et al. 2004; Cosgrove 2005; Cosgrove and Jarvis 2012). The secondary cell wall is generally less dynamic and malleable and may remain relatively static once deposited.
Most higher plants contain over 30 % pectic polysaccharides in their primary cell walls. Within the primary wall, pectin forms a hydrated gel phase in which the network of cellulose microfibrils and other cell wall components are embedded. Individual pectin polymers interact covalently and non-covalently with other cell wall components and other pectin molecules, contributing to cell wall structure and function.
Pectins are a group of complex polysaccharides whose defining features are 1,4-linked α-D-galacturonic acid residues. Pectins are classified into homogalacturonan (HG), the substituted galacturonan xylogalacturonan (XGA), and the two rhamnogalacturonans RG-I and RG-II (Ridley et al. 2001). HG is a polymer that contains only α-1,4-linked-d-galacturonic acid (Ridley et al. 2001). XGA has a HG backbone that it is substituted with single β-1,3-Xyl residues which can in turn be linked to an additional xylose residue. In contrast, RG-I has a backbone structure of [α-D-GalpA-1,2-α-L-Rhap-1,4]n repeating units; RG-I has been found to be largely unbranched in Arabidopsis seed mucilage (Western et al. 2001), but is frequently branched in other anatomical contexts with side chains of arabinan, galactan or arabinogalactan attached at the C-4 positions of backbone sugars (Lau et al. 1985). RG-II has a highly complex and conserved structure with a small HG backbone of only a few residues, and four well-defined side chains with a total of 12 different glycosyl residues in over 20 different linkages (Caffall and Mohnen 2009). RG-II is ubiquitous in the plant cell wall and represents up to 8 % of Arabidopsis plant cell wall polymers (Zablackis et al. 1995).
The different pectic polysaccharides can be covalently linked to each other, forming a massive pectin network. The known extent of this network has been expanded by several publications: HG can be covalently cross-linked to the pectic polysaccharides RG-I and RG-II (Willats et al. 2001), and also to the hemicellulose xyloglucan (XG) (Nakamura et al. 2002). In addition, HG can be covalently linked to arabinogalactan protein; it was recently demonstrated in Arabidopsis that a rhamnosyl residue in the arabinogalactan domain of the arabinogalactan protein ARABINOXYLAN PECTIN ARABINOGALACTAN PROTEIN1 (APAP1) was linked to RG-I and HG (Tan et al. 2013). Mutants lacking this protein and the corresponding wall structure showed an increased extractability of pectic and hemicellulosic polysaccharides, which indicates that the pectin–protein linkage plays an important structural role (Tan et al. 2013). The ratio of HG, XGA, RG-I, and RG-II differs between species, tissues, and developmental states, but HG is typically the most abundant pectin polymer and will remain our focus in this review.
HG biosynthesis and secretion
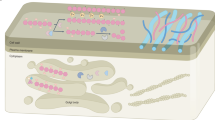
HG is synthesized in the Golgi apparatus and secreted into the cell wall. HG biosynthesis and processing are summarized in Fig. 1. In general, nucleotide sugars are transported from the cytosol to the Golgi, where glycosyl transferases assemble monosaccharides into polysaccharides. In reality, this process is complex; for example, it has been shown that a large number of glycosyl transferases are required to synthesize the pectic polysaccharides (Ridley et al. 2001; Caffall and Mohnen 2009; Rautengarten et al. 2014).
A complex of synthetic enzymes including Galacturonosyltransferase-1 (GAUT1) and -7 (GAUT-7) participates in the synthesis of the homogalacturonan (HG) pectin component (symbolized by black lines in the figure) in the Golgi apparatus. During synthesis, methylester groups and acetyl groups are added onto the HG by putative Methyl- or Acetyl-transferase. Subsequently, the HG pectin is delivered to the cell wall by the Trans Golgi Network inside secreted vesicles. Pectin Methyl Esterase (PME) enzymes and their inhibitors (PMEI) are also secreted into the cell wall. Unlike Group 1 PMEs, Group 2 PMEs, which include a pro-domain, require cleavage by Subtilisin-like serine protease (SBT) in the golgi for secretion to the cell wall. PMEIs may inhibit specific PMEs in muro. When not inhibited by PMEIs, the active PMEs will demethylesterify the HG pectin. This demethylesterification can lead to cell wall strengthening or loosening, likely depending on the pattern of demethylesterification. HG pectin can also be acetylated as well as deacetylated by pectin acetyl esterases (PAEs). Little is known about this process and its significance
According to the current model of HG synthesis, S-adenosyl methionine and UDP-GalA, precursors for pectin synthesis, are imported into the Golgi apparatus by specific transporters (Wolf et al. 2009a). Subsequently, a complex of HG galacturonosyltransferase (GAUT) and pectin methyl transferase (PMT) facilitates polymerization and methylesterification of HGs (Wolf et al. 2009a; Wang et al. 2013a). To date, GAUT1 is the only galacturonic acid transferase whose identity has been confirmed and characterized biochemically (Sterling et al. 2001). Two polypeptides of GAUT1 bind to one polypeptide of GAUT7, and this heterocomplex is responsible for retaining GAUT1 in the Golgi membranes (Atmodjo et al. 2011). Two further HG biosynthetic proteins are QUASIMODO1 (QUA1), which encodes the GalA transferase GAUT8 (Bouton 2002; Leboeuf et al. 2005), and QUA2 (also known as TUMOROUS SHOOT DEVELOPMENT2, TSD2), which has been proposed to be a pectin methyltransferase, (Krupková et al. 2007; Mouille et al. 2007). Due to their defects in HG biosynthesis, qua mutants have a decreased HG content. The length of the individual HG domains in the qua-2 mutant remains similar to that of WT. However, the number of HG domains is reduced, which enhances the flexibility of the pectin polymer, with consequences on morphology that are discussed below (Ralet et al. 2008).
Little is known about the secretion process of HG pectin. Nevertheless, HG-specific antibody labeling revealed a non-uniform distribution of this pectin component in pea parenchyma cells (Willats et al. 2001) as well as Nicotiana tabacum and Solanum chacoense pollen tubes (Bosch and Hepler 2005; Parre and Geitmann 2005). This supports the existence of a mechanism for localized HG secretion from the Golgi to the cell wall, or redistribution and concentration of HG in specific cell wall regions in muro after secretion. An example of directional pectin secretion is Arabidopsis seed coat mucilage, which is mainly composed of HG. The HG is deposited in seed coat cells specifically at the outward facing cell walls, through polarized secretion (Haughn and Western 2012). The trans-golgi network-localized ECHIDNA/YIP4a and 4b protein complex is required for the correctly localized secretion of cell wall polysaccharides, including HG, in the Arabidopsis seed coat (Gendre et al. 2013). The echidna mutant shows a highly reduced accumulation of pectineus mucilage in the cell walls of the seed coat, but mucilage can be detected in the vacuole, again highlighting the importance of a strict cellular control of the secretion mechanisms (McFarlane et al. 2013).
HG methylesterification and ion cross-links
HG GalA residues can be methylesterified at their C-6 carboxyl groups, or acetylated at their O-2 or O-3 (Ridley et al. 2001). The pattern and degree of these modifications is of central importance to the biochemical and biomechanical properties of the cell wall, and is therefore tightly regulated in a tissue-specific manner during development. Immunolabeling of un-esterified and highly methylesterified HG indicates that it is secreted in a methylesterified state into the cell wall (Zhang and Staehelin 1992; Staehelin and Moore 1995; Ha et al. 1997; Sterling et al. 2001; Mouille et al. 2007; Driouich et al. 2012) with as much as 80 % of the GalA residues being methylesterified before secretion (Mohnen 2008).
HG may then be de-methylesterified within the wall. De-methylesterified HG may encounter two general fates: (1) the formation of a stable structure with other HG molecules if at least ten consecutive GalA residues are de-esterified (Liners et al. 1989), or (2) degradation by polygalacturonases. The former, stable gel formation, will be our main focus. HG GalA residues are negatively charged and can ionically interact with divalent cations, mostly Ca2+, in a structure often referred to as the ‘egg-box’. Studies using antibodies against highly methylesterified HG (JIM7 and LM 20) or HG with a lower degree of methylesterification (DM; JIM5 and LM19) show that methylesterification patterns can vary on a very small spatial scale within tissues (Peaucelle et al. 2008; Lehner et al. 2010; Chebli et al. 2012). The non-homogeneous distribution of HG of various methylesterification degrees/patterns is thus a key factor contributing to spatial differences in the properties of the cell wall, even within a single cell (Zerzour et al. 2009). Within tissues, the distribution of stable pectin gels may also be spatially regulated, thus influencing mechanical properties on a tissue level, as well as growth.
In addition to HG, two pectin RG-II molecules can cross-link through ionic interaction by forming boron bridges (O’Neill et al. 2004). Contrary to the formation of Ca2+-bridges between HG strands, which are the consequence of linear de-methylesterification in muro, boron bridges are integrated when the RG-II molecule is synthesized and secreted (Chormova et al. 2014a, b). Disruption of the RG-II-boron complex causes a lack of wall expansibility in plants resulting in dwarfed stature, compromised cell adhesion, and defects in reproductive tissue function (Iwai et al. 2002, 2006). More recently, defects in boron transporters and subsequent decreases of cross-linked RG-II have been linked to developmental defects in Zea mays tassels (Durbak 2014; Chatterjee 2014). These data reinforce the important role of pectic polysaccharides in cell wall structure and plant growth and development.
HG acetylation
Pectin acetylation is not as well studied when compared to methylesterification (Whitcombe et al. 1995; Ishii 1997). Acetyl esters can be found on a number of cell wall polysaccharides including the pectin components (Manabe et al. 2011). The Golgi has been suggested to be the site for O-acetylation, with Acetyl-CoA as the putative donor substrate (Pauly and Scheller 2000). In support of this hypothesis, xyloglucan and putative xylan O-acetyltransferases were shown to be located in the Golgi (Xiong et al. 2013; Gille et al. 2011). In vitro assays using potato stem cell suspension cultured with [14C]acetyl-CoA led to the formation of radiolabeled polysaccharides including HG (Pauly and Scheller 2000), supporting the role of acetyl-CoA as a donor substrate for O-acetylation.
Reduced wall acetylation2 (RWA2) was the first protein shown to be involved in cell wall acetylation in planta (Manabe et al. 2011) and the rwa2 mutant of Arabidopsis has a 20 % decrease in cell wall O-Acetylation. RWA2 is involved in acetylation of both pectic and non-pectic polysaccharides and is suggested to be an O-acetyl-transferase, but its biochemical activity is yet to be characterized. In a quadruple rwa mutant covering four redundant family members, there is a 63 % decrease in overall O-Acetylation. The decrease is associated with severe dwarfism, absence of inter-fascicular fibers and abnormal xylem cells (Manabe et al. 2011). This phenotype points to a key, perhaps understudied role, for pectin acetylation in proper growth and development.
Pectin acetyl esterases (PAEs) catalyze the cleavage of the ester bond between a glycosyl carbon and an acetyl group, thus releasing acetate from a modified pectin polysaccharide (Gou et al. 2012). The first plant PAEs were isolated from Orange (Citrus sinensis) (Williamson 1991; Christensen et al. 1996) and mung bean (Vigna radiata) (Bordenave et al. 1995). However, the functional characterization of PAEs has remained rather scarce. Tobacco plants (Nicotiana tabacum) overexpressing a poplar PAE (PtPAE1) are impaired in the cellular elongation of floral organs as well as in the germination and growth of pollen tubes, inferring male sterility (Gou et al. 2012). Mutations in AtPAE8 and AtPAE9 led to a 20 % increase of acetate in the cell wall predominantly from a fraction rich in RGI (de Souza et al. 2014). The pae8 mutant also shows a slight, but significant, increase in acetate content in the low molecular weight fraction most likely corresponding to HG. The additive phenotype of the double mutant pae8/pae9 suggests that PAE8 has partial specificity for acetyl-esterified HG substrates (de Souza et al. 2014). The decrease of acetylation seen in rwa and the increase in pae8 and pae9 describe a complex relationship between growth and pectin acetylation: both display defects in elongation, which complicates their interpretation. Nevertheless, this remains an important puzzle to solve.
Pectin methylesterases (PMEs) and their inhibitors (PMEIs)
Pectin methylesterases (PMEs) catalyze the reaction by which methylesters are removed from an HG chain resulting in a free carboxyl group and the release of a proton and methanol (Wolf et al. 2009a). PMEs are antagonistically regulated in the cell wall by proteinaceous PME Inhibitors (PMEIs). PMEs are classified into two groups by their primary structures with the distinguishing feature being the presence or absence of an N-terminal PRO region (Louvet et al. 2006; Wolf et al. 2009b; Wang et al. 2013b). The group one/type II PMEs have only a short or absent N-terminal PRO region, whereas the group two/type I PMEs possess one to three long PRO domains (Micheli 2001; Sénéchal et al. 2014b). Several lines of evidence indicate that the PRO region is cleaved off from some or all PMEs before they are secreted into the cell wall. Cell-wall-extracted PMEs from mung bean hypocotyls do not have the PRO regions (Bordenave and Goldberg 1993), and only the mature form of AtPME3, i.e., the form without the PRO domain, was found in enriched cell wall samples (Guénin et al. 2011). Interestingly, the PRO region is highly similar in structure to specific domains of PMEIs. Mathematical modeling predicted that the major contribution of the PRO-containing group 2 PME family proteins to overall PME activity in different Lepidium sativum seed compartments is in de-methylesterification rather than in PMEI-like activity, supporting the hypothesis that the PRO region is generally cleaved off during protein maturation (Scheler et al. 2014).
Genome and EST sequencing projects have shown that PMEs belong to large multigene families in all plant species sequenced to date. In poplar (Populus trichocarpa), 89 ORFs have been annotated as putative full length PMEs compared to 41 ORFs in rice (Oryza sativa) (Coutinho et al. 2003; Wang et al. 2013b) Coutinho et al. (2003) listed 66 ORFs in Arabidopsis annotated as PME corresponding to 6.81 % of all carbohydrate-active enzymes in the species (Coutinho et al. 2003). Scheler et al. (2014) identified 67 putative PMEs in Arabidopsis, with 22 falling into group 1, and 45 into group 2. The smaller family size in rice relative to that of Arabidopsis and poplar is consistent with the finding that HG is less abundant in monocots than in dicots (Pelloux et al. 2007).
There are almost as many PMEI genes as PME genes in sequenced dicot species such as Arabidopsis and Populus. Database analysis has shown that 69 genes encode putative PMEIs/invertase inhibitors in Arabidopsis (Scheler et al. 2014). Monocots have fewer PMEIs, consistent with remarks above; 35 genes encoding putative PMEIs were found in rice (Wang et al. 2013b). The mode of action of PMEI is still unclear, but several studies have found that individual PMEIs can interact in a 1:1 ratio with a number of PMEs in a pH-dependent manner, sometimes over species boundaries. For example, a pepper PMEI can inhibit Arabidopsis PMEs (An et al. 2008), and Arabidopsis and kiwi PMEIs inhibit tomato PMEs (Hothorn et al. 2004). The three-dimensional structure of Arabidopsis and kiwi PMEIs interacting with purified PMEs from tomatoes has been resolved, revealing that PMEI prevents access for the substrate by covering the PME catalytic cleft (Hothorn et al. 2004; Di Matteo et al. 2005).
Regulation of PMEs and PMEIs at the posttranscriptional and posttranslational level
Given the strong influence of pectin on plant development detailed above, it is of vital importance for the plant to spatially and developmentally control pectin-modifying protein abundance and activity of enzymes such as PME and PMEI. Expression of a large number of genes belonging to the PME and PMEI families has been detected in microarrays at all analyzed developmental stages in Arabidopsis [eFP-browser, http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi (Winter et al. 2007)]. Thus, given the presence of large numbers of mRNAs for both PMEs and PMEIs, it is likely that the main part of PME activity regulation happens on the posttranscriptional or posttranslational level. Candidate mechanisms include RNA processing, protein maturation and protein stability, interactions of the active PMEs with PMEIs, and modulation of enzyme activities in muro. We will briefly discuss these possibilities in order.
Alternative splicing may regulate the organ specificity of the functional forms of two PMEIs in wheat. Mature and fully spliced transcripts that are translated into functional proteins were only found in anthers, but transcripts with retained introns were present in other tissues as well (Rocchi et al. 2012).
In addition to regulation at the transcript level, group 2 PMEs can be regulated posttranslationally through their maturation process. The PRO region of PMEs mediates retention of unprocessed group 2 PMEs in the Golgi (Wolf et al. 2009b). Subtilisin serine proteases (SBTs) are strong candidates for the enzymes that cleave off the PRO region in the Golgi have not been identified with certainty. The Arabidopsis SBT AtS1P was co-precipitated with the PME VDP1 from a microsome fraction, indicating interaction with unprocessed PMEs in the Golgi (Wolf et al. 2009b). It has furthermore been shown in Arabidopsis that AtSBT3.5 is not only co-expressed with PME 17, but also processes the PME for release of the mature form into the apoplast by cleaving off the PRO region at a specific motif (Sénéchal et al. 2014a). Because the PRO region is cleaved before secretion, it might be degraded or it could have a separate function in the cell, such as helping the correct folding of the PME, targeting of the PME to the cell wall, or inhibiting PME activity before secretion (Micheli 2001).
PME protein stability and degradation as regulatory factors of PME activity are just beginning to be explored. FLYING SAUCER1 1 (FLY1), a transmembrane RING E3 ubiquitin ligase, was recently proposed to regulate the DM of pectin by recycling pectin methylesterase enzymes in the endomembrane system of seed coat epidermal cells (Voiniciuc et al. 2013). FLY1 is orthologous to yeast E3 ligase involved in the quality control of membrane proteins in yeast. In Arabidopsis, the fly1 mutant causes a lower DM in the pectin-enriched seed mucilage (Voiniciuc et al. 2013). It is evident that the processing of PME proteins may have a significant effect on their activity, and thus the DM of the cell wall pectin.
PME activity is highly dependent on pH. The de-methylesterification patterns produced by PMEs depend on the pH of the cell wall environment, the pH optimum of the individual enzymes, and the degree of pectinification of the substrate (Catoire et al. 1998; Denès et al. 2000). The use of newly developed technologies for live imaging of extracellular pH might shed new light on the potential distribution of pH micro-domains in the wall of a given cell or within a given tissue (Gjetting et al. 2012). In addition, the type and concentration of cell wall cations can affect the activity of PMEs (Schmohl et al. 2000). Trivalent cations are more effective than bivalent cations which, in turn, are more effective than monovalent cations in increasing PME activity (Schmohl et al. 2000). The concentration of these cations is thought to modify the affinity of PMEs for their substrate (Moustacas et al. 1991). It is conceivable that localized micro-domains within the cell wall could differentially regulate PME and PMEI activity in spatial and temporal contexts.
Pectins in relation to plant development and plant interactions with the environment
The cell wall is a mediator of intrinsic processes such as growth, shape, and structural stability but also of interactions with extrinsic factors such as the environment and pests. It is a physical actor in these processes, and as such its physical properties become highly relevant. In recent years, methods used in the engineering sciences have been adapted for plant research to better characterize the biomechanical and biochemical properties of whole plant organs, tissues, and cell walls (Burgert and Keplinger 2013; Milani et al. 2013; Moulia 2013). Several cell wall properties influencing plant development, morphology, and interaction with the environment are strongly linked with pectin composition and modification. These include cell wall elasticity, hydration, porosity, and adhesion (Braybrook et al. 2012). In the following sections, we will attempt to describe how pectin and cell wall mechanical properties are related to cell elongation, stem structural integrity, tissue integrity, seed hydration, abiotic and biotic interactions.
There is mounting evidence that the mechanical properties of the primary cell wall matrix, primarily comprising pectins, are key in regulating developmental processes in plants. Pectin gels are capable of large changes in hydration and stiffness, which can alter the behavior of cells and tissues. As an example, increasing stiffness of cell wall’s pectin gel may result in decreased cell growth, or if stiffened enough in cell–cell separation by gel fracture. Alternatively, softening of a gel might lead to increases in growth or alteration in hydration effecting physiological processes such as seed germination. In the following examples, we will see that the story is not straightforward, but generally pectin softening is permissive of growth and hydration and stiffening restrictive of growth, required for strength and permissive of cell separation.
The softening and stiffening of a pectin-based cell wall matrix is largely achieved by alterations in pectin chemistry, here we will focus on HG pectin. Newly delivered HG, as mentioned earlier, is highly methylesterified (Zhang and Staehelin 1992; Staehelin and Moore 1995; Ha et al. 1997; Sterling et al. 2001; Mouille et al. 2007; Driouich et al. 2012). As high DM leads to a softer, more pliant gel in vitro and in planta, it may be assumed that new pectin is soft. The activity of PMEs on pectin can result in two major mechanical outcomes: further cell wall softening or cell wall stiffening. This apparently disparate set of outcomes results from the fate of low DM pectin: low DM pectin is a target for polygalacturonases which cleave it further and likely intensify gel softness; low DM pectin is also ready to cross-link with Ca2+ to increase gel rigidity (Vincent and Williams 2009). It is likely that the fate of low DM pectin is controlled by the presence/absence of polygalacturonases and/or calcium in a cell-specific manner. In line with this, the effect of PMEIs on cell wall matrix mechanics may also differ. In cases of cell adhesion and separation, we see the effect that PG can have on the mechanical outcome of PME activity. In most cases of growth and strength, as we will see below, the activity of PMEs leads to stiffening and PMEIs to softening. However, there are several cases of the opposite being true, which complicate our understanding of cell wall mechanics and growth.
Cell elongation growth processes
The strength of the cell wall counteracts the cell’s turgor pressure, and any growth process depends on tight spatial and temporal regulation of one of these two opposing forces (Schopfer 2006). At constant turgor pressure, cells grow in volume when the cell wall polymer network is loosened and extensibility increased through restructuring or dissolution of load-bearing bonds. The primary cell wall is a viscoelastic material that displays elastic properties below a stress threshold, which depends on its composition and state, but deforms like a viscous material when the threshold is crossed (Hayot et al. 2012). This can lead to the whole cell expanding isotropically, or more commonly in a tissue context, to anisotropic expansion or even bulging when only small domains in the cell wall deform (Schopfer 2006). Thus, in organs that grow particularly fast such as hypocotyls and stems, extensive cell wall modifications would be expected.
Several studies indicate a role for the DM in cell expansion and elongation. These studies are summarized in Table 1. In light-grown hypocotyls, low DM reduced cell elongation in GA-deficient mutants [ga (Derbyshire et al. 2007)]. Their shortened hypocotyl phenotype and low DM could be rescued by the application of exogenous GA (Derbyshire et al. 2007). Similarly in Arabidopsis, knocking out the ubiquitously expressed PME3 led to an increase in hypocotyl length in dark-grown hypocotyls (Guénin et al. 2011). Interestingly, not all evidence in dark-grown hypocotyls is congruent: Fourier Transform Infrared spectroscopy [FTIR, (Alonso-Simón et al. 2011)] analysis showed that their rapid growth was associated with a decrease of detectable pectin ester bonds suggested to be methylesters (Pelletier et al. 2010), and the transcription of a number of pectin-modifying enzymes, including PMEs and PMEIs, was upregulated during the growth phase (Pelletier et al. 2010). Overexpression of PMEI4 in dark-grown hypocotyls led to an increase in ester bonds in the cell wall pectin fraction, and delayed the growth acceleration (Pelletier et al. 2010).
The apparent contradiction that low DM in the cell wall has been documented to lead to opposite consequences, namely an increase (Pelletier et al. 2010) or decrease (Derbyshire et al. 2007; Guénin et al. 2011) in hypocotyl elongation, may result from several possible scenarios: differences in the pattern of de-methylesterification may yield different mechanical outcomes, and as mentioned earlier the presence of calcium and polygalacturonases may alter the fate of low DM pectin.
Changes in cell wall elasticity, brought about by changes in DM, have been shown to play a role in apical meristems (Peaucelle et al. 2008). Here, low DM led to cell wall relaxation in the meristem, which initiated organogenesis extemporally (Peaucelle et al. 2011a; Braybrook and Peaucelle 2013). This de-methylesterification could be induced through application of auxin, an accumulation of which is required for organ initiation. In line with this, auxin induction of initiation depended on the de-methylesterification process (Braybrook and Peaucelle 2013). Furthermore, in the Arabidopsis meristem, the transcription factor BELLRINGER (BLR) represses expression of PME5 (Peaucelle et al. 2011b). In the blr-6 mutant, ectopic expression of PME5 in the meristem leads to altered phyllotaxis, which is rescued in a blr/pme5 double mutant. The meristem is thus another example of a system in which a low DM leads to a decrease in cell wall stiffness, again pointing to the complexity of the connection between the DM and cell wall biomechanical properties.
Pectin modification also plays a key role in tip growth of plant cells, but here we return to evidence for high DM equaling softer walls. Pollen tubes elongate extremely fast through tip growth and PMEs have been shown to be involved, with a lower DM leading to slower elongation. VANGUARD1 (VGD1) encodes a pollen-specific PME required for the modification of the cell wall necessary for pollen tube growth (Jiang et al. 2005). PME activity and pollen tube growth were reduced in the vgd1 mutants, leading to an increase in male infertility. The closely related At2g47030 complemented the vgd1 mutant in contrast to the more distantly related At3g62170 (Jiang et al. 2005), which suggests different functions or biochemical activities of these isoforms. The pollen-specific AtPPME1 is involved in the determination of the shape and elongation of the pollen tube (Tian et al. 2006; Röckel et al. 2008), and can be inhibited by the equally pollen-specific AtPMEI2 in vitro (Röckel et al. 2008). The localisation of YFP fusion proteins in the pollen tube indicated that while AtPPME1 was present along the full length of the tube, AtPMEI2 was only detected in the apex, at the site of elongation (Röckel et al. 2008). This supports the hypothesis that PMEs localized at the pollen tube’s apex are specifically inhibited, and that the apex therefore has a higher DM, which is conducive to elongation.
The inverse situation occurs in the mature part of the pollen tube, which has less inhibition of PME activity and a lower DM. The de-methylesterification could lead to reduced extensibility through the formation of Ca2+ cross-links. The lower DM in the mature part of the pollen tube was confirmed in immunocytochemical assays (Dardelle et al. 2010). Supporting this hypothesis, the overexpression of AtPPME1 in tobacco pollen tubes led to a decrease in elongation, while the PME inhibitor AtPMEI2 increased the elongation rate (Röckel et al. 2008). It has recently been suggested that one of the biomechanical contributions of cell wall pectin to growth might be through its ability to undergo strain stiffening (Kierzkowski et al. 2012) as observed in vitro in pectin gels (Schuster et al. 2012). Strain stiffening is a rheological property of biological gels by which the stiffness of the gel increases under increasing strain, so that increasing force is required for increasing deformation. Strain stiffening in plant cell walls may limit growth to specific parts of tissues or cells, and could play a role in localized tip growth occurring during pollen tube elongation.
In addition to pollen tube elongation, PMEs play a role in pollen grain germination, as has recently been shown for PME48 (Leroux et al. 2015). pme48 mutant pollen grains showed a decrease in PME activity, slower imbibition of water and delayed or impaired germination both in vitro and in vivo. In the grains that did germinate, an increased diameter and increased proportion of bursting of the pollen tubes was observed in liquid germination media compared to the WT (Leroux et al. 2015). These pollen tube phenotypes could be rescued by the addition of Ca2+ to the germination medium (Leroux et al. 2015).
Stem morphology
Arabidopsis stems, such as hypocotyls, grow by fast cell elongation in internode regions, and a number of cell-wall-modifying and pectin-related enzymes are differentially expressed at different stages during the maturation process (Hall and Ellis 2013). Arabidopsis PECTIN METHYLESTERASE 35 (PME35) was shown to act on primary cell wall pectins in the stem (Hongo et al. 2012). The pme35 mutant has defects in de-methylesterification in cortex cells and inter-fascicular fibers, leading to a decrease in mechanical stem strength (Hongo et al. 2012). Stem morphology as well as stiffness is also affected by overexpression of AtPMEI5 in Arabidopsis (Wolf et al. 2012; Müller et al. 2013a, b). These plants show stem twists and loops as well as fusions. Furthermore, in the blr mutant, the upregulation of PME5 expression led to reduced cell expansion and defects in internode elongation (Peaucelle et al. 2011a). A decrease in stem length was also observed after mutation of AtPME3 in the same plants that showed an increased hypocotyl elongation as described above (Guénin et al. 2011).
In addition to depending on the DM, stem growth can be affected by the relative pectin content of cell walls, as is the case in the quasimodo (qua) mutants. qua1 (Bouton 2002; Leboeuf et al. 2005) and qua2 (Krupková et al. 2007; Mouille et al. 2007) have a decreased HG content, an increased flexibility of the pectin polymer dues to the increased ration of RG-I to HG domains, and altered stem growth (Ralet et al. 2008). In total, these findings point towards a further role of pectin modification in non-woody plants, regulating stem strength and structure.
In systems with more extensive wood formation, the DM is also important for strength. In hybrid aspen (Populus tremula x tremuloides), PtPME1 was expressed in developing woody tissues, and its overexpression decreased the DM and inhibited both symplastic and intrusive growth of wood fiber cells (Siedlecka et al. 2008). Interestingly, in a very distinct model, it was shown that PMEs are likely to influence solid wood properties in Eucalyptus, shedding new light on putative additional functions of PMEs (Sexton et al. 2012).
Cell adhesion and separation
Pectins are the main components of the middle lamellae connecting the cell walls of adjacent plant cells. Therefore, pectins play a major role in cell adhesion, and pectin modification may be necessary for the partial cell separation occurring at the end of cell division (Lord and Mollet 2002). Pectin Ca2+ bridges are thought to play a major role in cell adhesion.
In the Arabidopsis root cap, cells detach from the root tip and are released into the surrounding medium in layers of interconnected cells (Vicré et al. 2005). Although a large number of cell wall mutants have been tested, only qua1 and qua2, which have reduced amounts of HG in their cell walls, released individual cells instead of layers, indicating a reduced cell adhesion in the root cap (Durand et al. 2009). The qua1 mutant also showed drastic seedling defects, with tissues dissolving and falling apart on the living seedling (Bouton 2002). This confirms the importance of HG in cell adhesion.
There is evidence for a contribution of pectin-modifying enzymes to cell adhesion. The friable1 (frb1) mutant in Arabidopsis shows severe defects in cell adhesion, starting with the sloughing of outer cell layers and proceeding to the dissociation of tissues and organs, as well as developmental defects in embryos (Neumetzler et al. 2012). FRIABLE1 is a protein of unknown function, but localizes to the Golgi, and is hypothesized to be involved in secretory pathways. Seedlings were found to have a lower DM than WT as well as a significantly higher arabinose content. Interestingly, in addition to tissue dissolution, the frb1 plants displayed organ fusions. Organ fusions, but not tissue dissociation, were also observed in Arabidopsis plants with reduced PME activity due to overexpression of PMEI5 (Müller et al. 2013b).
The proteins Arabidopsis dehiscence zone polygalacturonase 1 (ADPG1) and ADPG2, which are closely related to one another, were shown to be crucial for seed pod opening in Arabidopsis, and showed strong expression in the abscission zones of floral organs (Ogawa et al. 2009). Leaf abscission was correlated with a reduced DM in the abscission zone of Impatiens, indicating that pectin de-methylesterification followed by polygalacturonase activity might also contribute to the separation of the cells necessary for the plant to shed its leaves as observed in pollen separation (Bowling and Vaughn 2011). The mesophyll of pme3 mutants, which had an increased DM, could be treated with cell wall-degrading enzymes to form protoplast with a higher efficiency than the WT, indicating that the cell wall is more susceptible to degradation and the cells separate more easily from each other (Lionetti et al. 2015). The same phenotype can be observed in overexpressors of PMEI1 or PMEI2.
Seeds
Pectin gels can hold large amounts of water, and the hydration state of the wall and the strength of the pectic polysaccharide gel influence its biomechanical properties (Ha et al. 1997). An impressive example of this ability is the seed mucilage of species such as Arabidopsis thaliana, Capsella bursa-pastoris, Lepidium sativum, and many more (Western et al. 2001; Harpaz-Saad et al. 2012; Deng et al. 2013). On contact with water, specialized cells in the seed coats, which die during development, release cellulose fibrils as well as large quantities of pectins (Haughn and Chaudhury 2005; Haughn and Western 2012). The seed mucilage probably serves as a short-term water reservoir for the seed and as an adhesive, but is not necessary for successful seed germination in Arabidopsis, as mutant seeds with reduced or nonexistent mucilage can also complete germination (Western et al. 2000). Ruthenium red staining of seed mucilage shows very clearly that the addition of Ca2+ leads to a denser mucilage as the Ca2+ forms cross-links between de-methylesterified carboxyl groups (Voiniciuc et al. 2013; Dean et al. 2007). The addition of EDTA, which chelates calcium away from the HG strands, leads to less dense, more voluminous mucilage, as the reduced number of network cross-links leads to less counteraction to the swelling force (Voiniciuc et al. 2013; Dean et al. 2007). PMEI6 was shown to be essential for full seed mucilage release, as an Arabidopsis ecotype deficient in PMEI6 only released very small quantities of mucilage (Saez-Aguayo et al. 2013). A strongly increased mucilage release could be achieved by overexpressing PMEI6 (Saez-Aguayo et al. 2013). This effect was independent of the similar effect of a mutation in the subtilisin-like protease SBT1.7 on mucilage release (Rautengarten et al. 2008), as a double mutation pmei6/sbt1.7 had an additive phenotype compared to the two independent mutants (Saez-Aguayo et al. 2013), indicating different target PMEs. These experiments provide interesting insights into the role of seed mucilage in particular, but also into the properties of plant pectin gels in general.
In addition to its importance in determining the properties of seed mucilage and its release from the testa, pectin methylesterification also plays a role in embryo development and seed germination in Arabidopsis (Müller et al. 2013b; Levesque-Tremblay et al. 2015). At least 7 PMEs are expressed during seed coat and embryo development in Arabidopsis in a highly temporally controlled manner (Louvet et al. 2006). However, knockout analyses for most of these genes did not reveal obvious seed phenotype, most likely due to functional redundancy within the PME family. Nevertheless, a PME renamed highly methylesterified seeds (HMS) was recently isolated based on its high expression in the seed coat and the embryo during seed development (Levesque-Tremblay et al. 2015). The disruption of the HMS gene in the knockout hms mutant causes a decrease in overall PME activity and an increase in DM in developing seeds, which is associated with altered embryo morphology, reduced cell expansion and softening of the seed tissue (Levesque-Tremblay et al. 2015). HMS may thus be required for cell wall loosening in the embryo to facilitate cell expansion during the accumulation of storage reserves.
In addition to seed development, PME activity is also tightly controlled during the germination process. Seeds overexpressing PMEI5 displayed lower PME activity in seeds during germination, and earlier onset and faster completion of germination compared to WT. This affected both stages of germination: testa rupture as well as endosperm rupture. PME activity in WT seeds peaked around testa rupture. The addition of ABA to the germination medium, which specifically delays endosperm rupture but does not affect testa rupture (Müller et al. 2006), led to a plateau phase of high PME activity, which only declined once the delayed endosperm rupture set (Müller et al. 2013b). High PME activity is thus associated with the ABA-induced delay in radicle elongation and endosperm weakening; two processes that take place prior to the completion of germination (Müller et al. 2006). In addition to the strong temporal regulation of PME and PMEI expression in seeds, PMEs and PMEIs have also been found to be differentially expressed in a tissue-specific manner during seed germination of the close Arabidopsis relative Lepidium sativum (Scheler et al. 2014). In support of a role for PME activity and the resulting changes in DM in the timing and process of testa rupture, changes in expression were temporally focused around testa rupture (Scheler et al. 2014).
The role of PMEs in seed germination is not limited to angiosperms: PME activity was also shown to be involved in the germination of a gymnosperm, the conifer yellow cypress (Callitropsis nootkatensis). PME activity increases in the megagametophyte and embryo in a tissue-specific manner during yellow cypress seed dormancy release and subsequent germination, with particularly high activity in the megagametophyte, where pectin is the predominant polysaccharide (Ren and Kermode 2000).
Abiotic interactions
As discussed above, the addition of divalent ions and chelators to the pectic mucilage gel of many seeds leads to the mucilage contracting and expanding, respectively (Voiniciuc et al. 2013; Dean et al. 2007), and to changes in its porosity and permeability. The same may, indeed, apply to the pectic gel in all plant cell walls. Porosity and permeability are crucial for any kind of transport or diffusion through the cell wall. The influence that the pectic polysaccharides have on cell wall porosity and density makes them strong candidates for cell wall components that are modified when drought or ice formation reduces the availability of free water in the plant cell. In support of this hypothesis, oilseed rape (Brassica napus) plants that underwent cold acclimation were found to have increased pectin levels in the cell wall, and higher PME activity as well as a lower DM and an increased tensile strength (Solecka et al. 2008).
Evidence for an accumulation of pectin during cold acclimation has also been found in other species. A promising Allium fistulosum epidermis testing system was recently developed which takes advantage of the large cell size and cold hardiness properties of this species for detailed investigations into cell changes during cold acclimation, freezing, and recovery from freezing (Tanino et al. 2013). Here, FTIR spectroscopy suggests that the pectin fraction of the cell wall increases when the plants are undergoing cold acclimation. This hypothesis is supported by findings in pea (Pisum sativum) plants, where a comparison between a frost-tolerant and a frost-sensitive genotype showed that during cold acclimation, the frost-tolerant plants—similar to the oilseed rape mentioned above—accumulated the pectic compounds HG, XG, and RG-I, while the frost-sensitive genotype accumulated xylans (Baldwin et al. 2014). However, contrary to oilseed rape, cold acclimation in the frost-tolerant pea plants led to a higher abundance of JIM7 epitopes, indicating a higher DM than in the frost-sensitive plants (Baldwin et al. 2014).
In addition to changes in the amount of pectin, changes in PME activity take place during cold acclimation. In accordance with the findings in rape seed described above, Arabidopsis plants exposed to cold stress showed an increase in PME activity. The expression and activity of PME41 were shown to increase in reaction to chilling stress as well as brassinosteroid application (Qu et al. 2011). Mutants deficient in brassinosteroid signaling did not show this increase, and only a reduced activity increase was observed in the mutant pme41. A lack of PME41 also led to increased electrolyte leakage in response to cold stress. The increase in PME activity in the WT in response to chilling stress could lead to an increase in the formation of Ca2+ bridges, thereby decreasing cell wall porosity. If temperatures fall below 0 °C, the altered cell wall density could impede the spread of ice (Qu et al. 2011).
PMEs might also play a role in water flow in woody plant vasculature under non-water limiting conditions. During water transport in the xylem of Fraxinus americana trees and a number of other woody species, the water flow through the pit membrane is regulated through volume changes of a hydrogel in response to the presence or absence of cations. If ions are present, the size of microchannels in the pit membrane increases as the gel shrinks, decreasing the resistance to water flow (Zwieniecki et al. 2001). Deionised water can reverse this effect. This may suggest that the hydrogel contains pectins. It remains unclear, however, if pectin is actually present in the pit membrane (van Doorn et al. 2011).
Pectin content and modifications have also been observed in connection with exposure to drought. An increased sensitivity to drought has been observed in some pectin defective mutants such as qua1 with their decreased HG content (Bouton 2002). Several PME isoforms are transcriptionally upregulated in crab apples (Malus pumila) under drought conditions after drought tolerance has been induced through application of DL-β-aminobutyric acid (Macarisin et al. 2009). The influence of pectin content and DM on drought sensitivity could be due to the effect of pectin on the water retention potential of the cell walls, and/or interactions of the pectin matrix with the cuticle, as a defective cuticle would lead to increased water loss through evaporation. PMEI5 overexpressors showed a defective cuticle, which allowed for the penetration of toluidine blue in patches on the leaves and stems (Müller et al. 2013b). This defect might be due to a role for pectin in the transport of cuticle wax components through the cell wall, or in their anchoring.
A further abiotic stress connected with changes in PME activity is heat. Heat acclimation of soybean seedlings induces an increase in PME activity (Wu et al. 2010). It can be reversed with the addition of a chelating agent during the recovery period after the heat shock, and can be reinstated by the addition of divalent cations (Wu et al. 2010). The addition of chelators increased electrolyte leakage, suggesting an increased cell wall porosity. The DM and availability of cations to form egg-box structures from de-methylesterified pectin thus seem to be important for cellular integrity during heat stress.
In addition to the porosity of the pectin gel, the DM and the pattern of methylesterification influence the number of negative charges on HG. A low DM and therefore a high level of negatively charged galacturonate was hypothesized to play a role in increased susceptibility to aluminum toxicity and higher levels of aluminum in the roots of the maize (Zea mays) cultivar Lixis compared to more resistant cultivars (Eticha et al. 2005). In addition, the overall pectin content in root tips was higher in the susceptible cultivar (Eticha et al. 2005). A very similar observation was made in a comparison of different buckwheat (Fagopyrum tataricum) cultivars, where the most aluminum-susceptible cultivars showed a higher pectin content and increased PME activity as well as a decrease in DM in response to aluminum exposure (Yang et al. 2011). Analogous findings were made in rice (Oryza sativa) (Yang et al. 2008). Increased PME activity was also correlated with increased aluminum sensitivity in potato plants (Solanum tuberosum) (Schmohl et al. 2000). The phenomenon of higher aluminum sensitivity of cells with an increased pectin content and decreased DM could be reproduced in cell cultures, in which the pectin content had been controlled by the addition of salt to the medium and the DM altered by different lengths of exposure to PMEs (Schmohl et al. 2000). This response in rice seemed to be specific for aluminum and did not extend to other potentially toxic metals such as cadmium or copper (Yang et al. 2008).
A screening for mutants with compromised root growth in the presence of Zn2+ led to the isolation of ozs2 carrying a mutation in the gene AtPME3 that prevents its efficient posttranslational processing and maturation (Weber et al. 2013). Interestingly, the hypersensitivity phenotype could be rescued by knocking out OZS2 with RNAi, thus demonstrating that the defects were associated with the presence of the unprocessed PME3. Hypersensitivity could also be induced by overexpressing WT PME3, and could be alleviated when excess Ca2+ was supplied to the plants. The ozs2 phenotype was hypothesized to cause hypersensitivity towards specific interference of Zn ions impacting cell wall-controlled growth processes (Weber et al. 2013).
Biotic interactions
PMEs play an important role in the biotic interactions of plants with pathogenic fungi and bacteria, as the cell wall forms a barrier that pathogens need to overcome to infect or spread an infection in a plant (Vorwerk et al. 2004; Cantu et al. 2008). Many pathogens secrete pectin-degrading enzymes that help break down the host’s cell walls as a necessary part of the infection process (Espino et al. 2010).
In addition to the pectin-related enzymes introduced into the plant by the pathogen, the infection can cause changes in the expression of pectin-modifying enzymes in the host plant, including PMEs and PMEIs. Table 2 summarizes findings on the relationship of PME activity, cell wall DM, and susceptibility to different biotic stress factors. The expression of a number of Arabidopsis PME and PMEI genes is altered in response to infection with both biotrophic and necrotrophic pathogens such as Golovinomyces cichoracearum, Pseudomonas syringae, Blumeria graminis, Phytophthora infestans and Botrytis cinerea (Lionetti et al. 2012). Interestingly, mutations in some of the upregulated PMEs can lead to an increased resistance to the pathogen, as was shown for B.cinerea and P. carotovorum infection of Arabidopsis. Infections with either pathogen leads to an upregulation of PME3, and the pme3 mutant shows increased resistance and reduced PME activity (Raiola et al. 2011). Similarly, transgenic Arabidopsis (Lionetti et al. 2007) and durum wheat (Volpi et al. 2011) with a reduced PME activity due to overexpressing PMEIs had a higher DM in the cell walls, and showed increased resistance to both fungal and bacterial pathogens. The pepper (Capsicum annuum) CaPMEI1 is expressed in response to infection of pepper leaves with Xanthomonas campestris pv. vesicatoria, and mutants lacking that protein show an increased susceptibility to the pathogen (An et al. 2008). Heterologous overexpression of CaPMEI1 in Arabidopsis conferred increased resistance to several biotic and abiotic stresses, indicating that the pepper PMEI could interact with Arabidopsis PMEs (An et al. 2008). All these studies have established a link between a decrease in PME activity and the resulting increased DM, and an increased level of resistance to the studied pathogens. Taken together with the observation that action of plant polygalacturonases expressed in ripening fruit on cell wall pectins was facilitated by a decrease in DM (Wakabayashi et al. 2003), it is possible that the increased resistance of the plants with a higher DM is due to the highly methylesterified pectin being less susceptible to being broken down by pathogen polygalacturonases.
However, in contrast to these findings, Bethke et al. showed that three out of four mutants lacking single PMEs that would be upregulated in response to an infection with a different pathogen, Pseudomonas, in the WT showed increased rather than decreased pathogen susceptibility (pme3, pme17, and At1g11580) (Bethke et al. 2014). The same was true for six out of 12 pme single mutants that have altered cell wall compositions compared to WT (pme35, pme39, ppme1, pme17, pme31, pme44), and to mutants deficient in more than one of these PMEs (Bethke et al. 2014). However, while baseline PME activity was reduced in many of the mutants, pathogen-induced PME activity in the majority of mutant lines including the higher order mutants was equal to WT or even increased, which could be related to compensation mechanism between isoforms upon infection. Jasmonic acid was required to increase overall PME activity, while ethylene and salicylic acid had no influence (Bethke et al. 2014).
The role of the DM in the immune response continues beyond the initial infection process. Once the cell wall pectin starts to be broken down in a pathogen attack, its fragments can be recognized by wall-associated kinase1 (WAK1) and WAK2 and elicit an immune response. These transmembrane proteins have pectin-binding domains in the cell wall and kinase domains in the cytoplasm. Elicitor activities of pectic fragments differ based on their length and DM (Osorio et al. 2008; Ferrari et al. 2013; Kohorn et al. 2014). Fragments with a low DM showed increased elicitor activity through formation of Ca2+-bridges and increased binding to WAK1 in Arabidopsis cell suspensions (Cabrera et al. 2008). In planta experiments in which a hyperactive allele of WAK1 was crossed into a pme3 knockout mutant showed that the reduced DM of the mutant led to a suppression of the WAK hyperactivity phenotype, indicating WAK1 requires HG with a low DM for activation (Kohorn et al. 2014). When exogenous low DM OGs were supplied to the pme3 plants expressing the hyperactive WAK1 allele, the WAK1 response was significantly stronger than in WT plants with the hyperactive WAK1 allele, possibly because of the decreased competition in the pme3 mutants between the exogenous low DM OGs and the endogenous high DM pectins (Kohorn et al. 2014).
The PMEI-mediated control of pectin methylesterification is also likely to play a role in plant–virus interactions, although this topic has seen very little studies until recently. Tobacco plants overexpressing a PMEI from kiwi (Actinidia chinensis) showed increased resistance to the tobacco mosaic virus, characterized by a decrease of systemic movement of the virus and a decrease in symptoms (Lionetti et al. 2014), thus confirming earlier report of an interaction of PMEs with virus movement proteins (Chen and Citovsky 2003). An analogous effect was observed in Arabidopsis plants overexpressing AtPMEI-2, which showed increased systemic resistance to the turnip vein clearing virus (Lionetti et al. 2014).
In a distinct pathosystem, Arabidopsis PME3 was shown in a yeast-two hybrid screen to interact with the Cellulose Binding Protein (CBP) secreted by the sugar beet cyst nematode Heterodera schachtii during the infection process (Hewezi et al. 2008). In accordance with a role of CBP in facilitating cyst parasitism, overexpression of CBP in Arabidopsis led to a decrease of resistance to nematode infection, as well as to a small increase in PME activity. A similar level of increase in PME activity was observed in plants overexpressing PME, which was also associated with a decrease of resistance to the nematode, while the pme3 mutant showed increased resistance (Hewezi et al. 2008). PME activity is thus a target of an infection-related pathogen-produced protein in this nematode–host interaction.
While it seems that PMEI upregulation and an increased overall DM are typical reactions to pathogen attack, PMEs are upregulated and the DM decreased in response to mechanical wounding by herbivores (von Dahl et al. 2006; Körner et al. 2009) as well as by artificial large-scale wounding of leaves (Dorokhov et al. 2012). When caterpillars of the moth Manduca sexta fed on Nicotiana attenuata plants, PMEs were upregulated in the attacked leaf, total PME activity increased, and the DM decreased (Körner et al. 2009). This response was brought about by the high pH of caterpillar oral secretions. In all cases where PME activity increases in response to a biotic interaction, the methanol released by PME activity can act as a signaling molecule to the plant that is being attacked, and potentially neighboring plants (von Dahl et al. 2006; Körner et al. 2009; Dorokhov et al. 2012; Dixit et al. 2013; Komarova et al. 2014). In the case of caterpillar feeding on Nicotiana attenuata, the release of methanol was significantly reduced in plants lacking a PME inducible by caterpillar oral secretions, and showed a lower expression of toxic defense proteins (Körner et al. 2009). It was also reported, however, that methanol treatment of Nicotiana attenuata reduced the level of defensive proteins when the methanol quantities were similar to those produced in response to herbivore attacks, and that larvae on methanol-treated plants gained more weight by feeding than did larvae on untreated plants (von Dahl et al. 2006). When PMEs were overexpressed in tobacco and methanol production thereby increased, the plants became more resistant to aphids, whiteflies, and two species of caterpillars (Dixit et al. 2013), possibly as a direct effect of methanol toxicity.
These opposite reactions in PME activity regulation to wounding and pathogen attack can contribute to an increased susceptibility of plants to pathogens following wounding, and changes in DM may play a large role in this effect. This has been illustrated in banana plants (Musa) (Ma et al. 2013). The pathogenic fungus Fusarium oxysporum enters banana plants through wounds in the roots, and has severe effects on the plants, ultimately leading to their death. Banana plants susceptible to the pathogen Fusarium showed a much larger increase in PME activity and subsequent decrease in the DM in response to wounding than did resistant banana plants (Ma et al. 2013). Fusarium secretes a number of pectinolytic enzymes, and it is likely that the resistance of the plants with the lower PME activity in response to wounding is caused by reduced lytic efficiency of those enzymes in cell walls with a higher DM.
Interplay of pectin modifications with overall cell wall composition
While it has been clear for some time that cell wall properties and composition may be changed through hormonal regulations in response to environmental conditions—a famous example being the auxin gradient in response to gravity that leads to unequal cell elongation on different sides of a hypocotyl—there is now also evidence that changes in cell wall properties might be able to trigger hormone signals that then lead to reactive cell wall modifications. Specifically, for pectins, changes in PME activity and the resulting changes in DM have been associated with changes in the brassinosteroid signaling pathway in Arabidopsis. In support of this association, several of the strong morphological effects of PMEI5 overexpression could be suppressed by a mutation in the brassinosteroid receptor protein (BRI) (Wolf et al. 2012).
The DM might also have an influence on the deposition of cellulose, which is one of the major fibrous load-bearing components of the cell wall (Yoneda et al. 2010). A chemical genetic screen showed that cobtorin {4-[(2-chlorophenyl)-methoxy]-1-nitrobenzene} interferes with cellulose microfibril deposition without disturbing the microtubule, although the microtubule network’s orientation is generally considered to be the deciding factor in cellulose microfibril orientation (Yoneda et al. 2007) Cobtorin treatment also increased the DM and disturbed the distribution of methylesterified HG (Yoneda et al. 2010). The effect of cobtorin on cellulose deposition is suppressed when the pectin methylesterase AtPME1 or a putative polygalacturonase is overexpressed, supporting a link between pectin modifications and cellulose deposition. Increased levels of HG domains have also been reported in several cellular systems in which cellulose synthesis is disrupted (Shedletzky et al. 1990; Diaz-Cacho et al. 1999; Manfield et al. 2004). In those systems, the accumulation of HG may have an effect on cell wall biomechanics which compensates for the loss of cellulose in the cell wall. This is supported by the finding that treatment of Arabidopsis cell suspension with the cellulose synthesis-inhibiting compound isoxaben leads to an increase in the transcription of HG synthesis- and modification-related genes including QUA1 and several PMEs, leading to an increase of HG and changes in DM. (Manfield et al. 2004). Ca2+-mediated cross-linking of HG is thought to be part of the mechanism of compensation for the diminished load-bearing capacity of the disrupted cellulosic network. As our technical and methodological options improve and allow for more detailed analyses of the cell wall with higher spatial and temporal resolution, the interactions between the different cell wall polymers which are currently still most often studied separately from each other, and the effects of those interactions will become more accessible for investigations.
Conclusions and outlook
Pectin composition and the degree of HG methylesterification have a major impact on the biochemical and biomechanical properties of plant cell walls, which in turn are crucial for plant development as well as for plants’ interactions with their abiotic and biotic environments. PMEs act on HG by either demethylesterifying residues in the GalA chain individually randomly, or linearly blockwise (Markovic and Kohn 1984). These two modes of action may have inverse consequences on the plant cell wall. When the PMEs act individually randomly, the de-esterification of HGs frees protons promoting the activity of polygalacturonases (Moustacas et al. 1991), but does not allow for the formation of Ca2+ bridges. The action of polygalacturonase can, in turn, break down the pectin and contribute to cell wall loosening. When the PMEs act linearly blockwise on HGs, long stretches of negatively charged carboxylate appear, which can interact with Ca2+ creating a denser gel structure that would be expected to lead to decreased porosity. Thus, PME activity can strengthen as well as loosen the cell wall. However, experimental observations of pme and pmei mutants have shown that the relationships between the DM, the pattern of de-methylesterification, its effect on cell wall elasticity, other biomechanical parameters, and growth are not straightforward. Working towards an understanding of the role of pectin and specifically HG methylesterification patterns on a molecular, biochemical and biomechanical level is one of the big tasks that PME biology is preparing to tackle. The current simple model will have to be reimagined and refined before we can begin to make detailed predictions of the effect of PME activity on plant development and environmental interactions. Further work should emphasize, at single cell resolution, how the fine-tuning of PME activity can affect pectin chemistry and regulate cell wall rheology to shape plant form.
An additional challenge to pectin research comes from the fact that pectin-modifying enzymes are very large families with significant functional redundancy, and little is known so far about the interactions between individual family members. It will be important to investigate individual PMEs, as well as to address the inhibitor target range of individual PMEIs and the putative polarized secretion of PMEs and PMEIs that could lead to their spatial separation in specific areas of the cell wall. A better understanding of these aspects will allow us to better predict the additional or contrary effect of a battery of PMEs and PMEIs expressed at the same time in the same tissues. It will also be important to take into account the different layers of regulation, in particular PME and PMEI posttranslational regulation and protein stability after secretion into the cell wall.
Author contribution statement
All authors wrote, read and approved the manuscript.
References
Alonso-Simón A, García-Angulo P, Mélida H, Encina A, Álvarez JM, Acebes JL (2011) The use of FTIR spectroscopy to monitor modifications in plant cell wall architecture caused by cellulose biosynthesis inhibitors. Plant Signal Behav 6:1104–1110
An SH, Sohn KH, Choi HW, Hwang IS, Lee SC, Hwang BK (2008) Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 228:61–78
Atmodjo MA, Sakuragi Y, Zhu X, Burrell AJ, Mohanty SS, Atwood JA, Orlando R, Scheller HV, Mohnen D (2011) Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan:galacturonosyltransferase complex. Proc Natl Acad Sci USA 108:20225–20230
Baldwin L, Domon J-M, Klimek JF, Fournet F, Sellier H, Gillet F, Pelloux J, Lejeune-Hénaut I, Carpita NC, Rayon C (2014) Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 104:37–47
Bethke G, Grundman RE, Sreekanta S, Truman W, Katagiri F, Glazebrook J (2014) Arabidopsis pectin methylesterases contribute to immunity against pseudomonas syringae. Plant Physiol 164:1093–1107
Bordenave M, Goldberg R (1993) Purification and characterization of pectin methylesterases from mung bean hypocotyl cell walls. Phytochemistry 33:999–1003
Bordenave M, Goldberg R, Huet JC, Pernollet JC (1995) A novel protein from mung bean hypocotyl cell walls with acetyl esterase activity. Phytochemistry 38:315–319
Bosch M, Hepler PK (2005) Pectin methylesterases, a regulator of pollen tube growth. Plant Phys 38:1334–1346
Bouton S (2002) Quasimodo1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in arabidopsis. Plant Cell Online 14:2577–2590
Bowling AJ, Vaughn KC (2011) Leaf abscission in Impatiens (Balsaminaceae) is due to loss of highly de-esterified homogalacturonans in the middle lamellae. Am J Bot 98:619–629
Braybrook SA, Peaucelle A (2013) Mechano-chemical aspects of organ formation in Arabidopsis thaliana: the relationship between auxin and pectin. PLoS One 8:e57813
Braybrook SA, Hofte H, Peaucelle A (2012) Probing the mechanical contributions of the pectin matrix: insights for cell growth. Plant Signal Behav 7:1037–1041
Burgert I, Keplinger T (2013) Plant micro- and nanomechanics: experimental techniques for plant cell-wall analysis. J Exp Bot 64:4635–4649
Cabrera JC, Boland A, Messiaen J, Cambier P, Van Cutsem P (2008) Egg box conformation of oligogalacturonides: the time-dependent stabilization of the elicitor-active conformation increases its biological activity. Glycobiology 18:473–482
Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344:1879–1900
Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell ALT (2008) Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci 13:610–617
Catoire L, Pierron M, Morvan C, du Penhoat CH, Goldberg R (1998) Investigation of the action patterns of pectinmethylesterase isoforms through kinetic analyses and NMR spectroscopy. Implications In cell wall expansion. J Biol Chem 273:33150–33156
Chatterjee M, Tabi Z, Galli M, Malcomber S, Buck A, Muszynski M, Gallavotti A (2014) The boron efflux transporter ROTTEN EAR is required for maize inflorescence development and fertility. Plant Cell 26:2962–2977
Chebli Y, Kaneda M, Zerzour R, Geitmann A (2012) The cell wall of the Arabidopsis pollen tube–spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol 160:1940–1955
Chen M-H, Citovsky V (2003) Systemic movement of a tobamovirus requires host cell pectin methylesterase. Plant J 35:386–392
Chormova D, Messenger DJ, Fry SC (2014a) Boron bridging of rhamnogalacturonan-II, monitored by gel electrophoresis, occurs during polysaccharide synthesis and secretion but not post-secretion. Plant J 77:534–546
Chormova D, Messenger DJ, Fry SC (2014b) Rhamnogalacturonan-II cross-linking of plant pectins via boron bridges occurs during polysaccharide synthesis and/or secretion. Plant Signal Behav, 9
Christensen TMIE, Nielsen JE, Mikkelsen JD (1996) Pectins and Pectinases, Proceedings of an International Symposium. Prog Biotechnol 14: 723–730
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Cosgrove DJ, Jarvis MC (2012) Comparative structure and biomechanics of plant primary and secondary cell walls. Front Plant Sci 3:204
Coutinho PM, Stam M, Blanc E, Henrissat B (2003) Why are there so many carbohydrate-active enzyme-related genes in plants? Trends Plant Sci 8:563–565
Dardelle F, Lehner A, Ramdani Y, Bardor M, Lerouge P, Driouich A, Mollet J-C (2010) Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiol 153:1563–1576
De Souza A, Hull PA, Gille S, Pauly M (2014) Identification and functional characterization of the distinct plant pectin esterases PAE8 and PAE9 and their deletion mutants. Planta 240:1123–1138
Dean GH, Zheng H, Tewari J, Huang J, Young DS, Hwang YT, Western TL, Carpita NC, McCann MC, Mansfield SD et al (2007) The Arabidopsis MUM2 gene encodes a beta-galactosidase required for the production of seed coat mucilage with correct hydration properties. Plant Cell 19:4007–4021
Denès JM, Baron A, Renard CM, Péan C, Drilleau JF (2000) Different action patterns for apple pectin methylesterase at pH 7.0 and 4.5. Carbohydr Res 327:385–393
Deng W, Iannetta PPM, Hallett PD, Toorop PE, Squire GR, Jeng D-S (2013) The rheological properties of the seed coat mucilage of Capsella bursa-pastoris L. Medik. (shepherd’s purse). Biorheology 50:57–67
Derbyshire P, McCann MC, Roberts K (2007) Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol 7:31
Di Matteo A, Giovane A, Raiola A, Camardella L, Bonivento D, De Lorenzo G, Cervone F, Bellincampi D, Tsernoglou D (2005) Structural basis for the interaction between pectin methylesterase and a specific inhibitor protein. Plant Cell 17:849–858
Diaz-Cacho P, Moral R, Encina A, Luis Acebes J, Alvarez J (1999) Cell wall modifications in bean (Phaseolus vulgaris) callus cultures tolerant to isoxaben. Physiol Plant 107:54–59
Dixit S, Upadhyay SK, Singh H, Sidhu OP, Verma PC (2013) Enhanced methanol production in plants provides broad spectrum insect resistance. PLoS One 8:79664
Dorokhov YL, Komarova TV, Petrunia IV, Frolova OY, Pozdyshev DV, Gleba YY (2012) Airborne signals from a wounded leaf facilitate viral spreading and induce antibacterial resistance in neighboring plants. PLoS Pathog 8:e1002640
Driouich A, Follet-Gueye M-L, Bernard S, Kousar S, Chevalier L, Vicré-Gibouin M, Lerouxel O (2012) Golgi-mediated synthesis and secretion of matrix polysaccharides of the primary cell wall of higher plants. Front Plant Sci 3:79
Durand C, Vicré-Gibouin M, Follet-Gueye ML, Duponchel L, Moreau M, Lerouge P, Driouich A (2009) The organization pattern of root border-like cells of Arabidopsis is dependent on cell wall homogalacturonan. Plant Physiol 150:1411–1421
Durbak AR, Phillips KA, Pike S, O’Neill MA, Mares J, Gallavotti A, Malcomber ST, Gassmann W, McSteen P (2014) Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 26:2978–2995
Espino JJ, Gutiérrez-Sánchez G, Brito N, Shah P, Orlando R, González C (2010) The Botrytis cinerea early secretome. Proteomics 10:3020–3034
Eticha D, Stass A, Horst WJ (2005) Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant Cell Environ 28:1410–1420
Ferrari S, Savatin DV, Sicilia F, Gramegna G, Cervone F, De Lorenzo G (2013) Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front Plant Sci 4:49
Fry SC (2004) Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytol 161:641–675
Gendre D, McFarlane HE, Johnson E, Mouille G, Sjödin A, Oh J, Levesque-Tremblay G, Watanabe Y, Samuels L, Bhalerao RP (2013) Trans-Golgi network localized ECHIDNA/Ypt interacting protein complex is required for the secretion of cell wall polysaccharides in Arabidopsis. Plant Cell 25:2633–2646
Gille S, de Souza A, Xiong G, Benz M, Cheng K, Schultink A, Reca I-B, Pauly M (2011) O-acetylation of Arabidopsis hemicellulose xyloglucan requires AXY4 or AXY4L, proteins with a TBL and DUF231 domain. Plant Cell 23:4041–4053
Gjetting KSK, Ytting CK, Schulz A, Fuglsang AT (2012) Live imaging of intra- and extracellular pH in plants using pHusion, a novel genetically encoded biosensor. J Exp Bot 63:3207–3218
Gou J-Y, Miller LM, Hou G, Yu X-H, Chen X-Y, Liu C-J (2012) Acetylesterase-mediated deacetylation of pectin impairs cell elongation, pollen germination, and plant reproduction. Plant Cell 24:50–65
Guénin S, Mareck A, Rayon C, Lamour R, Assoumou Ndong Y, Domon J-M, Sénéchal F, Fournet F, Jamet E, Canut H et al (2011) Identification of pectin methylesterase 3 as a basic pectin methylesterase isoform involved in adventitious rooting in Arabidopsis thaliana. New Phytol 192:114–126
Ha MA, Apperley DC, Jarvis MC (1997) Molecular Rigidity in Dry and Hydrated Onion Cell Walls. Plant Physiol 115:593–598
Hall H, Ellis B (2013) Transcriptional programming during cell wall maturation in the expanding Arabidopsis stem. BMC Plant Biol 13:14
Harpaz-Saad S, Western TL, Kieber JJ (2012) The FEI2-SOS5 pathway and CELLULOSE SYNTHASE 5 are required for cellulose biosynthesis in the Arabidopsis seed coat and affect pectin mucilage structure. Plant Signal Behav 7:285–288
Haughn G, Chaudhury A (2005) Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci 10:472–477
Haughn GW, Western TL (2012) Arabidopsis seed coat mucilage is a specialized cell wall that can be used as a model for genetic analysis of plant cell wall structure and function. Front Plant Sci 3:64
Hayot CM, Forouzesh E, Goel A, Avramova Z, Turner JA (2012) Viscoelastic properties of cell walls of single living plant cells determined by dynamic nanoindentation. J Exp Bot 63:2525–2540
Hewezi T, Howe P, Maier TR, Hussey RS, Mitchum MG, Davis EL, Baum TJ (2008) Cellulose binding protein from the parasitic nematode Heterodera schachtii interacts with Arabidopsis pectin methylesterase: cooperative cell wall modification during parasitism. Plant Cell 20:3080–3093
Hongo S, Sato K, Yokoyama R, Nishitani K (2012) Demethylesterification of the primary wall by PECTIN METHYLESTERASE35 provides mechanical support to the Arabidopsis stem. Plant Cell 24:2624–2634
Hothorn M, Wolf S, Aloy P, Greiner S, Scheffzek K (2004) Structural insights into the target specificity of plant invertase and pectin methylesterase inhibitory proteins. Plant Cell 16:3437–3447
Ishii T (1997) O-acetylated oligosaccharides from pectins of potato tuber cell walls. Plant Physiol 113:1265–1272
Iwai H, Masaoka N, Ishii T, Satoh S (2002) A pectin glucuronyltransferase gene is essential for intercellular attachment in the plant meristem. Proc Natl Acad Sci USA 99:16319–16324
Iwai H, Hokura A, Oishi M, Chida H, Ishii T, Sakai S, Satoh S (2006) The gene responsible for borate cross-linking of pectin Rhamnogalacturonan-II is required for plant reproductive tissue development and fertilization. Proc Natl Acad Sci USA 103:16592–16597
Jiang L, Yang S-L, Xie L-F, Puah CS, Zhang X-Q, Yang W-C, Sundaresan V, Ye D (2005) Vanguard1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17:584–596
Kierzkowski D, Nakayama N, Routier-Kierzkowska A-L, Weber A, Bayer E, Schorderet M, Reinhardt D, Kuhlemeier C, Smith RS (2012) Elastic domains regulate growth and organogenesis in the plant shoot apical meristem. Science 335:1096–1099
Kohorn BD, Kohorn SL, Saba NJ, Martinez VM (2014) Requirement for pectin methyl esterase and preference for fragmented over native pectins for wall-associated kinase-activated, EDS1/PAD4-dependent stress response in Arabidopsis. J Biol Chem 289:18978–18986
Komarova TV, Sheshukova EV, Dorokhov YL (2014) Cell wall methanol as a signal in plant immunity. Front Plant Sci 5:101
Körner E, von Dahl CC, Bonaventure G, Baldwin IT (2009) Pectin methylesterase NaPME1 contributes to the emission of methanol during insect herbivory and to the elicitation of defence responses in Nicotiana attenuata. J Exp Bot 60:2631–2640
Krupková E, Immerzeel P, Pauly M, Schmülling T (2007) The tumorous shoot development2 gene of Arabidopsis encoding a putative methyltransferase is required for cell adhesion and co-ordinated plant development. Plant J 50:735–750
Lau JM, McNeil M, Darvill AG, Albersheim P (1985) Structure of the backbone of rhamnogalacturonan I, a pectic polysaccharide in the primary cell walls of plants. Carbohydr Res 137:111–125
Leboeuf E, Guillon F, Thoiron S, Lahaye M (2005) Biochemical and immunohistochemical analysis of pectic polysaccharides in the cell walls of Arabidopsis mutant QUASIMODO 1 suspension-cultured cells: implications for cell adhesion. J Exp Bot 56:3171–3182
Lehner A, Dardelle F, Soret-Morvan O, Lerouge P, Driouich A, Mollet J-C (2010) Pectins in the cell wall of Arabidopsis thaliana pollen tube and pistil. Plant Signal Behav 5:1282–1285
Leroux C, Bouton S, Kiefer-Meyer M-C, Fabrice TN, Mareck A, Guénin S, Fournet F, Ringli C, Pelloux J, Driouich A et al (2015) Pectin methylesterase48 Is Involved in Arabidopsis Pollen Grain Germination. Plant Physiol 167:367–380
Levesque-Tremblay G, Müller K, Mansfield SD, Haughn GW (2015) Highly methyl esterified seeds is a pectin methyl esterase involved in embryo development. Plant Physiol 167:725–737
Liners F, Letesson JJ, Didembourg C, Van Cutsem P (1989) Monoclonal Antibodies against pectin: recognition of a conformation induced by calcium. Plant Physiol 91:1419–1424
Lionetti V, Raiola A, Camardella L, Giovane A, Obel N, Pauly M, Favaron F, Cervone F, Bellincampi D (2007) Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol 143:1871–1880
Lionetti V, Cervone F, Bellincampi D (2012) Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J Plant Physiol 169:1623–1630
Lionetti V, Raiola A, Cervone F, Bellincampi D (2014) Transgenic expression of pectin methylesterase inhibitors limits tobamovirus spread in tobacco and Arabidopsis. Mol Plant Pathol 15:265–274
Lionetti V, Cervone F, De Lorenzo G (2015) A lower content of de-methylesterified homogalacturonan improves enzymatic cell separation and isolation of mesophyll protoplasts in Arabidopsis. Phytochemistry 112:188–194
Lord EM, Mollet J-C (2002) Plant cell adhesion: a bioassay facilitates discovery of the first pectin biosynthetic gene. Proc Natl Acad Sci USA 99:15843–15845
Louvet R, Cavel E, Gutierrez L, Guénin S, Roger D, Gillet F, Guerineau F, Pelloux J (2006) Comprehensive expression profiling of the pectin methylesterase gene family during silique development in Arabidopsis thaliana. Planta 224:782–791
Ma L, Jiang S, Lin G, Cai J, Ye X, Chen H, Li M, Li H, Takác T, Samaj J et al (2013) Wound-induced pectin methylesterases enhance banana (Musa spp. AAA) susceptibility to Fusarium oxysporum f. sp. cubense. J Exp Bot 64:2219–2229
Macarisin D, Wisniewski ME, Bassett C, Thannhauser TW (2009) Proteomic analysis of β-aminobutyric acid priming and abscisic acid—induction of drought resistance in crabapple (Malus pumila): effect on general metabolism, the phenylpropanoid pathway and cell wall enzymes. Plant Cell Environ 32:1612–1631
Manabe Y, Nafisi M, Verhertbruggen Y, Orfila C, Gille S, Rautengarten C, Cherk C, Marcus SE, Somerville S, Pauly M et al (2011) Loss-of-function mutation of reduced wall acetylation2 in Arabidopsis leads to reduced cell wall acetylation and increased resistance to Botrytis cinerea. Plant Physiol 155:1068–1078
Manfield IW, Orfila C, McCartney L, Harholt J, Bernal AJ, Scheller HV, Gilmartin PM, Mikkelsen JD, Paul Knox J, Willats WGT (2004) Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: global transcript profiling and cellular analysis. Plant J 40:260–275
Markovic O, Kohn R (1984) Mode of pectin deesterification by trichoderma reseei pectinesterase. Experientia 40:842–843
McFarlane HE, Watanabe Y, Gendre D, Carruthers K, Levesque-Tremblay G, Haughn GW, Bhalerao RP, Samuels L (2013) Cell wall polysaccharides are mislocalized to the Vacuole in echidna mutants. Plant Cell Physiol 54:1867–1880
Micheli F (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6:414–419
Milani P, Braybrook SA, Boudaoud A (2013) Shrinking the hammer: micromechanical approaches to morphogenesis. J Exp Bot 64:4651–4662
Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11:266–277
Mouille G, Ralet M-C, Cavelier C, Eland C, Effroy D, Hématy K, McCartney L, Truong HN, Gaudon V, Thibault J-F et al (2007) Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. Plant J 50:605–614
Moulia B (2013) Plant biomechanics and mechanobiology are convergent paths to flourishing interdisciplinary research. J Exp Bot 64:4617–4633
Moustacas AM, Nari J, Borel M, Noat G, Ricard J (1991) Pectin methylesterase, metal ions and plant cell-wall extension. The role of metal ions in plant cell-wall extension. Biochem J 279 (Pt 2: 351–4
Müller K, Tintelnot S, Leubner-Metzger G (2006) Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol 47:864–877
Müller K, Levesque-Tremblay G, Fernandes A, Wormit A, Bartels S, Usadel B, Kermode AR (2013a) Overexpression of a pectin methylesterase inhibitor in Arabidopsis thaliana leads to altered growth morphology of the stem and defective organ separation. Plant Signal Behav 8:e26464
Müller K, Levesque-Tremblay G, Bartels S, Weitbrecht K, Wormit A, Usadel B, Haughn G, Kermode AR (2013b) Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol 161:305–316
Nakamura A, Furuta H, Maeda H, Takao T, Nagamatsu Y (2002) Structural studies by stepwise enzymatic degradation of the main backbone of soybean soluble polysaccharides consisting of galacturonan and rhamnogalacturonan. Biosci Biotechnol Biochem 66:1301–1313
Neumetzler L, Humphrey T, Lumba S, Snyder S, Yeats TH, Usadel B, Vasilevski A, Patel J, Rose JKC, Persson S et al (2012) The FRIABLE1 gene product affects cell adhesion in Arabidopsis. PLoS ONE 7:e42914
Ogawa M, Kay P, Wilson S, Swain SM (2009) ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are Polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21:216–233
O’Neill MA, Ishii T, Albersheim P, Darvill AG (2004) Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu Rev Plant Biol 55:109–139
Osorio S, Castillejo C, Quesada MA, Medina-Escobar N, Brownsey GJ, Suau R, Heredia A, Botella MA, Valpuesta V (2008) Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca). Plant J 54:43–55
Parre E, Geitmann A (2005) Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 220:582–592
Pauly M, Scheller HV (2000) O-Acetylation of plant cell wall polysaccharides: identification and partial characterization of a rhamnogalacturonan O-acetyl-transferase from potato suspension-cultured cells. Planta 210:659–667
Peaucelle A, Louvet R, Johansen JN, Höfte H, Laufs P, Pelloux J, Mouille G (2008) Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr Biol 18:1943–1948
Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Höfte H (2011a) Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr Biol 21:1720–1726
Peaucelle A, Louvet R, Johansen JN, Salsac F, Morin H, Fournet F, Belcram K, Gillet F, Höfte H, Laufs P et al (2011b) The transcription factor BELLRINGER modulates phyllotaxis by regulating the expression of a pectin methylesterase in Arabidopsis. Development 138:4733–4741
Pelletier S, Van Orden J, Wolf S, Vissenberg K, Delacourt J, Ndong YA, Pelloux J, Bischoff V, Urbain A, Mouille G et al (2010) A role for pectin de-methylesterification in a developmentally regulated growth acceleration in dark-grown Arabidopsis hypocotyls. New Phytol 188:726–739
Pelloux J, Rustérucci C, Mellerowicz EJ (2007) New insights into pectin methylesterase structure and function. Trends Plant Sci 12:267–277
Qu T, Liu R, Wang W, An L, Chen T, Liu G, Zhao Z (2011) Brassinosteroids regulate pectin methylesterase activity and AtPME41 expression in Arabidopsis under chilling stress. Cryobiology 63:111–117
Raiola A, Lionetti V, Elmaghraby I, Immerzeel P, Mellerowicz EJ, Salvi G, Cervone F, Bellincampi D (2011) Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol Plant Microbe Interact 24:432–440
Ralet M-C, Crépeau M-J, Lefèbvre J, Mouille G, Höfte H, Thibault J-F (2008) Reduced number of homogalacturonan domains in pectins of an Arabidopsis mutant enhances the flexibility of the polymer. Biomacromolecules 9:1454–1460
Rautengarten C, Usadel B, Neumetzler L, Hartmann J, Büssis D, Altmann T (2008) A subtilisin-like serine protease essential for mucilage release from Arabidopsis seed coats. Plant J 54:466–480
Rautengarten C, Ebert B, Moreno I, Temple H, Herter T, Link B, Doñas-Cofré D, Moreno A, Saéz-Aguayo S, Blanco F et al (2014) The Golgi localized bifunctional UDP-rhamnose/UDP-galactose transporter family of Arabidopsis. Proc Natl Acad Sci U S A 111:11563–11568
Ren C, Kermode AR (2000) An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiol 124:231–242
Ridley BL, O’Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57:929–967
Rocchi V, Janni M, Bellincampi D, Giardina T, D’Ovidio R (2012) Intron retention regulates the expression of pectin methyl esterase inhibitor (Pmei) genes during wheat growth and development. Plant Biol (Stuttg) 14:365–373
Röckel N, Wolf S, Kost B, Rausch T, Greiner S (2008) Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J 53:133–143
Saez-Aguayo S, Ralet M-C, Berger A, Botran L, Ropartz D, Marion-Poll A, North HM (2013) Pectin methylesterase inhibitor6 promotes Arabidopsis mucilage release by limiting methylesterification of homogalacturonan in seed coat epidermal cells. Plant Cell 25:308–323
Scheler C, Weitbrecht K, Pearce SP, Hampstead A, Büttner-Mainik A, Lee K, Voegele A, Oracz K, Dekkers B, Wang X et al (2014) Promotion of Testa Rupture during Lepidium sativum Germination Involves Seed Compartment-Specific Expression and Activity of Pectin Methylesterases. Plant Physiol 167:200–215
Schmohl N, Pilling J, Fisahn J, Horst WJ (2000) Pectin methylesterase modulates aluminium sensitivity in Zea mays and Solanum tuberosum. Physiol Plant 109:419–427
Schopfer P (2006) Biomechanics of plant growth. Am J Bot 93:1415–1425
Schuster E, Lundin L, Williams MAK (2012) Investigating the Relationship between Network Mechanics and Single-Chain Extension Using Biomimetic Polysaccharide Gels. Macromolecules 45:4863–4869
Sénéchal F, Graff L, Surcouf O, Marcelo P, Rayon C, Bouton S, Mareck A, Mouille G, Stintzi A, Höfte H et al (2014a) Arabidopsis PECTIN METHYLESTERASE17 is co-expressed with and processed by SBT3.5, a subtilisin-like serine protease. Ann Bot 114:1161–1175
Sénéchal F, Wattier C, Rustérucci C, Pelloux J (2014b) Homogalacturonan-modifying enzymes: structure, expression, and roles in plants. J Exp Bot 65:5125–5160
Sexton TR, Henry RJ, Harwood CE, Thomas DS, McManus LJ, Raymond C, Henson M, Shepherd M (2012) Pectin methylesterase genes influence solid wood properties of Eucalyptus pilularis. Plant Physiol 158:531–541
Shedletzky E, Shmuel M, Delmer DP, Lamport DT (1990) Adaptation and growth of tomato cells on the herbicide 2,6-dichlorobenzonitrile leads to production of unique cell walls virtually lacking a cellulose-xyloglucan network. Plant Physiol 94:980–987
Siedlecka A, Wiklund S, Péronne M-A, Micheli F, Lesniewska J, Sethson I, Edlund U, Richard L, Sundberg B, Mellerowicz EJ (2008) Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiol 146:554–565
Solecka D, Zebrowski J, Kacperska A (2008) Are pectins involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Ann Bot 101:521–530
Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T et al (2004) Toward a systems approach to understanding plant cell walls. Science 306:2206–2211
Staehelin LA, Moore I (1995) The Plant Golgi Apparatus: structure, Functional Organization and Trafficking Mechanisms. Annu Rev Plant Physiol Plant Mol Biol 46:261–288
Sterling JD, Quigley HF, Orellana A, Mohnen D (2001) The catalytic site of the pectin biosynthetic enzyme alpha-1,4-galacturonosyltransferase is located in the lumen of the Golgi. Plant Physiol 127:360–371
Tan L, Eberhard S, Pattathil S, Warder C, Glushka J, Yuan C, Hao Z, Zhu X, Avci U, Miller JS et al (2013) An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 25:270–287
Tanino KK, Kobayashi S, Hyett C, Hamilton K, Liu J, Li B, Borondics F, Pedersen T, Tse J, Ellis T et al (2013) Physiol Plant 147:101–111
Tian G-W, Chen M-H, Zaltsman A, Citovsky V (2006) Pollen-specific pectin methylesterase involved in pollen tube growth. Dev Biol 294:83–91
Van Doorn WG, Hiemstra T, Fanourakis D (2011) Hydrogel regulation of xylem water flow: an alternative hypothesis. Plant Physiol 157:1642–1649
Vicré M, Santaella C, Blanchet S, Gateau A, Driouich A (2005) Root border-like cells of Arabidopsis. Microscopical characterization and role in the interaction with rhizobacteria. Plant Physiol 138:998–1008
Vincent RR, Williams MAK (2009) Microrheological investigations give insights into the microstructure and functionality of pectin gels. Carbohydr Res 344:1863–1871
Voiniciuc C, Dean GH, Griffiths JS, Kirchsteiger K, Hwang YT, Gillett A, Dow G, Western TL, Estelle M, Haughn GW (2013) Flying saucer1 is a transmembrane RING E3 ubiquitin ligase that regulates the degree of pectin methylesterification in Arabidopsis seed mucilage. Plant Cell 25:944–959
Volpi C, Janni M, Lionetti V, Bellincampi D, Favaron F, D’Ovidio R (2011) The ectopic expression of a pectin methyl esterase inhibitor increases pectin methyl esterification and limits fungal diseases in wheat. Mol Plant Microbe Interact 24:1012–1019
Von Dahl CC, Hävecker M, Schlögl R, Baldwin IT (2006) Caterpillar-elicited methanol emission: a new signal in plant-herbivore interactions? Plant J 46:948–960
Vorwerk S, Somerville S, Somerville C (2004) The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci 9:203–209
Wakabayashi K, Hoson T, Huber DJ (2003) Methyl de-esterification as a major factor regulating the extent of pectin depolymerization during fruit ripening: a comparison of the action of avocado (Persea americana) and tomato (Lycopersicon esculentum) polygalacturonases. J Plant Physiol 160:667–673
Wang L, Wang W, Wang Y-Q, Liu Y-Y, Wang J-X, Zhang X-Q, Ye D, Chen L-Q (2013a) Arabidopsis galacturonosyltransferase (GAUT) 13 and GAUT14 have redundant functions in pollen tube growth. Mol Plant 6:1131–1148
Wang M, Yuan D, Gao W, Li Y, Tan J, Zhang X (2013b) A comparative genome analysis of PME and PMEI families reveals the evolution of pectin metabolism in plant cell walls. PLoS ONE 8:e72082
Weber M, Deinlein U, Fischer S, Rogowski M, Geimer S, Tenhaken R, Clemens S (2013) A mutation in the Arabidopsis thaliana cell wall biosynthesis gene pectin methylesterase 3 as well as its aberrant expression cause hypersensitivity specifically to Zn. Plant J 76:151–164
Western TL, Skinner DJ, Haughn GW (2000) Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol 122:345–356
Western TL, Burn J, Tan WL, Skinner DJ, Martin-McCaffrey L, Moffatt BA, Haughn GW (2001) Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiol 127:998–1011
Whitcombe AJ, O’Neill MA, Steffan W, Albersheim P, Darvill AG (1995) Structural characterization of the pectic polysaccharide, rhamnogalacturonan-II. Carbohydr Res 271:15–29
Willats WGT, McCartney L, Mackie W, Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47:9–27
Williamson G (1991) Purification and characterization of pectin acetylesterase from orange peel. Phytochemistry 30:445–449
Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2:e718
Wolf S, Mouille G, Pelloux J (2009a) Homogalacturonan methyl-esterification and plant development. Mol Plant 2:851–860
Wolf S, Rausch T, Greiner S (2009b) The N-terminal pro region mediates retention of unprocessed type-I PME in the Golgi apparatus. Plant J 58:361–375
Wolf S, Mravec J, Greiner S, Mouille G, Höfte H (2012) Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr Biol 22:1732–1737
Wu H-C, Hsu S-F, Luo D-L, Chen S-J, Huang W-D, Lur H-S, Jinn T-L (2010) Recovery of heat shock-triggered released apoplastic Ca2 + accompanied by pectin methylesterase activity is required for thermotolerance in soybean seedlings. J Exp Bot 61:2843–2852
Xiong G, Cheng K, Pauly M (2013) Xylan O-acetylation impacts xylem development and enzymatic recalcitrance as indicated by the Arabidopsis mutant tbl29. Mol Plant 6:1373–1375
Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146:602–611
Yang JL, Zhu XF, Zheng C, Zhang YJ, Zheng SJ (2011) Genotypic differences in Al resistance and the role of cell-wall pectin in Al exclusion from the root apex in Fagopyrum tataricum. Ann Bot 107:371–378
Yoneda A, Higaki T, Kutsuna N, Kondo Y, Osada H, Hasezawa S, Matsui M (2007) Chemical genetic screening identifies a novel inhibitor of parallel alignment of cortical microtubules and cellulose microfibrils. Plant Cell Physiol 48:1393–1403
Yoneda A, Ito T, Higaki T, Kutsuna N, Saito T, Ishimizu T, Osada H, Hasezawa S, Matsui M, Demura T (2010) Cobtorin target analysis reveals that pectin functions in the deposition of cellulose microfibrils in parallel with cortical microtubules. Plant J 64:657–667
Zablackis E, Huang J, Müller B, Darvill AG, Albersheim P (1995) Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol 107:1129–1138
Zerzour R, Kroeger J, Geitmann A (2009) Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev Biol 334:437–446
Zhang GF, Staehelin LA (1992) Functional compartmentation of the Golgi apparatus of plant cells : immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol 99:1070–1083
Zwieniecki MA, Melcher PJ, Michele Holbrook NM (2001) Hydrogel control of xylem hydraulic resistance in plants. Science 291:1059–1062
Acknowledgments
We thank Sam Taylor for his help with this manuscript. The financial support from the NSERC Postgraduate Scholarships (to G.L.-T.), the Institut Universitaire de France (IUF) to J.P. and a Marie Curie International Outgoing Fellowship to K.M. is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levesque-Tremblay, G., Pelloux, J., Braybrook, S.A. et al. Tuning of pectin methylesterification: consequences for cell wall biomechanics and development. Planta 242, 791–811 (2015). https://doi.org/10.1007/s00425-015-2358-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2358-5