Abstract
Main Conclusion
Systemic acquired resistance elicitors, BTH and BABA, reduce rust penetration in pea through phytoalexins pathway but differing in their mode of action.
It has been previously shown that rust (Uromyces pisi) infection can be reduced in pea (Pisum sativum) by exogenous applications of systemic acquired resistance elicitors such as BTH and BABA. This protection is known to be related with the induction of the phenolic pathway but the particular metabolites involved have not been determined yet. In this work, we tackled the changes induced in phytoalexin content by BTH and BABA treatments in the context of the resistance responses to pea rust. Detailed analysis through high-performance liquid chromatography (HPLC) showed qualitative and quantitative differences in the content, as well as in the distribution of phytoalexins. Thus, following BTH treatment, we observed an increase in scopoletin, pisatin and medicarpin contents in all, excreted, soluble and cell wall-bound fraction. This suggests fungal growth impairment by both direct toxic effect as well as plant cell wall reinforcement. The response mediated by BTH was genotype-dependent, since coumarin accumulation was observed only in the resistant genotype whereas treatment by BABA primed phytoalexin accumulation in both genotypes equally. Exogenous application to the leaves of scopoletin, medicarpin and pisatin lead to a reduction of the different fungal growth stages, confirming a role for these phytoalexins in BTH- and BABA-induced resistance against U. pisi hampering pre- and postpenetration fungal stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pea rust incited by Uromyces pisi (Pers.) Wint. is a widespread disease of pea (Pisum sativum L.), causing considerable losses in Europe, North and South America, Asia, Australia and New Zealand (EPPO 2013). During its cycle, U. pisi has five morphological stages, each one represented by a different type of spore. The uredial stage is the one causing the main agronomic losses in pea, being urediospores responsible for disease development under field conditions causing a multi-cycling infection (Barilli et al. 2009a). When urediospores germinate, a germ tube is formed to recognize the leaf stomata. When it occurs, the germ tube develops an appressorium that penetrates through the stomata. Then, a substomatal vesicle is formed and from them, haustoria mother cells elongate and attempt to invaginate into mesophyll cells. In the case of successful penetration, a nutrient-absorbing haustorium develops inside the mesophyll cell and fungal growth of subsequent hyphae is allowed. Rust infection can be hampered at early stages before stomatal penetration, although resistance based in reduced spore germination or stomata recognition is a rare event described only in few instances (Niks and Rubiales 2002). When the fungus enter through stomata and develop haustoria mother cells, penetration of mesophyll cells may be hampered by limiting intercellular growth of primary infection hyphae or by mesophyll cell penetration resistance based on the reinforcement of cell walls through papilla formation and/or protein crosslinking (Iglesias-García et al. 2015; Niks and Rubiales 2002). When penetration resistance fails and haustoria develop within the mesophyll host cells, the hypersensitive cell death response can be activated limiting fungal development (Niks and Rubiales 2002; Rubiales et al. 2011; Sillero et al. 2006). At present, complete resistance to U. pisi has not yet been found in peas, although partial resistance based on the activation of pre-haustorial mechanisms without host cell death has been described under field and controlled conditions (Barilli et al. 2009a, b).
One of the potential control methods for U. pisi consists of reducing disease severity through the induction of systemic acquired resistance (SAR) in pea plants (Barilli et al. 2010a), as previously found for other crops against pathogenic fungi, parasitic plants, oomycetes, viruses, and bacteria (Amzalek and Cohen 2007; Fu and Dong 2013; Gozzo and Faoro 2013; Pérez-de-Luque et al. 2004; Prats et al. 2002; Sillero et al. 2012). Enhancement of resistance can be accomplished by application of both abiotic and biotic elicitors on the plant before it is infected by a pathogen. Benzo[1,2,3]thiadiazole-7-carbothionic acid (BTH) and D,L-β-aminobutyric acid (BABA) are known by their general ability to induce resistance in treated plants (Gozzo and Faoro 2013), but knowledge of their underlying mechanisms of action for the same pathosystem are often missed or limited. We have recently proven that exogenous applications of BTH and BABA on pea leaves few days before inoculation with U. pisi reduced rust incidence reducing the percentage of appressorium and haustorium formation (Barilli et al. 2010a, b). This phenomenon was related to a significant increase in the activity of several defense-related enzymes such as peroxidases, β-1,3-glucanase and chitinase, as well as the activation of the phenylpropanoid pathway increasing accumulation of phenolic compounds (Barilli et al. 2010b, 2012). These were more abundant following both BTH and BABA application, but the methodology used did not allow identifying, quantifying and fully describing the particular phenolic compounds involved.
Phenolic compounds are a group of important plant secondary metabolites that have been suggested to play a variety of roles in defense mechanisms against pathogens. They were reported as phytoanticipins, phytoalexins, structural barriers, modulators of pathogenicity, and/or activators of plant defense genes (Arfaoui et al. 2007). In particular, phytoalexins are low molecular weight antimicrobial compounds synthesized de novo and accumulated in plants following pathogen challenge or other stresses (Ding et al. 2000; Prats et al. 2000, 2006; Serghini et al. 1996, 2001). Their accumulation is described also as one of the mechanisms induced during SAR responses (Gozzo and Faoro 2013). The phytoalexins pisatin and maackiain (Fig. 1a, b) are the major isoflavonoids found in pea (Ingham 1982), although others such as medicarpin (Fig. 1c), more abundant in Medicago spp. can also be found. All these three phytoalexins belong to the same pterocarpans subgroup of isoflavonoids and are well known for their antifungal activity (Dewick 2009). Recently, pisatin was found as principal metabolite in pea root exudates together with already know and new stilbene polyphenols which stimulates Orobanche seed germination (Evidente et al. 2010). Medicarpin was also previously isolated from heavy naturally Ascochyta rabiei-infected chickpea seeds (Evidente et al. 1996). Scopoletin has been isolated from many plants including leguminous (reviewed by Gnonlonfin et al. 2012) and belong, together with some analogs also incorporating an isoprene unit, to the coumarin subgroup of phenylpropanoids (Dewick 2009) (Fig. 1d).
The aim of the present work was to shed light on the underlying mechanisms operating during BTH and BABA-induced defense response in the pea-U. pisi interaction. For this purpose, we compared the effect of pea pre-treated with both elicitors on the content of phytoalexins in different leaf phenolic fractions of two pea genotypes in response to fungal infection. In addition, histological bioassays were performed testing the fungitoxic activity of phytoalexins on particular stages of U. pisi fungal infection progress.
Materials and methods
Plants and fungal material, growth conditions and inoculation
Uromyces pisi monosporic isolate UpCo-01 from the fungal collection belonging to the Institute for Sustainable Agriculture-CSIC (Córdoba, Spain) was used for the experiment. Spores were preserved in liquid nitrogen and multiplied on susceptible pea cv. Messire before use: urediospores were removed from storage, heat shocked at 40 °C for 5 min and then used for plant inoculation as described below. Eight days after infection, when pustules sporulate profusely, urediospores were recollected by means of a vacuum spore collection device, dried during 24 h in silica gel supplied by Merck (Darmstadt, Germany) and used for the experiment. Pea cv. Messire and accession PI347321 were used in the experiment. Messire is highly susceptible to U. pisi, while genotype PI347321 shows partial resistance based on pre-penetration mechanisms, that is the highest level of resistance available so far in pea (Barilli et al. 2009b). Plants were grown in pots (6 × 6 × 10 cm) filled with a 1:1 mixture of sand and peat in a growth chamber at 20 °C under a photoperiod at 14 h light and 10 h dark with light of 250 μmol m−2 s−1 photon flux density supplied by high-output white fluorescent tubes (Lumilux Cool White FQ 80 W//840 HO; Osram, Munich, Germany).
When the third leaves were fully expanded, plants were treated with the SAR elicitors benzo[1,2,3]thiadiazole-7-carbothionic acid-S-methyl ester (BTH, also named acibenzolar-S-methyl, ASM) and the nonprotein amino acid D,L-3-amino-n-butanoic acid (D,L-β-aminobutyric acid, BABA). BTH was purchased in the form of Bion 50 (50 % active ingredient) from Syngenta (Basel, Switzerland), and BABA was purchased from Sigma-Aldrich (St. Louis, MO, USA). Concentrations used were 10 mM and 50 mM for BTH and BABA, respectively, previously proven to be effective in this pathosystem (Barilli et al. 2010a). Solutions were prepared in sterile water to which Tween 20 (0.03 %, v/v) was added as a wetting agent. Then, 3 droplets (15 μl per drop) of each solution were applied on each leaflet at the first node from the base. Control plants were treated with sterile water plus Tween 20. Three replications, consisting of four plants each, per treatment and genotypes were analyzed and experiments were repeated three times. Five days after treatment with SAR elicitors, plants were inoculated by dusting the plants with rust urediospores (2 mg spores per plant) mixed with pure talc (1:10, w:w) using a spore settling tower, resulting in a spore deposition of about 30–35 spores/mm2. After inoculation, plants were incubated at 20 ± 2 °C in complete darkness and high humidity during the first 24 h. High humidity was provided by ultrasonic humidifiers operating for 15 min every 2 h. After that period, the humidifiers were turned off and plants returned to normal growth conditions. Controls, non-inoculated plants were maintained in the same environmental conditions as inoculated plants. Two leaves at the 3rd stages from non-inoculated, inoculated with and without SAR induction were harvested 48 h after inoculation (hai), weighted and used for biochemical analysis.
Extraction of phytoalexins
To quantify phytoalexins (both coumarins and isoflavonoids), leaves collected as mentioned above were washed with 5 ml of methanol by dripping the volume along the leaf surface. The methanol was recovered with a pipette and dripped again along the leaf. Then the same procedure was repeated using 5 ml of chloroform instead of methanol. The methanol and chloroform washes were combined, concentrated under vacuum, and then dissolved in 0.1 ml of methanol as described by Prats et al. (2007) for quantification of excreted coumarins. Then, the washed leaf was homogenized in 10 ml pre-chilled (−20 °C) acetone using a mortar. After filtering off the solvent extract, the residue was further sequentially extracted with a similar volume of acetone and acetone methanol (1:1, v:v). The combined solvent extract was concentrated under reduced pressure, dissolved in 1 ml of methanol and cleared by centrifugation (Prats et al. 2002) for quantification of soluble phytoalexin within the cell. For quantification of cell wall-bound coumarins, 0.2 g of the pellet was resuspended in 0.4 ml of 2 M NaOH and then incubated at 70 °C during 16 h. The suspension was chilled and neutralized adding 0.4 ml of 2 M HCl and then centrifuged at 15,557g during 15 min. The supernatant was collected, evaporated and resuspended in 0.25 ml of methanol. Prior to HPCL analysis, photosynthetic pigments were removed by adding to the methanolic solution 1:5 volumes of n-hexane ethyl ether (60:40, v:v). As a result, most pigments partitioned into the n-hexane ether phase. The aqueous phase was extracted three times with ethyl acetate, evaporated and resuspended in 0.1 ml of methanol. Total phytoalexin values were considered as sum of resulted excreted, soluble and cell wall-bound phytoalexins per genotype and treatment.
HPLC analysis of phytoalexins
Phytoalexins were identified and quantified by HPLC using an HPLC (Perkin Elmer, Series 200 System, Monza, Italy) equipped with a diode array detector set to 210 and 220 nm. Analyses were carried out in duplicate injecting 20 μl of all samples for each analysis. Standards (scopoletin, pisatin, medicarpin, maackiain, from Sigma-Aldrich) were prepared at concentration of 1 mg ml−1 in methanol and diluted in a 1:10 ratio in a C18 column 250 × 4 mm internal diameter, containing 5 mm diameter beads (LiChrospher 100 RP-18, Merck), using a flow rate of 1 ml min−1 and elution with CH3CN/H2O along a linear gradient of CH3CN from 20 to 33 % over a period of 90 min, and again from 33 to 20 % for 5 min. The column was re-equilibrated under isocratic conditions for 5 min before the next run. The data obtained were expressed in µg g−1 FW.
Fungal growth bioassay
Two leaves at the 2nd node per plant and genotype were used, with a total of six plants per treatment. Plants were treated with phytoalexins at concentrations previously found in the leaves (i.e. 8.0, 7.8, and 1.2 µg per leaf of scopoletin, pisatin, and medicarpin, respectively), by applying the solution with a pipette on the leaf surface and ensuring their complete absorption. As a control, BTH and BABA solutions were also applied to both genotypes (as reported above). Twenty-four hours after the treatments, plants were inoculated with U. pisi as reported above. Two days after inoculation (dai), leaves were harvested and stained according to Sillero and Rubiales (2002). The different stages of the infection process were assessed using a phase contrast Leica DM LS microscope at X400 magnification (Leica Microsystems, Wetzlar, Germany). Necrosis was identified by uptake of Trypan blue by the plant cells.
Statistical analyses
For statistical analysis, percentage data were transformed to arcsine square roots (transformed value = 180/π × arcsine [√(%/100)]) to normalize data and stabilize variances throughout the data range. Data were combined from the three experiments to determine average ratings. Transformed data were subjected to analysis of variance (ANOVA) using Statistix 8 (Analytical Software, Tallahassee, FL, USA), after which residual plots were inspected to confirm data conformity to normality. Significance of differences between means was determined by calculating least significant difference (LSD).
Results
Total phytoalexin content
Overall, 13 different compounds were detected distributed in the different fractions analyzed (Supplemental Table S1). Out of these, scopoletin, medicarpin and pisatin were identified by HPLC analysis carried out in comparison of commercial standard also by co-injection. Identification of the remaining compounds is currently being carried out; so in the present work, we will focus in these three important phytoalexins belong to two different groups of phenylpropanoids as coumarins and isoflavonoids. Scopoletin was by far the most abundant coumarin in both genotypes, followed by pisatin and medicarpin (Fig. 2). Whatever the genotype and treatment, the presence of maackiain was not detected. Scopoletin was constitutively most abundant in resistant PI347321, whereas similar levels of pisatin and medicarpin, were found in both genotypes. Total content of scopoletin and medicarpin did not changed significantly following rust inoculation. However, the total content in pisatin significantly increased in both genotypes following rust infection (P = 0.0026 and P = 0.049 in Messire and PI347321, respectively) (Fig. 2).
Total content of scopoletin, pisatin and medicarpin in phenolic fractions from pea accession Messire and PI347321. Values expressed as µg coumarin g−1 fresh weight are mean of 3 replications ± SE. White bars represent healthy genotypes, while black bars represents U. pisi inoculated genotypes. Different roman letters within an open bar indicate significant differences (P < 0.05) within the healthy plants. Different Greek letters within a solid bar indicate significant differences (P < 0.05) within the inoculated plants. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) between healthy and inoculated plants for each treatment
Interestingly, both, BTH and BABA induced changes in the total content of coumarins although not in the same manner. BTH treatment increased the level of pisatin and medicarpin (P = 0.0096 and P = 0.0214, respectively) (Fig. 2) in the resistant genotype PI347321 prior to pathogen challenge. After pathogen challenge, there was a marked accumulation of scopoletin, pisatin and medicarpin in PI347321 (more than fourfold respect to inoculated non-treated plants (Fig. 2). In contrast, BTH treatment did not affect the content of any of the coumarins in the susceptible Messire before or after rust challenge. Unlike BTH, BABA induced accumulation both, in the susceptible and resistant genotypes (Fig. 2). However, in the absence of pathogen challenge, BABA treatment did not affect coumarin content and following attack led to an increase only in the content of scopoletin (Fig. 2).
To correlate the changes of the particular phytoalexins with their possible role hampering specific stages of the rust infection process, we carried out an analysis of the different fractions: i.e., soluble phytoalexins excreted to the leaf surface, soluble phytoalexins within the cell, and cell wall-bound phytoalexins.
Changes on soluble phytoalexins excreted to the leaf surface
As for the total content, in this fraction, scopoletin was the most abundant phytoalexin, followed by pisatin and medicarpin (Fig. 3). Similar constitutive levels of scopoletin and medicarpin were found in the susceptible and resistant genotypes, whereas pisatin was present constitutively only in the latter. The content of scopoletin and medicarpin excreted to the leaf surface did not change following rust inoculation. However, the total content in pisatin significantly increased in both genotypes following pathogen attack (P = 0.0001 and P = 0.0208 in Messire and PI347321, respectively) (Fig. 3).
Scopoletin, pisatin and medicarpin content in excreted phenolic fraction from pea accession Messire and PI347321. Values expressed as µg coumarin g−1 fresh weight are mean of 3 replications ± SE. White bars represent healthy genotypes, while black bars represents U. pisi inoculated genotypes. Different roman letters within an open bar indicate significant differences (P < 0.05) within the healthy plants. Different Greek letters within a solid bar indicate significant differences (P < 0.05) within the inoculated plants. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) between healthy and inoculated plants for each treatment
BTH did not modify the content of any of the phytoalexins in the susceptible genotype Messire, either constitutively or after pathogen attack. However, in resistant PI347321, it significantly increased the pisatin and medicarpin contents prior to fungal inoculation (P = 0.0358 and P = 0.0300, respectively) (Fig. 3). In addition, in genotype PI347321, BTH also induced accumulation of all phytoalexins following pathogen attack in more than fivefold (P = 0.0002, 0.0002 and 0.0066 for scopoletin, pisatin and medicarpin, respectively) (Fig. 3). BABA treatment did not affect significantly the constitutive content of any of the phytoalexins in both genotypes. However, excreted scopoletin increased significantly in both BABA-treated genotypes following U. pisi inoculation (Fig. 3).
Changes on soluble phytoalexins within the cell
Scopoletin and pisatin were the most abundant phytoalexins of this fraction, followed by medicarpin (Fig. 4) as observed in the excreted fraction. A similar constitutive level of pisatin was found in the susceptible and resistant genotypes, while constitutive levels of scopoletin and medicarpin were higher in the resistant PI347321 (P = 0.050 and P = 0.034, respectively). The content of any of the three phytoalexins did not change significantly in the susceptible genotype following rust inoculation. However, the pisatin content significantly increased in resistant PI347321 following pathogen attack (P = 0.0491) (Fig. 4).
Scopoletin, pisatin and medicarpin content in soluble mesophyll fraction from pea accession Messire and PI347321. Values expressed as µg coumarin g−1 fresh weight are mean of 3 replications ± SE. White bars represent healthy genotypes, while black bars represents U. pisi inoculated genotypes. Different roman letters within an open bar indicate significant differences (P < 0.05) within the healthy plants. Different Greek letters within a solid bar indicate significant differences (P < 0.05) within the inoculated plants. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) between healthy and inoculated plants for each treatment
Both BTH and BABA induced changes in the content of the phytoalexins albeit these changes depend on the particular treatment applied. Prior to pathogen attack, elicitors did not affect the content of scopoletin and medicarpin. However, pisatin content was significantly reduced following BABA treatment in the susceptible genotype Messire (Fig. 4). On the other hand, BTH significantly increased the constitutive level of pisatin (P = 0.0018) (Fig. 4). After U. pisi inoculation, both elicitors led to scopoletin accumulation in both genotypes (P = 0.007 and 0.0052 for Messire and PI347321, respectively), values being higher in BABA-treated genotypes. As previously found, BTH also induced pisatin in inoculated PI347321 (P = 0.0017) and, to a minor extent, medicarpin accumulation (P = 0.0063) (Fig. 4).
Changes on cell wall-bound phytoalexins
Phytoalexin quantification revealed only the presence of scopoletin in this fraction. Similar constitutive levels of this phytoalexin were found in both susceptible and resistant genotypes, and its content did not significantly increase following rust inoculation (Fig. 5). Elicitor treatments did not have any significant effect in the susceptible cv. Messire. However, BTH treatment significantly induced the accumulation of scopoletin in the resistant genotype following pathogen attack more than twofold (P = 0.0001) (Fig. 5). By contrary, BABA treatment reduced the content of the cell wall-bound scopoletin of inoculated plants (P = 0.0009).
Scopoletin content in wall cell bound phenolic fraction from pea accession Messire and PI347321. Values expressed as µg coumarin g−1 fresh weight are mean of 3 replications ± SE. White bars represent healthy genotypes, while black bars represents U. pisi inoculated genotypes. Different roman letters within an open bar indicate significant differences (P < 0.05) within the healthy plants. Different Greek letters within a solid bar indicate significant differences (P < 0.05) within the inoculated plants. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) between healthy and inoculated plants for each treatment
Fungal growth bioassay
The different phytoalexins affected fungal growth at different stages of the infection process, in some cases to a similar extent observed for the BTH and/or BABA treatment (Fig. 6). As expected, control plants from the resistant PI347321 showed a lower infection rate than the susceptible control Messire.
Histological components of pre- and post-penetration resistance measured against Uromyces pisi in pea accessions, at 48 h after infection (hai) under controlled conditions. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) between treatments and their respective control for each genotype studied
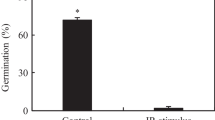
Urediospore germination was high in both genotypes (Fig. 6). BTH and BABA did not significantly reduce fungal germination compared to the water control. Interestingly scopoletin reduced spore germination in both genotypes (P = 0.0240 and P = 0.0162 for Messire and PI347321, respectively) (Fig. 6).
When germination succeeded, the germ tube grew towards the stoma, over which it formed an appressorium. The percentage of germ tubes that failed to form an appressorium was significantly higher in the resistant genotype PI347321 than in cv. Messire (P < 0.001). Treatment with BTH and BABA significantly increased the percentage of germlings that failed in forming appressoria in both genotypes (Fig. 6). Appressoria formation was also reduced in both genotypes after application of any of the phytoalexins tested, scopoletin being the most effective substance in both Messire and PI347321 (P = 0.0010 and P = 0.0004) (Fig. 6). In addition, plants treated with scopoletin reduced the number of germlings associated with haustoria to a similar level as those treated with BABA although the effect was not as strong as in plants treated with BTH (P = 0.0321 and P = 0.0283 for Messire and PI347321, respectively) (Fig. 6).
The resistant genotype PI347321 showed also a reduction of the colony size observed as a reduced number of haustoria and hyphal tips per colony (P = 0.0025 and P = 0.0371, respectively), compared to cv. Messire (Fig. 6). Both parameters were strongly reduced in the presence of BTH, whereas results obtained with BABA treatment were not always significant. All phytoalexins tested reduced the number of haustoria per colony and the number of hyphal tips per colony in the susceptible genotype Messire but the effect was not as strong as in the resistant genotype.
Discussion
As reported (Barilli et al. 2010a, b, 2012), resistance to leaf infection by U. pisi in pea was enhanced by prior treatments with BTH and BABA, prompting lower infection frequency. This induced resistance has been previously associated with the phenylpropanoid pathway. In fact, Maffi et al. (2011) showed an accumulation of phenylpropanoids in BTH-treated Phaseolus vulgaris plants upon Uromyces appendiculatus infection, coupled with increased activities of phenylalanine ammonia-lyase, the key enzyme of the phenylpropanoid pathway. In addition, Prats et al. (2002) described phytoalexins accumulation after BTH application in sunflower (Helianthus annuus) increasing the resistance against the sunflower rust (Puccinia helianthi). On the other hand BABA has been related to an accumulation of stilbene phytoalexins, including resveratrol and viniferins upon Plasmopara viticola infection (Slaughter et al. 2008). Our previous work (Barilli et al. 2010b) showed an increase in total phenolic contents in pea following BTH and BABA treatments. Here, our detailed analysis further showed quantitative, qualitative and distribution differences in phytoalexin composition between BTH and BABA treatments in relation with the resistance to the pea rust.
In terms of total content, scopoletin was the most highly induced phytoalexin by both elicitors. Scopoletin accumulation has been correlated with resistance to microbial attack and other stresses (reviewed by Gnonlonfin et al. 2012). Our data are also in agreement with other works that showed this phytoalexin as the most abundant in pathogen challenged plants compared with other phytoalexins or phytoalexin glycosides (Prats et al. 2006, 2007; Gnonlonfin et al. 2012). In the present study pisatin, and to a lesser extent, medicarpin were also promoted in BTH- and BABA-treated plants. Previous studies demonstrated that pea inoculated with various pathogenic fungi had an increased pisatin content, and this was related to inhibition of fungal growth (Cruickshank 1962; Daniels and Hadwiger 1976). Medicarpin and other pterocarpans were also synthesized in response to fungal or bacterial infections (Ingham 1982). As example, Phoma medicaginis attack led to the accumulation of medicarpin in alfalfa leaves (Jasinski et al. 2009) and medicarpin was also described in other legumes such as chickpea (Cicer arietinum) for its direct antimicrobial activity against Fusarium oxysporum f. sp. ciceris (Arfaoui et al. 2007). As above cited, medicarpin was also found in naturally Ascochyta rabiei-infected chickpea seeds (Evidente et al. 1996). Interestingly, the response induced by BTH, with regard to the quality, quantity and distribution of the phytoalexins differed from that observed for BABA.
BTH and BABA differed in their induction of phytoalexins in a genotype-depended manner
Considering total phytoalexin content, BABA induced mainly scopoletin accumulation following pathogen attack compared to non-treated plants in both genotypes, whereas BTH induced the level of all phytoalexins but its effect focussed only in the resistant genotype. Similarly, following BTH treatment and challenge by Magnaporthe oryzae, Baldoni et al. (2013) found higher expression of particular resistance-related genes in resistant rice accessions than in the susceptible ones. On the contrary, BABA was found to induce resistance against Plasmopara viticola to both susceptible and resistant cultivars of grapevine (Hamiduzzaman et al. 2005). These data suggest a genotype-dependent mode of action for BTH that probably needs a certain threshold of inherent resistance for its action. In the present work the application of both elicitors to the same plant–pathogen interaction highlighted the differences between BTH and BABA with respect to their mode of action.
According to the fungal bioassays, different phytoalexins impaired fungal growth at different stages of the infection process. Thus, the differential accumulation of specific phytoalexins following each elicitor treatment might explain the differences observed with respect to the fungal growth following the treatment with either BTH or BABA.
Phytoalexin distribution following BTH and BABA application
We found that differences in phytoalexins accumulation following BTH and BABA treatment were not only related to the quality or quantity of the specific phytoalexins but interestingly also to where they accumulate within the leaf. BTH induced the excretion of the three phytoalexins to the leaf surface whereas BABA induced mainly scopoletin accumulation and only in the fraction extracted from the cells compared with inoculated but non-treated plants. Further, our bioassays showed that exogenous scopoletin application significantly reduced the percentage of U. pisi appressoria formation. Similar reduction of appressorium formation by scopoletin has been reported in sunflower against P. heliantii (Prats et al. 2002). This suggests an involvement for this excreted phytoalexin in the reduction of spore germination and appressoria formation following BTH treatment. The fact that BABA treatment also reduces appressoria formation, suggests that other compounds (different from phytoalexins) might also contribute to the resistance at that stage. In this sense, it has been reported that polyamines have an effect, impairing appressorium formation in M. grisea (Choi et al. 1998). An effect for pisatin or medicarpin reducing appressorium formation had not been previously described so far. However, our results show that both phytoalexins also contributed to the reduction of the rust infection by hampering appressoria formation.
In addition, BTH induced high levels of cell wall-bound scopoletin, not observed in BABA-treated plants. Scopoletin can be incorporated to the cell wall by specific scopoletin peroxidases contributing to the cell wall strengthening by cross-linking with polysaccharides and extensin monomers (Jorrín and Prats 1999). We have previously observed that BTH-treated plants induced total and scopoletin peroxidase activity (Barilli et al. 2010b), which might explain the increased level of scopoletin accumulated in the cell wall in the BTH treated plants. The cell wall strengthening might contribute to the higher penetration resistance observed and would explain the lower number of germlings associated with haustoria that we found following BTH treatment compared with BABA. Peroxidase activity has been also reported in pea-U. viciae–fabae pathosystems, after BTH treatment (Dann and Deverall 2000) suggesting that the coupled induction of peroxidases and their corresponding substrates might be a common mode of action of BTH.
On the other hand, BABA highly induced the scopoletin content in the soluble fraction compared with a lower induction observed for BTH. In a recent proteomic profile of BABA-induced peas in response to U. pisi inoculation (Castillejo et al. 2009), we have observed that the chalcone–flavanone isomerase significantly increased in BABA-induced plants. This protein is the second enzyme of the flavonoid–isoflavonoid biosynthesis and might be responsible for the high levels of scopoletin observed. Since BABA treatment did not elicit total peroxidase nor scopoletin peroxidase activities in peas (Barilli et al. 2010b), most of the scopoletin produced would not be incorporated within the cell wall, as our results showed, increasing its content in the soluble fraction extracted from the cells. It is not surprising that a high quantity of the phytoalexins was found in this latter fraction. In fact, many phenylpropanoids have been shown to accumulate in the vacuole, or in vesicles formed from the vacuole that eventually coalesce (Dixon and Paiva 1995). The vacuolar sequestration is a well-known method of detoxification in plant cells by using transporters localized in the plasma membrane to ensure the extrusion of metabolites out of the cell (Jasinski et al. 2001). It has been demonstrated that in Nicotiana spp., a high level of terpenoids induced the ATP-binding cassette (ABC) transporters, which pump the metabolites out of the cells when its concentration within the cell solution reaches a certain level and before it becomes toxic (Jasinski et al. 2001).
Overall, our data showed that both elicitors induced defense in pea against the rust fungus likely through induction of different phytoalexins. Interestingly, BTH constitutively induced the accumulation of phytoalexins of specific fractions albeit the higher phytoalexin changes in BTH-treated plants were observed following pathogen attack, highlighting the priming of the defensive response. Interestingly the mode of action of BABA was more related to the priming of defenses, since elicitation of phytoalexins by the elicitor in absence of the pathogen was not observed. This priming that accelerates and increases the plant’s ability to activate defenses under disease pressure was known for BTH and BABA (Fu and Dong 2013; Gozzo and Faoro 2013). However, the priming response depends on a largely unknown defense pathway. Our work integrated the role of phytoalexins, particularly scopoletin, pisatin and medicarpin in this pathway, although it does not exclude the possibility that other metabolites may operate in BTH- and BABA-induced peas in conjunction with phytoalexins. In this respect, HPLC analyses have revealed the existence of other compounds which were present at a higher concentration in BTH-treated plants after fungal inoculation. These compounds have been purified and their chemical structure and antifungal activity is now being studied.
Author contribution
E. Barilli carried out most of the experimental work and data analysis and contributed to the writing of the manuscript. D. Rubiales contributed to the disease resistance aspects. C. Amalfitano and A. Evidente contributed to the more chemical aspects. E. Prats designed experiments and contributed to the interpretation of results and writing of the manuscript. D. R. and A. E. also contributed to critical reading.
Abbreviations
- BABA:
-
(D,L)-3-amino-n-butanoic ((D,L)-β-aminobutyric) acid
- BTH:
-
Benzo[1,2,3]thiadiazole-7-carbothionic acid-S-methyl ester (Bion®)
- SAR:
-
Systemic acquired resistance
References
Amzalek E, Cohen Y (2007) Comparative efficacy of systemic acquired resistance-inducing compounds against rust infection in sunflower plants. Phytopathology 97:179–186
Arfaoui A, El Hadrami A, Mabrouk Y, Sifi B, Boudabous A, El Hadrami I, Daayf F, Chérif M (2007) Treatment of chickpea with Rhizobium isolates enhances the expression of phenylpropanoid defense-related genes in response to infection by Fusarium oxysporum f. sp. ciceris. Plant Physiol Bioch 45:470–479
Baldoni E, Mattana M, Locatelli F, Consonni R, Cagliani LR, Picchi V, Abbruscato P, Genga A (2013) Analysis of transcript and metabolite levels in Italian rice (Oryza sativa L.) cultivars subjected to osmotic stress or benzothiadiazole treatment. Plant Physiol Bioch 70:492–503
Barilli E, Serrano A, Sillero JC, Rubiales D (2009a) Differential response of pea (Pisum sativum) to rusts incited by Uromyces viciae-fabae and U. pisi. Crop Prot 28:980–986
Barilli E, Sillero JC, Moral A, Rubiales D (2009b) Characterization of resistance response of pea (Pisum spp.) against rust (Uromyces pisi Pers.) Wint. Plant Breed 128:665–670
Barilli E, Sillero JC, Rubiales D (2010a) Induction of systemic acquired resistance in pea against rust (Uromyces pisi) by exogenous application of biotic and abiotic inducers. J Phytopathol 158:30–34
Barilli E, Prats E, Rubiales D (2010b) Benzothiadiazole and BABA improve resistance to Uromyces pisi (Pers.) Wint. in Pisum sativum L. with an enhancement of enzymatic activities and total phenolic content. Eur J Plant Pathol 128:483–493
Barilli E, Rubiales D, Castillejo MA (2012) Comparative proteomic analysis of BTH and BABA-induced resistance in pea (Pisum sativum) toward infection with pea rust (Uromyces pisi). J Proteomics 75:5189–5205
Castillejo MA, Maldonado AM, Dumas-Gaudot E, Fernández-Aparicio M, Susín R, Rubiales D (2009) Differential expression proteomics to investigate responses and resistance to Orobanche crenata in Medicago truncatula. BMC Genom 10:1–17
Choi WB, Kang SH, Lee YW, Lee YH (1998) Cyclic AMP restores appressorium formation inhibited by polyamines in Magnaporthe grisea. Phytopathology 88:58–62
Cruickshank IAM (1962) Studies on phytoalexins IV. The antimicrobial spectrum of pisatin. Aust J Biol Sci 15:147–159
Daniels DL, Hadwiger LA (1976) Pisatin-inducing components in filtrates of virulent and avirulent Fusarium solani cultures. Physiol Plant Pathol 8:9–19
Dann EK, Deverall BJ (2000) Activation of systemic disease resistance in pea by an avirulent bacterium or a benzothiadiazole, but not by a fungal leaf spot pathogen. Plant Pathol 49:324–332
Dewick PM (2009) Medicinal natural products—a biosynthetic approach. Wiley, Chichester
Ding H, Lamb RJ, Ames N (2000) Inducible production of phenolic acids in wheat and antibiotic resistance to Sitodiplosis mosellana. J Chem Ecol 26:969–985
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
EPPO (2013) Data sheet for standards pea. Available at http://archives.eppo.org/EPPOStandards/PP2_GPP/pp2-14-e.doc. Accessed 10 August 2013)
Evidente A, Capasso R, Motta A, Andolfi A, Vurro M, Zonno MC, Bottalico A (1996) Toxic metabolites from phytopathogenic Ascochyta species. Boll Chim Farm 135:552–555
Evidente A, Cimmino A, Fernández-Aparcio M, Andolfi A, Rubiales D, Motta A (2010) Polyphenols, including the new peapolyphenols A-C, from pea root exudates stimulate Orobanche foetida seed germination. J Agric Food Chem 58:2902–2907
Fu ZQ, Dong X (2013) Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64:839–863
Gnonlonfin GJB, Sanni A, Brimer L (2012) Review scopoletin: a coumarin phytoalexin with medicinal properties. Crit Rev Plant Sci 31:47–56
Gozzo F, Faoro F (2013) Systemic acquired resistance (50 years after discovery): moving from the lab to the field. J Agr Food Chem 61:12473–12491
Hamiduzzaman MM, Jakab G, Barnavon L, Neuhaus J, Mauch-Mani B (2005) β-Aminobutyric acid-induced resistance against downy mildew in grapevine acts through the potentiation of callose formation and jasmonic acid signalling. Mol Plant Microbe In 18:819–829
Iglesias-García R, Rubiales D, Fondevilla S (2015) Penetration resistance to Erysiphe pisi in pea mediated by er1 gene is associated with protein cross-linking but not with callose apposition or hypersensitive response. Euphytica 201:381–387
Ingham JL (1982) Phytoalexins from the Leguminosae. In: Bailey JA, Mansfield JW (eds) Phytoalexins. Blackie, London, pp 21–80
Jasiński M, Stukkens Y, Degand H, Purnelle B, Marchand-Brynaert J, Boutry M (2001) A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell 13:1095–1107
Jasiński M, Kachlicki P, Rodziewicz P, Figlerowicz M, Stobiecki M (2009) Changes in the profile of flavonoid accumulation in Medicago truncatula leaves during infection with fungal pathogen Phoma medicaginis. Plant Physiol Bioch 47:847–853
Jorrín JV, Prats E (1999) Allelochemicals, phytoalexins and insect-feeding deterrents: different definitions for 7-hydroxylated coumarins. In: Macías FA, Galindo JCG, Molinillo JMG, Cutler HG (eds) Recent advances in allelopathy, vol I. A science for the future. Universidad de Cádiz, pp 179–191
Maffi D, Iriti M, Pigni M, Vannini C, Faoro F (2011) Uromyces appendiculatus infection in BTH-treated bean plants: ultrastructural details of a lost fight. Mycopathologia 171:209–221
Niks RE, Rubiales D (2002) Potentially durable resistance mechanisms in plant to specialized fungal pathogens. Euphytica 124:201–216
Pérez-de-Luque A, Jorrín JV, Rubiales D (2004) Crenate broomrape control in pea by foliar application of benzothiadiazole (BTH). Phytoparasitica 32:21–29
Prats E, Bazzalo ME, León A, Jorrín J (2000) Agronomic aspects of the sunflower 7-hydroxylated simple phytoalexins. Helia 23:105–111
Prats E, Rubiales D, Jorrín J (2002) Acibenzolar-S-methyl-induced resistance to sunflower rust (Puccinia helianthi) is associated with an enhancement of phytoalexins on foliar surface. Physiol Mol Plant 60:155–162
Prats E, Bazzalo ME, León A, Jorrín JV (2006) Fungitoxic effect of scopolin and related phytoalexins on Sclerotinia sclerotium. A way to overcome sunflower head rot. Euphytica 147:451–460
Prats E, Llamas MJ, Jorrín JV, Rubiales D (2007) Constitutive phytoalexin accumulation on sunflower leaf surface prevents rust germ tube growth and appressorium differentiation. Crop Sci 47:1119–1124
Rubiales D, Castillejo MA, Madrid E, Barilli E, Rispail N (2011) Legume breeding for rust resistance: lessons to learn from the model Medicago truncatula. Euphytica 180:89–98
Serghini K, Gutierrez-Mellado MC, Prats E, Werck-Reicchart D, Cabello-Hurtado F, Jorrin J (1996) Induction of simple 7-hydroxylated phytoalexins: a universal response to biotic and abiotic stresses in cultivated sunflower. Colloq INRA 96:365–366
Serghini K, Pérez de Luque A, Castejón-Muñoz M, García-Torres L, Jorrín JV (2001) Sunflower (Helianthus annuus L.) response to broomrape (Orobanche cernua Loefl.) parasitism: induced synthesis and excretion of 7-hydroxylated simple phytoalexins. J Exp Bot 52:2227–2234
Sillero JC, Rubiales D (2002) Histological characterization of resistance to Uromyces viciae-fabae in faba bean. Phytopathology 92:294–299
Sillero JC, Fondevilla S, Davidson J, Vaz Patto MC, Warketin TD, Thomas J, Rubiales D (2006) Screening techniques and sources of resistance to rusts and mildews in grain legumes. Euphytica 147:255–272
Sillero JC, Rojas-Molina MM, Avila CM, Rubiales D (2012) Induction of systemic acquired resistance against rust, ascochyta blight and broomrape in faba bean by exogenous application of salicylic acid and benzothiadiazole. Crop Prot 34:55–69
Slaughter AR, Hamiduzzaman MM, Gindro K, Neuhaus JM, Mauch-Mani B (2008) Beta-aminobutyric acid-induced resistance in grapevine against downy mildew: involvement of pterostilbene. Eur J Plant Pathol 122:185–195
Acknowledgments
The authors are greatly indebted to the Spanish AGL2014-52871-R and Spanish-Italian HI2007-0233 projects for financial support. Dr. E. Barilli was granted by JAE-Doc program, co-financed by the European Social Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barilli, E., Rubiales, D., Amalfitano, C. et al. BTH and BABA induce resistance in pea against rust (Uromyces pisi) involving differential phytoalexin accumulation. Planta 242, 1095–1106 (2015). https://doi.org/10.1007/s00425-015-2339-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2339-8