Abstract
The mechanisms of BTH [benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester]-induced resistance against bean rust caused by Uromyces appendiculatus have been explored in Phaseolus vulgaris by light and transmission electron microscopy, following the infection progression in plants challenged 7 days after treatment. While BTH did not affect uredospore germination and fungal penetration in the substomatal cavity, a first impairment to the colonization appeared evident about 48–96 h after inoculation, with alterations of infection hypha structure and reduction in mycelium expansion. No differences were found in this phase regarding the formation and ultrastructure of haustoria in untreated and BTH-treated plants, except for the deposition of electron-opaque material in the extrahaustorial matrix of the latter. A second and decisive impairment in fungal progression was observed at 7–10 days after inoculation when host cell penetrated, or in close contact with the fungal hyphae, were impregnated by phenolic compounds. The same was observed in fungal walls, particularly around haustoria, thus hampering the biotrophic habitus of the fungus and further mycelium spreading. This, in turn, prevented the evasion of fungal reproductive structures, the uredinia, and the appearance of visible symptoms. No particular ultrastructural alterations were observed in most of the penetrated cells, even at late stages of infection, indicating that BTH treatment does not induce host cells to respond with a hypersensitive reaction (HR). A parallel time course of the expression of phenylalanine ammonia lyase (PAL) gene, the key enzyme for the synthesis of phenylpropanoidic phytoalexins and many other phenolics, has shown that PAL mRNA is strongly and persistently transcripted in BTH-treated plants since the 6th h after treatment, though no apparent ultrastructural alterations were detectable up to some days after pathogen challenging. This indicates that BTH, at the employed concentration of 0.3 mM, directly activates the plant’s own defences, thus accounting for the observed full protection against bean rust.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants possess an innate immune system consisting of an array of either physical and chemical constitutive and inducible defences, the latter being activated following pathogen sensing [1]. As a result, host pattern recognition receptors (PRRs), similar to Drosophila toll and mammalian toll-like receptors (TLRs), may recognize microbe-associated molecular patterns (MAMPs) or pathogen-associated avirulence (avr) factors, i.e. general or specific elicitors, respectively [2]. Whatever the recognition type, it may trigger a hypersensitive response (HR), which is the rapid and programmed cell death at the sites of the attempted pathogen invasion, in order to impair pathogen spreading [3, 4]. Furthermore, pathogen recognition, either associated or not with HR, establishes a state of immunity in plant tissues nearby the infection site (local acquired resistance, LAR) and very often also in distal organs (systemic acquired resistance, SAR) [5–7]. Conversely, when the pathogen is able to overcome plant sensing and other defence mechanisms, it establishes a compatible interaction with its host, thus triggering the infection.
An array of both natural and synthetic compounds mimicking endogenous molecular signals, or acting as MAMPs, can also elicit plant defence response [reviewed in 8]. These compounds, also known as plant activators or SAR inducers, deserve particular interest in agriculture, because of their long-lasting ability to protect crops against a broad spectrum of pathogens, in addition to their low environmental impact. Furthermore, the action mechanism of these molecules can be different, because of the diverse signal transduction pathways elicited at the onset of SAR [6, 9].
Benzothiadiazole [benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester] (BTH), synonymous acybenzolar-S-methyl (ASM), is one of the more effective plant resistance inducers, able to prime the plant defence machinery against the pathogens [10, 11]. It is a functional analogue of the plant hormone-like molecule salicylic acid (SA), the key player of SAR. Similarly to SA, although more efficiently, BTH inhibits at the biochemical level hydrogen peroxide (H2O2) scavenger enzymes ascorbate peroxidases (APXs) and catalases (CATs), whereas at the molecular level, it activates the NIM (non-induced immunity)/NPR1 (non-pathogenesis-related protein 1 inducer) genetic pathway [12, 13]. Accordingly, BTH modifies the redox homoeostasis in plant cells and tissues, thus triggering the plethora of defence mechanisms orchestrated by H2O2, besides upregulating defence genes codifying for pathogenesis-related (PR) proteins and enzymes of phytoalexin biosynthesis [11, 14, 15].
The basidiomycete Uromyces appendiculatus (Pers.) Unger is the aetiological agent of bean rust, a chronic, yield-limiting and worldwide disease. Particularly in Central America, Caribbean and Southern Africa, bean rust is endemic, with epidemics occurring every 2–3 years [16, 17]. Rust fungi are obligate, biotrophic pathogens that extract their nutrients from living plant cells by means of haustoria, specialized feeding structures differentiated within plant cells and developed from the haustorium mother cell, a specialized cell belonging to the extracellular mycelium [18]. In this way, the pathogen delays the plant cell death, thus avoiding the associated defence responses. Typically, the haustorium is composed of a haustorial body, spherical or lobated in rust fungi, and a haustorium neck, so that different plant cell–haustorium interfaces can be recognized. In particular, the extrahaustorial membrane is the invaginated portion of the plant plasmalemma, surrounding the haustorium through an extrahaustorial matrix between the fungal cell wall and the extrahaustorial membrane. The main pathogen-induced modification of extrahaustorial membrane includes the impairment of ATPase activity in order to abolish the plant’s ability of retrieving nutrients from the extrahaustorial compartment and to direct the nutrient flux from plant to fungus [19].
In a previous work, we showed that BTH is a powerful SAR inducer in bean plants against U. appendiculatus, by stimulating H2O2 accumulation in leaf tissues, though in absence of any cell death phenomena [14]. Therefore, it was apparent that BTH treatment did not modify the compatible plant–pathogen interaction into an incompatible one, i.e. triggering HR, as observed in other pathosystems [20–22], but other defence mechanisms were operating. It was proposed that the maintenance of the tissue H2O2 concentration under the cell death threshold contributed to create an hostile environment before the U. appendiculatus challenging, i. e. during the induction phase (IP), the time required for the effective priming of plant own defences. However, it was also shown that H2O2 was no more detectable after 48 h from BTH treatment, while a full resistance to U. appendiculatus was found even inoculating the fungus after a 7-day IP [14]. In order to further elucidate the plant resistance mechanisms involved in the different stages of fungal infection, we have followed, step by step and with the aid of light and electron microscopy, the fate of U. appendiculatus infection in BTH-treated and untreated bean plants. The pathogen was inoculated after a 7-day IP to achieve a full protection from bean rust in treated plants. In parallel, the expression profile of phenylalanine ammonia lyase (PAL) mRNA was analysed to verify whether BTH, in our pathosystem and at the employed concentration, only primes or directly activates plant defences [23] and whether one of these effects correlates with the observed pattern of fungal infection impairment.
Materials and Methods
Plant Material and Treatment
Phaseolus vulgaris L. plants, cv. Borlotto Nano Lingua di Fuoco, were seeded in 12-cm pots and grown in a greenhouse at 24 ± 2°C temperature, 60 ± 5% relative humidity (HR) and 16 h/8 h light/dark period. Approximately 10–12 days after seeding, when the primary leaves were completely expanded, plants were sprayed with a water solution of 0.3 mM benzothiadiazole [benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester] (BTH, trade name Bion®, Syngenta, CH) prepared from a wettable formulation containing 50% (w/w) active ingredient. BTH was applied as single treatment, by spraying the adaxial leaf surface, sufficient to fully protect bean plants from rust [14]. Control plants were sprayed with the wettable powder alone.
Pathogen Inoculation
Seven days after treatment with BTH, plants were challenged by spraying the abaxial leaf surface of both primary and trifoliate leaves with a spore suspension of Uromyces appendiculatus in distilled water containing 0.05% Tween 20. Spore concentration in the suspension was adjusted to 105 ml−1. Plants were then incubated for 24 h at 20°C and 100% relative humidity (RH) and finally transferred into growth chambers at 21 ± 2°C, 70–80% RH, 14-h photoperiod with artificial illumination of 2,500 Lux, as measured at leaf surface.
Effect of BTH Treatment on Uredospore Germination
A suspension of uredospores (105 ml−1) was prepared in 0.3 mM BTH, or in water as a control, added of 0.01% Tween 20 and sprayed in the lid of a Petri dish. After 3 and 5 h, the lid was examined at the light microscope equipped with interference contrast. One hundred uredospores were observed for each one of three replicated experiments. Uredospores were considered normally germinated when showing a germ tube as long as spore diameter.
In other experiments, bean leaf fragments (5 × 5 mm) were excised, floated over water and sprayed with the same uredospore suspension as above to assess BTH effect on uredospore germination in vivo. After 3 and 5 h, the germination rate of uredospores was assessed as above.
Data of each experiment were subjected to one-way analysis of variance (ANOVA), and comparison among means was determined according to Tukey’s test. Significant differences were accepted at P < 0.05.
Light Microscopy
To investigate fungal development and the presence of dead cells in bean tissues, trypan blue staining was carried out by boiling leaf fragments (1 cm2) for 1 min in a mixture of phenol, lactic acid, glycerol and distilled water containing 1 mg/ml trypan blue (1:1:1:1), prepared immediately before use. Tissues were then clarified overnight in a solution of 2.5 mg/ml chloral hydrate [24].
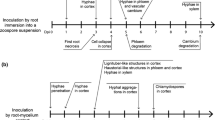
Fungal development stages were recorded day by day after inoculation, starting from 24 h to 10 days. In the first 96 h, 100 infection sites were examined for each thesis, and the development stage of each infection site (represented in Fig. 1) was recorded. Measurements on spreading hyphae were taken on digitalized images by GLOBAL LAB® Image analyzer (DATA TRANSLATION®, USA).
Developmental stages of Uromyces appendiculatus infection in untreated and BTH-treated plants. The graphic stereoscopic reconstruction has been made at the light microscope on trypan blue-stained fragments by manual drawing instruments and is representative of 1 out of 100 infection sites (starting from a penetrated stomata) examined at different time after inoculation
Detection of lignin and callose deposition was carried out by clarifying leaf fragments in 95% boiling ethanol followed by staining with toluidine blue and aniline blue, respectively [25, 26]. All samples were examined with an Olympus BX50 (Olympus, Japan) light microscope, equipped with a differential interference contrast (DIC) and epifluorescence.
Electron Microscopy
Tissue fragments (1 mm2) were excised from the leaves and fixed in 3% (v/v) glutaraldehyde, 3% paraformaldehyde in 100 mM phosphate buffer (PB), pH 7.2 for 2 h. Samples were then washed for 1 h in PB and post-fixed for 2 h in 1% osmium tetroxide in PB. Finally, they were dehydrated in an ethanol series and embedded in Spurr resin. Ultrathin sections were cut and conventionally stained with uranyl acetate and lead citrate and examined with a Jeol 100SX transmission electron microscope (TEM) (Jeol, Japan).
Probe Preparation: Isolation and Labelling
Primers for the PAL-1 (phenylalanine ammonia lyase) encoding gene were designed from the sequence available at NCBI (National Centre for Biotechnology Information, Betheseda, MD). Primers were designed to amplify a fragment of 930 bp from genomic DNA isolated from bean using PCR (polymerase chain reaction). The amplified 930-bp PCR product was purified by means of a DNA gel extraction kit (Qiagen). This 930-bp bean PAL-1 fragment was used as probe in Northern blot analysis. The probe was radioactively labelled with [32P]dCTP, using a nick-translation DNA labelling kit.
Northern Blot Analysis of PAL-1 Transcript Levels
Total RNA was extracted using Trizol Reagent (GibcoBRL), according to the manufacturer’s instructions, from leaves of bean plants (i) untreated and not inoculated (control), (ii) only BTH-treated, (iii) untreated and inoculated and (vi) treated and inoculated 7 days later. Total RNAs (10 μg per lane) were size fractionated by electrophoresis on a 1, 2% formaldehyde agarose gel and blotted onto Hybond-N+ membrane (Amersham), using standard techniques as described by [27]. The membranes were pre-hybridized at 68°C in a high SDS (sodium dodecyl sulphate) hybridization buffer. The membranes were then hybridized in the same solution with the addition of the radioactively labelled [32P]dCTP (deoxycytidine triphosphate) PAL-1 probe. After hybridization, membranes were washed twice in 2 × SSC (3 M NaCl plus 0.3 M sodium citrate) and 0.1% SDS at room temperature for 15 min, and twice in 0.5 × SSC and 0.1% SDS at 68°C for 15 min. Hybridization signals were quantified using Phosphorimager Storm system (Molecular Dynamics).
Results
BTH Does not Inhibit Uredospore Germination
Two preliminary experiments were carried out to verify the possible inhibitory effect of BTH on uredospore germination. In the first, uredospores were kept in contact with 0.3 mM BTH in a Petri dish for 3 or 5 h, then the % germination determined as detailed in materials and methods. In the second experiment, the % germination was assessed in planta on BTH-sprayed leaves with the same timing as above. In both cases, no significant difference was found between BTH and water-exposed uredospores, indicating that this compound does not affect their germination (Table 1).
BTH Impairs the First Steps of Colonization but not Stomatal Penetration
Light microscope observations at 24, 48, 72 and 96 h after inoculation (hpi) of ten leaf fragments randomly chosen from BTH-treated and untreated plants were carried out to follow the first steps in the infection process, which are represented in Fig. 1. In particular, after 24 hpi, no difference was observed between treated and untreated plants regarding the number of penetrated stomata (afterwards named infection sites), the number of appressoria and substomatal vesicles and the length of the infection hyphae originated from vesicles. Average length of infection hyphae was 52 ± 28.5 and 42 ± 9.6 μm in untreated and treated plants, respectively, while haustoria were very rarely observed in both cases and showed a diameter of 6–7 μm.
At 48 hpi, hyphae had already started to grow into the intercellular spaces, radially from the infection sites, bifurcating regularly and forming new haustoria. However, some differences were appreciable between untreated and treated tissues. In the latter, hyphal growth was less linear, and hyphae were more distorted and swollen than in untreated controls, though the size of haustoria, now larger than 10 μm, was similar. The maximum extension of infection hyphae from the penetrated stomata was 260 ± 38.6 μm in the untreated control and 175 ± 37.7 μm in treated leaves (Fig. 1). Another noticeable difference resided in the number of infection sites showing an early stage of development with short infection hyphae just appearing from the substomatal vesicles. These sites were almost five times more frequent in treated than in untreated plants (Fig. 2), suggesting that many of them were likely blocked by BTH treatment in the first stages of infection.
Percentage of infection sites showing the 4 development stages of fungal infection illustrated in Fig. 1, at different times after inoculation, in untreated and BTH-treated plants
The differences between the developing mycelium in treated and untreated samples became more evident at 72 hpi inoculation, being the treated much more branched than the untreated and with distorted hyphae (Fig. 1). The number of infection sites blocked in stage 1 of infection was 17% in treated plants, against 2.5% of untreated ones, indicating a significant impairment of the infection in this phase (Fig. 2). Moreover, in BTH-treated leaves, mycelium tended to develop in all directions, with a maximum extension of 610 ± 97.6 μm from the infection centre, while in untreated ones radial growth was almost planar, with a maximum hyphal length of 822 ± 128 μm. Again, no appreciable difference was observed in haustoria formation as in both cases, they were similar in size (about 14 μm) and, possibly, also in number.
At 96 hpi, the growing mycelium in untreated plants continued its radial growth by linear and branched hyphae with dense cytoplasm and generating large multilobate haustoria of 14–15 μm in the mesophyll cells (Fig. 3h, i). Conversely, in BTH-treated plants, mycelium was less expanded, with distorted and highly branched hyphae, often swollen and vacuolated (Fig. 3a, b). Furthermore, the number of infection sites showing stage 1 of infection was unchanged, ranging around 16.5% (Fig. 2). These sites were characterized by yellow autofluorescence of cell wall under UV light, indicating phenolic deposition in the cells around penetrated stomata (Fig. 3c, d). Haustoria were present in a reduced number and size in respect to those in control plants, and rarely appeared lobated (Fig. 3e, f). Only rare dead mesophyll cells were present, not necessarily close to the hyphae or penetrated by the fungus while forming the haustorium.
Light (trypan blue staining) and electron microscopy (ultrathin sections) of bean leaves treated with BTH (a–f) or untreated (g–i) at 96 h after inoculation with Uromyces appendiculatus. a Numerous infection sites showing a similar stage of development are visible; one of them (framed) is enlarged in b and shows distorted and highly branched hyphae and a reduced growth in comparison with the control g growing in untreated plant. The epidermal cell walls around the penetrated stomata (arrow) in an infection site, enlarged in c, are autofluorescent under UV light d, indicating phenolic deposition. e Haustoria (arrow) are present at mycelium periphery and appear roundish, while in the control, they are often lobated h (arrows); f the extrahaustorial matrix (arrow) is thicker and more electron-dense than in the control i growing on untreated plant. A collar of a similar electro-dense material, with patches of black osmiophilic deposits of phenolics (asterisk), is visible around the haustorial neck f. Osmiophilic deposits (arrowhead) are present also in the wall of the haustorium mother cell (M) and start to appear in the outer layer of the infected host cell f. The protoplasm of both the penetrated host cell and the haustorium do not appear particularly altered (CH = chloroplast)
From the above observations, it seems that stomatal penetration is not influenced by BTH treatment, and the first impairment to fungal colonization starts between 24 and 72 h, when a significant number of infection sites are blocked in their growth.
Polyphenol Deposition and Cell Wall Strengthening Eventually Stop the Infection
Microscopical observations were also carried out at 7 and 10 days after inoculation (dpi), to follow the fate of infection sites not blocked by BTH treatment in the first 96 h. These time courses corresponded to the first appearance of macroscopic symptoms and to pustule complete formation, respectively, in untreated control plants.
At 7 dpi, no visible symptoms of infection were present in BTH-treated plants, while numerous chlorotic spots started to appear in the control ones, indicating that uredinia were forming underneath epidermis in the infection sites. Light microscope observations confirmed the presence of a differentiating uredinium in the centre of these spots, surrounded by an extended radial mycelium composed of masses of hyphae, mostly linear, with numerous lobate haustoria (not shown). Instead, in BTH-treated leaves, mycelium spreading was very limited, with vacuolated hyphae, and a lower number of dark globular haustoria that rarely appeared collapsed at ultrastructural level (Fig. 4a), with the collar surrounded by dark deposits of oxidized phenolic compounds and less electron-dense material similar to callose (Fig. 4a, b). However, this material was not fluorescent when stained with aniline blue and observed under UV light (not shown). Hyphal cell walls in the intercellular spaces were darker than in untreated controls, possibly because impregnated with phenolics as well (Fig. 4a). Also, the outer layer of host cell walls showed a slight phenolic deposition in those tissue sectors colonized by the fungus. A similar deposition was never observed in infected control plants, at least at this stage of infection development (Fig. 4c).
Ultrathin sections of bean leaves from BTH-treated (a, b) and untreated plants (c) at 7 days after inoculation with Uromyces appendiculatus. a most of the haustoria (H) do not show appreciable alterations of their protoplasm, though their neck is often surrounded by a large collar of slight electron-dense material with patches of dark osmiophilic deposits of phenolics; one of these haustoria is enlarged in b. Osmiophilic deposition is also present all over the wall of the haustorium mother cells (M) and in the outer layer of the host cell wall a. The ultrastructure of penetrated cells a does not appear particularly altered with apparently normal organelles (CH = chloroplast). c In untreated controls, infectious hyphae (F), haustoria (H) and host cells show normal ultrastructure, and no phenolic deposits are visible in their walls or in the extrahaustorial matrix
Rust pustules were already present at 10 dpi all over the inoculated control leaves (Fig. 5a), but not in BTH-treated ones that remained symptomless. Light microscope observations with trypan blue staining showed that, in spite of the lack of macroscopic symptoms, in BTH-treated plants, some of the infection centres visible in Fig. 3 had developed in larger colonies, however unable to sustain the formation of a proper pustule with the consequent extrusion from the epidermis (Fig. 5b). Besides being composed by vacuolated hyphae with an irregular profile (Fig. 5d), the mycelium of these colonies was much less extended than in untreated control leaves (Figs. 5a, c) and presented very dark haustoria when observed in the light microscope. Ultrastructurally, these haustoria appeared completely surrounded by a huge deposition of electron-dense material (Fig. 5f, g), mainly composed by phenolics, being autofluorescent with UV light (not shown). Cell walls of infected cells (Fig. 5f, g) and of cells just contacted by the fungus (Fig. 5h) showed a similar phenolic deposition that was neither observed in leaf tissues not colonized by the fungus nor in the infected untreated controls (Fig. 5e) or in treated but not inoculated plants (not shown). Again, such depositions were present also in the cell wall of hyphae in the nearby intercellular spaces. Both in treated and untreated plants, no cell death was observed in the tissues colonized by the fungus except for the area of pustule development.
Light (trypan blue staining) and electron microscopy (ultrathin sections) of bean leaves untreated (a, c, e) or treated with BTH (b, d, f–h) at 10 days after inoculation with Uromyces appendiculatus. a Pustule (P) extruded from the epidermis and already releasing uredospores (arrow); c around the pustule the mycelium is largely spread and formed by hyphae normally shaped; the ultrastructure of both haustoria and penetrated cells e does not show particular alterations due to the activation of defence mechanisms. b In BTH-treated plants, mycelium spreading is much more reduced and rare microscopic pustules (P) are present, however not extruding from the epidermis. d Hyphae appear heavily stained, possibly because of the phenolic apposition on their wall, and show an irregular profile. f–h Huge osmiophilic deposition is visible on the wall of haustorial mother cell (M) and on the host cell wall; the haustoria (H) are completely encapsulated by similar depositions in the extrahaustorial matrix, together with other less electron-dense material; the protoplasm of the haustoria and of the host cells does not appear particularly altered, only showing a slight plasmolysis
These observations suggest that in BTH-treated plants, the infection sites not blocked in the first stages of infection continue to develop a low functional mycelium, mainly because of a huge phenolic deposition both in host and fungal cell walls, particularly around haustoria. This mycelium cannot afford for pustule development and thus for macroscopic symptom manifestation.
PAL Expression Pattern Indicates a BTH Direct Activation of Plant Defences
As no sign of phenolic deposition and other apparent ultrastructural alterations were found in BTH-treated plants before pathogen inoculation nor in untreated but inoculated plants, we wanted to verify whether BTH had only primed defence responses or directly activated them. For this purpose, we examined the expression of PAL mRNA at different times after treatment and/or pathogen inoculation. In treated leaves, PAL transcripts were already high at 6 h after BTH treatment and steadily increased up to 7 days, partially because of leaf age (see lanes 3 and 9 of Fig. 6). Fungal infection, carried out at 7 days after treatment, did not increase appreciably PAL expression, possibly because already high (compare lane 9 and 13). Furthermore, this expression level remained quite high in the following days (lane 14, Fig. 6). Instead, in untreated plants, pathogen inoculation induced a slight enhancement of PAL mRNA at 24 h after inoculation, possibly during the stomata penetration phase (compare lanes 3 and 10 of Fig. 6), then PAL transcripts decreased to a level similar to those found in untreated and non-inoculated plants of the same age.
This finding demonstrates that BTH treatment directly activates the phenylpropanoids pathway that however does not lead to phenolic deposition until pathogen challenging.
Discussion
We demonstrated in a previous work [14] that a single BTH application on bean plants completely prevented rust infection by U. appendiculatus, when the fungus was inoculated after a 7 day IP. In that study, we also showed that soon after BTH spray, the level of H2O2 increased in the tissues and was cytochemically detectable up to 48 h after treatment, however without triggering cell death [14]. The lack of cell death in spite of high H2O2 accumulation and the increased activity of anionic peroxidases suggested that this compound is actively utilized as a substrate by these enzymes, typically associated with the cell wall and relatively abundant in beans [28]. Anionic peroxidases promote cell wall strengthening by cross-linking proteins and lignin deposition that are long-term defence responses [29–31], though lignin deposition before pathogen challenging was actually detectable only after multiple BTH treatments [14]. Based on those results, it was speculated that the great efficacy of BTH treatments after a long IP, as well as the long-lasting effect of treatments, could be due to the time required for cell wall fortification, which represents a main barrier for U. appendiculatus infection, both during the penetration phase to form haustoria and in the emergence from epidermis to develop the pustule [18].
The findings of the present study cast further light on the mechanisms of rust infection restraint in BTH-treated plants, at least in the middle and late stages of infection. In fact, comparing step by step the fungal development in treated and untreated leaves both at histo-cytological and ultrastructural level, it appears that many infection sites are blocked starting at stage 2 (48 hpi) and ahead. Thus, it seems that plant defences become significantly active 1–2 days after pathogen challenging, in spite of the fact that PAL mRNA expression was high also during the preceding 7 days. Possibly, the supposed cell wall strengthening mediated by the initial oxidative burst and/or the phytoalexin and pathogenesis-related protein synthesis induced by BTH [11] were actually less intense than expected and/or not enough effective to block the infection immediately. In this regard, Lin et al. (2009) have recently shown that in BTH-treated cucumber plants, the gradual enhancement of phytoalexin synthesis is due to the increasing growth stages of the plants, irrespective of BTH treatment [32].
Indeed, the persistence of a high PAL mRNA level for several days indicates a direct activation of phenylpropanoid pathway rather than its priming, as observed in other BTH-treated plants in which PAL transcription steadily increased only after pathogen challenging [reviewed in 33]. The reason for direct activation may be due to the BTH concentration used in this study (0.3 mM) to obtain full protection, though the same, or even higher concentrations, only primed PAL in other plant species. That is the case of Arabidopsis, cotton and cucumber, in which PAL transcription was upregulated in 0.3–0.6 mM BTH-treated plants only after pathogen challenging [13, 34, 35], confirming that the threshold concentration that shifts PAL priming into direct activation is species specific and also dependant on the physiological conditions of the plant, particularly the age [36]. It should be noted that direct activation of plant defence in an enemy-free system can incur in fitness cost for the plant [37], though in a previous field study addressing this problem we did not observe reduction in bean seed production and quality after treatment with the same BTH concentration used in this work [38].
Nevertheless, the PAL direct activation we observed neither produced apparent ultrastructural alterations in the host cells until pathogen challenging nor led to a significant impairment of fungal development in the first stage of infection. A possible explanation resides in the fact that low molecular weight phenylpropanoids, precursors of lignin, that are synthesized at first, including 4-coumaric and ferulic acid, could be esterified to the cell wall only following pathogen elicitation, as already observed in many pathosystems, including bean [39]. Thus, a possible accumulation of precursors during the induction phase can lead to massive esterification in the host walls with lignin production after pathogen challenging, accounting for the heavy polyphenol deposition observed from 94 hpi and ahead in host walls, particularly around haustoria that would considerably reduce nutrient uptake through extrahaustorial matrix. Similarly, Benhamou and Belanger (1998) provided evidence that the beneficial effect of 1.5 mM BTH foliar application in reducing the extent of Fusarium oxysporum colonization in tomato root tissues is primarily associated with a massive accumulation of structural barriers in the cortical cells and these defence reactions occurred with a much higher magnitude after fungal challenge, confirming, once again, that the contact with the pathogen is essential for the plant to mobilize its defence strategy [40]. Interestingly, in untreated tomato roots, the infection of F. oxysporum did not trigger apparent defence mechanisms, at least at ultrastructural level, as in our case.
Nevertheless, in the first 96 hpi, cell wall strengthening only partially justifies the reduction of mycelium growth and the complete block of about 17% of infection sites. In this time interval, the possible presence of toxic compounds in the intercellular spaces, such as phytoalexins and other simple phenols, could be much more effective in inhibiting hyphal growth, which, in fact, appeared more vacuolated and distorted than in controls. In the same way, direct inhibitory effect of fungal growth by phenolics was detected in cucumber plant treated with BTH and infected by Pythium ultimum [41]. Another intriguingly observation was the fact that in the first days after infection, in untreated control plants, the mycelium growth was almost planar, while in the treated plants, hyphae elongated in all directions and were much more branched. This could be due to the difficulty of generating haustoria, i.e. because of the fortified host cell walls, and, in turn, to the necessity of exploring new tissue areas.
Except for the presence of electron-opaque material around haustoria and in the wall of hypha mother cells, for the shape of haustoria that rarely appeared lobated and for the collar around haustorial neck, at ultrastructural level no other difference was observed in comparison with untreated controls. About the haustorial collar, it is likely formed by callose, besides phenolics, though aniline blue-specific staining for callose was generally negative. This could be due to a quenching effect of polyphenols, masking aniline blue fluorescence. In any case, callose deposition does not seem to play a significant role in impairing U. appendiculatus growth in BTH-treated bean, differently from what observed in other pathosystems [15, 40]. Instead, at 7–10 days after infection, the histology and ultrastructure of infected tissues appeared very different in treated and untreated plants. In particular, in the former, large patches of very electron-dense materials, mainly composed by phenolics, being autofluorescent under UV light, were apposed to the cell wall of cells contacted or penetrated by the fungus and on fungal walls, as well. The structure and osmiophilic properties of the accumulating material suggest that it may be enriched with phenolic compounds containing O-dihydroxy groups [42]. Considering that the adjacent uninfected tissues appeared similar to the correspondent leaf tissues of healthy plants, this confirms that the consistent phenolic deposition is elicited only by the presence of the pathogen. Such an intense deposition is possibly the consequence of the long-lasting effect of BTH in the activation of phenylpropanoid pathway. On the other hand, the absence of similar depositions in infected untreated controls matches with the very low expression of PAL mRNA in these plants. Interestingly, penetrated cells with thick black walls and showing haustoria completely surrounded by depositions in the extrahaustorial matrix did not appear particularly damaged, being their organules and membranes well preserved. This indicates that BTH does not switch the compatible interaction Phaseolus vulgaris–U. appendiculatus in an incompatible one, i.e. leading to HR, as observed in other pathosystems [20–22]. In fact, the infected cells apparently remain functional, while the fungus loses viability along the way, unable to absorb nutrient, neither through haustoria nor by intercellular hyphae with their thick impregnated walls. The presence of highly vacuolated and distorted hyphae observed at 7–10 days after infection supports this view, particularly if compared with those still rich in cytoplasm and organelles present in the untreated controls. In these conditions, the mycelium hardly can sustain the effort of sporulation and, in fact, pustule formation is impaired.
In conclusion, by merging the results from the previous and the present study, we can draw a clearer picture of the mechanisms by which BTH is able to control bean rust disease. At first, the treatment induces an oxidative burst with a rising of H2O2 level within 24 h, and PAL mRNA transcription that continues for several days, possibly inducing the accumulation of low molecular weight phenylpropanoids. The oxidative burst enhances the pool of anionic peroxidases that eventually induce cross-linking of cell wall proteins leading to wall fortification. However, the bulk of defence compound synthesis appears activated after pathogen inoculation, with intense esterification of polyphenols to both fungal and cell walls that in the end starve and, possibly, poison the fungus.
References
Iriti M, Faoro F. Review of innate and specific immunity in plants and animals. Mycopathologia. 2007;164:57–64.
Király L, Barna B, Király Z. Plant resistance to pathogen infection: forms and mechanisms of innate and acquired resistance. J Phytopathol. 2007;155:385–96.
Greenberg JT. Programmed cell death in plant-pathogen interactions. Annu Rev Plant Physiol Mol Biol. 1997;48:525–45.
Király L, Király Z. To die or not to die—is cell death dispensable for resistance during the plant hypersensitive response? Acta Phytopathol Entomol Hung. 2006;41:11–21.
Mauch-Mani B, Métraux J-P. Salicylic acid and systemic acquired resistance to pathogen attack. Ann Bot. 1998;82:535–40.
Conrath U. Systemic acquired resistance. Plant Signal Behav. 2006;1:179–84.
Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–9.
Schreiber K, Desveaux D. Message in a bottle: chemical biology of induced resistance in plants. Plant Pathol J. 2008;24:245–68.
Gozzo F. Systemic acquired resistance in crop protection: from nature to a chemical approach. J Agric Food Chem. 2003;51:4487–503.
Friedrich L, Lawton K, Ruess W, Mesner P, Speker N, Gut Rella M, Meier B, Dincher S, Staub T, Uknes S, Métraux JP, Kessmann H, Ryals J. A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J. 1996;10:61–70.
Görlach J, Volrath S, Knauf-Beiter G, Hengy G, Beckhove U, Kogel KH, Oostendorp M, Staub T, Ward E, Kessmann H, Ryals J. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell. 1996;8:629–43.
Wendehenne D, Durner J, Chen Z, Klessig F. Benzothiadiazole, an inducer of plant defences, inhibits catalase and ascorbate peroxidase. Phytochemistry. 1998;47:651–7.
Kohler A, Schwindling S, Conrath U. Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding and infiltration of water into leaves requires the NPR1/NIM1 gene in arabidopsis. Plant Physiol. 2002;128:1046–56.
Iriti M, Faoro F. Benzothiadiazole (BTH) induces cell-death independent resistance in Phaseolus vulgaris against Uromyces appendiculatus. J Phytopath. 2003;151:171–80.
Faoro F, Maffi D, Cantu D, Iriti M. Chemical-induced resistance against powdery mildew in barley: the effects of chitosan and benzothiadiazole. Biocontrol. 2008;53:387–401.
Lindgren DT, Eskridge KM, Steadman JR, Schaaf DM. A model for dry bean yield loss due to rust. HortTechnol. 1995;5:35–7.
Coyne DP, Steadman JR, Godoy-Lutz G, Gilbertson R, Arnaud-Santana E, Beaver JS, Myers JR. Contributions of the bean/cowpea CRSP to management of bean diseases. Field Crop Res. 2003;82:155–68.
Maheshwari R, Allen PJ, Hildebrandt AC. Physical and chemical factors controlling the development of the infection structures from urediospores germ tubes of rust fungi. Phytopathology. 1967;57:855–62.
Heat MC, Skalamera D. Cellular interaction between plants and biotrophic fungal parasites. In: Tommerup IC, Andrews JH. editors. Advanced in botanical research vol. 24. San Diego: Academic Press; 1997. p. 195–225.
Latunda-Dada AO, Lucas JA. The plant defence activator acibenzolar-S-methyl primes cowpea [Vigna unguicolata (L.) Walp.] seedlings for rapid induction of resistance. Physiol Mol Plant Pathol. 2001;58:199–208.
Buzi A, Chilosi G, Magro P. Induction of resistance in melon seedlings against soil-borne fungal pathogens by gaseous treatments with methyl jasmonate and ethylene. J Phytopatol. 1994;152:491–7.
Boava LP, Kuhn OJ, Pascholati SF, di Piero RM, Furtado EL. Effect of acibenzolar-S-methyl and Saccharomyces cerevisiae on the activation of Eucalyptus defences against rust. Australas Plant Pathol. 2009;38:594–602.
Conrath U, Beckers GJM, Flors V, García-Agustín P, Jakab G, Mauch F, Newman MA, Pieterse CMJ, Poinssot B, Pozo MJ, Pugin A, Schaffrath U, Ton J, Wendehenne D, Zimmerli L, Mauch-Mani B. Priming: getting ready for battle. Mol Plant Microbe Interact. 2006;19:1062–71.
Keogh RC, Deverall BJ, Mcleod S. Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Trans Br Mycol Soc. 1980;74:329–33.
O’Brien TP, Feder N, Mc Cully ME. Polychromatic staining of plant cell walls by toluidine blue. Protoplasma. 1964;59:367–73.
Eschrich W, Currier HB. Identification of callose by its diachrome and fluorochrome reactions. Stain Technol. 1964;39:303–7.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Ford N editor. NY: Cold Spring Harbor Laboratory Press; 1989.
Wojtaszek P, Trethowan J, Bolwell P. Reconstitution in vitro of the components and conditions required for the oxidative cross-linking of extracellular proteins in French bean (Phaseolus vulgaris L). FEBS Lett. 1997;405:95–8.
Kauss H, Jeblick W. Pretreatment of parsley suspension cultures with salicylic acid enhances spontaneous and elicited production of H2O2. Plant Physiol. 1995;108:1171–8.
Katz VA, Thulke OU, Conrath U. A benzothiadiazole primes parsely cells for augmented elicitation of defence responses. Plant Physiol. 1998;117:1333–9.
Alvarez ME, Pennell RI, Meijer PJ, Ishikatwa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–84.
Lin TC, Ishizaka M, Ishii H. Acibenzolar-S-methyl-induced systemic resistance against anthracnose and powdery mildew diseases on cucumber plants without accumulation of phytoalexins. J Phytopathol. 2009;157:40–50.
Goellner K, Conrath U. Priming: it’s all the world to induced disease resistance. Eur J Plant Pathol. 2008;121:233–42.
McFadden HG, Chapple R, de Feyter R, Dennis E. Expression of pathogenesis related genes in cotton stems in response to infection by Verticillum dahaliae. Physiol Mol Plant Pathol. 2001;58:119–31.
Cools HJ, Ishii H. Pre-treatment of cucumber plants with acibenzolar-S-methyl systemically primes a phenylalanine ammonia lyase gene (PAL1) for enhanced expression upon infection with a pathogenic fungus. Physiol Mol Plant Pathol. 2002;61:273–80.
Whan JA, Dann EK, Smith LJ, Aitken EAB. Acybenzolar-S-methyl-induced alteration of defence gene expression and enzyme activity in cotton infected with Fusarium oxysporum f. sp. vasinfectum. Physiol Mol Plant Pathol. 2009;73:175–82.
Walters DR, Fountaine JM. Practical application of induced resistance to plant diseases: an appraisal of effectiveness under field conditions. J Agric Sci. 2009;147:523–35.
Iriti M, Faoro F. Does benzothiadiazole induced resistance increase fitness cost in bean? J Plant Pathol. 2003;85:265–70.
Nicholson RL, Hammerschmidt R. Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol. 1992;30:369–89.
Benhamou N, Bélanger RR. Benzothiadiazole-mediated induced resistance to Fusarium oxysporum f. sp. radicis-lycopersici in tomato. Plant Physiol. 1998;118:1203–12.
Benhamou N, Bélanger RR. Induction of systemic resistance to Pythium damping-off in cucumber plants by benzothiadiazole: ultrastructure and cytochemistry of the host response. Plant J. 1998;14:13–21.
Scalet M, Crivaletto E, Mallardi F. Demonstration of phenolic compounds in plant tissues by an osmium-iodide postfixation procedure. Stain Technol. 1989;64:273–90.
Acknowledgments
This paper is dedicated to the memory of Dr. Massimo Pigni who suddenly died on 25th September 2009, soon after finishing his doctoral thesis of which this work is a fundamental part.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Maffi and M. Iriti equally contributed to this work.
Rights and permissions
About this article
Cite this article
Maffi, D., Iriti, M., Pigni, M. et al. Uromyces appendiculatus Infection in BTH-Treated Bean Plants: Ultrastructural Details of a Lost Fight. Mycopathologia 171, 209–221 (2011). https://doi.org/10.1007/s11046-010-9350-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-010-9350-1