Abstract
Main conclusions
A comparative study of isolated vs. lichenized Trebouxia sp. showed that lichenization does not influence the survival capability of the alga to the photo-oxidative stress derived from prolonged desiccation.
Coccoid algae in the Trebouxia genus are the most common photobionts of chlorolichens but are only sporadically found in soil or bark outside of a lichen. They all appear to be desiccation tolerant, i.e. they can survive drying to water contents of below 10 %. However, little is known about their longevity in the dry state and to which extent lichenization can influence it. Here, we studied the longevity in the dry state of the lichenized alga (LT) Trebouxia sp. in the lichen Parmotrema perlatum, in comparison with axenically grown cultures (CT) isolated from the same lichen. We report on chlorophyll fluorescence emission and reactive oxygen species (ROS) production before desiccation, after 15–45 days in the dry state under different combinations of light and air humidity and after recovery for 1 or 3 days in fully hydrated conditions. Both the CT and the LT were able to withstand desiccation under high light (120 µmol photons m−2 s−1 for 14 h per day), but upon recovery after 45 days in the dry state the performance of the CT was better than that of the LT. By contrast, the quenching of excess light energy was more efficient in the LT, at high relative humidities especially. ROS production in the LT was influenced mostly by light exposure, whereas the CT showed an oxidative burst independent of the light conditions. Although lichenization provides benefits that are essential for the survival of the photobiont in high-light habitats, Trebouxia sp. can withstand protracted periods of photo-oxidative stress even outside of a lichen thallus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Green microalgae are typical inhabitants of marine and freshwater environments, but they also colonize terrestrial habitats, from tree bark (Lüttge and Büdel 2010) to rocks (Matthes-Sears et al. 1999) to the soil of hot and cold deserts (Gray et al. 2007). Habitat occupancy is also extended through lichenization, i.e. the formation of a stable, extracellular symbiosis between a carbon-heterotrophic fungus (generally an ascomycete, the mycobiont), and one or more green algae or cyanobacteria (the photobiont). Lichenization has been considered as a life-style that creates favourable conditions for both symbiotic partners, and particularly for the photobiont, that would be put in the best conditions as for light, gas and water exchange (Nash 2008). All photobionts and all their fungal partners appear to be desiccation tolerant, i.e. they can survive drying to below 10 % water content, and resume normal metabolism within minutes as soon as water becomes available again (Kranner et al. 2008).
Desiccation tolerance occurs in phylogenetically unrelated taxa, such as tardigrades, fungi, mosses and ferns (Alpert 2006), and it may be extended to the whole life cycle of the organism, or just to some stages, as it happens, e.g. in flowering plants. Here pollen grains and seeds are often desiccation tolerant, whereas sporophytes only exceptionally (Berjak et al. 1990). Desiccation tolerant organisms generally can survive long periods in the dry state, with time spans that range from days to several decades, as in the extreme case of some seeds (Buitink and Leprince 2004). This is possible because in desiccation tolerant tissues the combination of oligosaccharides and/or polyols, proteins and other cytoplasmic molecules with low water contents leads to the intracellular formation of glasses, which are thermodynamically unstable solids with high viscosity (Franks et al. 1991). In this condition molecular mobility and the diffusion within the cytoplasm are extremely reduced and, therefore, all the chemical reactions are slowed down. However, deleterious processes such as lipid peroxidations, de-esterifications and Maillard reactions still take place and over long periods, they can cause viability loss (Buitink et al. 1998). Even lichens show an exceptional ability to survive long periods in the dry state. For example, Lange (1953) was able to register their reviviscence after more than 90 weeks in the dry state at laboratory conditions, whereas Honegger (2003) documented the germination of ascospores and vegetative propagules (soredia) after 9.25 years stored desiccated at −20 °C. However, it is still not clear to which extent this capacity is an emerging character of lichenization. To date, comparative research dealing with the physiology of isolated vs. lichenized partners provided some indications, but the results are not fully congruent. For example, Lange et al. (1990) did not observe significant differences in the photosynthetic performance of isolated vs. lichenized algae under water stress, whereas Kranner et al. (2005) found that molecular photoprotection is more efficient in the lichen than in its isolated symbionts. Kosugi et al. (2009, 2013) also suggested that lichenization enhanced the desiccation tolerance of isolated photobionts.
In this work, we studied the influence of lichenization on the longevity in the dry state of a Trebouxia species, i.e. a representative of a green algal genus that occurs in c. 50 % of the known lichens (Ahmadjian 1993). Trebouxia belongs to the order Trebouxiales (Chlorophyta), best known as the “lichen algae group” (Leliaert et al. 2012), which includes several other lichen symbionts (e.g. Asterochloris, Myrmecia) and soil algae (e.g. Myrmecia). In this study the alga was isolated from the lichen Parmotrema perlatum (Huds.) M. Choisy, a mid-temperate foliose species of humid habitats, and was identified as a still undescribed species of the T. arboricola clade (L. Muggia, in litt.). The resistance of the lichenized and the isolated and axenically cultured Trebouxia to long-term storage in the dry state was tested at increasing photo-oxidative conditions under different light and air relative humidity (RH) regimes. Chlorophyll a fluorescence (Chl a F) was used to assess the physiological state of the algae, and semi-quantitative histochemical localization of reactive oxygen species (ROS) was carried out to test the influence of the exposure conditions on ROS production.
Materials and methods
Lichen sampling and pre-treatment of samples
North-exposed thalli of the lichen Parmotrema perlatum (Huds.) M.Choisy were collected from oak [Quercus petraea (Matt.) Liebl.] trunks in a dolina wood of the Classic Karst (Trieste, NE Italy) far from known pollution sources. A detailed description of the collection site with characterization of the epiphytic lichen vegetation is given by Carvalho (1996). The material was dried in the laboratory at room temperature for 24 h under dim light, and mosses and bark fragments were carefully removed with stainless tweezers under a stereomicroscope. Marginal lobes (50 ± 3 mg DW) without necrotic spots, soralia or parasites were randomly selected for the experiments. Before the treatments, the samples were subjected to a 2-day conditioning process: they were immersed in distilled water for 3 min every 12 h, and during the remaining time were placed on Petri dishes within plastic boxes containing water at the bottom; the boxes were covered, but not sealed, with transparent plastic wrap (>95 % RH) and placed in a thermostatic chamber at 18 ± 1 °C with a light/dark regime of 14/10 h and a light intensity of 20 µmol photons m−2 s−1. This procedure was applied to reduce potential photoinhibition phenomena due to differences in the light environment of the collection sites.
Isolation and culture of the lichen photobiont
An axenic strain of the photobiont from a thallus of P. perlatum was isolated according to Yamamoto et al. (2002). The isolated photobiont was inoculated in sterile plastic tubes filled with ≈5 mL of slanted solid Trebouxia medium (TM; 1.5 % agar) (Ahmadjian 1973), and kept in a thermostatic chamber at 18 ± 1 °C and 17 ± 2 μmol photons m−2 s−1 with a light/dark regime of 14/10 h until abundant biomass was produced. The photobiont was subcultured every 30 days and kept at the same conditions.
The cultures used in the experiments were prepared by inoculating 100 μL of a cell water suspension (density: 3.5 × 106 cells mL−1) on hand-cut sterile filter paper discs (Whatman, 60 ± 5 g m−2, diam. 25 mm), placed on solid TM (1.5 % agar) in Petri dishes. Four filters were placed one close to the other in each plate. The cultures were grown at the same controlled conditions as described above for 30 days before exposure. The cultured algal biomass was estimated by measuring the chlorophyll content in crude extracts (Table 1). The pigment extraction was carried out using whole discs (n = 6) immersed in DMSO for 24 h (Tretiach et al. 2007a). The supernatant was analysed spectrophotometrically, and the equations of Wellburn (1964) were applied. The chlorophyll content was then expressed on an area basis. Reference algal material was cryo-conserved according to Dahmen et al. (1983) and is available upon request.
Experimental design
Two separate experiments, A and B, were performed, with Chl a F measurements (A and B) and histochemical localization of ROS (A) carried out before exposure, after exposure and after recovery under optimal conditions.
Experiment A
The lichen lobes and the discs bearing the axenically cultured photobiont were exposed for 15 days to 3 % or 80 % RH, and to 0, 40 or 120 µmol photons m−2 s−1 with a light/dark regime of 14/10 h. After this exposure, lobes and cultures underwent a recovery period of 3 days at the respective pre-exposure conditions, and the growth medium of the algal cultures was changed from TM to BBM, which does not contain organic nutrients. In this way it was possible to limit the growth of fungi and/or bacteria after exposure in non-axenic conditions (see below).
Experiment B
The lichen lobes and the discs bearing the axenically cultured photobiont were exposed for 15, 30 or 45 days to 3 % RH and to 0 or 120 µmol photons m−2 s−1 with a light/dark regime of 14/10 h. After this exposure, lobes and cultures were subjected to a recovery period of 1 day at the same conditions described for experiment A.
Exposure conditions
The lobes and the discs bearing the algal cultures were placed in desiccators with transparent lids (polypropilene-polycarbonate, vol. 9.2 L, diam. 25 cm, h 30 cm; Kartell®, Milan, Italy) inside an environmentally controlled chamber at 20 ± 1 °C. Low (3 %) and high (80 %) RHs were obtained using, respectively, silica gel and a saturated solution of NaCl, at the bottom of the desiccators. In order to permit gas exchange with the external atmosphere while maintaining a constant RH, the desiccators were modified with an open funnel inserted at the top of the lid and filled with silica gel (3 % RH) or wet paper (80 % RH) that was changed daily. This procedure was adopted to maintain a constant concentration of oxygen in the desiccators to enable respiration and photosynthesis at 80 % RH.
The lichen samples were illuminated using a Gavita Superagro GAN 4–550 (GAVITA AS, Andebu, Norway) with a high-pressure sodium lamp (Lucalox PSL 230 V 400 W). The thermal infrared emission was reduced with a home-made glass filter chamber (40 × 40 × 4 cm) filled with running tap water, placed at c. 7 cm from the light source. The desiccators were covered by aluminium foil. During exposure, RH and air temperature (°C) inside the desiccators were constantly monitored with data loggers (EL-USB-2, Lascar Electronics Inc, Whiteparish, UK). The temperature of the samples was checked with a contact thermocouple (Digi-Sense® Dual J-T-E-K™ Thermocouple Thermometer, Eutech Instruments®, Landsmeer, Netherlands) in a preliminary test and never exceeded 22 °C. PPFD values higher than 150 µmol photons m−2 s−1 causing significant increases in sample temperature were not applied.
Chl a F measurements
Chl a F measurements on LT were carried out for each exposure condition on the same set of samples before and after exposure and after the recovery period (exp. A: n = 10; exp. B: n = 6). Measurements of CT were carried out on two independent sets of discs bearing the photobionts, one for the pre-exposure measurements and one for the post-exposure and post-recovery measurements (exp. A: n = 10; exp. B: n = 5). This option was adopted to limit contaminations during the recovery period.
Before measurements the lobes and the algal cultures were rehydrated by submerging the lobes in distilled water for 3 min, and by adding two drops of distilled water to each culture disc and then by placing it in a Petri dish filled with solid BBM medium. Lobes and cultures were then dark adapted for 30 min.
Chl a F measurements were carried out with a pulse-amplitude-modulated fluorimeter Mini-PAM (Walz, Effeltrich, Germany), positioning the measuring optic fibre (length: 100 cm; active diameter: 5.5 mm) at 60° on the upper surface of terminal parts of the lobes, because these portions have considerably higher Chl a F emission than the central ones (Tretiach et al. 2007b), and on the centres of the culture discs, because of the higher cell density. The modulated light was turned on to obtain F 0 (minimal Chl a F level). A saturating light pulse of ca. 8000 μmol photons m−2 s−1 for 0.8 s was emitted to obtain F m (transient maximum Chl a F level) and to calculate Fv (variable Chl a F level, i.e. F m − F 0) and F v/F m (maximum quantum efficiency of PSII photochemistry) (Genty et al. 1989). An external actinic light provided by a light unit FL-460 (Walz) with a halogen lamp was turned on to record the Kautsky effect at 108 μmol photons m−2 s−1 (light intensity consistent with the species-specific PPFDIk value of P. perlatum as described by Piccotto and Tretiach 2010). Once the peak F p was achieved, saturating light pulses were applied at 60 s intervals during actinic illumination to determine non photochemical quenching (NPQ) (e.g. Roháček 2002; Baker 2008). NPQ was calculated as (F m − F′ m)/F′ m where F′ m is the maximum quantum yield of PS II in illuminated samples. Maximum quantum yield and NPQ were selected because both are influenced by light during prolonged desiccation periods (Štepicová et al. 2008; Gauslaa et al. 2012).
Histochemical localization of ROS production
Immediately after each treatment three lobes and three cultures were frozen in liquid nitrogen and stored at −80 °C until use. The histochemical localization of ROS production in the lichen lobes was conducted according to Pellegrini et al. (2014). Before the analysis, several tests were performed to assess the possible interference of lichen substances on the assay by removing them with repeated washes in pure (99.9 %) acetone (Sigma-Aldrich, St. Louis, MO, USA). As will be discussed later on, no effect was detected at cortical and algal layer but the removal from the medullar layer resulted in a better detection of ROS in the mycobiont hyphae. However, the washing treatment with acetone was later avoided since acetone is a strong dehydrating agent and its application could have thus compromised the results. Lobes taken from the freezer were left to warm up at room temperature for 10 min and then rectangular fragments of 4 × 3 mm were cut with a stainless blade, rehydrated in distilled water for 5 min and patted dry with absorbent paper to remove the excess of water. The fragments were mounted in a cryostat embedding medium (Killik, Bio-Optica, Milan, Italy) and then cut with a cryotome LEICA CM 1510 S (Leica microsystems®, Wetzlar, Germany) to obtain 30 μm thick transversal sections. These were immersed in 15 mL of an aqueous solution 10 μM of 2′,7′-dichlorofluorescin diacetate (DCFH-DA, HPLC grade, Sigma-Aldrich) inside a vacuum chamber in the dark for 90 min. DCFH-DA is a non-polar molecule that can enter the cell and is deacetylated by intracellular esterases to 2′,7′-dichlorofluorescin (DCFH), a polar molecule unable to cross membranes. DCFH is then oxydized by H2O2 or other ROS to 2′,7′-dichlorofluorescein (DCF), a fluorescent molecule, with an exciting/emission wavelength of 488/525 nm. After this treatment, seven transversal sections were put on glass slides and observed; image acquisitions were taken only for three randomly selected sections.
Frozen cultures were thawed at room temperature for 10 min, rehydrated with two drops of water for 5 min and re-suspended in 1 mL of the DCFH-DA 10 μM solution. The culture suspension was incubated for 30 min in the dark on a shaker and then centrifuged at 1400g for 1 min. The DCFH-DA supernatant was discharged and the pellet re-suspended in distilled water (400 μL). One drop of the water suspension was put on a glass slide and observed with a confocal laser scanning microscope (CLSM) Nikon C1-si (Nikon, Tokyo, Japan). Image acquisitions were carried out for three randomly selected fields after careful observation of the whole sample surface.
Lobe sections and culture suspensions were excited with an argon laser at 488 nm with an intensity of 10.5 %. The signal from the excited DCF was acquired with a 515/30 nm band pass filter. Emission of the autofluorescence from chlorophyll was acquired with a 650 nm long pass filter (λ ≥ 650 nm). Each acquired field was made by a variable number of focal planes, depending on the thickness of the sections and on the algal abundance, to permit the ROS localization at the intracellular level. Acquisitions were elaborated with the Nikon EZ-C1 FreeViewer software (Nikon, Tokyo, Japan) and with the freeware suite ImageJ 1.46r (National Institutes of Health, Washington DC, USA). A unification algorithm (Z-projection) was applied to each confocal acquisition to obtain bi-dimensional images. The entire set of images was analysed to provide a semi-quantitative estimation of the ROS production in the photobiont cells. A cell count was performed to calculate the percentage of cells that developed ROS. Mature cells (diam > 6 μm, according to Tschermak-Woess 1989) were distinguished from aplano- or autospores, and dead cells or cells with a collapsed chloroplast were excluded. Production of ROS was considered to occur when the signal of intracellular excited DCF was higher than the background noise due to DCFH-DA.
RH equilibration during exposure
The samples were introduced into the desiccators in the fully hydrated state (Table 2) and this resulted in a different dehydration rate that was faster at 3 % than at 80 % RH; the RH monitored within the desiccators equilibrated slower in the latter RH (see Online Resource S1 and S2). However, a preliminary experiment based on Chl a F measurements and the histochemical localization of ROS showed that the different times of equilibration to 3 and 80 % RH did not affect the Chl a F emission but caused a slight increase in ROS production in the samples equilibrated to 80 % RH.
Additional data
Measurements of cortex transmittance were carried out on dry and wet lobe fragments (4 × 3 mm; n = 8) to characterize the shielding effect of the cortical layer of the mycobiont that protects in the lichen thallus the algae from direct light. Moistened fragments of lichen lobes were flattened between two paper sheets, gently pressed, left to dry out for 1 day and then stuck with double-sided transparent adhesive on single microscope slides with the lower surface up. The lower cortex, the medulla, and most of the algal layer were then carefully removed with a blade under a stereo-microscope working at high magnification (115×). The removal process was interrupted when the upper cortex was visibly intermingled with clusters of algae. The samples were placed under the 5× objective of a Zeiss Axioplan microscope (Carl Zeiss, Jena, Germany). Light was set at the maximum intensity and the PPFD passing through the sample was measured by placing the probe of a quantum radiometer HD 2302.0 (Delta Ohm, Padua, Italy) directly on the microscope ocular. In dry and wet fragments cortex transmittance through the first algal layer was 14 ± 2 and 22 ± 3 % of the PPFD, respectively, when passing through an empty glass covered with the adhesive.
The algal layer thickness in the lichen thalli and in the culture discs was also measured (Table 1). In the first case the measurements were performed directly on a subset of confocal samples (see above; n = 6) using the measuring tool of the program Nikon EZ-C1 FreeViewer, whereas in the second case they were performed under the light microscope on sections (10 µm thick) of culture disc fragments (4 × 3 mm) embedded in Technovit 7100 resin (Heraeus-Kulzer).
The relative water content (% RWC) of lobes was calculated as (FWt − DW)/DW × 100, where FWt is the sample weight after each treatment (t: pre-, post- exposure, recovery) and DW is the sample weight after drying for 48 h in silica and 24 h in an oven at 70 °C. The water potential of fully hydrated (pre-exposure and recovery, n = 6) and desiccated lobes (3 and 80 % RH, n = 3) was measured with a dew-point water potential meter WP4 (Decagon Devices Inc., Pullman, WA, USA) as detailed in Nardini et al. (2013).
Statistics
All calculations were performed with Microsoft Office Excel 2003 SP3 (Microsoft corporation, Redmond, WA, USA) and STATISTICA 6.0 (StatSoft Inc., Tulsa, OK, USA). A one-way ANOVA was performed to verify the significance of differences before and after exposure and after the recovery period, whereas a factorial ANOVA was performed to test the influence of the environmental descriptors (light and RH) and “lichenization” on the fluorimetric parameters measured in experiment A. Scheffè’s post hoc test was then applied to verify significant differences between datasets. Other statistical analyses were conducted applying the non-parametric Mann–Whitney U test, also known as Wilcoxon non-paired test, as suggested by Lazár and Nauš (1998) and Baruffo and Tretiach (2007).
Results
Chl a F
The F v/F m values of cultured (CT) and lichenized (LT) Trebouxia sp. after the initial conditioning process ranged from 0.633 to 0.708 (both experiments; Figs. 1, 2), confirming that the samples were healthy before exposure.
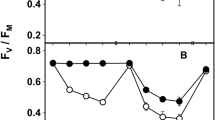
Maximum quantum yield of photosystem II (F v/F m) (a, b) and non photochemical quenching (NPQ) (c, d) at 3 % RH (a, c) and 80 % RH (b, d) measured in lichenized (grey bars) and cultured (white bars) Trebouxia sp. before exposure (Pre-), after 15 days of exposure (Post-) at 0, 40 and 120 μmol photons m−2 s−1 and after 3 days of recovery (Rec-) at 20 μmol photons m−2 s−1 and 100 % RH with morning and evening watering. Values are means ± 1 standard error; significant differences (One-way ANOVA, Scheffé’s post hoc test) against Pre- and Post-values for P < 0.05 are marked a and b, respectively, whereas for P < 0.001 they are marked aa and bb (n = 10 in a, b; n = 5 in c, d)
Maximum quantum yield of photosystem II (F v/F m) (a, b) and non photochemical quenching (NPQ) (c, d) at 120 (a, c) and 0 (b, d) μmol photons m−2 s−1 measured in lichenized (grey bars) and cultured (white bars) Trebouxia sp. before exposure (Pre-), after exposure (Post-) of 15, 30 and 45 days at 3 % RH and after 24 h of recovery (Rec-) at 20 μmol photons m−2 s−1 and 100 % RH with morning and evening watering. Values are means ± 1 standard error; significant differences (Mann–Whitney U test) against Pre- and Post-values for P < 0.05 are marked a and b, respectively (n = 4–6)
After the 15 days of exposure in exp. A, Chl a F emission was impaired. F v/F m of LT decreased proportionally to PPFD at both % RHs values (Fig. 1), although the decline was significantly more pronounced at 3 % RH (−60 %) than at 80 % RH (−30 %; Fig. 1) at the highest PPFD. F v/F m of CT also decreased proportionally at 3 % RH, but apparently not at 80 % RH, for the pronounced decrease observed in the dark-exposed samples. Light was actually the most important factor affecting F v/F m, with an F value almost ten times higher than the other factors (Table 3). NPQ changed significantly (P < 0.001) in CT but not in LT. In the former, NPQ decreased proportionally to PPFD at 3 % RH, whereas at 80 % RH an intense decrease was observed only at the highest PPFD (P < 0.001; Fig. 1d). In this case the principal factor influencing NPQ was lichenization, immediately followed by RH and light (Table 3).
The recovery period allowed a general re-establishment of standard Chl a F emission. Lichenized Trebouxia recovered F v/F m totally at both % RH values, confirming that no permanent damage to the photosynthetic apparatus occurred. Interestingly, the post-recovery NPQ values were higher than the pre-exposure ones, but the increase was proportional to PPFD only in the samples exposed at 3 % RH (Fig. 1). Cultured Trebouxia recovered the pre-exposure values of F v/F m and NPQ (Fig. 1c, d), and at 3 % RH, in particular, the increasing trend was the same observed in LT.
The extension of the exposure period at 3 % RH (exp. B) caused an increasing photo-inhibition over time in both light-exposed LT and CT (Fig. 2). F v/F m decreased more in LT than in CT and was absent after 45 days. F v/F m of CT strongly decreased, too (final median: 0.192; Fig. 2). The exposure in the dark did not cause any statistically significant decline of F v/F m in both LT and CT (Fig. 2b).
Also in this case, NPQ showed a different trend in LT vs. CT: a significant decrease was observed after 15 days in both light-exposed LT and CT, but it became more intense over time only in CT (Fig. 2). In the dark, after 30 and 45 days, a significant decrease was observed in CT, but not in LT.
In this experiment, the recovery period allowed only a partial re-establishment of Chl a F emission. After 30 and 45 days, the dark-exposed samples recovered pre-exposure F v/F m values, whereas the light-exposed ones did not. In the latter case, LT was less efficient than CT (Fig. 2). On the contrary, LT could completely recover the initial NPQ, whereas CT recovered only partially at both dark- and light-regime (Fig. 2).
Histochemical localization of ROS
Before exposure, ROS were detected only in a small percentage of cells in both LT and CT (Table 4), being mostly restricted to the cytoplasm; sporadically an intense ROS staining was observed in the central portion of the chloroplast corresponding to the pyrenoid (Fig. 3b). In the LT samples, ROS were generally observed also in the mycobiont cells, particularly in the paraplectenchymatous upper cortex, in the melanized lower cortex and in the appressoria, i.e. the specialized hyphal cells in physical contact with single photobiont cells (Fig. 3f). No reaction was observed in the aerial medullar layer (Fig. 3 e–g, k–m).
Micrographs of Parmotrema perlatum (a, e–g, k–m) cross sections and Trebouxia sp. cultures (c, h–j, n–p) stained with DCFH-DA and observed with a confocal laser scanning microscope before exposure (a, c), after 15 days of exposure (e–j) at 0 (e, h), 40 (f, i) and 120 (g, j) μmol photons m−2 s−1 at 3 % RH and, respectively, after 3 days of recovery at 20 μmol photons m−2 s−1 and 100 % RH with morning and evening watering (k–p). Green signal emitted by DCF, red signal by chlorophyll a after an excitation with lasers at λ = 488 and 637 nm, respectively. In b, cross section of a lichen lobe with ROS diffused in both photobiont and mycobiont cells (arrows pyrenoids). In d, Trebouxia sp. mature cells observed at the light microscope (differential interference contrast). Bar 15 µm (a, e–g, k–m); 30 µm (b–d, h–j, n–p)
After exposure, cells producing ROS increased proportionally in LT from 0 to 120 µmol photons m−2 s−1 to a maximum of four times as much as pre-exposure (P < 0.05; Table 4), whereas in CT samples ROS production was differently influenced: it increased statistically after the exposure, with the exception of low-PPFD-exposed samples at 80 % RH (Table 4): in this case light induced only a slight increase of ROS which was not, however, statistically significant (Table 4). Air humidity was never influential.
In the LT samples the recovery period did not allow a reduction of the number of cells producing ROS, whose presence remained relatively frequent in PPFD-exposed samples at both % RH values (Table 4). On the contrary, the CT samples had a ROS production very similar to the post-exposure one(s), with two exceptions: dark-exposed at 3 % RH, which recovered totally, and low PPFD-exposed at 80 % RH, in which the percentage of cells with ROS production increased (Table 4).
Discussion
In this study the effect of lichenization on the desiccation tolerance of a Trebouxia alga was tested under increasing photo-oxidative conditions at different environmental moisture regimes. The Trebouxia culture (CT) used for this experimentation is functionally different from the population inside a lichen lobe (LT, Fig. 3) (see Online Resource S3), and even from the isolated photobionts used by previous authors (see, e.g. Kosugi et al. 2009, 2013) for very short experiments and recovery times. Nonetheless, our comparison offers some interesting results, which allows suggesting new hypotheses regarding the mutual benefits of lichenization for the two partners.
Experiment A showed that light was the environmental factor with the most negative effects in both desiccation regimes. Consistent with earlier studies (Solhaug and Gauslaa 1996; Gauslaa et al. 2012) light induced a photo-inhibitory effect proportional to the illumination regime in both LT and CT. It is important to note that the light intensity was not the same for LT and CT, due to the presence of the thalline upper cortex in LT, which has a shielding effect (Dietz et al. 2000; Kosugi et al. 2010). Notwithstanding the low PPFD to which LT was actually exposed, LT showed an increased “oxidative burst” upon rehydration. This phenomenon was already observed in lichens (Weissman et al. 2005; Catalá et al. 2010) and in mosses (Minibayeva and Beckett 2001; Cruz de Carvalho et al. 2012), but the influence of the illumination in the time span prior to rehydration was not tested before. Protracted periods under photo-oxidative conditions cause an inevitable impairment of the antioxidant machinery (Kranner et al. 2005; Vráblíková et al. 2005) which can leave the cells unprotected against the subsequent oxidative burst derived from the sudden metabolism reactivation (Weissman et al. 2005). This scenario agrees with our experimental evidence, because after a period under photo-oxidative conditions the number of algal cells affected by oxidative burst significantly increased (Table 4). The same behaviour was observed in the fungal cells of the upper cortex, but not in the cells of the medulla, because the cortical layers are hydrophilic, whereas the medulla is hydrophobic (Honegger 1991) due to the deposition of lichen substances on the hyphal cell walls (Scherrer et al. 2000) that prevent DCFH-DA diffusion into the cells (data not shown).
By contrast, the ROS burst in CT was caused proportionally more by desiccation than by light because it was observed also in dark exposed samples. In agreement with the results of Kranner et al. (2005), that will be further discussed below, this suggests the hypothesis that prolonged periods in the dry state cause a more intense ROS production upon rehydration in CT than in LT. In vascular plants it is known that in certain circumstances ROS are purposefully produced as signalling molecules to modulate growth, defence against pathogens and stomatal aperture (Apel and Hirt 2004). Therefore, we cannot exclude a priori a possible positive role played by this increased ROS burst.
Interestingly, the LT samples in equilibrium with 80 % RH could mitigate the negative effect of light on the photosynthetic apparatus. This result is consistent with the findings of Gauslaa et al. (2012), who observed the same phenomenon in two other chloro-lichens, Lobaria pulmonaria and Platismatia glauca. A possible explanation can be found in the quantity of water still present, which was small (Table 2) but evidently sufficient to permit minimal metabolic activity (Lange et al. 1990; Nash et al. 1990), with some protection mechanisms still partly functioning during the exposure period. This hypothesis is reinforced by the recent experimental evidence of Fernández-Marín et al. (2013), who demonstrated that an enzymatic activity is still detectable in a moss with very low water content. However, it is worth noting that the mitigating effect due to the equilibrium with high RH was not sufficient to avoid photo-inhibitory phenomena in CT. Our study showed that statistically (Table 3) lichenization has a clear influence on the algal partner. The NPQ after the exposures of CT was always different from that of LT, with CT showing a more pronounced decrease in response to light intensity and exposure length. Considering that NPQ is related to the xanthophyll cycle activity (Gilmore et al. 1994; Müller et al. 2001; Fernández-Marín et al. 2010), the differences between CT and LT match those observed by Kranner et al. (2005) who demonstrated that the antioxidant machinery and the xanthophyll cycle activity of a lichenized alga provide better protection against photo-oxidative stress than that of the isolated one. In addition, at the biochemical level the beneficial effect of lichenization is also supported by the findings of Kosugi et al. (2013) who recently observed that the increased NPQ occurring in the desiccated status (d-NPQ; Heber et al. 2007; Heber 2008) benefits from an apparent movement of arabitol from the mycobiont to the photobiont upon dehydration. On the other hand, after 3 days of recovery both LT and CT showed an increase in NPQ proportional to the light intensity applied during the exposure and triggered only by complete desiccation (Fig. 1c). Acclimation to changes in the environmental light conditions via NPQ is reported for lichens but at a different time scale. MacKenzie et al. (2002) observed a seasonal increase in NPQ whereas in our case it occurred just in 3 days. Such fast acclimation was previously observed in vascular plants (Zia et al. 2011) and mosses (Gerotto et al. 2011) and, depending of the stress type (e.g. osmotic stress, light), it involves the increase in the xanthophylls pool or the overexpression of the Psbs protein. Considering that both NPQ mechanisms are present in lichens (Schofield et al. 2003) we cannot exclude their combined contribution to this increase as a response to the increased ROS burst.
Experiment B was intended to test the extent of CT desiccation tolerance by extending the exposure period. This experiment demonstrated that Trebouxia is not as “delicate” as generally considered. It survived a desiccation period of 45 days under photo-oxidative conditions, although it suffered increased photo-inhibition over time. Interestingly, the performance of Trebouxia was comparable to that of six desiccation-tolerant green algae isolated from soil-crusts of desert environments (Gray et al. 2007) and subjected to experimental conditions very similar to those applied in this study. On the other hand, LT recovered less than CT. This was possibly due to the loss of vitality of the mycobiont that could have further impaired the recovery capacity of the photobiont. As a matter of fact, the lichen lobes showed bleaching of the upper cortex and became brittle after 30 days of light exposure, and even more so after 45 days. Both phenomena were not observed in the dark exposed samples. Bleaching of the upper cortex due to light exposure is commonly related to a loss of photosynthetic pigments by the photobiont layer (Gauslaa and Solhaug 2000). However, in this case, the rehydration of samples revealed the typical bright green colour of a healthy algal layer, and not the expected brownish colour caused by the degradation of chlorophylls into phaeophytins.
Taken together, our results suggest that the weak partner of this lichen symbiosis is the mycobiont, not the photobiont. This is in good agreement with the known ecology and chemistry of the lichen. Parmotrema perlatum is rather hygrophilous (Nimis and Martellos 2008) and for this study the samples were collected in a woody habitat with high moisture and frequent dew events (Carvalho 1996). For these reasons this lichen is neither adapted to withstand long periods of drought nor to face high PPFD for several hours a day.
In conclusion, this study confirmed that light is a particularly important environmental factor for Trebouxia in both its isolated and lichenized state. It was demonstrated that the symbiosis benefits the algal partner by increasing its photo-protective mechanisms. However, under our relatively mild experimental conditions, this influence was not a determinant for the survival of the algal partner. Free-living Trebouxia can withstand even prolonged periods of photo-oxidative stress without the protection provided by the fungus. However, we cannot exclude that the distribution of the free-living alga in natural habitats could be eventually limited by high-light regimes harsher than those applied here.
The results of this study allow considering the influence of the mycobiont on the algal partner also from another perspective. The lichenized Trebouxia had a photosynthetic performance very similar to that of the isolated counterpart grown on a culture medium rich in all the inorganic and organic nutrients required for optimal development (Ahmadjian 1993). This could mean that the mycobiont is capable to recover and to provide its partner with all the mineral and organic nutrients essential to a sub-optimal growth, even though it occurs in a relatively nutrient-poor environment (Carvalho 1996). This exchange is well known in several other cases of fungal symbioses, e.g. in mycorrhiza (Govindarajulu et al. 2005), and it occurs to such a great extent to be fundamental for the survival of entire biomes (Malloch et al. 1980). To date, the movement of solutes from the mycobiont to the photobiont has not been fully circumstantiated yet (Nash 2008), although it represents a very promising field for further research.
In conclusion our work confirms that lichenization can influence the physiology of the photobiont, but it also shows that this species of Trebouxia is perfectly apt to withstand prolonged periods of time in the desiccated status under mid photo-oxidative conditions also outside a lichen thallus. In the future, from the comparison of the physiological performance of diverse isolated lichen photobionts under photo-oxidative conditions, we could understand why Trebouxia is one of the most common photobionts in the lichen symbiosis.
Author contribution
FCC wrote the manuscript. He performed the histochemical localization of ROS and took part in the experimental design development, Chl a F measurements and data analysis. SB took part in the Chl a F measurements. DZ performed the majority of the Chl a F measurements and carried out the data analysis. MT is the project supervisor. He took part in the experimental design development, contributed actively with his expertise in each step of the work and co-edited the manuscript.
Abbreviations
- Chl a F:
-
Chlorophyll a fluorescence
- CLSM:
-
Confocal laser scanning microscopy
- CT:
-
Cultured Trebouxia
- DCF:
-
2′,7′-Dichlorofluorescein
- DCFH:
-
2′,7′-Dichlorofluorescin
- DCFH-DA:
-
2′,7′-Dichlorofluorescin diacetate
- F 0 and F m :
-
Minimal and maximal Chl fluorescence intensity in dark-adapted samples, respectively
- F′ m :
-
Maximum quantum yield of photosystem II in illuminated samples
- F p :
-
Maximal Chl fluorescence under non-saturating actinic light
- F v :
-
Variable Chl fluorescence (F v = F m − F 0)
- F v/F m :
-
Maximum quantum yield of photosystem II
- LT:
-
Lichenized Trebouxia
- NPQ:
-
Non photochemical quenching
- PPFDIk :
-
Photosynthetic photon flux density corresponding to the effective maximum value of quantum yield CO2 assimilation
- RH:
-
Air relative humidity
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water content
References
Ahmadjian V (1973) Methods of isolation and culturing lichen symbionts and thalli. In: Ahmadjian V, Hale ME (eds) The lichens. Academic Press, New York, pp 653–659
Ahmadjian V (1993) The lichen symbiosis. Wiley, Oxford
Alpert P (2006) Constraints of tolerance: why are desiccation tolerant organisms so small or rare? J Exp Biol 209:1575–1584
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Baruffo L, Tretiach M (2007) Seasonal variations of F 0, F m, and F v/F m in an epiphytic population of the lichen Punctelia subrudecta (Nyl.) Krog. Lichenologist 39:555–565
Berjak P, Farrant JM, Mycock DJ, Pammenter NW (1990) Recalcitrant homoiohydrous seeds: the enigma of their desiccation-sensitivity. Seed Sci Technol 18:297–310
Buitink J, LePrince O (2004) Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology 48:215–228
Buitink J, Claessens MMAE, Hemminga MA, Hoekstra FA (1998) Influence of water content and temperature on molecular mobility and intracellular glasses in seeds and pollen. Plant Physiol 118:531–541
Carvalho P (1996) Microclimate and diversity of cryptogamic epiphytes in a Karst doline (Trieste, NE Italy). Gortania 18:41–68
Catalá M, Gasulla F, Pradas del Real AE, García-Breijo F, Reig-Armiñana J, Barreno E (2010) Fungal-associated NO is involved in the regulation of oxidative stress during rehydration in lichen symbiosis. BMC Microbiol 10:297
Cruz de Carvalho R, Català M, Marques da Silva J, Branquinho C, Barreno E (2012) The impact of dehydration rate on the production and cellular location of reactive oxygen species in an acquatic moss. Ann Bot Lond 110:1007–1016
Dahmen H, Staub T, Schwinn FJ (1983) Technique for long-term preservation of phytopathogenic fungi in liquid nitrogen. Phytopathology 73:241–246
Dietz S, Büdel B, Lange OL, Bilger W (2000) Transmittance of light through the cortex of lichens from contrasting habitats. Bibl Lichenol 75:171–182
Fernández-Marín B, Becerril JM, García-Plazaola JI (2010) Unravelling the roles of desiccation-induced xanthophyll cycle activity in darkness: a case study in Lobaria pulmonaria. Planta 231:1335–1342
Fernández-Marín B, Kranner I, San Sebastían M, Artexte U, Laza JM, Vilas JL, Pritchard HW, Nadajaran J, Míguez F, Becerril JM, García-Plazaola JI (2013) Evidence for the absence of enzymatic reactions in the glassy state. A case study of xanthophyll cycle pigments in the desiccation-tolerant moss Syntrichia ruralis. J Exp Bot 64:3033–3043
Franks F, Hatley RHM, Mathias S (1991) Materials science and the production of shelf-stable biologicals. Biopharmaceuticals 4:38–42
Gauslaa Y, Solhaug KA (2000) High-light-intensity damage to the foliose lichen Lobaria pulmonaria within natural forest: the applicability of chlorophyll fluorescence methods. Lichenologist 32:271–289
Gauslaa Y, Coxson DS, Solhaug KA (2012) The paradox of higher light tolerance during desiccation in rare old forest cyanolichens than in more widespread co-occurring chloro- and cephalolichens. New Phytol 195:812–822
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Gerotto C, Alboresi A, Giacometti GM, Bassi R, Morosinotto T (2011) Role of PSBS and LHCSR in Physcomitrella patens acclimation to high light and low temperature. Plant Cell Environ 34:922–932
Gilmore AM, Hazlett TL, Govindjee (1994) Xanthophyll cycle-dependent quenching of photosystem II chlorophyll a fluorescence: formation of a quenching complex with a short fluorescent lifetime. Proc Natl Acad Sci USA 92:2273–2277
Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Gray DW, Lewis LA, Cardon ZG (2007) Photosynthetic recovery following desiccation of desert green algae (Chlorophyta) and their aquatic relatives. Plant Cell Environ 30:1240–1255
Heber U (2008) Photoprotection of green plants: a mechanism of ultra-fast thermal energy dissipation in desiccated lichens. Planta 228:641–650
Heber U, Azarkovich M, Shuvalov V (2007) Activation of mechanisms of photoprotection by desiccation and by light: poikilohydric photoautotrophs. J Exp Bot 58:2745–2759
Honegger R (1991) Functional aspects of the lichen symbiosis. Annu Rev Plant Biol 42:553–578
Honegger R (2003) The impact of different long term storage conditions on the viability of lichen-forming ascomycetes and their green algal photobiont, Trebouxia spp. Plant Biol 5:324–330
Kosugi M, Arita M, Shizuma R, Moriyama Y, Kashino Y, Koike H, Satoh K (2009) Responses to desiccation stress in lichens are different from those in their photobionts. Plant Cell Physiol 50:879–888
Kosugi M, Kashino Y, Satoh K (2010) Comparative analysis of light response curves of Ramalina yasudae and freshly isolated Trebouxia sp. revealed the presence of intrinsic protection mechanisms independent of upper cortex for the photosynthetic system of algal symbionts in lichen. Lichenology 9:1–10
Kosugi M, Miyake H, Yamanakawa H, Shibata Y, Miyazawa A, Sugimura T, Satoh K, Itoh S, Kashino Y (2013) Arabitol provided by lichenous fungi enhances ability to dissipate excess light energy in symbiotic green alga under desiccation. Plant Cell Physiol 54:1316–1325
Kranner I, Cram JW, Zorn M et al (2005) Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. Proc Natl Acad Sci USA 102:3141–3146
Kranner I, Beckett RP, Hochman A, Nash TH III (2008) Desiccation tolerance in lichens: a review. Bryologist 111:576–593
Lange OL (1953) Hitze- und Trockenresistenz der Flechten in Beziehung zu ihrer Verbreitung. Flora 140:39–47
Lange OL, Pfanz H, Killian E, Mayer A (1990) Effect of low water potential on photosynthesis in intact lichens and their liberated algal components. Planta 182:467–472
Lazár D, Nauš J (1998) Statistical properties of chlorophyll fluorescence induction parameters. Photosynthetica 35:121–127
Leliaert F, Smith DR, Moreau H, Herron MD, Verbruggen H, Delwiche CF, De Clerck O (2012) Phylogeny and molecular evolution of the green algae. Crit Rev Plant Sci 31:1–46
Lüttge U, Büdel B (2010) Resurrection kinetics of photosynthesis in desiccation-tolerant terrestrial green algae (Chlorophyta) on tree bark. Plant Biol 123:437–444
MacKenzie TDB, Król M, Huner NPA, Campbell DA (2002) Seasonal changes in chlorophyll fluorescence quenching and the induction and capacity of the photoprotective xanthophyll cycle in Lobaria pulmonaria. Can J Bot 80:255–261
Malloch DW, Pirozynski KA, Raven PH (1980) Ecological and evolutionary significance of mycorrhizal symbioses in vascular plants (a review). Proc Natl Acad Sci USA 77:2113–2118
Matthes-Sears U, Gerrath JA, Gerrath JF, Larson DW (1999) Community structure of epilithic and endolithic algae and cyanobacteria on cliffs of the Niagara Escarpment. J Veg Sci 10:587–598
Minibayeva F, Beckett RP (2001) High rates of extracellular superoxide production in bryophytes and lichens, and an oxidative burst in response to rehydration following desiccation. New Phytol 152:333–341
Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Nardini A, Marchetto A, Tretiach M (2013) Water relation parameters of six Peltigera species correlate with their habitat preferences. Fungal Ecol 6:397–407
Nash TH III (2008) Lichen biology, 2nd edn. Cambridge University Press, New York
Nash TH III, Reiner A, Demmig-Adams B, Kilian E, Kaiser WM, Lange OL (1990) The effect of osmotic water stress on photosynthesis and dark respiration of lichens. New Phytol 116:269–276
Nimis PL, Martellos S (2008) ITALIC—the information system on Italian lichens. Version 4.0. http://dbiodbs-univ.trieste.it. Accessed 1 Apr 2012
Pellegrini E, Bertuzzi S, Candotto Carniel F, Lorenzini G, Nali C, Tretiach M (2014) Ozone tolerance in lichens: a possible explanation from biochemical to physiological level using Flavoparmelia caperata as test organism. J Plant Physiol 171:1514–1523
Piccotto M, Tretiach M (2010) Photosynthesis in chlorolichens the influence of the habitat light regime. J Plant Res 123:763–775
Roháček K (2002) Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 40:13–29
Scherrer S, De Vries OMH, Dudler R, Wessels JGH, Honegger R (2000) Interfacial self-assembly of fungal hydrophobins of the lichen-forming ascomycetes Xanthoria parietina and X. ectaneoides. Fungal Genet Biol 30:81–93
Schofield SC, Campbell DA, Funk C, MacKenzie TDB (2003) Changes in macromolecular allocation in nondividing algal symbionts allow for photosynthetic acclimation in the lichen Lobaria pulmonaria. New Phytol 159:709–718
Solhaug KA, Gauslaa Y (1996) Parietin, a photoprotective secondary product of the lichen Xanthoria parietina. Oecologia 108:412–418
Štepigová J, Gauslaa Y, Cempírková-Vráblíková H, Solhaug KA (2008) Irradiance prior to and during desiccation improves the tolerance to excess irradiance in the desiccated state of the old forest lichen Lobaria pulmonaria. Photosynthetica 46:286–290
Tretiach M, Adamo P, Bargagli R, Baruffo L, Carletti L, Crisafulli P, Giordano S, Modenesi P, Orlando S, Pittao E (2007a) Lichen and moss bags as monitoring devices in urban areas. Part I: influence of exposure on sample vitality. Environ Pollut 146:380–391
Tretiach M, Piccotto M, Baruffo L (2007b) Effect of ambient NOx on chlorophyll a fluorescence in transplanted Flavoparmelia caperata (Lichen). Environ Sci Technol 46:2978–2984
Tschermak-Woess E (1989) Developmental studies in trebouxioid algae and taxonomical consequences. Plant Syst Evol 164:161–195
Vráblíková H, Barták M, Wonish A (2005) Changes in glutathione and xanthophyll cycle pigments in the high light-stressed lichens Umbilicaria antartica and Lasallia pustulata. J Photochem Photobiol, B 79:35–41
Weissman L, Garty J, Hochman A (2005) Rehydration of the lichen Ramalina lacera results in production of reactive oxygen species and nitric oxide and a decrease in antioxidants. Appl Environ Microbiol 71:2121–2129
Wellburn AR (1964) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Yamamoto M, Kinoshita Y, Yoshimura I (2002) Photobiont culturing. In: Kranner I, Beckett RP, Varma AK (eds) Protocols in lichenology. Culturing, biochemistry, ecophysiology and use in biomonitoring. Springer, Berlin, pp 34–42
Zia A, Johnson MP, Ruban AV (2011) Acclimation- and mutation-induced enhancement of PsbS levels affects the kinetics of non-photochemical quenching in Arabidopsis thaliana. Planta 233:1253–1264
Acknowledgments
We thank A. Montagner for help in the laboratory, G. Baj for assistance at the confocal microscopy, D. Kodnik for field work, L. Muggia for assessing the phylogenetic position of our photobiont and P. Crisafulli for the culture inclusions. A special thanks goes to Professor I. Kranner (University of Innsbruck) for the critical comments to the manuscript and the constructive discussion about this work. This study was supported by the Italian Ministry of Education, University and Research (20082WWM9A to M.T.), and by the University of Trieste (F.R.A. 2011 to M.T.).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special topic: Desiccation Biology.
Guest editors: Olivier Leprince and Julia Buitink.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Candotto Carniel, F., Zanelli, D., Bertuzzi, S. et al. Desiccation tolerance and lichenization: a case study with the aeroterrestrial microalga Trebouxia sp. (Chlorophyta). Planta 242, 493–505 (2015). https://doi.org/10.1007/s00425-015-2319-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2319-z