Abstract
In order to survive sunlight in the absence of water, desiccation-tolerant green plants need to be protected against photooxidation. During drying of the chlorolichen Cladonia rangiformis and the cyanolichen Peltigera neckeri, chlorophyll fluorescence decreased and stable light-dependent charge separation in reaction centers of the photosynthetic apparatus was lost. The presence of light during desiccation increased loss of fluorescence in the chlorolichen more than that in the cyanolichen. Heating of desiccated Cladonia thalli, but not of Peltigera thalli, increased fluorescence emission more after the lichen had been dried in the light than after drying in darkness. Activation of zeaxanthin-dependent energy dissipation by protonation of the PsbS protein of thylakoid membranes was not responsible for the increased loss of chlorophyll fluorescence by the chlorolichen during drying in the light. Glutaraldehyde inhibited loss of chlorophyll fluorescence during drying. Desiccation-induced loss of chlorophyll fluorescence and of light-dependent charge separation are interpreted to indicate activation of a highly effective mechanism of photoprotection in the lichens. Activation is based on desiccation-induced conformational changes of a pigment–protein complex. Absorbed light energy is converted into heat within a picosecond or femtosecond time domain. When present during desiccation, light interacts with the structural changes of the protein providing increased photoprotection. Energy dissipation is inactivated and structural changes are reversed when water becomes available again. Reversibility of ultra-fast thermal dissipation of light energy avoids photo-damage in the absence of water and facilitates the use of light for photosynthesis almost as soon as water becomes available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Active life depends on the presence of water. In higher plants, desiccation tolerance of metabolically active organs is rare. Only about 350 of more than 235,000 species of vascular plants have been found to survive desiccation (Raven et al. 1992; Proctor and Tuba 2002). In lower plants, the situation is different. Within some 9,500 species of mosses, more than 10% are desiccation-tolerant (Alpert 2000). Among the about 13,500 species of lichens, desiccation-tolerance is common. Almost immediately after water has become available, lichens resume active metabolism (Aubert et al. 2007). Chlorophyll fluorescence increases in response to hydration. Photosynthetic pigments harvest light and convert the energy of light quanta into redox energy by creating a strong oxidant and a strong reductant in specialized reaction centers according to
where P represents a reactive chlorophyll dimer within the reaction center of PS II and Pheo the tetrapyrrol pigment pheophytin. During active metabolism, which requires the presence of water, redox energy is stabilized in reactions following charge separation in PSII and PSI. Energy is stored in the form of oxidizable carbohydrates and other organic compounds. However, when water is absent, charge separation is either not possible or is followed by charge recombination that reverses Eq. 1. Spin reversal during charge recombination yields long-lived excited triplet 3P680 in PSII reaction centers which, by reacting with oxygen, gives rise to highly destructive singlet oxygen according to
(Krieger-Liszkay 2005). Significant formation of singlet oxygen in strong light threatens survival of photosynthetic organisms.
In 1987, Barbara Demmig recognized that the xanthophyll pigment zeaxanthin is important for the photoprotection of many hydrated plants by facilitating the controlled radiationless dissipation of excess light energy as heat (Demmig-Adams 1990). In this way, formation of destructive radicals is minimized. Activation of this mechanism of energy dissipation requires in addition to the presence of zeaxanthin the protonation of a special protein, the PsbS protein, in the thylakoid membrane of the photosynthetic apparatus (Li et al. 2004; Takizawa et al. 2007). Protons for protonation are provided by light-dependent electron transport, but are also consumed during the synthesis of the ATP that is needed for carbon reduction. The preferential channeling of protons into ATP synthesis favors photosynthesis over energy dissipation (Heber et al. 2006a). Only excess protons are diverted to the PsbS protein. Dissociation of protons from the protonated protein in low light or darkness inactivates zeaxanthin-dependent energy dissipation.
Importantly, the sensitive regulation by a light-dependent protonation reaction raises questions regarding the role of zeaxanthin-dependent energy dissipation to protect photosynthetic organisms against photooxidation when water is absent. Light is not always present when organisms dry out. Also, sensitive regulation ascertains that energy dissipation does not compete with energy conservation in photosynthesis. As long as photoinhibition by excess light can be avoided, zeaxanthin-dependent energy dissipation proceeds simultaneously with, and does not inhibit, photosynthetic energy conservation in hydrated plants.
As water is lost from poikilohydric autotrophs, photosynthesis decreases as does stable charge separation (Heber et al. 2006a). Fluorescence also decreases. Hydration increases fluorescence. It also re-establishes stable charge separation. At first sight, quenching of fluorescence during desiccation might be viewed as a simple consequence of drying chlorophyll-containing photosynthetic membranes. However, light use for photosynthesis, fluorescence emission and radiationless energy dissipation are, according to the first law of thermodynamics, in competition to one another. Owing to this, the concurrent loss of charge separation and fluorescence during drying actually indicates activation of energy dissipation. Conversely, the increased fluorescence caused by hydration shows inactivation of energy dissipation.
In recent publications, evidence has been presented for new mechanisms of energy dissipation which protect desiccated mosses and lichens against photoinactivation (Heber and Shuvalov 2005; Heber et al. 2007; Veerman et al. 2007). These mechanisms do not require a protonation reaction for activation of energy dissipation. The present communication shows that a main mechanism which does not require light for activation is based on desiccation-induced conformational changes of a chlorophyll protein complex. By permitting ultrafast thermal energy dissipation within the pigment–protein complex, functional reaction centers are deprived of energy and thereby protected against photoinactivation. When light is present during desiccation, the conformational changes are modified so as to provide increased photoprotection particularly to desiccated chlorolichens.
Material and methods
The fruticose lichen Cladonia rangiformis Hoffm. (Cladoniaceae) was obtained from a sun-exposed habitat on calcareous soil near Leinach, 20 km from Würzburg, Germany, and the foliose chlorolichen Parmelia sulcata Ach. (Parmeliaceae) from the bark of trees in the Botanical Garden of the University of Würzburg. The cyanolichen Peltigera neckeri Hepp ex Müll. Arg. (Peltigeraceae) was from a shaded site under trees in the Botanical Garden of the University of Würzburg. Dark adaptation of hydrated lichens (intended to minimize or eliminate zeaxanthin by its conversion to violaxanthin) was achieved by exposing thalli for prolonged times (several hours to a few days) to darkness before drying them either rapidly or slowly in the dark. Rapid drying was done by placing the superficially dried hydrated organisms into a desiccator over silica gel or P2O5. This was followed by rapid evacuation. Alternatively, fast drying was also achieved by directing a stream of dry air at about 30°C over the hydrated lichens. A thermocouple was used to follow drying by recording the decrease and subsequent increase of temperature of a desiccating sample. Slow drying was done at room temperature in air of a relative humidity below 60% or a water potential below −70 MPa either in darkness or under conditions of near-darkness, while fluorescence was recorded in the presence of a very low intensity modulated measuring beam of an averaged photosynthetically active photon flux density PPFD = 0.02 or 0.04 μmol m−2 s−1. Absence of zeaxanthin-dependent energy dissipation after prolonged incubation of hydrated lichens in darkness or near-darkness was checked by making sure that quenching of basal or F o chlorophyll fluorescence was absent immediately after illumination with strong light pulses (Katona et al. 1992; Heber et al. 2007). Modulated chlorophyll fluorescence was measured after excitation at about 650 nm as fluorescence emission beyond 700 nm (using the far-red transmitting filter RG 9 of Schott, Mainz, Germany) by the pulse amplitude modulation fluorometer 101 (PAM, Walz, Effeltrich, Germany) (Schreiber et al. 1986). Short pulses (usually 1 s) of white light (filters: Calflex c and DT-Cyan of Balzers, Liechtenstein) from a halogen lamp (KL 1500 electronic, Schott) were brought to the cuvette by fiber optics to probe for ΔF = F m − F o (see Fig. 1), which originates from PSII reaction centers. The PPFD of the light pulses was usually 11,000 or 12,000 μmol m−2 s−1 (equivalent to six or seven times full sunlight) but occasionally only 2 or 4 μmol m−2 s−1, when high light fluxes had to be avoided (see legends to figures). Whenever necessary, temperature of the samples was monitored by a thermocouple. Light-dependent absorption changes at 800 nm were measured in reflection using the PAM instrument (Walz) in combination with an ED800 T emitter/detector unit. This attachment was modified for the measurement of absorption changes at 950 nm by replacing the original LED of the emitter/detector unit with a LED with peak emission at 950 nm.
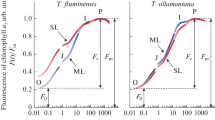
Modulated chlorophyll fluorescence of the chlorolichen Cladonia rangiformis (a) and the cyanolichen Peltigera neckeri (b) before, during and after two hydration phases. Fluorescence was elicited by extremely low modulated light (PPFD = 0.004 μmol m−2 s−1). During the first hydration, continuous actinic light was absent. Stationary fluorescence increased on addition of water to a level termed Fo. Initially, strong light pulses (1 s; PPFD = 11,000 μmol m−2 s−1, equivalent to seven times full sunlight) were given every 500 s. They increased fluorescence transiently to maximum levels, termed Fm. Later, they were replaced by weak pulses (1 s; PPFD = 2 μmol m−2 s−1; see smaller fluorescence peaks). During the second hydration, actinic light of PPFD = 300 μmol m−2 s−1 was present throughout. All short light pulses were now strong. For further information, see text
Glutaraldehyde and the protonophore nigericin were obtained from Sigma-Aldrich/Fluka (Seelze, Germany).
Results
Figure 1 shows responses of chlorophyll fluorescence of two lichens to hydration and desiccation. One of the lichens, Cladonia rangiformis, is associated with a green alga as the photobiont, the other, Peltigera neckeri, with a cyanobacterium. A first hydration/desiccation cycle was performed under conditions of near darkness to avoid activation of light-requiring mechanisms of photoprotection. During a second hydration/desiccation cycle, continuous background actinic illumination was present to permit activation of light-dependent mechanisms of energy dissipation. Pulses of either strong or very weak light lasting 1 s each were given every 500 s to check for light-dependent fluorescence responses which reveal charge separation according to Eq. 1. Such responses were suppressed while the lichens were dry. After hydration, fluorescence increased. Pulse-induced transient increases in fluorescence revealed charge separation. Small post-illumination fluorescence quenching was seen immediately after strong light pulses only in the chlorolichen Cladonia during the first hydration and not in the cyanolichen Peltigera. Cyanolichens are known to lack the zeaxanthin-dependent mechanism of energy dissipation, which is present in chlorolichens (Demmig-Adams et al. 1990). The post-illumination F o quenching following strong light pulses during the first hydration in the Cladonia experiment in Fig. 1 indicates activity of zeaxanthin-dependent energy dissipation (Katona et al. 1992; Heber et al. 2007). The quinone acceptor QA in the reaction center of photosystem II was oxidized under these conditions. Quenching seen during the second hydration in both the lichens immediately after light pulses does not reflect F o quenching but rather post-illumination oxidation of reduced QA. Ratios of maximum pulse-induced fluorescence \( F_{{{\text{m}}_{{{\text{hydrated}}}} }} \) to \( F_{{{\text{o}}_{{{\text{desiccated}}}} }} \) were often higher than 20 in Cladonia, but were generally lower in Peltigera. Different ratios are indicative of differences in the extent of photoprotection (Heber et al. 2007).
Fluorescence of desiccated Cladonia was lower before than after the first hydration/desiccation cycle (Fig. 1a). It was low again after the second cycle. Such differences were much smaller, but still noticeable in the case of the cyanolichen Peltigera (Fig. 1b). Apparently, slow desiccation in near darkness had been less effective to decrease fluorescence (i.e., to activate energy dissipation) in Cladonia than desiccation in the field or under illumination. After the second hydration/desiccation cycle in the presence of light, residual fluorescence of Cladonia was below than that seen after the first cycle by a factor of two.
Since the recordings of Fig. 1 document single observations, they raise questions regarding generalization and reproducibility. In a set of parallel experiments with different materials collected in the field, hydrated lichens were after prolonged preincubation in darkness either dried slowly in darkness or under illumination. The results are shown in Table 1. Not surprisingly, they reveal considerable variability of fluorescence emission in different experiments with material of different origin, but confirm that fluorescence is quenched more when desiccation proceeds under illumination than in darkness. They also show that desiccation in the light causes more fluorescence quenching in the chlorolichen Cladonia than in the cyanolichen Peltigera.
Figure 2a shows inhibition of desiccation-induced fluorescence quenching by glutaraldehyde, a crosslinking agent used as a fixative in electron microscopy. Hydrated Cladonia had been predarkened and then incubated in 0.25% glutaraldehyde. Light-dependent charge separation was decreased by glutaraldehyde, but not fully inhibited. In strong contrast to the desiccation-induced quenching of fluorescence shown in the experiments in Fig. 1, loss of water during drying did not result in fluorescence quenching when glutaraldehyde was present. Because glutaraldehyde is capable of reacting with proteins of the photosynthetic electron transport system (Coughlan and Schreiber 1984), loss of desiccation-induced energy dissipation in the presence of glutaraldehyde strongly suggests conformational changes of a protein as the basis of desiccation-induced energy dissipation.
a Modulated chlorophyll fluorescence of the chlorolichen Cladonia rangiformis after incubation with 0.25% glutaraldehyde. Strong light pulses PPFD = 11,000 μmol m−2 s−1were given every 500 s. Desiccation did not quench fluorescence. b Modulated chlorophyll fluorescence of the chlorolichen Cladonia rangiformis after hydration with 5 μM nigericin. Arrows indicate reduction in the sensitivity of recording. Strong light pulses of PPFD = 11,000 μmol m−2 s−1 were given every 500 s. Desiccation quenched fluorescence. In a, fluorescence was excited by extremely low modulated light (PPFD = 0.004 μmol m−2 s−1); in b by PPFD = 2.5 μmol m−2 s−1
In attempts to find out whether the effect of light on desiccation-induced fluorescence quenching, as shown in Fig. 1a and Table 1, is caused by the activation of zeaxanthin-dependent energy dissipation, inhibitors known to interfere with zeaxanthin-dependent energy dissipation were used. Dithiothreitol inhibits zeaxanthin formation from violaxanthin (Yamamoto and Kamite 1972). It proved to be ineffective to inhibit the light-dependent part of desiccation-induced fluorescence quenching in Cladonia (data not shown). The protonophore nigericin inhibits not only de-epoxidation of violaxanthin to zeaxanthin but also the protonation of the PsbS protein. Cladonia that had been hydrated in darkness to permit inactivation of zeaxanthin-dependent energy dissipation was desiccated in darkness to activate only the mechanism of desiccation-induced energy dissipation, which does not require the presence of light. Subsequently, the lichen was hydrated with a 5 μM solution of the protonophore nigericin. It was then slowly dried under illumination with PPFD of 300 μmol m−2 s−1. Desiccation still caused extensive fluorescence quenching (Fig. 2b). The ratio of \( F_{{{\text{m}}_{{{\text{hydrated}}}} }} \) to \( F_{{{\text{o}}_{{{\text{desiccated}}}} }} \) was 20. This is very similar to the \( {\raise0.7ex\hbox{${F_{{{\text{m}}_{{{\text{hydrated}}}} }} }$} \!\mathord{\left/ {\vphantom {{F_{{{\text{m}}_{{{\text{hydrated}}}} }} } {F_{{{\text{o}}_{{{\text{desiccated}}}} }} }}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${F_{{{\text{o}}_{{{\text{desiccated}}}} }} }$}} \) ratio in the Cladonia experiment of Fig. 1 after the second hydration/desiccation cycle. Thus, dithiothreitol and nigericin experiments failed to explain the effect of light on desiccation-induced fluorescence quenching as activation of zeaxanthin-dependent energy dissipation.
Also, no evidence was obtained for a close kinetic relationship between light-dependent quenching of chlorophyll fluorescence and light-dependent absorption changes at around 800 nm (caused by chlorophyll radicals) or 950 nm (attributable to carotenoid radicals), which would be expected if the light effect on desiccation-induced fluorescence quenching is based on the light-dependent formation of a radical quencher. A close kinetic relationship between fluorescence quenching and 800 nm absorption changes had been observed during desiccation of a poikilohydric moss (Heber et al. 2006b; see also Holt et al. 2005).
Heating experiments might give information on the effect of light on fluorescence quenching during desiccation. Desiccated Cladonia, which had been dried either in the dark (Fig. 3a) or in the light (Fig. 3b; PPFD 130 μmol m−2 s−1), was first cooled to below room temperature. It was then heated (Fig. 3). Fluorescence responded to increasing temperature. Two phases of increased fluorescence are discernable. A first small phase was followed by a steep increase in fluorescence emission. Initially, the second increase in fluorescence was fully reversible when the temperature was lowered again (Heber and Shuvalov 2005). Fluorescence emission became irreversible as the temperature approached 80°C.
Importantly, the heat-induced increase in fluorescence was smaller, as shown in Fig. 3a when compared to Fig. 3b. Figure 1a and Table 1 had shown that quenching of fluorescence during desiccation was larger when Cladonia was dried in the light than in darkness. It thus appears that heating was capable of reversing the stronger fluorescence quenching that had occurred during slow desiccation of Cladonia in the light.
Effects of heating on fluorescence emission, as shown in Fig. 3, for Cladonia were also observed with other chlorolichens such as Parmelia sulcata (data not shown). Always slow desiccation in the presence of light increased quenching when compared to slow drying in darkness. Also, in all cases fluorescence emission during heating of desiccated chlorolichens, which had been dried under illumination, was larger than when the lichens had been dried in darkness.
The cyanolichen Peltigera appeared to be somewhat different from chlorolichens in its response to heating. Although heating increased fluorescence also of desiccated Peltigera (starting from about 50°C; data not shown), the increase was not appreciably different whether the thalli were dried in darkness or under illumination. It has already been shown in Fig. 1b and in Table 1 that desiccation-induced fluorescence quenching was not very different when Peltigera was dried in darkness or under illumination.
Lichens respond to strong light even when desiccated. Figure 4 shows effects of three very strong light pulses on chlorophyll fluorescence of desiccated Cladonia, which had been dried slowly either in darkness (Fig. 4a) or in light (Fig. 4b). During short illumination of a thallus, which had been dried in the dark, a quencher accumulated (Fig. 4a). Darkening reversed the light reaction only partially. In thalli, which had been dried in the light, quencher formation was either much reduced (not shown) or absent. Even small light-dependent increases in fluorescence were observed (Fig. 4b) revealing charge separation followed by some reduction of the quinone acceptor QA in the reaction center of PSII. Light-dependent QA reduction was also seen as reversible light-dependent fluorescence increase in the chlorolichen Parmelia sulcata after thalli had been desiccated in the light (not shown, but see Heber et al. 2007).
Effect of three consecutive illumination periods of 20 s with PPFD = 11,000 μmol m−2 s−1 on modulated chlorophyll fluorescence of desiccated Cladonia rangiformis. a The predarkened hydrated lichen had been slowly desiccated in darkness. b The predarkened hydrated lichen had been slowly desiccated under continuous actinic illumination with PPFD = 300 μmol m−2 s−1. Scales indicate changes in fluorescence in percent of the level of fluorescence remaining after desiccation
In desiccated Peltigera, strong actinic illumination produced small reversible quenching responses similar to those shown in Fig. 4a for Cladonia, whether or not the Peltigera thalli had been dried in dark or light.
After the experiment in Fig. 2a had shown that activation of the desiccation-induced mechanisms of energy dissipation by desiccation can be fully inhibited by glutaraldehyde, a somewhat less drastic and perhaps more physiological method to interfere with energy dissipation than shown in the heating experiments of Fig. 3, is demonstrated in Fig. 5. Predarkened lichens were dried rapidly in vacuo (Heber et al. 2007) or in a stream of dry air (data not shown). Fast drying resulted in less fluorescence quenching than slow drying. Initial fluorescence levels were therefore higher in rapidly dried Cladonia (Fig. 5a) than in slowly dried thalli (Fig. 5b). Similar relations were true for Peltigera (Fig. 5c vs. d). During exposure of rapidly dried lichens to strong light, equivalent to six times full sunlight, more fluorescence was lost (Fig. 5a, c) than that in slowly dried control samples (Fig. 5b, d; see also Table 2). Irreversible loss of fluorescence is considered as a sign of light-induced radical damage to the photosynthetic apparatus. Darkening reversed a small part of the loss of fluorescence indicating some reversibility of quencher formation. Hydration increased fluorescence strongly by inactivating desiccation-induced energy dissipation. However, the extent of the recovery of fluorescence during hydration differed between rapidly and slowly dried lichens. Damage to reaction centers of photosystem II was indicated by the loss of light-induced charge separation as indicated by decreased (F m − F o)/F m ratios in Table 2. It was almost complete in the cyanolichen Peltigera, but was less pronounced in Cladonia.
Modulated chlorophyll fluorescence of the desiccated chlorolichen Cladonia rangiformis (a, b) and of the desiccated cyanolichen Peltigera neckeri (c, d) before and after a 25 min illumination period with PPFD = 11,000 μmol m−2 s−1. The hydrated predarkened lichens had been dried in darkness either rapidly in vacuo (a, c) or slowly in air (b, d). After illumination of the desiccated thalli (25 min) and subsequent darkening (10 min), water was added. Fluorescence increased after a small decline (which had optical reasons). Sensitivity of measurements was reduced as indicated by arrows by a factor of about 21 (a and b) and of 5.2 (c and d) in order to follow the time course of fluorescence. Sensitivity of recordings was identical in a and b and also in c and d: slow drying (b, d) increased quenching and protection of reaction centers against photoinactivation. After hydration, strong short light pulses were given every 300 s to monitor light-dependent charge separation
When light-stress experiments, such as shown in Fig. 5, were done not in air but under nitrogen, irreversible loss of fluorescence was much reduced. There was less damage to photosystem II reaction centers (data not shown), confirming that the activation of oxygen is an important, although not the only, aspect of photoinduced damage (see also Heber et al. 2007).
Discussion
The significance for photoprotection of the loss of chlorophyll fluorescence and of stable charge separation during desiccation of lichens can best be understood by a consideration of relative rates of competitive reactions involved in energy conservation or energy dissipation. For the sake of simplicity, it is assumed in the following argument that time scales of energy migration within the pigment body of the photosynthetic apparatus and of energy trapping in functional reaction centers are not very different in hydrated and desiccated lichens.
In very low light, photosynthesis of hydrated plants is known to have maximum quantum efficiency. Zeaxanthin-dependent energy dissipation is absent and fluorescence emission represents less than 1% of absorbed light energy. This means that energy capture by reaction centers accounts for 99% or more of absorbed light energy. Trapping of energy and subsequent charge separation in the reaction centers have been reported to take place within 1–3 ps (Zinth and Kaiser 1993; Holzwarth et al. 2006). As fluorescence and energy conservation compete directly for absorbed light energy when energy dissipation is not active, the level of Fo fluorescence seen under very low light is therefore in quasi-equilibrium with and indicative of effective energy trapping within 1–3 ps.
On hydration of desiccated lichens, fluorescence increases and light-dependent charge separation becomes active revealing progressive inactivation of desiccation-induced thermal energy dissipation (Fig. 1). After energy dissipation has been lost, the level of Fohydrated now indicates energy trapping within a picosecond time domain. Fluorescence of desiccated lichens far below Fohydrated not only indicates effective energy dissipation, but also shows that energy dissipation in newly formed dissipation centers is faster than energy capture by open reaction centers, that is, faster than the trapping time of a few picoseconds within the reaction centers. Faster energy trapping in dissipation centers deprives open reaction centers of energy. It is a main basis of protection against photoinactivation by the formation of singlet oxygen according to Eqs. 1 and 2 in the reaction centers or elsewhere. Very strong fluorescence suppression as shown by \( {\raise0.7ex\hbox{${F_{{{\text{o}}_{{{\text{hydrated}}}} }} }$} \!\mathord{\left/ {\vphantom {{F_{{{\text{m}}_{{{\text{hydrated}}}} }} } {F_{{{\text{o}}_{{{\text{desiccated}}}} }} }}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${F_{{{\text{o}}_{{{\text{desiccated}}}} }} }$}} \) ratios close to 10 or more in Cladonia then indicate energy dissipation in the subpicosecond, that is, in the femtosecond time domain. This results in photoprotection of reaction centers.
The above argument remains valid even if time scales and of energy trapping in reaction centers of energy migration change during desiccation as suggested by Bilger et al. (1989). Ratios of energy trapping in reaction centers to trapping in dissipation centers are important, not absolute reaction rates. The ratios change during desiccation by the activation of energy dissipation.
After Heber and Shuvalov (2005) had suggested a low energy form of a chlorophyll species to act as long-wavelength fluorescence quencher in desiccated lichens and mosses, Veerman et al. (2007) have recently shown that the excited state lifetime of a quencher, which weakly emits at 740 nm is shortened during desiccation of the lichen Parmelia by a factor of about 8. Assuming that energy trapping in functional reactions centers takes about 300 ps and not about 3 ps as reported by Zinth and Kaiser (1993) and Holzwarth et al. (2006), the authors consider the observed fluorescence lifetime of 40 ps sufficient for effective photoprotection. Their argument is similar to that developed above: trapping of light energy in dissipation centers, if faster than trapping in reaction centers, deprives the latter of energy thereby providing protection.
The very small reversible increase in the residual fluorescence displayed by light-dried desiccated Cladonia (Fig. 4b) and the stronger increase observed in desiccated Parmelia (Heber et al. 2007) in response to very strong illumination reveal charge separation in fully protected functional reaction centers with some reduction of the quinone acceptor QA. The quenching of fluorescence by strong illumination in dark-dried desiccated Cladonia shows the formation of radicals in less protected reaction centers that act as quenchers of fluorescence (Fig. 4a). Such quenching has also been observed in the cyanolichen Peltigera (not shown) and the moss Rhytidiadelphus squarrosus (Heber et al. 2006b). It may be the result of a janus-headed reaction. As a quencher, a radical may contribute to photoprotection. As a reactive radical, it could contribute to damage. Radical formation is indicated by light-dependent reversible changes in 800 and 950 nm absorption (not shown, but see carotenoid oxidation in desiccated leaves; Heber et al. 2006a).
Differences between \( F_{{{\text{o}}_{{{\text{desiccated}}}} }} \) after desiccation in near-darkness (first hydration/desiccation cycle) and after desiccation under illumination (second hyration/desiccation cycle) as shown particularly for Cladonia in Fig. 1 and Table 1 suggest the existence of more than one mechanism of photoprotection. The Quantum yield of fluorescence F may be written as
where k values denote reaction constants for different competing reactions. F stands for fluorescence, EC for energy conservation and ED1 for the mechanism of energy dissipation which does not require light for activation. ED2 denotes quenching based on a light reaction. Conditions for the activation ED1 are provided only during the first hydration/desiccation cycle (Fig. 1), conditions for activation of all mechanisms including light-requiring mechanisms were present during the second hydration/desiccation cycle. In the desiccated state, k′EC can be neglected because charge separation is not noticeable under sunlight conditions. The same is true for k′′′ED2 after the first hydration/desiccation cycles. Only ED1 had been active. The ratio of \( {\raise0.7ex\hbox{${F_{{{\text{m}}_{{{\text{hydrated}}}} }} }$} \!\mathord{\left/ {\vphantom {{F_{{{\text{m}}_{{{\text{hydrated}}}} }} } {F_{{{\text{o}}_{{{\text{desiccated}}}} }} }}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${F_{{{\text{o}}_{{{\text{desiccated}}}} }} }$}} \) had been 9.6 in Cladonia and, initially, 14.3 in Peltigera after the first hydration/desiccation cycle. With both k′′ED1 and k′′′ED2 active after the end of the second cycle, \( {\raise0.7ex\hbox{${F_{{{\text{m}}_{{{\text{hydrated}}}} }} }$} \!\mathord{\left/ {\vphantom {{F_{{{\text{m}}_{{{\text{hydrated}}}} }} } {F_{{{\text{o}}_{{{\text{desiccated}}}} }} }}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${F_{{{\text{o}}_{{{\text{desiccated}}}} }} }$}} \) had been 20.4 in Cladonia and 16.2 in Peltigera. This means that ED1 which did not require light activation had been responsible for about half of the photoprotection observed under illumination conditions in Cladonia, whereas ED2 was responsible for the other half. In Peltigera, which does not possess the zeaxanthin-dependent mechanisms of energy dissipation (Demmig-Adams et al. 1990), about 90% of photoprotection was attributable to ED1.
In previous work (Heber et al. 2007), differences between the extent of desiccation-induced fluorescence quenching after fast and slow desiccation in combination with the results of heating experiments similar to those shown in Fig. 3 led to the conclusion that a desiccation-induced conformational change of a pigment protein close to or in the core of photosystem II is responsible for ED1. The question as to the nature of ED2 had remained unresolved. Now the heating experiments (Fig. 3) suggest a molecular basis for ED2. Heating was shown to lead to increased fluorescence emission from desiccated Cladonia and other chlorolichens. Apparently, heating had inactivated energy dissipation. It is thought to have reversed quenching by unfolding and finally inactivating the protein that is responsible for fluorescence quenching. The observation of heat sensitivity of energy dissipation is incompatible with the assumption, that ED2 is based on the formation of a quencher, such as a chlorophyll radical or carotenoid radical during desiccation in the light. Such radicals are expected to be heat-stable. Rather, the observation of heat-lability is compatible with the assumption that not only ED1 but also ED2 reflects the desiccation-induced conversion of a light-harvesting chlorophyll protein complex into an effective quencher. In fact, the kinetic similarity of the heat-induced increase in fluorescence in Fig. 3a (where ED1 is active), and in Fig. 3b (where ED2 is also active) suggests that ED2 is based on conformational changes of the same chlorophyll–protein complex which is responsible for ED1. The only difference is that in Fig. 3a loss of water is sufficient for altering the protein conformation. Figure 3b shows that the protein responds to light. This gives high flexibility to the regulation of quenching. Not much light is required. No appreciable difference was found in the activating effect on quenching during desiccation of Cladonia in the presence of PPFDs as different as 300, 130 and 25 μmol m−2 s−1 (data not shown).
Recently, Pascal et al. (2005) have described a molecular mechanism of photoprotective energy dissipation in higher plants,which involves a conformational change of the main light-harvesting protein of chloroplasts, LHCII. The transition into a dissipative state of LHCII is accompanied by a change in the configuration of bound neoxanthin (Ruban et al. 2007). Energy dissipation was facilitated by fast energy transfer from chlorophyll to low-lying excited states of carotenoids such as lutein. Although the experiments with dithiothreitol and nigericin (Fig. 2b) have failed to give evidence of appreciable participation of PsbS-regulated zeaxanthin-dependent energy dissipation in photoprotection of desiccated Cladonia, this does not rule out another role of zeaxanthin in desiccation-induced energy dissipation. In combination, the recent findings of Ruban et al. (2007) on the role of lutein in LHCII of higher plants and the results shown in Figs. 1,3 and 5 suggest that in lichens a conformational change of a chlorophyll protein brought about by desiccation permits energy transfer from 1Chl* to optically forbidden low-lying excited states of bound carotenoids thus facilitating ultra-fast energy dissipation. It has previously been suggested that the responsible protein is located within or close to the core of photosystem II where the cyanolichen Peltigera has its chlorophyll (Heber et al. 2007). Peltigera lacks the LHCII complex of higher plants.
In summary, the observations recorded in Figs. 1, 2, 3, 4 and 5 show that photoprotection of desiccated photoautotrophs, such as lichens, is largely based on desiccation-induced conformational changes of a pigment–protein complex. Removal of structural water is suggested to alter the structure of the protein so as to change the position of bound pigment molecules to one another within the protein. Quenching centers are formed. Light present during slow desiccation affects the structural changes giving considerable flexibility to the extent of fluorescence quenching under different external conditions. In fact, seasonal differences in phototolerance have been noted before in field experiments with poikilohydric photoautotrophs (Heber et al. 2006b). During desiccation, normal energy transfer to functional reaction centers is replaced by much faster energy dissipation within the pigment system of the protein complex. Reaction centers are thus deprived of energy.
Abbreviations
- F o :
-
Basal modulated chlorophyll fluorescence of hydrated photoautotrophs indcating oxidation of the primary quinone acceptor QA of PSII
- F m :
-
Maximum modulated chlorophyll fluorescence elicited by saturating light pulses indicating reduction of the primary quinone acceptor QA of PSII
- ΔF/F m :
-
(F m − F o)/F m: Quantum efficiency of stable charge separation in PSII
- PPFD:
-
Photosynthetically active photon flux density
- PSII, PSI:
-
Photosystems II or I
References
Alpert P (2000) The discovery, scope and puzzle of desiccation tolerance in plants. Plant Ecol 151:5–17
Aubert S, Juge C, Boisson A-M, Gout E, Bligny R (2007) Metabolic processes sustaining the reviviscence of lichen Xanthoria elegans (Link) in high mountain environments. Planta 226:1287–1297
Bilger W, Rimke S, Schreiber U, Lange OL (1989) Inhibition of energy transfer to photosystem II in lichens by dehydration: different properties of reversibility with green and blue–green photobionts. J Plant Physiol 134:261–268
Coughlan SJ, Schreiber U (1984) The differential effects of short-time glutaraldehyde treatments on light-induced thylakoid membrane conformational changes, proton pumping and electron transport properties. Biochim Biophys Acta 767:606–617
Demmig-Adams B (1990) Carotenoids and photoprotection of plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020:1–24
Demmig-Adams B, Máguas C, Adams WWIII, Meyer A, Kilian E, Lange OL (1990) Effect of high light on the efficiency of photochemical energy conversion in a variety of lichen species with green and blue–green phycobionts. Planta 180:400–409
Heber U, Shuvalov VA (2005) Photochemical reactions of chlorophyll in dehydrated photosystem II: two chlorophyll forms (680 and 700 nm). Photosynth Res 84:85–91
Heber U, Lange O-L, Shuvalov VA (2006a) Conservation and dissipation of light energy by plants as complementary processes involved in sustaining plant life: homoiohydric and poikilohydric autotrophs. J Exp Bot 57:1211–1223
Heber U, Bilger W, Shuvalov VA (2006b) Thermal energy dissipation in reaction centers of photosystem II protects desiccated poikilohydric mosses against photooxidation. J Exp Bot 57:2993–3006
Heber U, Azarkovich M, Shuvalov (2007) Activation of mechanisms of photoprotection by desiccation and by light: poikilohydric photoautotrophs. J Exp Bot 58: 2745–2759
Holt NE, Tigmantas D, Valkunas L, Li X-P, Niyogi KK, Fleming GR (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307:433–436
Holzwarth AR, Muller MG, Reus M, Nowazyk M, Saner J, Rogner M (2006) Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: pheophytin is the primary electron acceptor. Proc Natl Acad Sci USA 103:6895–6900
Katona E, Neimanis S, Schönknecht G, Heber U (1992) Photosystem I-dependent cyclic electron transport is important in controlling photosystem II activity in leaves under conditions of water stress. Photosynth Res 34:449–464
Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56:337–346
Li X-P, Gilmore AM, Caffari S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279:22866–22874
Pascal AA, Liu Z, Broess K, van Oort B, van Amerongen H, Wang C, Horton P, Robert B, Chang W, Ruban A (2005) Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436:134–137
Proctor MCF, Tuba Z (2002) Poikilohydry and homoihydry: antithesis or spectrum of possibilities. New Phytol 156:327–349
Raven PH, Evert RF, Eichhorn SE (1992) Biology of plants. Worth Publishers, New York
Ruban AV, Berera R, Ilioaia C, van Stokkum IHM, Kennis JTM, Pascal AA, van Amerongen H, Robert B, Horton P, van Grondelle R (2007) Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450:575–578
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Takizawa K, Cruz JA, Kanazawa A, Kramer DM (2007) The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim Biophys Acta 1767:1233–1244
Veerman J, Vasil’ev S, Paton GD, Ramanauskas J, Bruce D (2007) Photoprotection in the lichen Parmelia sulcata: The origins of desiccation-induced fluorescence quenching. Plant Physiol 145:997–1005
Yamamoto HY, Kamite L (1972) The effects of dithiothreitol on violaxanthin de-epoxidation and absorbance changes in the 500 nm region. Biochim Biophys Acta 267:538–543
Zinth W, Kaiser W (1993) Time-resolved spectroscopy of the primary electron transfer in reaction centers of Rhodopacter sphaeroides and Rhodopseudomonas viridis. In: Deisenhofer J, Norris JR (eds) The photosynthetic reaction center. Academic Press, San Diego, pp 71–88
Acknowledgments
I am grateful to anonymous reviewers who have read and criticized a first version of this contribution. Special thanks are due to reviewer 4. Professor R. Hedrich, head of the chair of Molecular Plant Physiology and Biophysics of the Julius-von-Sachs-Institute of the University of Würzburg, has provided laboratory space and facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Otto Ludwig Lange, Würzburg, on the occasion of his 80th birthday.
Rights and permissions
About this article
Cite this article
Heber, U. Photoprotection of green plants: a mechanism of ultra-fast thermal energy dissipation in desiccated lichens. Planta 228, 641–650 (2008). https://doi.org/10.1007/s00425-008-0766-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0766-5