Abstract
Main conclusion
A fungal gene encoding a transcription factor is expressed from its own promoter in Arabidopsis phloem and improves drought tolerance by reducing transpiration and increasing osmotic potential.
Horizontal gene transfer from unrelated organisms has occurred in the course of plant evolution, suggesting that some foreign genes may be useful to plants. The CtHSR1 gene, previously isolated from the halophytic yeast Candida tropicalis, encodes a heat-shock transcription factor-related protein. CtHSR1, with expression driven by its own promoter or by the Arabidopsis UBQ10 promoter, was introduced into the model plant Arabidopsis thaliana by Agrobacterium tumefaciens-mediated transformation and the resulting transgenic plants were more tolerant to drought than controls. Fusions of the CtHSR1 promoter with β-glucuronidase reporter gene indicated that this fungal promoter drives expression to phloem tissues. A chimera of CtHSR1 and green fluorescence protein is localized at the cell nucleus. The physiological mechanism of drought tolerance in transgenic plants is based on reduced transpiration (which correlates with decreased opening of stomata and increased levels of jasmonic acid) and increased osmotic potential (which correlates with increased proline accumulation). Transcriptomic analysis indicates that the CtHSR1 transgenic plants overexpressed a hundred of genes, including many relevant to stress defense such as LOX4 (involved in jasmonic acid synthesis) and P5CS1 (involved in proline biosynthesis). The promoters of the induced genes were enriched in upstream activating sequences for water stress induction. These results demonstrate that genes from unrelated organisms can have functional expression in plants from its own promoter and expand the possibilities of useful transgenes for plant biotechnology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fact that horizontal gene transfer from unrelated organisms has occurred in the course of plant evolution (Bock 2009; Talianova and Janousek 2011; Schönknecht et al. 2014) indicates that some foreign genes had been useful to plants. This also suggests that complete foreign genes, with their own promoter, may be useful for plant biotechnology, in particular genes from organisms surviving under severe stress conditions because the encoded proteins could be special for stress tolerance (Reed et al. 2013; Schönknecht et al. 2014).
Some time ago we reported that a gene from the halotolerant yeast Candida tropicalis improved salt stress tolerance in the model yeast Saccharomyces cerevisiae. This gene, named CtHSR1, up-regulated in S. cerevisiae the ENA1 gene, encoding a cation-extrusion pump important for sodium stress tolerance (Ali et al. 2001). CtHSR1 has at its N-terminus a domain of about 80 amino acids with homology to the DNA binding domain of transcription factors of the heat-shock response (HSFs) and several low-complexity regions at its C-terminus. The latter are regions characterized by a high level of amino acid (and nucleotide) sequence repetition and tend to occur in disordered regions of transcription factors that could act as activation domains (Alba et al. 2007). CtHSR1, like other HSF-related transcription factors of the Ets family (Landsman and Wolffe 1995), has no homologous regions to the trimerization and activation domains of true HSFs. HSF trimers bind to contiguous arrays (at least 2) of the 5 bp sequence XGAAX arranged in alternating orientation (heat-shock element, HSE) and activate transcription of downstream genes (Sorger 1991). The HSF-related proteins bind as monomers to the GAA trinucleotide of the HSE and activate genes without repeats of this element (Landsman and Wolffe 1995).
Considering that CtHSR1 improves tolerance to salt stress in S. cerevisiae although this yeast has no orthologous transcription factors (Ali et al. 2001), it is plausible that CtHSR1 could improve tolerance to some stresses in unrelated organisms such as plants. To test this hypothesis we transformed Arabidopsis thaliana plants with a genomic fragment containing the CtHSR1 gene and our results indicate that this fungal gene improves drought tolerance. The promoter of CtHSR1 drives expression at phloem tissues and the CtHSR1 protein localizes to the nuclei of plant cells. Physiological and transcriptomic studies indicated that CtHSR1 increases jasmonic acid concentration, reduces stomatal aperture and transpiration and increases osmotic potential and proline concentration.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana (L.), Heynh. plants (ecotype Columbia-0 or Col-0; Nottingham Arabidopsis Stock Centre, NASC N6673) were grown in the greenhouse and in vitro culture as previously described (Alejandro et al. 2007). The conditions were: 23 °C, 70 % relative humidity and 16-h light (130 µmol photons m−2 s−1)/8-h dark. Plants of Nicotiana tabacum L. (cv. Samsun, kindly donated by Prof. Angelo Spena, Verona, Italy) and Nicotiana benthamiana Domin. (kindly donated by Dr. Diego Orzaez, IBMCP, Valencia) were grown in the greenhouse under same conditions as that of Arabidopsis.
Plant transformation with a genomic fragment containing the fungal CtHSR1 gene
A 5829 bp BamHI–XbaI genomic fragment from Candida tropicalis containing the CtHSR1 gene (3003 bp of 5′-upstream promoter region, 2187 bp ORF with stop codon and 639 bp 3′-downstream region; see Ali et al. (2001) and GenBank AJ296093.1) was inserted into pBIN19 binary vector (Bevan 1984) and the recombinant plasmid (P CtHSR1 ::CtHSR1) used for transformation of Agrobacterium tumefaciens strain LBA4404 (Hoekema et al. 1983) to transform tobacco and strain GV3101::pMP90 (Koncz and Schell 1986) to transform Arabidopsis.
For tobacco transformation, seeds of N. tabacum cultivar Samsun were surface sterilized and germinated on 1/2 strength MS with 3 % sucrose and 1 % agar. Fully expanded leaves were cut in pieces for transformation. The A. tumefaciens LBA4404 containing P CtHSR1 ::CtHSR1 (see above) was co-cultivated with leaf discs and transgenic plants were selected on medium containing 25 mg/l kanamycin. Transgenic plants were confirmed by PCR with primers HSR1Forward (5′-GCACCGCCAGTTCCATCTGG, starting at position 3862 of the genomic fragment) and HSR1Reverse (5′-CACTGGCGTTTCCATTCATAGG, ending at position 4969 of the genomic fragment), amplifying a fragment of 1.1 kb within the coding region. Six transgenic lines segregating 3:1 upon self-pollination (suggesting a single insertion) were raised for three successive generations to get homozygosis and three CtHSR1 lines were selected for further analyses.
Arabidopsis thaliana Col-0 was transformed with A. tumefaciens GV3101::pMP90 containing plasmid P CtHSR1 ::CtHSR1 (see above) by flower infiltration (Bechtold et al. 1993). Transgenic seedlings were selected in medium with 50 mg/l kanamycin and confirmed by PCR as described above. Seven transgenic lines segregating 3:1 upon self-pollination (suggesting a single insertion) were raised for three successive generations to get homozygosis and four CtHSR1 lines were selected for further analyses and designed H1–H4.
Arabidopsis transformation with the coding region of CtHSR1 expressed from the AtUBQ10 promoter
The ORF with stop codon of CtHSR1 (2187 bp) was amplified from the 5829 bp BamHI–XbaI genomic fragment (see above) by PCR using Phusion High-Fidelity DNA polymerase (New England BioLabs, Ipswich, MA, USA) and primers HSR1CDSFwBamHI (5′-CGGGATCCATGAGTAAGAAAAGCAACAGTACC, BamHI site before start codon underlined) and HSR1CDSRvHistKpnI (5′-GGGGTACCCTAGTGGTGGTGGTGGTGGTGATTTTCTTCTTTTTCAATAGTACTATCC, KpnI site underlined before stop codon and a tail of six CAC histidine codons). PCR-derived constructs were verified by DNA sequencing. After digestion with BamHI and KpnI, the fragment was cloned into plasmid pGPTVII.Hyg.P UBQ10 ::MCS digested with the same enzymes giving plasmid P UBQ10 ::CtHSR1. PCR-derived constructs were verified by DNA sequencing. pGPTVII.Hyg.P UBQ10 ::MCS is a derivative of pGPTVII.Hyg (Walter et al. 2004) where the uidA (gusA) gene was removed and the AtUBQ10 promoter (Grefen et al. 2010) inserted before the multi-cloning site. The recombinant plasmid was used for transformation of A. tumefaciens strain GV3101::pMP90 to transform Arabidopsis as described above. Seven transgenic lines tolerant to 25 mg/l hygromycin with a single insertion were obtained and three were selected for analyses (designed as U1–U3).
Arabidopsis transformation with the coding region of CtHSR1 expressed from the 35S promoter with or without GFP fusion
The ORF of the CtHSR1 gene was PCR amplified with high-fidelity DNA polymerase as described above with primers: HSR1CDSFw + 1 5′-ATGAGTAAGAAAAGCAACAGTACC (start codon underlined) and either HSR1CDSRv + stop 5′-CTAATTTTCTTCTTTTTCAATAGTAACTATCC (stop codon underlined) or HSR1CDSRv-stop 5′-ATTTTCTTCTTTTTCAATAGTAACTATCC (no stop codon) and cloned in the pCR8/GW/TOPO Gateway entry plasmid (Invitrogen-Life Technologies, Carlsbad, CA, USA). The ORF with stop codon was cloned by in vitro recombination into binary plasmids pMDC32 (no GFP) and pMDC43 (GFP at N-terminus of CtHSR1 ORF) while the ORF without stop codon was cloned into pMDC83 (GFP at C-terminus of CtHSR1 ORF) (Curtis and Grossniklaus 2003). PCR-derived constructs were verified by DNA sequencing. The P 35S ::CtHSR1, P 35S ::GFP–CtHSR1 and P 35S ::CtHSR1–GFP plasmids were used for transformation of A. tumefaciens strain GV3101::pMP90. The Agrobacterium containing the P 35S ::CtHSR1 plasmid was used to transform Arabidopsis as described above and three hygromycin-tolerant homozygous lines with a single insertion (S1–S3) were selected for analysis. The Agrobacterium transformants containing plasmids P 35S ::CtHSR1–GFP and P 35S ::GFP–CtHSR1 were used for transient expression in leaves of N. benthamiana and confocal laser scanning fluorescence microscopy as described below. Transformation of Arabidopsis with these plasmids resulted in no detection of GFP.
Transient expression of CtHSR1–GFP fusions in leaves of N. benthamiana and confocal laser scanning fluorescence microscopy
Agrobacterium tumefaciens GV3101::pMP90 containing GFP-tagged constructs (P 35S ::CtHSR1–GFP or P 35S ::GFP-CtHSR1; see above) were incubated with shaking at 28 °C in Luria–Bertani (LB) broth for 24 h. Cells were harvested by centrifugation (2500g 15 min 4 °C) and resuspended (10 mM MES-KOH, pH 5.6, 10 mM MgCl2, 200 μM acetosyringone) to obtain an absorbance at 600 nm of about 1. These cells were mixed with an equal volume of culture at similar absorbance of strain C58C1 (pBin61 P 35S ::p19), which expresses the silencing suppressor p19 of tomato bushy stunt virus (Voinnet et al. 2003). Bacteria were then incubated for 3–4 h at 28 °C before being injected into young, fully expanded leaves of 20-day-old N. benthamiana plants as described (Yang et al. 2000). Epidermal cell layers of agro-infiltrated leaves were examined for fluorescence under a TCS LS confocal laser scanning inverted microscope (Leica Microsystems, Wetzlar, Germany) 3–5 days after infiltration. The samples were treated with DAPI (4′,6-diamino-2-phenylindole; 1 mM) for 5 min prior to obtaining the microphotographs. GFP was excited at 488 nm and emission collected between 500 and 520 nm. DAPI was excited at 358 nm and emission collected at 461 nm.
Arabidopsis transformation with the GUS reporter expressed from the promoter of the CtHSR1 gene (P CtHSR1 ::GUS)
We constructed a promoter–reporter fusion with the promoter of the CtHSR1 gene driving expression of the GUS gene (gusA) encoding β-glucuronidase. The genomic region upstream of the CtHSR1-coding region (3003 bp) was PCR amplified from the 5829 bp BamHI–XbaI genomic fragment (see above) by high-fidelity DNA polymerase as described above with primers pHSR1-Fw-BamHI (5′-CGGGATCCGGATCCATAATAGACAGAGGCAC, including the genomic BamHI site, underlined) and pHSR1-Rv-NcoI (5′-CATGCCATGGTTCTTTTTTGCAATCTTGGGA, with an NcoI site, underlined, added after the first nucleotide before the starting codon). After digestion with BamHI and NcoI the 3 kb promoter fragment was cloned into binary plasmid pCAMBIA1303 (http://www.cambia.org/daisy/cambia/585.html) digested with the same enzymes, giving recombinant plasmid pCAMBIA1303-P CtHSR1 ::GUS. This plasmid and the original pCAMBIA1303 were used for transformation of A. tumefaciens strain GV3101::pMP90 to transform Arabidopsis as described above. Five transgenic lines tolerant to 25 mg/l hygromycin with a single insertion were obtained from each construct and three were selected for analyses.
Determination of gene expression by reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using NucleoSpin RNA II kit (Macherey–Nagel, Düren, Germany) and 3 µg RNA was reverse transcribed using the Maxima first-strand cDNA synthesis Kit for RT-qPCR (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. PCR amplifications were performed on first cDNA strand corresponding to 150 ng of total RNA and each reaction was performed in triplicate in a total volume of 25 µl. For semi-quantitative PCR analysis of CtHSR1 expression, the primers HSR1Forward and HSR1Reverse described above were used, the reference gene was PP2AA3 (see primers given in Supplementary Table S1) and the number of cycles was 25 (S lines with 35S promoter) or 30 (H and U lines with CtHSR1 and AtUBQ10 promoters, respectively, and reference gene). Quantitative (real-time) PCR (qRT-PCR) was performed using an Applied Biosystems 7500 Real-Time PCR System (Thermo Fisher Scientific) with the 5× PyroTaq EvaGreen qPCR Mix Plus (ROX) (Cultek S.L.U., Madrid, Spain) according to the manufacturer’s protocol. Data are the mean of three biological samples. PCR amplification specificity was confirmed with a heat-dissociation curve (from 60 to 95 °C). Efficiency of the PCR was calculated and different internal standards (PP2AA3 or At5g55840) were selected depending on the efficiency of the primers (Czechowski et al. 2005). Relative mRNA abundance was calculated using the comparative ΔC t method according to Pfaffl (2001). Primers for the different genes are described in Supplementary Table S1.
Assay of GUS activity
For qualitative histochemical GUS assays different plant organs from seedlings or adult plants were incubated in buffer with 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-GlcU, Duchefa, Haarlem, The Netherlands) overnight at 37 °C (Naleway 1992). The plant material was cleared in 70 % (v/v) ethanol solution before microscopic observation in a binocular loupe of fluorescence MacroFluo MZZ16F (Leica) or a microscope Eclipse E-600 (Nikon, Chiyoda, Tokyo, Japan). All independent transgenic lines showed identical GUS-staining patterns and only differed in the expression level of GUS.
In the quantitative assay of GUS activity, 25 seedlings of each line were grown in solid MS medium under long-day conditions during 7 days. The samples were ground under liquid nitrogen and resuspended in 250 μl of extraction buffer (100 mM KPO4 pH7.8, 1 mM EDTA, 1 % Triton X-100, 10 % glycerol and 1 mM DTT). Following centrifugation of the crude extract (14,000g, 15 min, 4 °C) proteins were quantified with the Bradford assay (Bradford 1976). For the fluorescent reaction, 5 μl extract was incubated at 37 °C in 100 μl of buffer with 4-methylumbelliferyl β-d-glucuronide (4-MUG) and after 15 min the reaction was stopped with 2 ml of 0.2 M Na2CO3 pH 10.3 (Naleway 1992). Fluorescence of the 4-methylumbelliferone (4-MU) product was quantified with a fluorometer (LS 50B, Perkin Elmer CytoFluor II, Waltham, MA, USA) with an excitation wavelength of 365 nm and an emission wavelength of 445 nm. The activity was given in pmol of 4-MU min−1 µg of protein−1.
Transcriptomic analysis with long oligonucleotide microarrays
Two transgenic lines (H1 and H3) with very similar phenotypes (see below under “Results”) were utilized together with the control, non-transformed line (Col-0). Three biological replicas were made for each transgenic line versus the control and only genes significantly altered in expression in at least four replicas were considered. For each experiment 30 seedlings of each line were grown in solid MS medium under long-day conditions during 7 days and all samples harvested at the same time of the day (middle of the light cycle). RNA extraction, amplification, labeling, and hybridization were performed as described previously (Bueso et al. 2007), and the analysis of microarray data was as described by Bissoli et al. (2012). Long oligonucleotide microarrays were provided by Dr. David Galbraith (University of Arizona; http://www.ag.arizona.edu/microarray/). Oligonucleotides were from the Operon Arabidopsis Genome Oligo Set version 3.0 (https://www.operon.com/), which contains 29,110 70-mer probes, representing 26,173 protein-coding genes, 28,964 protein-coding gene transcripts, and 87 microRNAs. The design is based on ATH1 release 5.0 of The Institute for Genomic Research Arabidopsis genome annotation database (http://www.tigr.org/tdb/e2k1/ath1/) and release 4.0 of the microRNA registry at the Sanger Institute (http://www.sanger.ac.uk/Software/Rfam/mirna/index.shtml).
Identification of preferentially located motifs
The methodology has been described in detail (Bernard et al. 2010) and recently utilized in Bueso et al. (2014). We studied a set of 199 known motifs coming from the AGRIS database (Yilmaz et al. 2011), and for each motif, we extracted all occurrences in a promoter set. We defined promoters for 20,687 genes of Arabidopsis and for 105 genes out of 108 up-regulated in CtHSR1 transgenic lines (see above). To define an overrepresented motif we performed a binomial probability to compare percentages between these 105 promoters and the whole promoter set of Arabidopsis. Each motif with a probability of less than 5 % was considered significant.
Assay of drought tolerance
Individual Arabidopsis plants were grown in square pots of 7 × 7 × 6.5 cm placed in trays, irrigated twice a week to reach water field capacity and drained. After 4 weeks, and with the pots at their water field capacity, irrigation was stopped in half of the samples but continued for the other half. After 2 weeks, pictures were taken and fresh and dry weight of aerial part of 10 plants were determined.
Assay of plant growth in presence of abscisic acid (ABA)
Seedlings were grown on vertically plates for 7 days. Afterwards, plants were transferred to new plates with or without 10 µM of ABA. To score plant growth, fresh weight of each plant was measured after 21 days.
Stomata assays
For stomata assays, leaves of 7-day seedlings were used. Measurements were performed on microphotographs of epidermal cells, which were first incubated for 2 h at 20 °C in agitation and constant light in stomata opening buffer containing 10 mM KCl, and 10 mM MES/Tris, pH 6.2, supplemented or not with 0.5 µM ABA. Then, samples were incubated for 5 min in 0.001 % propidium iodide. Leaves were rinsed with distilled water and placed with the lower surface facing down on a microscope slide and samples were examined for fluorescence under a TCS LS confocal microscope (Leica) with excitation at 535 nm and emission collected at 617 nm. The stomata area and aperture (width/length), and number of stomata were measured with the ImageJ/Fiji software (http://fiji.sc/Fiji). Data were expressed as the average of four experiments with 30–40 stomata measured in each one.
Photosynthesis measurements
For photosynthesis measurements, leaves of 40-day-old plants grown in the greenhouse were used. The conditions were: 23 °C, 70 % relative humidity and 8-h light (130 µmol photons m−2 s−1)/16-h dark. Instantaneous determinations of net CO2 assimilation (A n; μmol CO2 m−2 s−1), stomatal conductance to water vapor (g s; mol H2O m−2 s−1), transpiration rate (E; mmol H2O m−2 s−1) and quantum yield of photosystem II (Φ SII) were carried out under conditions of saturating light (1200 μmol m−2 s−1), a vapor pressure difference of 1–2 kPa, and at 400 ppm CO2 with an LI-6400 (Li-Cor, Lincoln, NE, USA).
Osmotic potential and cellular solutes
Leaves of 30-day-old plants grown in the greenhouse were used. Samples were ground in liquid nitrogen, centrifuged (14,000g for 15 min) and the supernatant used for different measurements. Osmotic potential was measured by the freeze point depression method using an osmometer (Osmomat 030, Gonotec GmbH, Berlin, Germany). K+ and Na+ determination was by atomic absorption spectrophotometry (Alejandro et al. 2007), proline content was estimated using ninhydrin acid reagent according to Bates et al. (1973) and soluble sugars by high-performance liquid chromatography (HPLC; see Supplementary Methods). All solutes were expressed as mM in tissue water.
Quantification of indoleacetic acid (IAA), abscisic acid (ABA), and jasmonic acid (JA)
Hormones were quantified in the aerial part of 30-day-old plants grown in the greenhouse. Each sample consisted of material from five plants that was frozen immediately, ground under liquid nitrogen and aliquots of 100 mg of fresh weight were stored at −80 °C until analysis. The detailed procedures of the Plant Hormones Quantification Service of IBMCP (Universidad Politécnica de Valencia-CSIC, Spain) are described under Supplementary Methods.
Reproducibility and statistical analysis of the results
All the experiments were repeated at least three times and samples from Arabidopsis lines included 10–30 plants. We calculated the mean and the standard error (SE) and considered differences of the means to be statistically significant when P < 0.05 by Student test. In the analysis of 3:1 segregation for homozygosis of transgenic lines, the χ 2 test was employed.
Results
Arabidopsis plants transformed with the CtHSR1 gene with its own promoter region or with the UBQ10 promoter are more drought tolerant than controls
The initial experiment of plant transformation with a genomic fragment including the CtHSR1 gene was done in tobacco. As indicated in Supplementary Fig. S1, transgenic plants have bigger leaves than control plants under irrigation conditions (Supplementary Fig. S1a), have a better aspect than control plants after drought treatment (Supplementary Fig. S1b) and could recover from drought stress upon re-watering while control plants could not survive (Supplementary Fig. S1c). As similar results were obtained with three transgenic tobacco lines (data not shown) we decided to further investigate the phenomenon in the model plant, A. thaliana.
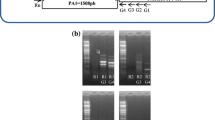
As indicated in the left part of Fig. 1, four lines of Arabidopsis transformed with a genomic fragment including the CtHSR1 gene exhibited better drought tolerance than the control line (untransformed Col-0 or wild type). The aspect of transgenic plants grown under irrigation conditions was indistinguishable form that of control plants (Fig. 1a, left panel). After drought treatment, however, the leaves of control plants were brownish while in the transgenic lines most leaves remained green (Fig. 1b, left panel). Under irrigation conditions both the fresh and dry weights of the aerial parts of the transgenic plants were slightly smaller (20–35 %) than those of control plants (Fig. 1d, left part). After drought treatment the fresh weight of transgenic aerial parts was 50–80 % greater than that of control plants while the dry weight of transgenic aerial parts was 5–15 % smaller than that of control plants (Fig. 1e, left part). These results indicate that transgenic plants have slightly less growth than control plants under irrigation conditions but retain much more water during drought treatment. The water content under irrigation conditions was similar in control and transgenic plants (9.4–9.8 g water per g dry weight) but after drought treatment transgenic plants retained about twice as much water (4.0 versus 2.3 g water per g dry weight, respectively). Actually, control plants could not recover from the drought treatment (irreversible wilting) while transgenic plants continued growth upon irrigation (data not shown). As indicated in Fig. 1c, the phenotypes observed in the transgenic lines are similar despite some differences in expression levels of CtHSR1. This can be explained if a threshold level of expression already results in maximal drought tolerance.
Drought tolerance of Arabidopsis lines transformed with a genomic fragment containing the CtHSR1 gene (left part, P CtHSR1 ::HSR1, transgenic lines H1 to H4) and with the AtUBQ10 promoter driving expression of the coding region of CtHSR1 (right part, P AtUBQ10 ::CtHSR1, transgenic lines U1 to U3). Results with the control line (Col-0 or WT) and 3–4 transgenic lines are shown. a Aspect of representative plants of the indicated lines after 6 weeks of irrigation. b Aspect of representative plants of the indicated lines after 4 weeks of irrigation and 2 weeks without irrigation. c Expression of CtHSR1 gene in control (Col-0 or WT) and transgenic plants as determined by semi-quantitative RT-PCR with 30 cycles of amplification. d Average fresh and dry weight (mg) of the aerial part of plants of the indicated lines after 6 weeks of irrigation. e Average fresh and dry weight (mg) of the aerial part of plants of the indicated lines after 4 weeks of irrigation and 2 weeks without irrigation. Bars indicate standard error and asterisks significant differences (P ≤ 0.05) with control line according to the Student’s t test. It must be indicated that in the experiment of the right part of the figure the growth of control and transgenic plants was smaller than in the experiment of the left part due to different periods of growth
To demonstrate that the phenotype of drought tolerance conferred by a genomic fragment including the CtHSR1 gene is due to expression of the coding region of this gene, we have expressed this 2184 bp ORF from two types of promoters driving expression in most plant tissues: the strong 35S promoter (Benfey and Chua 1990) and the moderate Arabidopsis AtUBQ10 promoter (Norris et al. 1993; Grefen et al. 2010). As indicated at the right part of Fig. 1, transgenic plants from three different lines expressing the CtHSR1 ORF from the AtUBQ10 promoter were more drought tolerant than control plants, reproducing the results obtained when CtHSR1 ORF was expressed from its own fungal genomic promoter. On the other hand, three transgenic lines expressing the CtHSR1-coding region from the 35S promoter exhibited no phenotype (Supplementary Fig. S2). This suggests that moderate expression of CtHSR1, but not strong expression, confers the drought tolerance phenotype.
The fungal gene CtHSR1 is expressed in phloem tissue of Arabidopsis from its own promoter
Having confirmed a phenotypic effect in transgenic plants expressing the CtHSR1 gene from its own genomic promoter region and from the AtUBQ10 promoter, we investigated if the gene is actually expressed in the transgenic plants. First we performed semi-quantitative RT-PCR and compared expression from the genomic promoter region (P CtHSR1 ::CtHSR1), from the UBQ10 promoter (P AtUBQ10 ::CtHSR1) and from the 35S promoter (P 35S ::CtHSR1). As indicated in Fig. 1c and in Supplementary Fig. S2c, using semi-quantitative RT-PCR we could detect expression in the aerial part of transgenic lines with the three promoters but not in control plants (Col-0 or wild type). However, in the case of the P CtHSR1 ::CtHSR1 and P AtUBQ10 ::CtHSR1 plants 5 more amplification cycles (30 versus 25) were needed to obtain similar levels of PCR product than in P 35S ::CtHSR1 plants. This indicates that expression from the first two promoters was about 32-fold lower than from the 35S promoter.
To identify tissues where the CtHSR1 promoter is active, we constructed transgenic plants with the 3 kb genomic region upstream of the CtHSR1-coding region (containing the fungal gene promoter) driving the expression of the GUS reporter gene. The reporter was expressed at vascular bundles of different organs (Fig. 2a) and specifically at phloem tissue (Fig. 2b).
The promoter of the fungal gene CtHSR1 drives expression of GUS at phloem tissue. Transgenic Arabidopsis plants containing the GUS reporter gene expressed from the promoter of the CtHSR1 gene were analyzed for β-glucuronidase activity by the histochemical method described in “Materials and methods”. a Expression at vasculature in a plantlet of 2 days and in hypocotyl, leaf, stamen, root and petal of adult plants. b Expression at phloem tissue in root, hypocotyl and stem of adult plants
We have quantified the level of GUS expression driven by the CtHSR1 promoter by determining in extracts of transgenic plants the specific activity of β-glucuronidase and compared with the activity of plants transformed with the GUS gene under the 35S promoter (plasmid pCAMBIA1303). In the P CtHSR1 ::GUS plants the activities of three lines were in the range of 0.5–5 pmol 4-methylumbelliferone min−1 µg protein−1 (Supplementary Fig. S3) while in the P 35S ::GUS plants the activity range of four lines in the same units was 30–100 (data not shown). Therefore, the fungal promoter works in Arabidopsis at levels 1–5 % than the 35S promoter, in agreement with the RT-PCR results discussed above.
The effect of stresses on the activity of the CtHSR1 promoter was investigated in P CtHSR1 ::GUS transgenic plants. The activity of β-glucuronidase was not significantly affected by 2-h treatment of 7-day plantlets with 125 mM NaCl or by increasing temperature to 42 °C but 250 mM mannitol resulted in a modest induction of 1.2–1.5-fold in 2 of the three lines investigated (Supplementary Fig. S3).
The fungal HSR1 protein fused to GFP localizes to the cell nuclei in leaves of N. benthamiana
We have used transient expression in leaves of N. benthamiana of an AtHSR1–GFP protein fusion driven by the 35S promoter to determine the subcellular localization of the fungal transcription factor in transgenic plants. As indicated in Fig. 3, the CtHSR1–GFP fusion protein concentrates in spots coincident with those stained with the nuclear dye DAPI. This indicates a nuclear location as expected from a transcription factor recognized by the nuclear localization machinery. Similar results were obtained with GFP–CtHSR1 fusions (data not shown). It must be indicated that GFP alone, without a targeting sequence, is known to accumulate diffusely in the cytoplasm and nucleus and only concentrates in nuclei when fused to proteins containing nuclear localization signals (Chiu et al. 1996), as seems to be case with GFP–CtHSR1 fusions.
Physiological basis of drought tolerance in transgenic plants expressing CtHSR1
To understand the physiological mechanisms of drought tolerance conferred by expression of CtHSR1, we have determined in transgenic and control plants several physiological parameters. Arabidopsis plants transformed with a genomic fragment including the CtHSR1 gene were used for these experiments.
The inhibition of seedling growth by abscisic acid (ABA) was similar in control and transgenic plants (Supplementary Fig. S4), suggesting that ABA sensitivity was not affected by expression of CtHSR1. On the other hand, both in the absence and presence of 0.5 µM ABA, the opening of stomata in the leaves of CtHSR1 transgenic plants is 13–14 % smaller than in the leaves of control (non-transgenic) plants (Fig. 4a). Stomata density in transgenic leaves is slightly less than in control leaves but the differences are not significant (Fig. 4b) and the stomata surface is very similar in control and transgenic leaves (Fig. 4c).
Stomatal aperture, but not density or size, is decreased by expression of CtHSR1. Results with the control line (Col-0 or WT) and 3 transgenic lines (H1 to H3) expressing CtHSR1 from its own promoter are shown. a Stomata aperture in the absence (filled bars) and presence (empty bars) of 0.5 µM ABA as measured by the ratio wide/length of the opening cavity determined by microscopy. b Stomata density. c Stomata size without ABA. d Micrographs of the stomata of the different lines without ABA utilized to determine the above data. Values are the mean of 4 determinations from micrographs as in d with 20–30 stomata. Bars indicate standard error and asterisks significant differences (P ≤ 0.05) with control line according to the Student’s t test
These results were corroborated by measurements of gas exchange with an LI-6400 Portable Photosynthesis System. As indicated in Table 1, both the stomatal conductance and the transpiration rate were significantly less in transgenic CtHSR1 lines than in control plants (on average 20–25 % less both parameters) and this was expected from the smaller stomatal opening of the transgenic lines indicated above. The photosynthetic rate and the quantum yield of photosystem II were not significantly different between control and transgenic lines, although we observed a tendency for less photosynthetic rate in transgenic lines (Table 1). This could explain the slightly reduced growth rate of transgenic plants as compared with controls.
The osmotic potential of transgenic lines was significantly greater than that of controls (Table 1). The observed 5 % increase in osmotic potential corresponds to a solute concentration of about 18 mM. Looking for candidate solutes we found that the concentrations of neither soluble sugars (sucrose, glucose and fructose; Supplementary Table S2) nor major cations (sodium and potassium; Supplementary Fig. S5) were significantly different between control and transgenic leaves. A different situation, however, was encountered by measuring proline concentrations, which were twofold greater in transgenic lines than in controls, both under irrigation and under drought conditions (Fig. 5).
The concentration of proline is higher in leaves of CtHSR1 transgenic plants than in control plants (Col-0 or WT). Values are the mean of three experiments where proline was determined in leaf samples from 10 plants of every line. Open bars correspond to plants grown under irrigation conditions while filled bars after a drought treatment as described in Fig. 1. Bars indicate standard error and asterisks significant differences (P ≤ 0.05) with control line under the same conditions, according to the Student’s t test
We have also determined the concentration of some hormones important for drought tolerance. As indicated in Table 2, the concentration of ABA was similar in control and transgenic lines but in the case of JA and auxin transgenic lines contained concentrations 2.1-fold and 1.3-fold greater, respectively, than control plants.
Expression of CtHSR1 alters the transcriptome of Arabidopsis
To identify molecular mechanisms for the drought tolerance conferred by expression of CtHSR1, we have performed a transcriptomic analysis of two CtHSR1 transgenic lines with similar phenotypes (H1 and H3) versus control (non-transformed) Arabidopsis. No significantly repressed genes were found but 108 genes were up-regulated at least 1.5-fold as compared with the control, with a maximum increase of fourfold (Supplementary Table S3). The validation by qRT-PCR of the up-regulation of some of these genes is shown in Supplementary Fig. S6.
This set of genes is enriched in some functional categories related to biotic and abiotic stress such as “Response to wounding”, “Response to jasmonic acid”, “Response to ethylene”, “Response to abscisic acid”, “Response to water deprivation”, “Response to oxidative stress” and “Regulation of transcription” (Supplementary Table S4). It includes relevant genes to the physiology of the CtHSR1 transgenic plants such as P5CS1 and LOX4. P5CS1 encodes isoform 1 of Δ-1-pyrroline-5-carboxylate synthetase, the rate-limiting step of proline biosynthesis in vegetative tissues that is required for proline accumulation under osmotic stress (Székely et al. 2008). LOX4 encodes an isoform of 13-lipoxygenase, the rate-limiting step for JA biosynthesis during plant defense against herbivores (Ozalvo et al. 2014).
A comparison was made between the set of genes up-regulated by expression of CtHSR1 in Arabidopsis and microarray experiments in the NASCArrays data base (http://affymetris.arabidopsis.info/narrays/experimentbrowse.pl). The most significant overlaps of the 108 CtHSR1-induced genes were found with genes induced by osmotic stress (43), JA (23) and heat stress (12) (Supplementary Table S5a–d).
Motifs enriched in the promoters of CtHSR1-induced Arabidopsis genes
We have studied preferentially located motifs in the promoter of CtHSR1 up-regulated genes as compared with all Arabidopsis genes. The two most enriched motifs are the CGCG box of genes involved in ethylene and ABA signaling (Yang and Poovaiah 2002) and the ABRE element of genes responding to ABA (Yamaguchi-Shinozaki and Shinozaki 2005). Although less significant, some enrichment of the WRKY transcription factor-binding box was observed (Table 3).
Discussion
Our results indicate that a fungal gene with its own promoter is expressed in plants and confers a useful phenotype such as drought tolerance. As this may be relevant for horizontal gene transfer from fungi to plants in the course of evolution (Bock 2009; Talianova and Janousek 2011; Schönknecht et al. 2014) and for biotechnological applications, we investigated the functionality of the fungal promoter in plants, the expression of the encoded protein and the mechanisms of the conferred drought tolerance.
We have shown that the promoter region of the CtHSR1 gene drives expression of the GUS reporter at the Arabidopsis phloem. This fungal promoter functions in plants with moderate strength, similar to the AtUBQ10 promoter but much less active than the 35S promoter. The fact that similar phenotypes are observed in transgenic plants with either the CtHSR1 promoter (phloem specific) or with the AtUBQ10 promoter (general expression) indicates that phloem expression is sufficient for the phenotypes and that expression in other tissues is not deleterious. Although cis-elements determining phloem expression are not completely characterized (Yin and Beachy 1997; Saha et al. 2007), some potential sequences such as the ASL (GCAX17–18GCA), GATA (AX3GATA) and CCCC boxes are present in the CtHSR1 promoter (Supplementary Fig. S7). It is interesting that expression of CtHSR1 from moderate promoters such as its own one or the AtUBQ10 promoter results in drought tolerance but expression from the strong 35S promoter results in no phenotype. No silencing at the level of mRNA was observed with the strong promoter (Supplemental Fig. S2) and, therefore, a plausible mechanism is aggregation and degradation of the overproduced protein (Tyedmers et al. 2010). A similar situation has recently been found by overexpressing the transcription factor ATHB25 in Arabidopsis with AtUBQ10 and 35S promoters, when only in the first case was a phenotype observed (Bueso et al. 2014). This may also explain that transgenic Arabidopsis plants with plasmids P 35S ::CtHSR1–GFP and P 35S ::GFP-CtHSR1 exhibited no fluorescence signal (see “Materials and methods”).
Transient expression in leaves of N. benthamiana of a fusion protein CtHSR1–GFP indicates that CtHSR1 localizes in plant cells at the nucleus, as expected from a transcription factor. Therefore, we investigated the transcriptome of the transgenic Arabidopsis plants expressing CtHSR1. Only about hundred genes were altered in expression by CtHSR1, all of them up-regulated. Some of these genes could be direct targets of the fungal transcription factor but as there are 16 transcription factors induced by CtHSR1 (Supplemental Table S3), some of the up-regulated genes may be secondary targets. For example, expression of CtHSR1 up-regulates several ERF transcription factors and it has been described that ERF1 induces the expression of defense genes such as P5CS1 (Cheng et al. 2013). Also, two transcription factors up-regulated by CtHSR1, BZIP1 and ZFP1, induce the expression of several genes involved in drought tolerance (Sun et al. 2012; Han et al. 2014). The most enriched motifs in the promoters of up-regulated genes are the CGCG box (Yang and Poovaiah 2002) and a partial version of the ABRE box (GCCAC; Yamaguchi-Shinozaki and Shinozaki 2005). This motif of only 5 nucleotides is probably not a real ABRE because it lacks two crucial nucleotides (the last GT; compare the complete ABRE motif in Table 3 with the partial one enriched in the promoter of genes up-regulated by CtHSR1). The primary targets of CtHSR1 can only be speculated. It probably binds a single repeat (GAA) of the heat-shock element (Landsman and Wolffe 1995), a sequence too short for specificity. A plausible mechanism is that CtHSR1 interacts with some other transcription factor and the proximity of the two recognized sequences determines the specific binding to a few promoters.
We have characterized the physiological parameters of the CtHSR1 transgenic plants and the observed drought tolerance may be explained by two observations. In the first place, transgenic plants have decreased stomatal opening and conductance, decreased transpiration and better water retention (Figs. 1, 4; Table 1). This also explains the slightly reduced growth of the transgenic plants under irrigation conditions because of slightly reduced photosynthetic rate. This stomata behavior could be the consequence of the doubling of JA concentration in transgenic plants (Table 2) because this hormone induces closing of stomata (Acharya and Assmann 2009; Montillet and Hirt 2013). The increase in JA correlates with increased expression of LOX4, a gene encoding a lipoxygenase of leaf mesophyll cells involved in JA synthesis (see http://bbc.botany.utoronto.ca/efp/). The small increase in auxin concentration may be a secondary effect of the increase in JA (Hentrich et al. 2013).
The second clue to the drought tolerance conferred by CtHSR1 was the increase in osmotic potential of the transgenic plants (Table 1). One of the solutes contributing to this increase is proline, whose concentration is doubled in transgenic with respect to control plants (Fig. 5). Although this can only explain a small part of the change in osmotic potential, proline is a special protecting solute (osmolyte) whose levels correlate with stress tolerance (Ramanjulu and Bartels 2002; Kavi Kishor and Sreenivasulu 2014). The increase in proline in the CtHSR1 transgenic plants correlates with the increased expression of the P5CS1 gene, encoding Δ1-pyrroline-5-carboxylate synthase, the rate-limiting enzyme in the biosynthesis of proline (Székely et al. 2008). Additional up-regulated genes that could directly contribute to drought tolerance include those encoding the chaperones DNAJ11 and DJC22 and one LEA2 protein (At2g27080) (Ramanjulu and Bartels 2002).
In conclusion, a fungal transcription factor gene expressed in plants from its own promoter confers drought tolerance by altering the transcriptome and some physiological parameters related to water stress. Clearly, this is a case of serendipity but it cannot be discarded that expression of other foreign transcription factors may also result in beneficial effects for plants under certain conditions, paving the way for biotechnological applications and, in the evolutionary timescale, horizontal gene transfer.
Author contribution
A. A., R. A. and C. M. performed the initial molecular and physiological experiments in tobacco. F. M., S. G. N., E. B. and J. M.-B. conducted the molecular and physiological work on Arabidopsis. V. B. analyzed the preferential located motifs. R. S. and J. M.-B. coordinated the work and wrote the manuscript. All authors read and approved the manuscript.
Abbreviations
- ABA:
-
Abscisic acid
- DAPI:
-
4′,6-diamino-2-phenylindole
- GFP:
-
Green fluorescent protein
- GUS:
-
β-Glucuronidase
- HSE:
-
Heat-shock element
- HSF:
-
Heat-shock transcription factor
- IAA:
-
Indolacetic acid
- JA:
-
Jasmonic acid
References
Acharya BR, Assmann SM (2009) Hormone interactions in stomatal function. Plant Mol Biol 69:451–462
Alba MM, Tompa P, Veitia RA (2007) Amino acid repeats and the structure and evolution of proteins. Genome Dyn 3:119–130
Alejandro S, Rodríguez PL, Bellés JM, Yenush L, García-Sanchez MJ, Fernández JA, Serrano R (2007) An Arabidopsis quiescin-sulfhydryl oxidase regulates cation homeostasis at the root symplast-xylem interface. EMBO J 26:3203–3215
Ali R, Pascual-Ahuir A, Marquez JA, Malik KA, Serrano R (2001) Identification of Candida tropicalis HSR1, a gene of the heat-shock factor-related family, which confers salt tolerance in Saccharomyces cerevisiae. Yeast 18:605–610
Bates L, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci Paris/Life Sci 316:1194–1199
Benfey PN, Chua NH (1990) The cauliflower mosaic virus 35S promoter: combinatorial regulation of transcription in plants. Science 250:959–966
Bernard V, Lecharny A, Brunaud V (2010) Improved detection of motifs with preferential location in promoters. Genome 53:739–752
Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12:8711–8721
Bissoli G, Niñoles R, Fresquet S, Palombieri S, Bueso E, Rubio L, García- Sánchez MJ, Fernández JA, Mulet JM, Serrano R (2012) Peptidyl-prolyl cis-trans isomerase ROF2 modulates intracellular pH homeostasis in Arabidopsis. Plant J 70:704–716
Bock R (2009) The give-and-take of DNA: horizontal gene transfer in plants. Trends Plant Sci 15:11–22
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bueso E, Alejandro S, Carbonell P, Perez-Amador MA, Fayos J, Bellés JM, Rodriguez PL, Serrano R (2007) The lithium tolerance of the Arabidopsis cat2 mutant reveals a cross-talk between oxidative stress and ethylene. Plant J 52:1052–1065
Bueso E, Muñoz-Bertomeu J, Campos F, Brunaud V, Martínez L, Sayas E, Ballester P, Yenush L, Serrano R (2014) ARABIDOPSIS THALIANA HOMEOBOX25 uncovers a role for gibberellins in seed longevity. Plant Physiol 164:999–1010
Cheng MC, Liao PM, Kuo WW, Lin TP (2013) The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol 162:1566–1582
Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6:325–330
Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133:462–469
Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139:5–17
Grefen C, Donald N, Hashimoto K, Kudla J, Schumacher K, Blatt MR (2010) A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J 64:355–365
Han G, Wang M, Yuan F, Sui N, Song J, Wang B (2014) The CCCH zinc finger protein gene AtZFP1 improves salt resistance in Arabidopsis thaliana. Plant Mol Biol 86:237–253
Hentrich M, Böttcher C, Düchting P, Cheng Y, Zhao Y, Berkowitz O, Masle J, Medina J, Pollmann S (2013) The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation of YUCCA8 and YUCCA9 gene expression. Plant J 74:626–637
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoot RA (1983) A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303:179–180
Kavi Kishor PB, Sreenivasulu N (2014) Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant, Cell Environ 37:300–311
Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204:383–396
Landsman D, Wolffe AP (1995) Common sequence and structural features in the heat- shock factor and Ets families of DNA binding domains. Trends Biochem Sci 20:225–226
Montillet J-L, Hirt H (2013) New checkpoints in stomatal defense. Trends Plant Sci 18:295–297
Naleway JJ (1992) Histochemical, spectrophotometric, and fluorometric GUS substrates. In: Gallagher SR (ed) GUS protocols. Using the GUS gene as a reporter of gene expression. Academic Press, San Diego, pp 61–76
Norris SR, Meyer SE, Callis J (1993) The intron of Arabidopsis thaliana polyubiquitin genes is conserved in location and is a quantitative determinant of chimeric gene expression. Plant Mol Biol 21:895–906
Ozalvo R, Cabrera J, Escobar C, Christensen SA, Borrego EJ, Kolomiets MV, Castresana C, Iberkleid I, Brown Horowitz S (2014) Two closely related members of Arabidopsis 13-lipoxygenases (13-LOXs), LOX3 and LOX4, reveal distinct functions in response to plant-parasitic nematode infection. Mol Plant Pathol 15:319–332
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Ramanjulu S, Bartels D (2002) Drought- and desiccation-induced modulation of gene expression in plants. Plant, Cell Environ 25:141–151
Reed CJ, Lewis H, Trejo E, Winston V, Evilia C (2013) Protein adaptations in archaeal extremophiles. Archaea 2013:373275. doi:10.1155/2013/373275
Saha P, Chakraborti D, Sarkar A, Dutta I, Basu D, Das A (2007) Characterization of vascular-specific RSs1 and rolC promoters for their utilization in engineering plants to develop resistance against hemipteran insect pests. Planta 226:429–442
Schönknecht G, Weber AP, Lercher MJ (2014) Horizontal gene acquisitions by eukaryotes as drivers of adaptive evolution. BioEssays 36:9–20
Sorger PK (1991) Heat shock factor and the heat shock response. Cell 65:363–366
Sun X, Li Y, Cai H, Bai X, Ji W, Ding X, Zhu Y (2012) The Arabidopsis AtbZIP1 transcription factor is a positive regulator of plant tolerance to salt, osmotic and drought stresses. J Plant Res 125:429–438
Székely G, Abrahám E, Cséplo A, Rigó G, Zsigmond L, Csiszár J, Ayaydin F, Strizhov N, Jásik J, Schmelzer E, Koncz C, Szabados L (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53:11–28
Talianova M, Janousek B (2011) What can we learn from tobacco and other solanaceae about horizontal gene transfer? Am J Bot 98:1231–1242
Tyedmers J, Mogk A, Bukau B (2010) Cellular strategies for controlling protein aggregation. Mol Cell Biol 11:777–778
Voinnet O, Rivas S, Mestre P, Baulcombe D (2003) An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 33:949–956
Walter M, Chaban C, Schütze K, Batistic O, Weckermann K, Näke C, Blazevic D, Grefen C, Schumacher K, Oecking C, Harter K, Kudla J (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40:428–438
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10:88–94
Yang T, Poovaiah BW (2002) A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J Biol Chem 277:45049–45058
Yang Y, Li R, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22:543–551
Yilmaz A, Mejia-Guerra MK, Kurz K, Liang X, Welch L, Grotewold E (2011) AGRIS: the Arabidopsis gene regulatory information server, an update. Nucleic Acids Res 39:D1118–D1122
Yin Y, Beachy R (1997) Promoter elements required for phloem-specific gene expression from the RTBV promoter in rice. Plant J 12:1179–1188
Acknowledgments
We acknowledge support by Grants BFU2011-22526 of the Spanish MICINN (Madrid, Spain) and PROMETEO II 2014-041 of Generalitat Valenciana (Valencia, Spain). J. M.-B. was supported by a Juan de la Cierva contract of the Spanish MICINN. A. A. was supported by a short-term EMBO fellowship to visit the laboratory of R. Serrano. We thank Dr. Jose María Bellés (IBMCP, Valencia, Spain) for assistance in the determination of sugars, Dr. Isabel Lopez-Diaz and Dr. Esther Carrera for the hormone analysis carried out at the Plant Hormone Quantification Service of IBMCP and Prof. Jörg Kudla (Westfalische Wilhelms-Universitat, Münster, Germany) for the pGPTVII.Hyg.P UBQ10 ::MCS plasmid.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martínez, F., Arif, A., Nebauer, S.G. et al. A fungal transcription factor gene is expressed in plants from its own promoter and improves drought tolerance. Planta 242, 39–52 (2015). https://doi.org/10.1007/s00425-015-2285-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2285-5