Abstract
Brassinosteroids (BRs) are an important group of plant steroidal hormones that are actively involved in a myriad of key growth and developmental processes from germination to senescence. Moreover, BRs are known for their effective role in alleviation of stress-induced changes in normal metabolism via the activation of different tolerance mechanisms. Efforts to improve plant growth through exogenous application of BRs (through different modes such as foliar spray, presowing seed treatment, or through root growing medium) have gained considerable ground world over. It has been widely demonstrated that the exogenous application of BRs to stressed plants imparts the stress tolerance mechanisms. In BR-induced regulation of physio-biochemical processes in plants, interaction (crosstalk) of BRs with other phytohormones has been reported. This crosstalk may fine-tune the effective roles of other hormones in regulating stress tolerance. The multifaceted role of BRs consolidated so far has reflected their immense potential to help plants in counteracting the stress-induced changes. The effects of introgression and up- and down-regulation of BR-related genes reported so far to improve crop productivity have been presented here. Strong evidence exists that BRs are involved in signal transduction particularly in the regulation of the mitogen-activated protein kinase (MAPK) cascade, which in turn is involved in controlled development, cell death, and the perception of pathogen-associated molecular pattern (PAMP) signaling. How far BRs are involved in signal transduction pathways operative under stressful environments has also been comprehensively discussed in this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stresses (both abiotic and biotic) have been recognized as the prime potential threats to the normal growth maintenance of plants and agricultural productivity. Stresses including water, salinity, alkalinity, lodging, metal(loid)s, UV radiation, ozone, and temperature (high and low) alter plant growth by affecting physiological, biochemical, and molecular aspects of metabolism. Climate change and the anthropogenic interferences have aggravated the situation leading to conversion of arable lands to unproductive wastelands and also reduce the yield of most crops below their potential (Ahanger et al. 2014, 2015, 2017; Ahmad et al. 2010, 2011, 2015, 2016a, b, 2017a, b, 2018a, b). Stresses affect germination, growth pattern, and the yield productivity significantly and it has been proposed that by 2050 there will be a severe scarcity of staple food for the progressively growing human population (Ahanger et al. 2014). In view of this changing scenario, agriculturalists and plant biologists have made enough strides to meet the challenges. Several growth-promoting alternatives and agricultural practices like proper mineral supplementation, water management, drainage, use of pesticides and fungicides have been tested and employed to meet the cumbersome challenges of increasing crop productivity for feeding the ever-increasing human population. However, understanding of the genetic, molecular, and hormonal regulation of growth in response to stresses has remained under intensive research trials for the last few decades, and some satisfactory and fruitful achievements have been attained (Divi et al. 2010, 2016; Deng et al. 2016; Sahni et al. 2016; Ahanger et al. 2017a, b).
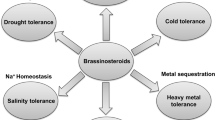
Phytohormones play an important role in plant growth regulation and are actively involved in counteracting the abiotic and biotic stresses by mediating signaling cascades for better response elicitation (Fig. 1). BRs are involved in growth regulation through their active involvement in processes like photosynthesis, antioxidant metabolism, osmolyte accumulation, nitrogen metabolism, and plant–water relations under normal and stressful conditions (Ali et al. 2007, 2008a, b; Hayat et al. 2012; Krumova et al. 2013; Fariduddin et al. 2014; Ahmad et al. 2018a, b). It has been demonstrated that plants defective in BR biosynthesis exhibit abnormalities in developmental phenotypes and the underlying signaling path ways regulating such processes (Sahni et al. 2016). Genetic and molecular studies have witnessed their active role in development of root, anther and pollen, stem elongation, vasculature differentiation, and cellulose biosynthesis suggesting involvement of BR is far beyond promoting the cell elongation (Yang et al. 2011; Clouse 2015).
Stress tolerance mechanisms and the underlying pathways triggered by brassinosteroids (Brs) in plants. Moderate stress can initiate the synthesis of phytohormones for controlling growth which is, however, negatively affected after stress crosses certain levels. BRs (generated either after moderate stress exposure or supplemented exogenously) can either show antagonistic or synergistic interactions with other endogenous phytohormones for elicitation of stress response. (Arrows and lines ending in bars indicate positive and negative regulation, respectively)
BRs are known to exist in a wide range of organisms including lower and higher plants. These steroidal plant hormones mediate growth elevation via activation of different mechanisms (Bajguz and Hayat 2009; Liu et al. 2017). BRs have been shown to be beneficial for growth regulation by imparting the induction of genes related to a stress and other defense mechanism for better growth adaptability (Yang et al. 2004; Zhao et al. 2016) (Fig. 1). Effective stress amelioration due to application of BRs has been reported in crops exposed to water/drought (Talaat et al. 2015), salinity, high temperature (Hayat et al. 2010), cold (Singh et al. 2012), and metal stress (Zhao et al. 2016). Commercial use of BRs for improving growth through exogenous application is attracting the interests of researcher’s world over. It has been reported that BRs impart growth stimulation and stress mitigation in a concentration-dependent manner. Application of BRs to stressed plants exogenously either through spraying, priming, or feeding along with nutrient solution has been reported to impart and strengthen the stress tolerance mechanisms (Anuradha and Rao 2007; Hayat et al. 2007, 2010; Ali et al. 2008a, b; Fariduddin et al. 2009; Talaat et al. 2015; Sharma et al. 2016; Ahmad et al. 2018a, b). However, induction or inhibition of growth is determined by the concentration applied and such variation in plant responses are reported to be dependent on the type of plant species and the intensity of stress. For example, Ali et al. (2008a, b) have reported that application of 24-epibrassinolide (EBL) and 28-homobrassinolide (HBL) to aluminum (1 and 10 mM)-stressed bean seedlings mitigated the stress more significantly in 1 mM Al-treated plants as compared to 10 mM Al-treated counterparts. Application of EBL improved cold tolerance of Zea mays up to 1 µM, while declined growth was observed with higher EBL (10 µM) concentration (Singh et al. 2012). However, in addition to a concentration applied, the effect of exogenous BR application, inhibitory or stimulatory, can vary with the developmental stage, plants species, and the plant organ treated (Bao et al. 2004). Some studies have also revealed the involvement of BRs in expression of genes having an important role in regulation of various processes of stress tolerance. For example, BRs induce expression of late anthocyanin biosynthetic genes including those of dihydroflavonol reductase, leucoanthocyanidin dioxygenase, and UDP-glucose: flavonoid-3-O-glucosyl transferase and the transcription factors like production of anthocyanin pigment 1 (PAP1), glabra 3 (GL3), and enhancer of glabra 3 (EGL3) involved in their regulation (Yuan et al. 2015). It is imperative to note here that excessive application of BRs can interfere with the endogenous BRs biosynthesis by inhibiting gene expression concomitant with the up-regulation of BR-inactivation genes, thereby causing growth reduction (Tanaka et al. 2005; Zhu et al. 2013). Recent studies have evidenced the involvement of BRs in the regulation of the mitogen-activated protein kinase (MAPK) cascade (Kim et al. 2011; Khan et al. 2013) and possible interaction of the BR receptor (BRI1), for example, BRI1-associated protein receptor kinase (BAK1), by its role as co-receptor for several receptor-like kinases (RLKs) to bring controlled development, cell death, and the perception of pathogen-associated molecular pattern (PAMP) signaling (Li 2003, 2005; Kemmerling and Nurnberger 2008; Greeff et al. 2012).
Research on exploring the role of BRs is currently gaining ground due to their role in plant growth modulation and more importantly their integration with other signaling molecules including auxins, abscisic acid (ABA), ethylene, salicylic acid (SA), and gibberellic acid. With respect to this, developing ideotypes for acquiring the agricultural sustainability by improving plant performance under changing climatic conditions, it would be imperative to exploit the existing knowledge about the involvement of BRs in plant stress tolerance. So in the present review, efforts have been made to examine that how far BRs play an effective role in regulating growth and physio-biochemical processes in plants exposed to abiotic stresses. In addition, the BR-mediated signaling and crosstalk with other hormones are also briefly summarized here.
BRs and Plant Stress Tolerance
It is now evident that BRs can induce considerable resistance in plants against a variety of abiotic stress factors including salinity, water logging, drought, metals, high and low temperature (Anuradha and Rao 2007; Hayat et al. 2007, 2010; Ali et al. 2008a, b; Fariduddin et al. 2009; Talaat et al. 2015; Sharma et al. 2016; Ahmad et al. 2018a, b). However, the effects of BRs in counteracting the adverse effects of different stresses may vary and it depends on the type of a stress, plant species, BR dose, and plant ontogenic phase at which BRs are applied. Thus, manipulation of intrinsic BR levels either through genetic engineering or exogenous application results in enhanced yield in crops exposed to stressful environments.
Drought Stress
One of the main challenges to sustainable agricultural production is the frequently occurring chronic or sporadic drought stress particularly in arid and semi-arid regions of the globe. Often characterized by reduced relative water content (RWC), drought stress causes considerable reduction in crop growth and ultimate yield (Jatav et al. 2014; Ahanger et al. 2014, 2015; Ahanger and Agarwal 2017a, b). Drought stress-induced growth reduction generally occurs due to reduced photosynthetic rate, and alteration in nitrogen and antioxidant metabolism, secondary metabolite accumulation, and mineral nutrition (Jatav et al. 2014; Ahanger et al. 2015, 2017). However, drought-induced adverse effects on plant growth and metabolism can be mitigated by exogenous application of a variety of growth regulating substances including BRs. For example, in Phaseolus vulgaris, application of EBL or HBL to water-stressed plants resulted in improved growth and yield by enhancing root nodulation and nitrogenase activity, thereby providing strength to the plants to withstand drought (Upreti and Murti 2004). Similarly, in another study with Brassica juncea, application of 0.01 µM HBL to drought-stressed plants at two different developmental stages enhanced the photosynthetic rate by improving RWC, stomatal conductance, and water use efficiency by increasing the accumulation of proline (Fariduddin et al. 2009). Kocova et al. (2010) have also demonstrated improved growth performance in field grown maize due to application of EBL (10−12 M) through improved chlorophyll synthesis, Hill reaction, and PSI activity, and specific leaf mass production. Exogenous application of HBL has been reported to counteract the detrimental effects of drought in Zea mays by causing improvement in the activity of antioxidant enzymes, soluble protein content, and water uptake (Anjum et al. 2011). Drought-induced improvement in antioxidant activity and photosynthetic functioning was observed in Capsicum annum due to exogenously applied EBL (0.01 mg L−1) (Hu et al. 2013). Similarly, Raphanus sativus plants subjected to simulated drought stress using PEG-6000 exhibited considerable growth retardation, but presoaking with EBL and HBL caused a significant stress amelioration by enhancing the activity of antioxidant enzymes concomitant with reduction in RNase activity depicting the protective role of BRs for RNA (Mahesh et al. 2013).
It has been widely reported that exogenous BR application up-regulates the activity of antioxidant enzymes and the levels of non-enzymatic antioxidants for mediating efficient removal of reactive oxygen species (ROS) and thereby providing protection to membrane lipids for maintaining membrane integrity and functioning (Li et al. 2012a, b; Shahana et al. 2015; Ahmad et al. 2017a). For example, application of EBL reduced drought-induced oxidative damage in tomato plants by down-regulating lipoxygenase activity and up-regulating the antioxidant defense system by enhancing the expression of antioxidant isozymes (Behnamnia 2015). A study carried out by Talaat et al. (2015) has shown a significant modulation of the antioxidant system and enhanced osmolyte accumulation due to exogenous EBL application in drought-stressed Zea mays cultivars. EBL when applied in combination with spermine up-regulated the oxidative defensive mechanisms by reducing the production of superoxide and hydrogen peroxide radicals reflecting lower lipid peroxidation and higher membrane stability (Talaat et al. 2015). Similarly, in maize, application of EBL to drought-stressed plants showed enhanced production of polyamines by up-regulating the activity of biosynthetic enzymes, while it down-regulated the catabolizing enzymes resulting in enhanced drought stress tolerance by protecting Rubisco enzymes through enhanced production of osmolytes (Talaat and Shawky 2016).
BRs are believed to play a vital role in expressing genes involved in the mechanism of drought tolerance. For example, Sahni et al. (2016) observed enhancement in drought stress tolerance in transgenic Brassica napus over-expressing the BR biosynthetic gene, DWF4. It has also been observed that application of EBL augments drought tolerance by up-regulating the transcription factors regulating the expression of the drought-responsive element, DRE, as observed in Arabidopsis thaliana and Brassica napus (Kagale et al. 2007). Gruszka et al. (2016) reported that barley genotypes defective in BR synthesis display less drought stress tolerance, and drought induced accumulation of castasterone and EBL imparted regulation of the hormonal profile at the whole plant level. Crosstalk between drought and BR signaling is regulated by the transcription factor RD26 which when over-expressed can inhibit BR-regulated growth. Such negative regulation is under direct control of BRASSINOSTERIOD INSENSITIVE1-EMS-SUPPRESSOR 1/ BRASSINAZOLE-RESISTANT 1 (BES1/BZR1) repressing the expression of RD26 at transcriptional levels; however, RD26 protein interacts with BES1 and inhibits its transcriptional activity. RD26 antagonistically regulates BR as revealed by the over-expression and knockout studies of RD26 leading to repressed and increased BR response (Ye et al. 2017). It is believed that BR signaling controls the activities of BES1/BZR1 transcription factors. Additionally, other transcription factors like WRKY (46, 54, and 70) have been shown to regulate both BR-regulated plant growth as well as drought response. As reported, wrky46 wrky54 wrky70 triple mutants having defects in BR-regulated growth are more tolerant to drought stress; however, their global role in promoting BR-mediated gene expression and inhibition of drought responsive genes often regulated by the direct interaction between WRKY54 and BES1 has been recently established (Chen et al. 2017). Phosphorylation and destabilization of WRKY54 by GSK3-like kinase BR-INSENSITIVE2 lead to negative regulation of the BR pathway. Therefore, it can be concluded that elucidating the factors involved in controlling the antagonistic regulation of BR and drought-responsive genes can be interesting. Sahni et al. (2016) concluded that enhanced drought tolerance in transgenic plants over-expressing BR biosynthesis is likely mediated by BES1/BZR1, and therefore further studies using genomics integrated with proteomics can be helpful in dissecting the exact pathway.
Salt Stress
Increasing salt concentrations in soils have rendered most of the agricultural lands as unproductive wastelands resulting in an increased food crisis. In view of some projections, about 45 million hectares of irrigated land are salt affected (Munns and Tester 2008; Machado and Serralheiro 2017). Salinity reduces plant productivity by adversely affecting growth through induction of osmotic and ionic imbalances (Ahanger and Agarwal 2017; Ahmad et al. 2017b, 2018b; Kaur et al. 2018). Salinity-induced deleterious effects include ionic toxicity, osmotic stress, reduced nitrogen metabolism, elevated production of ROS leading to oxidative damage, hampered photosynthetic functioning and photosynthate translocation, impeded uptake and translocation of mineral nutrients (Turkan and Demiral 2009; Ahmad et al. 2010, 2016a, b; Iqbal et al. 2015; Ahanger and Agarwal 2017) (Fig. 1). Although plants employ various mechanisms to counteract salinity stress, often they fail to fully counteract the negative effects. Therefore, devising strategies and management techniques for achieving improved salt tolerance is imperative. Significant contributions of exogenously supplied BRs to assuaging the salinity-induced growth retardation have been reported from time to time. The role of BRs in regulating salinity tolerance mechanisms has been reported in several crops like Oryza sativa (Anuradha and Rao 2003), Cicer arietinum (Ali et al. 2007), Brassica juncea (Ali et al. 2008), Vigna sinensis (El-Mashad and Mohamed 2012), and Mentha piperita (Coban and Baydar 2016). The most studied metabolic mechanism with respect to the effect of BRs is the oxidative defense. A number of reports can be deciphered from the literature wherein researchers have shown a marked regulatory effect of BRs on the antioxidative defense system. For example, in a study with wheat, foliar application of EBL counteracted the adverse effects of salinity by enhancing the activity of peroxidase and catalase in wheat cultivars with contrasting degrees of salt tolerance (Shahbaz and Ashraf 2007). Similarly, in salt-stressed Cucumis sativus, supplementation of EBL caused a marked enhancement in growth by up-regulating the activity of superoxide dismutase (SOD), peroxidase, and catalase (CAT) concomitant with reduced electrolyte leakage and malondialdehyde (MDA) content (Song et al. 2006). In the same vegetable crop, Lu and Yang (2013) have demonstrated NaCl-accrued production of ROS like O2−, H2O2 imparting considerable loss in membrane functioning, but application of EBL significantly mitigated the oxidative damage by strengthening the antioxidative system. Application of 0.01 µM EBL to Vigna radiata (Hayat et al. 2010) and Cucumis sativus (Fariduddin et al. 2013) proved significant in inducing enhancement in the photosynthetic attributes by modulating the antioxidant defense system and carbonic anhydrase activity, and such growth-promoting effect of exogenous EBL was maintained under NaCl treatments.
Despite using foliar application of BRs, some researchers have employed seed priming techniques to achieve enhanced stress tolerance. For example, the seeds of Medicago sativa pretreated with 5.0 µM EBL and then exposed to salinity of 13.5 dS m−1 showed enhanced seed germination and reduced oxidative damage by improving the activity of SOD, POD, and CAT (Zhang et al. 2007). Moreover, exogenously (foliar) sourced EBL (10−8 M) has been reported to enhance the polyamine (putrescine, 10−3 M)-induced NaCl tolerance (100 mM) in Cucumis sativus by mediating quick elimination of ROS and the accumulation of osmolytes (Fariduddin et al. 2014). A number of key enzymes of different metabolic processes are also believed to be inhibited in salt-stressed plants, but their inhibited activity can be effectively recovered by the exogenous application of BRs. For example, salt stress can hamper nitrogen metabolism by inhibiting the activity of key nitrogen metabolizing enzymes including nitrate reductase that mediates the first and rate limiting step in nitrogen metabolism (Ahanger and Agarwal 2017). The activity of nitrate reductase is impeded by salt-induced restriction of nitrate uptake and xylem loading having a direct impact on its transport to shoot tissues (Cerezoa et al. 1997). For example, in Cicer arietinum the activities of some key enzymes of N metabolism were reported to be decreased due to saline stress, but exogenously applied EBL improved nitrogen metabolism by improving the nitrogen fixing and mobilizing capacity by increasing the number of nodules and associated biochemical attributes including nitrogenase activity and leghemoglobin content (Ali et al. 2007). In another study with Vigna radiata, application of HBL improved nitrate reductase activity and the nitrogen content with a concomitant enhancement in carbonic anhydrase activity and photosynthetic efficiency under salt stress (Hayat et al. 2010).
BRs are also known to regulate a variety of genes involved in some key metabolic processes in plants exposed to saline stress. For example, EBL application enhanced the expression of stress-responsive genes and the OsBRI1 conferred stress tolerance in Oryza sativa (Sharma et al. 2013). In another study with Arabidopsis thaliana, application of EBL enhanced the expression of phytohormone marker genes and it rescued the ethylene-insensitive ein2 mutant and the ABA-deficient aba-1 mutants of Arabidopsis from salt stress, suggesting that BRs share transcriptional targets with other hormones (Divi et al. 2010). Further they suggested that the master regulator of salicylic acid-mediated defense genes—the redox-sensitive protein NPR1 (NON-EXPRESSOR OF PATHOGENESIS-RELATED GENES1)—has a critical role in EBL-mediated salt tolerance. In another study with wheat, application of EBL (0.4 nM and 0.4 mM) induced enhancement in the synthesis of wheat germ agglutinin (WGA). WGA is a classical lectin that belongs to the rab gene family and its expression is controlled by endogenous ABA levels; however, experiments with EBL have shown the ABA-independent control of WGA expression in Triticum aestivum imparting enhanced salt tolerance (Shakirova and Bezrukova 1998; Shakirova et al. 2002). Over-expression of sterol glycosyl transferases (WsSGTL1) from Withania somnifera increased salt tolerance of Arabidopsis thaliana by enhancing expression of genes coding antioxidant, salt sequestration pathways, LEA proteins, and photosynthetic system (Mishra et al. 2013).
From the above-reported studies, it is evident that exogenously applied BRs can effectively mitigate the salt-induced adverse effects on crop growth and metabolic processes by improving the activities of key enzymes, the antioxidative defense mechanism, and expression of a number of genes involved in some key metabolic processes behind salt tolerance.
Metal/Metalloid Stress
In the contemporary era, metal(loid)-induced growth inhibition has become a subject of great concern for sustainable agricultural productivity. With continuous addition of highly metal-polluted industrial effluents, sewage waste and sludge, agricultural toxic chemicals, and garbage dumping, productive agricultural soils are converted into unproductive lands, thereby affecting crop growth adversely. Once taken up by the plant system, metal(loid)s restrict growth by interfering with the membrane structure, enzyme activity, and transport, distribution, and metabolism of mineral elements (Ahmad et al. 2011, 2015, 2016a, b, 2018a). In addition, the most toxic effect of metal(loid)s is the free radical-induced oxidative stress causing genotoxic stress by triggering the formation of exocyclic DNA/RNA adducts, which have been reported to alter the cellular redox homoeostasis leading to restriction in the electron transport (Asgher et al. 2014; Khan et al. 2015; Ahmad et al. 2010, 2018a). Metal(loid)s once taken by plants can be deleterious for humans as well. Plants have a remarkable ability to withstand metal-induced changes to some extent by the induction/regulation of several indigenously existing tolerance mechanisms (Ahmad et al. 2015). Use of BRs for averting metal(loid)s-induced oxidative stress has been under intense research for many years, and the results of the research reported so far favor strongly the use of exogenous BR for metal(loid)s stress amelioration. For example, Hayat et al. (2007) demonstrated Cd-induced oxidative stress amelioration in Brassica juncea by foliar application of 0.01 µM HBL through enhancement in the activity of antioxidant enzymes and the contents of some key osmolytes. Cadmium is known to affect photosynthesis by (a) down-regulating the activity of chlorophyll synthesizing enzymes and such a negative effect of Cd has been attributed to Cd-induced inactivation of the sulfhydryl site of proteins (Ernst 1980), and (b) by down-regulating the expression of the Rubisco protein (Asgher et al. 2014). However, a significant role of exogenous BR in assuaging the metal(loid)-induced growth inhibition has been reported time and again. For example, enhancement in RWC and membrane stability due to EBL application was evident due to increased activity of antioxidant enzymes and the accumulation of osmolytes in Raphanus sativus (Anuradha and Rao 2007; Sharma et al. 2010; Ramakrishna and Rao 2015), Phaseolus vulgaris (Rady 2011), Solanum lycopersicum (Hayat et al. 2012), Brassica juncea (Kanwar and Poonam Bhardwaj 2015), and Oryza sativa (Sharma et al. 2016). In radish (Raphanus sativus), treatment of EBL for 8 h significantly neutralized the Cd- and Hg-induced oxidative stress by causing considerable elevation in the activity of antioxidant enzymes including SOD, CAT, polyphenol oxidase (PPO), glutathione-S-transferase (GST) and the enzymes of the ascorbate–glutathione pathway (ascorbate peroxidase, APX; dehydroascorbate reductase, DHAR; monodehydroascorbate reductase, MDHAR; glutathione reductase, GR), and the accumulation of flavonoid content (Kapoor et al. 2014). They also observed that the EBL-induced antioxidative defense system protected photosynthetic pigments from the individual as well as combined effects of these metals. HBL and EBL application has been reported to nullify the adverse effects of Cd on the photosynthetic attributes and the antioxidant machinery in Cicer arietinum (Hasan et al. 2008) and tomato cultivars (Hasan et al. 2011). A study by Arora et al. (2010a, b) showed a strong growth-promoting effect of exogenously applied EBL on Brassica juncea exposed to chromium (Cr) stress and at all concentrations of EBL applied stimulated growth by up-regulating the activities of the enzymes of the ascorbate–glutathione pathway. In Raphanus sativus, Choudhary et al. (2011) reported that exogenous application of EBL suppressed Cr-induced oxidative damage by enhancing the activity of SOD, GR, and APX and the accumulation of GSH and AsA leading to photo-protection by maintaining the cellular redox and osmotic balance. In addition, supplementation of EBL triggered the synthesis of indole-3-acetic acid (IAA), polyamines (putrescine and cadaverine), and phytochelatins, thereby mediating better signaling events and the subsequent chelation under Cr stress. They also reported the growth-promoting interactive role of EBL and polyamine (spermidine), which was reflected in enhancement in the accumulation of secondary metabolites and the non-enzymatic components of antioxidants leading to improved free radical scavenging potential (measured as DPPH radical scavenging). Treatment of BRs (BL; EBL; castasterone, CS; 24-epicastasterone, ECS; 28-homoCS) to Chlorella vulgaris enhanced the synthesis of phytochelatins (Bajguz 2002). Fariduddin et al. (2009) showed that soaking Brassica juncea with HBL (10−10, 10−8, and 10−6 M) for 8 h significantly improved growth and photosynthetic parameters under copper (50, 100, and 150 mg kg−1 sand) stress by enhancing SOD, POD, and CAT activity, thereby reducing the formation of H2O2-induced lipid peroxides together with improved nitrate reductase activity and proline accumulation. They also observed that HBL at 10−8 M proved to be most effective in imparting tolerance in Brassica juncea to externally added copper. Exogenous application of EBL (10−6 M) to Vigna radiata cultivars with contrasting nickel (Ni) stress tolerance was shown to improve the nitrogen-fixing attributes (number of nodules, leghemoglobin content, etc.) and the nitrogen metabolism by mediating improvement in the synthesis of osmolytes and the activities of SOD, CAT, and POD, hence resulting in improved yield and biomass productivity (Yusuf et al. 2012, 2014). Application of EBL to Ni-stressed Solanum nigrum enhanced the growth by causing a significant enhancement in relative content of Rubisco, proline, ascorbate acid (AsA) and the activities of SOD and APX accompanied with the decline in the generation of superoxide, H2O2 and MDA (Soares et al. 2016). They also reported EBL-induced post-translational level regulation of antioxidant enzymes. Application of EBL protected chickpea from Hg-induced oxidative damage and nutrient uptake by up-regulating the antioxidant system and osmolyte accumulation (Ahmad et al. 2018a, b).
Zinc stress was mitigated in Brassica juncea by EBL treatment (10−7, 10−9, 10−11 M), which improved the antioxidant defense system and restricted uptake and accumulation of excess Zn in plant tissues (Sharma et al. 2007). In another study, supplementation of EBL to Zn (0.5, 1.0, 1.5, and 2.0 mM)-treated Brassica juncea ameliorated oxidative stress and growth inhibition by mediating up-regulation of ROS scavenging system including SOD, CAT, and enzymatic and non-enzymatic components of the ascorbate–glutathione cycle (Arora et al. 2010). Ramakrishna and Rao (2015) have demonstrated that foliar application of EBL and HBL at the concentrations of 0.5, 1.0, or 2.0 µM effectively alleviated the deleterious effects of zinc toxicity on Raphanus sativus primarily by protecting the membranes by reducing the production of ROS and stabilizing the protein structures by reducing the formation of protein oxidative products, carbonyls. Moreover, both BRs improved RWC by triggering the synthesis of osmolytes like proline, thereby providing protection to nitrate reductase activity. Supplementation of EBL (5 nM) to tomato seedlings raised on Murashige and Skoog medium supplemented with ZnO nanoparticles (0, 10, 20, 50, and 100 mg/L) exhibited reduced oxidative damage due to enhanced activity and expression of SOD, CAT, APX, and GR isozymes, and the contents of AsA and GSH, thereby protecting the cellular redox homeostasis (Li et al. 2016). It shall be noted here that the exact mechanisms underlying BR-induced growth regulation under metal(loid) stress is largely unknown. Understanding the possible genetic controls regulating BR-mediated changes and tolerance potential needs more research.
Temperature Extremes
Climate change is being widely witnessed in the current era resulting in considerable fluctuation in the mean temperatures. Both high and low (cold and chilling) temperatures are potential environmental factors affecting both physiological and biochemical aspects of metabolism, and such changes are regulated at the molecular as well as genetic levels (Kazemi-Shahandashti et al. 2014; Siboza et al. 2014; Hatfield and Prueger 2015; Calzadilla et al. 2016). However, BR application has been reported to benefit crop plants by enhancing the efficiency of growth mediating key metabolic pathways. Exogenous application of BR has been widely reported to mitigate the adverse effects of high (Zhou et al. 2004; Ogweno et al. 2008; Hayat et al. 2010; Mazorra et al. 2011; Sahni et al. 2016; Yadava et al. 2016) and low (Janeczko et al. 2007; Liu et al. 2009; Aghdam et al. 2012; Aghdam and Mohammadkhani 2014; Calzadilla et al. 2016) temperature regimes to considerable extent in different crops by regulating a variety of metabolic processes. Both high and low temperatures can effectively retard plant growth but the effects of low and high temperatures differ considerably in plants. Also, plant responses to high and low temperatures vary significantly. For example, low temperature particularly below freezing can freeze the cells, thereby causing a marked disruption in uptake and translocation of both water and nutrients. However, the ameliorative effect of BRs has been assessed in different crops exposed to low temperature stress. For example, in a fruit crop, grapevines, application of BR was reported to reduce the cold-induced ion leakage by maintaining membrane integrity through improvement in antioxidant and osmoregulatory components (Xi et al. 2013). In Cucumis sativus, foliar spray of HBL (10−8 or 10−6 M) mediated growth enhancement under chilling stress (10/8 °C, 5/3 °C) by improving the activities of antioxidant enzymes (SOD, CAT, and POD), thereby providing protection to the photosynthetic system from the ROS-induced oxidative damage (Fariduddin et al. 2011). Such BR-induced growth enhancement was well correlated with enhancement in the activities of nitrate reductase, carbonic anhydrase and the accumulation of proline, and the quantum yield of PSII. Pepper fruit supplied with BR (5, 10, and 15 µM) and stored for 18 days at 3 °C had reduced electrolyte leakage, lipid peroxidation with a concomitant increment in the activities of antioxidant enzymes like POD, APX, and GR, as well as enhanced chlorophyll and AsA levels (Wang et al. 2012). Experiments with tomato have inferred that supply of BR (3 and 6 µM) enhanced the chilling tolerance (1 °C) by elevating antioxidant metabolism and osmolyte accumulation together with up-regulating phenylalanine ammonia lyase activity leading to improved phenol accumulation, thereby reducing electrolyte leakage and lipid peroxidation (Aghdam et al. 2012). Exogenous application of EBL (0.1 mg L−1) to Cucumis sativus subjected to chilling under low light increased the activity of ROS neutralizing antioxidant system imparting quick recovery after the stress release (Hu et al. 2010). They also evidenced that EBL application proved significantly protective for the photosynthetic system by restricting the production of ROS including superoxide and H2O2. Pectin methylesterases (PMEs) are considered among key cell wall enzymes having beneficial roles in plant tolerance against chilling and/or freezing and BRs regulate the expression of PME in chilling-stressed Arabidopsis (Qu et al. 2011). Exogenous application of BR activated Calvin cycle enzymes and antioxidant enzymes leading to improved photosynthesis through the alleviation of chilling-induced pho-oxidative damage (Jiang et al. 2013). Treatment of EBL (3 and 6 µM) to stored tomato fruits at 1 °C for 21 days resulted in reduced chilling injury by causing a decline in electrolyte leakage and lipid peroxidation leading to maintained membrane integrity by down-regulating the activities of phospholipase-d and lipoxygenase (Aghdam and Mohammadkhani 2014). However, despite having such a plethora of literature available, no research work has been published depicting the role of BRs in regulation of cold-responsive genes. Thus, future research in this direction could be worthwhile.
Like for cold stress, assessment of the influence of BRs on high-temperature mitigation has also been under intensive research with different crops. For example, banana plants when exposed to high (34 °C) temperature showed a marked necrosis, but the application of the trihydroxylated spirotane analogue of BR significantly enhanced growth by improving the accumulation of proline and the photosynthetic efficiency (Gonzalez-Olmedo et al. 2005). In Indica rice, Cao and Zhao (2008) demonstrated that exogenous application of BR (0.005 mg L−1 foliar spray) mitigated the deleterious impact of high temperature (38/30 °C) in two cultivars differing in stress tolerance by improving the expression of antioxidant isozymes resulting in enhanced antioxidant protection and reduced lipid peroxidation. In another study with tomato, in three lines, that is, BR-deficient mutant, BR-insensitive mutant, and a line over-expressing the BR-biosynthesis gene, exogenous application of EBL (1 µM) under heat stress (40 °C) was correlated with reduced lipid peroxidation and membrane leakage more in the BR-deficient mutant as compared to that in BR-insensitive mutant. However, in the cultivar over-expressing the BR-biosynthesis gene, exogenously sourced BR did not show a significant benefit evidencing the active involvement of endogenously produced BR in heat stress amelioration and growth promotion (Mazorra et al. 2011). This means if the endogenous concentration of BR is high, then exogenously supplied BR may not be effective in promoting growth. However, in contrast in another study with tomato, exogenous application of EBL protected photosynthetic efficiency of tomato plants from heat stress (40/30 °C for 8 days) by improving photosynthetic electron transport and carboxylation efficiency of Rubisco concomitant with reduction in lipid peroxidation and production of high amounts of H2O2 which was well correlated with increased activity of antioxidant enzymes like SOD, CAT, POD, and APX (Ogweno et al. 2008). In the same crop, Ogweno et al. (2010) reported that treatment of EBL protected the photosynthetic system in detached leaf tissues from mild light intensity (400 µmol m−2 s −1 PPFD) at 28 °C by preventing the photoinhibition and increasing the quantum yield by stimulating the antioxidant system. In another crop, Vigna radiata, exogenous application of HBL enhanced membrane stability and leaf water potential by increasing the activities of antioxidant enzymes and accumulation of proline (Hayat et al. 2010). In Ficus concinna, foliar spray of EBL (0.25 µM) mitigated the high (40 °C) and moderate (35 °C) temperature-induced oxidative damage by elevating the activities of antioxidant enzymes (SOD, CAT, APX, GR, MDHAR, DHAR, and GST), the glyoxylase system, and the synthesis of GSH and AsA leading to maintenance of redox homoeostasis, thereby causing a significant reduction in lipid peroxidation (Jin et al. 2015). Recently, it has been demonstrated that over-expressing the BR biosynthetic gene DWF4 in Brassica napus imparted high temperature (40 °C) and dehydration stress tolerance, and these transgenic lines maintained higher yield and biomass accumulation. In heat-stressed (30/40 °C) Oryza sativa L. var Pathum Thani 1, Thussagunpanit et al. (2015) reported that exogenous application of EBL and the BR-mimic compound, 7,8-dihydro-8α-20-hydroxyecdysone (DHECH), at the flowering (reproductive) stage protected the photosynthetic system by improving water use efficiency, stomatal conductance, and the quantum yield of PSII, and EBL- and DHECH-treated plants showed higher synthesis of free sugars, starch, and yield.
Heat shock proteins have been widely studied in plants because of their potential role in high-temperature tolerance. However, in view of some reports it is evident that BRs can promote the expression of heat shock proteins in heat-stressed plants. For example, exogenously supplied EBL improved growth of Brassica napus and tomato seedlings by enhancing the expression of heat shock proteins (hsp) including hsp100, hsp90, hsp70 and the low molecular weight hsps under thermal stress (Dhaubhadel et al. 1999). Involvement of EBL in protection of the hsp-translational machinery during heat stress has been suggested via EBL-mediated increase in the number of several translation initiation and elongation factors in spite of having a negative correlation with mRNA levels leading to improved basic thermotolerance after the stress release (Dhaubhadel et al. 2002). Such EBL-induced increase in the expression levels of hsps during recovery triggers rapid resumption of cellular protein synthetic machinery after heat stress exposure, hence enhancing the survival rates. Singh and Shono (2005) reported that foliar application of EBL (5 µM) induced the expression of mitochondrial-small hsp (Mt-shsp) more at 38 °C as compared to that at 25 °C causing a significant improvement in photosynthesis, pollen germination, and pollen tube growth with prevention of pollen bursting, indicating an important role of EBL in reproduction and yield. In Cucumis sativus, manipulating BR synthesis through a chemical genetics approach affected tolerance to photo-oxidative, cold stresses and cucumber mosaic virus, and treatment of BR optimized the H2O2 levels inducing the expression of both regulatory (RBOH, MAPK1, and MAPK3) and stress defensive and antioxidant genes (Xia et al. 2009). However, the exact mechanisms mediating tolerance to extreme temperatures are still being investigated and experimental evidence is needed to consolidate the protective role of BR whether it is regulated independently or in combination with the stress-triggered mechanisms. An investigation of the possible link between the expression of temperature responsive genes and endogenous BR levels in model crops can lead to identification, enhancement, and better understanding of the tolerance mechanisms controlling plant growth.
Nutrient Stress
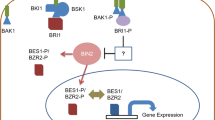
Optimal availability of mineral nutrients is believed to regulate growth by mediating changes at physiological, biochemical, and molecular levels (Ahanger et al. 2015, 2016, 2017a), and deficiency of essential inorganic nutrients affects growth adversely. Although phytohormones including BRs cannot replace nutrients in regulating the physiological and biochemical aspects of plant metabolism, they can compensate the need for nutrients to some extent. For example, foliar application of EBL caused a significant enhancement in the uptake of potassium and calcium, thereby enabling Triticum aestivum cultivars S-24 and MH-97 to counteract salt stress by maintaining the K/Na ratio (Janeczko et al. 2010). In addition, exogenous application of EBL (foliar and soaking) increased uptake of potassium, magnesium, and calcium, and reduced sodium and iron in wheat (Janeczko et al. 2010). In Cucumis sativus L., cv. ‘Jinyou No. 4’ adverse effects of excess calcium [80 mM Ca(NO3)2] on the uptake of important mineral elements, like potassium, phosphorous, magnesium, and manganese, were mitigated by foliar spray of EBL (0.01 M). EBL-treated plants maintained a higher K+/Na+ ratio and improved activity of Ca2+-ATPase, which mediated extrusion of excess Ca2+ from the cells to prevent toxicity (Yuan et al. 2015a, b). Song et al. (2016) demonstrated that application of EBL to Arachis hypogea mitigated the Fe-deficiency-induced oxidative stress by up-regulating the activity of nitrate reductase, the antioxidant system, and the accumulation of osmolytes, thereby reducing the production of ROS including superoxide and H2O2. An enhancement in the activity of H+, and Ca2+ ATPase and ferric(III)-chelate reductase (FCR) was also noticed in EBL-treated plants, and such EBL-induced increase in FCR activity mediated improved uptake of Fe(II) ions across the root plasma membrane under Fe-deficient conditions (Song et al. 2016). In Arabidopsis, Zhao et al. (2016) demonstrated that treatment of BR reduced ammonium toxicity by down-regulating the expression of ammonium transporter-1 (AMT1) expression in roots, which was confirmed by its reversion using the BR receptor BRI1 mutant bri1-5. The expression of AMT1 transporters (AMT1;1, AMT1;2, AMT1;3) is directly regulated by BR signaling transcription factor, BES1, and NH4+-mediated repression of AMT1 transporters was observed to suppress in a gain-of-function in ammonium-sensitive BES1 mutant (bes1-D). Further, by supplying exogenous glutamine, they confirmed that NH4+-mediated suppression of AMT1 was induced by nitrogen metabolites rather than NH4+ reflecting the activation of BES1 causing inhibition of the NH4+-mediated induction of GS/GOGAT. They concluded that BR-induced regulation of nitrogen uptake and assimilation occurs via the BR signaling pathway. 14-3-3 proteins are believed to regulate many cellular processes by binding to phosphorylated proteins and hence assist in plant nutrient signaling and hence can modulate nutrient metabolic pathways (Shin et al. 2011). This protein has been reported to control the nuclear location of key transcription factors BZR1/BZR2 involved in BR-mediated signaling via its interaction with cell-surface receptors (BRI1 and BAK1) and a GSK3 kinase (BIN2) in transgenic Arabidopsis (Gampala et al. 2007). Therefore, integrating the transgenic approach with the efficient uptake and compartmentation of beneficial mineral ions shall be a fruitful future exercise for biofortification in essential food crops. Additionally, the exclusion and sequestration of toxic ions can be achieved by visiting the role of BR in transport proteins involved therein.
Interplay Between BRs and Other Phytohormones, and the Underlying Signaling Events
For displaying regulated and continuous growth patterns, a controlled, integrated, and regulated coordination forms a preeminent part, of which the interaction between phytohormones forms an essential part. Like other phytohormones, BRs perform various important functions by interacting with other phytohormones including auxins, ABA, cytokinins, ethylene, gibberellic acid, polyamines, SA, and jasmonic acid (Bajguz and Hayat 2009; Saini et al. 2015). Such coordination between BRs and other phytohormones is regulated by several intriguing networking mechanisms including transcription and protein–protein interaction. Studies carried out so far have provided evidence about the underlying interactions between BRs and other phytohormones, and several studies have shown antagonistic as well as synergistic roles of BRs with other hormones.
Auxins
Several aspects of growth have been observed to be under regulated interaction between BRs and auxins; however, the actual mechanisms are still illusive (Maharjan et al. 2011; Saini et al. 2013, 2015; Liu et al. 2014; Ma et al. 2016). For example, in Arabidopsis, Mouchel et al. (2006) have demonstrated that auxin-induced root growth is regulated by BRAVIS RADIX (BRX) which they observed to be dependent on the threshold BR level. This BRX phenotype results due to root-specific deficiency of BRs and affects expression of other genes. However, such inhibition can be restored by exogenous treatment of BRs. Moreover, BR treatment rescued the brx phenotype fully or partially at the embryonic and post-embryonic stages, respectively. Exogenous application of auxins has been reported to promote the BR synthesis gene DWF4 (Maharjan and Choe 2011). Interestingly, positive regulation of DWF4 and CPD by BRX (Tanaka et al. 2005) has been reported, thereby demonstrating a direct linkage of auxin signaling with BR synthesis (Mouchel et al. 2006; Chung et al. 2011). Zhang et al. (2014) have demonstrated the synergistic role of BR and auxins in leaf lamina inclination. They reported that over-expression of OsGH3.5 and OsARF19 exhibited reduced free auxin content when lamina joint tissues were analyzed, altering the lamina inclination. OsBRI1 has been reported to be up-regulated under the positive regulation imparted by binding of OsARF19 to its promoter, and finally OsBRI1 activates the expression of OsBZR1, and subsequently the downstream signaling genes affect the lamina inclination (Zhang et al. 2014). Chaiwanon and Wang (2015) have reported an antagonistic interaction of auxins and BRs in Arabidopsis. Such interaction was reported to be linked by the BZR1 transcription factor and the optimal expression of BZR1 was dependent on BR catabolism, auxin biosynthesis, and BR-mediated signaling leading to activation of genes in the elongation zone concomitant with the repression in the quiescent center. Such spatio-temporal gene expression is regulated in a totally opposite way by auxins. IAA and BR regulate the dwarfism in autotetraploid apple (Malus × domestica) by up-regulating the expression of microRNA390 which contrary to diploids maintained shorter vertical length of cortical parenchyma cells. The dwarf phenotype in autotetraploid apple plants has been suggested due to the miR390 accumulation following genome doubling, and inducing up-regulated expression of trans-acting short-interfering RNA 3 (MdTAS3) which imparts down-regulation of MdARF3. This has been suggested to disturb the IAA and BR-mediated signal transduction pathway (Ma et al. 2016).
The phytochrome-mediated induction of shade avoidance syndrome in Arabidopsis thaliana has been reported to be under direct regulation of auxin/BR responses in the petiole and further evidence has been provided by using auxin-deficient (doc1/big) and BR-deficient (rot3/cyp90c1) mutants indicating that auxin and BR contribute equally for a normal petiole elongation response to the shade stimulus (Kozuka et al. 2010). Therefore, interactions between auxins and BRs are emerging as key regulators in the major growth and developmental processes being implicated at physiological, biochemical, and genetic levels. However, studying the involvement of other molecules in such interactions can be useful in categorizing them as independent or dependent regulatory crosstalk.
Gibberellic Acid
BRs regulate biosynthesis of gibberellins (GA) and it has been evidenced that BR-regulated transcription factor BES1 regulates GA biosynthesis by binding to the promoters. Arabidopsis thaliana deficient in BR signaling has been posited to exhibit severe impairment in the synthesis of bioactive GA which has been directly correlated with altered GA biosynthetic gene expression (Unterholzner et al. 2015). Quite strong evidence showing the involvement of BRs in GA-mediated growth regulation under normal as well as stressful conditions is available. For example, in rice while studying the interactive role of BRs and GA against the root oomycete Pythium graminicola, De Vleesschauwer et al. (2012) demonstrated the BR–GA antagonistic interaction in which P. graminicola made use of BRs as virulence factors taking over the rice BR machinery to counteract the disease. Using inhibitors of GA (uniconazole) and BR (brassinazole) synthesis, they demonstrated suppression of SA-mediated defenses by BRs occurs downstream of SA biosynthesis, but upstream of NPR1 and OsWRKY45, the master defense regulators. However, exogenously supplied BR enhanced GA-controlled immunity by interfering with GA metabolism ensuing oblique stabilization of protein and the central GA repressor, DELLA and SLENDER RICE1 (SLR1), respectively, indicating that the BR pathway is co-opted by Pythium graminicola to tempt antagonistic effect of SA- and GA-mediated defenses. The findings of Tong et al. (2014) have shown that an unknown mechanism underlying BR–GA crosstalk becomes operative depending on tissues and hormone levels. They reported that under physiological conditions, BR promotes cell elongation by inducing the synthesis of GA through up-regulating the expression of GA biosynthesis genes D18/GA3ox-2 in rice and consequently d18, and loss-of-function GA-signaling mutant exhibited decreased BR sensitivity. Exogenously supplied high concentrations of BR suppressed GA biosynthesis by up-regulating the expression of inactivation gene GA2ox-3 concomitant with repression of BR synthesis, thereby causing growth retardation by reducing endogenous hormone levels. They suggested that GA inhibits BR biosynthesis and signaling through a feedback mechanism. Direct signaling crosstalk between BR and GA results from the interaction between BZR1/BES1 and DELLAs—key transcriptional regulators of BR and GA signaling pathways, leading to controlled cell elongation in Arabidopsis. Such elongations occur after DELLA proteins affect protein stability leading to inhibition of the transcriptional activity of BZR1 and GA releases DELLA-mediated inhibition of BZR1 thereby inducing cell elongation (Li et al. 2012a, b; Li and He 2013).
Cytokinins
Cytokinins (CK) are another broad group of phytohormones involved in regulating a myriad of plant developmental and metabolic processes. Recent research has evidenced its crosstalk with other growth hormones either through direct interaction for eliciting a common growth response or imparting indirect interactive effect reflecting the role of other phytohormones. For example, Bao et al. (2004) reported the indirect crosstalk of CK–BR in regulating lateral root development by modulating the transport of auxins in which BR over-expresses the efflux carrier of auxins including the PIN gene. Bajguz and Piotrowska-Niczyporuk (2013, 2014) have reported the interactive effect of BR and CK on the growth and metabolite accumulation in Chlorella vulgaris and also observed a concentration-dependent increase due to application of BR and CK with a maximal positive obvious impact when BR and CK were supplemented together compared to their individual involvement, depicting the synergistic operation of BRs and CK activities. They also observed a positive influence of BR–CK interaction on auxin levels. BR has been reported to maintain the levels of CK in wheat (Yuldashev et al. 2012). Peleg et al. (2011) have reported that transgenic rice over-expressing the isopentenyltransferase (IPT) gene driven by P(SARK), a stress and maturation-induced promoter, resulted in enhanced synthesis of CK, and IPT-overexpressing plants were observed to exhibit increased water stress tolerance by displaying enhanced expression of BR-related genes including those implicated in the synthesis (DWF4, DWF5, HYD1) and signaling (BRI1, BZR1, BAK1, SERK1, BRH1). BR–CK crosstalk was observed to show a positive influence on the yield by modifying the source/sink relationships. Interaction of BRs with CK have been evidenced to regulate the CK-deficiency-induced excess root growth which is under direct influence of the CYTOKININ OXIDASE/DEHYDROGENASE-3 (CKX3) controlled by root-specific PYK10 promoter (Vercruyssen et al. 2011). Plants harboring PYK10-CKX3 exhibited higher leaf and root growth, and such synergism in growth enhancement was reported due to the ectopic expression of CKX3 and BRASSINOSTEROID INSENSITIVE-1, indicating crosstalk between CK and BR (Vercruyssen et al. 2011). Transcriptomic analysis of Gerbera hybrida has revealed the involvement of CK in the BR-mediated petal growth (Huang et al. 2017). Further studies employing gain/loss-of-function procedures can assist in unraveling the exact targets for enhancing the growth promoting signaling crosstalk between BR and CKs.
Abscisic Acid
ABA is known as an inhibitor of germination and a major factor of causing seed dormancy throughout the embryo maturation. In contrast, BRs are known to promote germination depicting the antagonistic interaction between ABA and BRs (Hu and Yu 2014). Such regulation of ABA-mediated growth inhibition by BR is regulated by the interaction of glycogen synthase kinase 3-like kinase BRASSINOSTEROID INSENSITIVE2 (BIN2; a critical repressor of BR signaling) with ABSCISIC ACID INSENSITIVE5 (ABI5; a bZIP transcription factor) in Arabidopsis. Furthermore, genetic analysis has revealed that the ABA-hypersensitive phenotype of BIN2-overexpressing plants requires ABI5, which is phosphorylated and stabilized by BIN2 in the presence of ABA, mediates ABA responses during seed germination and contrarily the exogenous sourced EBL inhibited the BIN2-induced regulation of ABI5 by repressing the BIN2-ABI5 cascade for antagonizing the ABA-mediated growth inhibition in plants (Hu and Yu 2014). Zhang et al. (2009) have also evidenced the involvement of BIN2 in ABA signaling in det2-1 and bri1 mutants of BR synthesis and signaling. However, while analyzing the ABA signaling mutants ABI1 and ABI2, PP2C family serine/threonine phosphatases were observed to be the major regulators of BR signaling in addition to BIN2. These ABA signaling components act downstream and upstream of BRI1 and BIN2 receptors, respectively (Zhang et al. 2009). In another study, growth regulation by BRs has been reported due to the activation of the BRASSINAZOLE-RESISTANT 1 (BZR1) family transcription factor, mediating partial crosstalk between BRs and ABA signaling (Yang et al. 2016). They have also reported that the bzr1-1D dominant mutant exhibited enhanced BR signaling and less sensitivity to ABA-induced primary root growth inhibition by suppressing the expression of the major ABA signaling component ABI5 by binding of BRZ1 strongly with several G-box cis-elements in the promoter of ABI5. However, the accurate underlying mechanisms are largely unknown. Recently, KIB1 has been identified as an F-box E3 ubiquitin ligase and believed to be an active player in BR signaling after inhibiting BIN2 by blocking its access to substrate (BZR1) through dephosphorylation-triggered degradation (Zhu et al. 2017).
BR regulated positively the stress responses in the ABA-deficient aba1-1 mutant of Arabidopsis leading to elevated thermo and salt tolerance (Divi et al. 2010). BR induces rapid and transient production of H2O2 and expression of NADPH oxidase and RBOH1 genes triggering generation of ABA in ABA biosynthetic mutants (notabilis; not), thereby leading to enhanced tolerance to cold and paraquat-induced oxidative damage (Zhou et al. 2014).
Ethylene
Like interactions with other phytohormones, more or less similar interactions in a different way are depicted between BRs and ethylene for growth regulation. For example, while studying the role of BRs and ethylene in regulation of gravitropism, it was found that auxins are involved in signaling (Vandenbussche et al. 2013). They reported the involvement of BRs in activation of AUX/IAA gene, causing a negative regulation of auxin signaling by inactivating the auxin response factors, ARF7 and ARF19 (the positive regulators of auxin signaling), resulting in inhibition of shoot gravitropism, which is however regulated positively by ethylene by down-regulating AUX/IAA and up-regulating ARF7 and ARF19. However, during root gravitropism an antagonistic regulatory role of BRs and ethylene has been reported (Buer et al. 2006; Vandenbussche et al. 2013). Moreover, exogenous BR treatment enhanced the synthesis of ethylene by enhancing the activity of the 1-aminocyclopropane-1-carboxylate synthase gene (Muday et al. 2012) and also preventing ubiquitination of ACS proteins (including ACS5, ACS6, ACS9) by 26S proteasome, thereby providing stability to ACS proteins depicting the BR-induced post-translational regulation. Further involvement of BRs in sharing crosstalk with ethylene-mediated defense responses has been confirmed by its involvement in attenuating the ethylene-induced xylanase (Eix), an effective elicitor of plant defense responses after silencing BAK1. The receptors for Eix belonging to the leucine-rich receptor-like protein (RLP) group and bearing signals for receptor-mediated endocytosis with the ability to bind Eix include LeEix1 and LeEix2; however, only LeEix2 has been recognized for its key role in inducing the defensive responses. Application of Eix is believed to induce heterodimerization of LeEix1 with LeEix2 resulting in considerable reduction in Eix-triggered internalization and signaling. However, in BAK1-silenced plants, Leix1 no longer attenuated the plant responses to Eix indicating the interaction between BRs and ethylene (Bar et al. 2010). Over-expression of the BR transcription factor brassinazole-resistant 1 (BZR1) has been reported to mediate BR signaling leading to regulation of many specific developmental processes like fruit ripening and the accumulation of carotenoids, sugars, and ascorbic acid, thereby improving the nutritional quality of ethylene-insensitive tomato in addition to the up-regulation of SIGLK2 involved in chloroplast development (Liu et al. 2014). Growth regulation induced by BRs is also controlled by the co-ordinated effect of ethylene and H2O2 with application of BR triggering ethylene signaling pathways leading to greater stress tolerance contrary to this silencing ethylene signaling that inhibited BR-mediated salt tolerance (Zhu et al. 2016). Further analysis confirmed the involvement of H2O2 in overall crosstalk between BR and ethylene. As already pointed out, it is not clear whether it is dependent or independent of ROS, and therefore further studies are needed to make final confirmation.
Jasmonic Acid
BRs have been reported to interact (antagonistic or synergistic) with jasmonic acid (JA) for developmental regulation during normal as well as stress conditions. For instance, Kitanaga et al. (2006) reported enhancement in endogenous levels of JA by BR application reflecting synergistic crosstalk of JA and BR in enhancing the expression of antimicrobial peptide coding thionin genes. BRs negatively regulate JA signaling in root growth inhibition. In Arabidopsis thaliana, F-box protein CORONATINE INSENSITIVE1 (COI1) plays a central role in JA signaling and partially suppressing COI1 mutants (psc1) has been demonstrated as a leaky mutation of DWARF4 (DWF4) encoding a key enzyme involved in BR biosynthesis thereby inhibiting root growth. JA-induced root growth inhibition is due to COI1-dependent JA-mediated down-regulation of DWF4 located downstream of COI1 in the JA signaling pathway (Ren et al. 2009). In tomato while studying the role of JA and BRs in framing an interactive defense against the insect Spodoptera frugiperda and pest Tuta absoluta, it was confirmed that BRs and JA affect the trichome density and allelochemical content in opposite fashion with increased pubescence, zingiberene biosynthesis, and expression of the proteinase inhibitor in the BR-deficient mutant dpy compared to the JA-insensitive jai1-1 mutant which displayed an opposite trend, causing enhanced creation of defensive traits. Additionally, experiments with the double mutant, dpy × jai1-1 revealed that jai1-1 is epistatic to dpy indicating the action of BR upstream of the JA signaling. This study has confirmed the negative regulation of JA-induced anti-herbivory by BR (Campos et al. 2009). BR mutants in Arabidopsis exhibited reduced JA-mediated anthocyanin accumulation and similar evidence has been claimed in wild type treated with the BR biosynthetic inhibitor, brassinazole, and exogenous BR reversed this effect significantly. Such effects have been attributed to down-regulation of ‘late’ anthocyanin biosynthesis genes like DFR, LDOX, and UF3GT and the Myb/bHLH transcription factors like PAP1, PAP2, and GL3 which form key components of the WD-repeat/Myb/bHLH transcriptional complexes implemented in regulation of ‘late’ anthocyanin biosynthesis genes (Peng et al. 2011). Therefore, it needs to be clarified whether it is negative or positive crosstalk that dominates in plants in response to different stresses and developmental transitions.
Salicylic Acid
Salicylic acid has been reported to mediate the expression of a master regulator of systemic acquired resistance usually promoted through expression or induction of pathogenesis-related genes—non-expressor of pathogenesis-related genes-1 (NPR1also called NIM1), and in Arabidopsis it has been reported that NPR1 is active only after induction of SA (Maier et al. 2011). Mutants defective in the NPR1 gene are sensitive to stresses (Clarke et al. 2009). Divi et al. (2010) demonstrated that EBL triggers the NPR1 gene expression to mediate the SA-induced defense against salt and thermal stress depicting EBL–SA interaction. However, EBL mediates induction of PATHOGENESIS-RELATED 1 (PR1) genes independent of SA. They further reported that BR shares transcriptional targets with other hormones. Not many reports are available pertaining to the transcriptomic and molecular genetic approaches for unraveling the BR–SA-mediated stress tolerance. Thus, future research work in this direction can be meaningful.
Conclusion and Future Prospects
From the present review, it can be concluded that BRs are effectively involved in diverse metabolic processes. BRs can regulate processes including photosynthesis, antioxidant metabolism, osmotic regulation, nitrogen metabolism, and plant–water relations under normal and stressful conditions, but limited information is available within the existing literature on how BR-induced regulation of these plant processes varies from species to species with BR application. From the past two decades, much progress has been made in identifying and characterizing the biosynthetic and metabolizing enzymes of BRs. However, research work regarding the interactive role of BRs with other phytohormones is currently being intensively executed for making the elusive interactive events clear which is expected to add to the current understanding of the BR-induced growth regulation mechanisms. In addition, much work is to be done in identifying the receptors involved in BR signaling while regulating the metabolic processes from germination to senescence. Identification and characterization of key genes regulating the hormonal crosstalk and the underlying signaling also need to be explored. Involvement of transcription factors in integrating the beneficial crosstalk of BR with multiple phytohormones and the subsequent identification of gene and gene products posing a negative regulatory effect on growth need clarification. Knocking out such growth inhibitory regulating gene products shall be a way forward in understating and improving plant growth. In addition, optimizing the endogenous levels of phytohormones for maximizing the stress-responsive crosstalk mechanisms between multiple hormones needs attention.
References
Aghdam MS, Mohammadkhani N (2014) Enhancement of chilling stress tolerance of tomato fruit by postharvest brassinolide treatment. Food Bioproc Technol 7:909–914
Aghdam MS, Asghari M, Farmani B, Mohayeji M, Moradbeygi H (2012) Impact of postharvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. Sci Hort 144:116–120
Ahanger MA, Agarwal RM (2017a) Potassium improves antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L.). Protoplasma 254:1471–1486
Ahanger MA, Agarwal RM (2017b) Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol Biochem 115:449–460
Ahanger MA, Tyagi SR, Wani MR, Ahmad P (2014) Drought tolerance: roles of organic osmolytes, growth regulators and mineral nutrients. In: Ahmad P, Wani MR (eds) Physiological mechanisms and adaptation strategies in plants under changing environment Volume Ist. Springer, New York, pp 25–56
Ahanger MA, Agarwal RM, Tomar NS, Shrivastava M (2015) Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L. cultivar Kent). J Plant Int 10:211–223
Ahanger MA, Moad-Talab N, Abd-Allah EF, Ahmad P, Hajiboland R (2016) Plant growth under drought stress: Significance of mineral nutrients. In Ahmad P. (eds) Water stress and Crop Plants: A sustainable approach Wiley Blackwell, New York 649–668
Ahanger MA, Akram NA, Ashraf M, Alyemni MN, Wijaya L, Ahmad P (2017a) Plant responses to environmental stresses – from gene to biotechnology. Annals Bot (AoB) Plants. https://doi.org/10.1093/aobpla/plx025
Ahanger MA, Tomar NS, Tittal M, Argal S, Agarwal RM (2017b) Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol Plants 23:731–744
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of Enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30(3):161–175
Ahmad P, Nabi G, Ashraf M (2011) Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. & Coss.] plants can be alleviated by salicylic acid. S Afr J Bot 77:36–44
Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran LSP (2015) Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS ONE 10(1):e0114571
Ahmad P, Latef AA, Abd_Allah EF, Hashem A, Sarwat M, Anjum NA, Gucel S (2016a) Calcium and potassium supplementation enhanced growth, osmolytes, secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.). Front Plant Sci 7:513
Ahmad P, Latef AA, Hashem A, Abd_Allah EF, Gucel S, Tran LSP (2016b) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Egamberdieva D, Bhardwaj R, Ashraf M (2017a) Zinc application mitigates the adverse effects of NaCl stress on mustard [Brassica juncea (L.) Czern & Coss] through modulating compatible organic solutes, antioxidant enzymes, and flavonoid content. J Plant Inter 12:429–437
Ahmad P, Ahanger MA, Egamberdieva D, Alam P, Alyemeni MN, Ashraf M (2017b) Modification of osmolytes and antioxidant enzymes by 24-epibrassinolide in chickpea seedlings under mercury (hg) toxicity. J Plant Growth Regul 37:309–322
Ahmad P, Ahanger MA, Alam P, Alyemeni MN, Wijaya L, Ali S, Ashraf M (2018a) Silicon (Si) supplementation alleviates NaCl toxicity in mung bean [Vigna radiata (L.) Wilczek] through the modifications of physio-biochemical attributes and key antioxidant enzymes. J Plant Growth Regul. https://doi.org/10.1007/s00344-018-9810-2
Ahmad P, Ahanger MA, Alam P, Alyemeni MN (2018b) Modification of osmolytes and antioxidant enzymes by 24-epibrassinolide in chickpea seedlings under mercury (Hg) toxicity. J Plant Growth Regul 37:309–322
Ali B, Hayat S, Ahmad A (2007) 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinumL.). Environ Exp Bot 59:217–223
Ali B, Hasan SA, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A (2008a) A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ Exp Bot 62:153–159
Ali B, Hayat S, Fariduddin Q, Ahmad A (2008b) 24-Epibrassinolide protects against the stress generated by salinity and nickel in. Brassica juncea Chemosphere 72:1387–1392
Anjum SA, Wang LC, Farooq M, Hussain M, Xue LL, Zou CM (2011) Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci 197:177–185
Anuradha S, Rao SSR (2003) Application of brassinosteroids to rice seeds (Oryza sativa L.) reduced the impact of salt stress on growth, prevented photosynthetic pigment loss and increased nitrate reductase activity. Plant Growth Regul 40:29–32
Anuradha S, Rao SSR (2007) The effect of brassinosteroids on radish (Raphanus sativus L.) seedlings growing under cadmium stress. Plant Soil Environ 53:465–472
Arora P, Bhardwaj R, Kanwar MK (2010a) 24-epibrassinolide regulated diminution of Cr metal toxicity in Brassica juncea L. plants. Braz J Plant Physiol 22:159–165
Arora P, Bhardwaj R, Kanwar MK (2010b) 24-epibrassinolide induced antioxidative defense system of Brassica juncea L. under Zn metal stress. Physiol Mol Biol Plants 16:285–293
Asgher M, Khan NA, Khan MIR, Fatma M, Masood A (2014) Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotoxicol Environ Saf 106:54–61
Bajguz A (2002) Brassinosteroids and lead as stimulators of phytochelatins synthesis in Chlorella vulgaris. J Plant Physiol 159:321–324
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Bajguz A, Piotrowska-Niczyporuk A (2013) Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol Biochem 71:290–297
Bajguz A, Piotrowska-Niczyporuk A (2014) Interactive effect of brassinosteroids and cytokinins on growth, chlorophyll, monosaccharide and protein content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol Biochem 80:176–183
Bao F, Shen J, Brady SR, Muday GK, Asami T, Zhenbiao Y (2004) Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol 134:1624–1631
Bar M, Sharfman M, Ron M, Avni A (2010) BAK1 is required for the attenuation of ethylene-inducing xylanase (Eix)-induced defense responses by the decoy receptor LeEix1. Plant J 63:791–800
Behnamnia M (2015) Protective roles of brassinolide on tomato seedlings under drought stress. Int J Agri Crop Sci 8:455–462
Buer CS, Sukumar P, Muday GK (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol 140:1384–1396
Calzadilla PI, Maiale SJ, Ruiz OA, Escaray FJ (2016) Transcriptome response mediated by cold stress in Lotus japonicus. Front Plant Sci 7:374. https://doi.org/10.3389/fpls.2016.00374
Campos ML, deAlmeida M, Rossi ML, Martinelli AP, Junior CGL, Figueira A, Rampelotti-Ferreira FT, Vendramim JD, Benedito VA, Peres LEP (2009) Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. J Exp Bot 60:4347–4361
Cao YY, Zhao H (2008) Protective roles of brassinolide in rice seedlings under heat stress. Rice Sci 15:63–68
Cerezoa M, Garcia-Agustina P, Sernab MD, Primo-Millob E (1997) Kinetics of nitrate uptake by Citrus seedlings and inhibitory effects of salinity. Plant Sci 126:105–112
Chaiwanon J, Wang ZY (2015) Spatio-temporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in Arabidopsis roots. Curr Biol 25:1031–1042
Chen J, Nolan TM, Ye H, Zhang M, Tong H, Xin P, Chu J, Chu C, Li Z, Yin Y (2017) Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 29:1425–1439
Choudhary SP, Kanwar M, Bhardwaj R, Gupta BD, Gupta RK (2011) Epibrassinolide ameliorates Cr(VI) stress via influencing the levels of indole-3-acetic acid, abscisic acid, polyamines and antioxidant system of radish seedlings. Chemosphere 84:592–600
Chung Y, Maharjan PM, Lee O, Fujioka S, Jang S, Kim B, Takatsuto S, Tsujimoto M, Kim H, Cho S, Park T, Cho H, Hwang I, Choe S (2011) Auxin stimulates DWARF4 expression and brassinosteroid biosynthesis in Arabidopsis. Plant J 66(4):564–578
Clarke SM, Cristescu SM, Miersch O, Harren FJ, Wasternack C, Mur LA (2009) Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol 182:175–187
Clouse SD (2015) A history of brassinosteroid research from 1970 through 2005: thirty-five years of phytochemistry, physiology, genes, and mutants. J Plant Growth Regul 34:828–844
Coban O, Baydar NG (2016) Brassinosteroid effects on some physical and biochemical properties and secondary metabolite accumulation in peppermint (Mentha piperita L.) under salt stress. Ind Crops Prod 86:251–258
De Vleesschauwer D, Van Buyten E, Satoh K, Balidion J, Mauleon R, Choi IR, Ver-Cruz C, Kikuchi S, Hofte M (2012) Brassinosteroids antagonize gibberellin– and salicylate-mediated root immunity in rice. Plant Physiol 158:1833–1846
Deng XG, Zhu T, Peng XJ, Xi DH, Guo H, Yin Y, Zhang DW, Lin HH (2016) Role of brassinosteroid signaling in modulating Tobacco mosaic virus resistance in Nicotiana benthamiana. Sci Rep 6:20576. https://doi.org/10.1038/srep20579
Dhaubhadel S, Chaudhary KS, Dobinson KF, Krishna P (1999) Treatment of 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol 40:333–342
Dhaubhadel S, Browning KS, Gallie DR, Krishna P (2002) Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J 29:681–691
Divi UK, Rahman T, Krishna P (2010) Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol 10:151
Divi UK, Rahman T, Krishna P (2016) Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. Plant Biotech J 14:419–432
El-Mashad AAA, Mohamed HI (2012) Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 249:625–635
Ernst WHO (1980) Biochemical aspects of cadmium in plants. In: Nriagu JO (ed) Cadmium in the environment, Wiley, New York, pp 639–653
Fariduddin Q, Khanam S, Hasan SA, Ali B, Hayat S, Ahmad A (2009) Effect of 28-homobrassinolide on the drought stress-induced changes in photosynthesis and antioxidant system of Brassica juncea L. Acta Physiol Plant 31:889–897
Fariduddin Q, Yusuf M, Chalkoo S, Hayat S, Ahmad A (2011) 28-Homobrassinolide improves growth and photosynthesis in Cucumis sativus L. through an enhanced antioxidant system in the presence of chilling stress. Photosynthetica 49:55–64
Fariduddin Q, Khalil RRAE, Mir BA, Yusuf M, Ahmad A (2013) 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ Monit Assess 185:7845–7856
Fariduddin Q, Mir BA, Yusuf M, Ahmad A (2014) 24-epibrassinolide and/or putrescine trigger physiological and biochemical responses for the salt stress mitigation in Cucumis sativus L. Photosynthetica 52:464–474
Gampala SS, Kim TW, He JX, Tang W, Deng Z, Bai MY, Guan S, Lalonde S, Sun Y, Gendron JM, Chen H, Shibagaki N, Ferl RJ, Ehrhardt D, Chong K, Burlingame AL, Wang ZY (2007) An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev Cell 13:177–189
Gonzalez-Olmedo JL, Cordova A, Aragon CE, Pina D, Rivas M, Rodrıguez R (2005) Effect of an analogue of brassinosteroid on FHIA-18 plantlets exposed to thermal stress. InfoMusa 14:18–20
Greeff C, Roux M, Mundy J, Petersen M (2012) Receptor-like kinase complexes in plant innate immunity. Front Plant Sci 3:209
Gruszka D, Janeczko A, Dziurka M, Pociecha E, Oklestkova J, Szarejko I (2016) Barley brassinosteroid mutants provide an insight into phytohormonal homeostasis in plant reaction to drought stress. Front Plant Sci 7:1824. https://doi.org/10.3389/fpls.2016.01824
Hasan SA, Hayat S, Ali B, Ahmad A (2008) 28-Homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidants. Environ Poll 151:60–66
Hasan SA, Hayat S, Ahmad A (2011) Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 84:1446–1451
Hatfield JL, Prueger JH (2015) Temperature extremes: Effect on plant growth and development. Weather Clim Ext 10:4–10
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41
Hayat S, Hasan SA, Yusuf M, Hayat Q, Ahmad A (2010) Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ Exp Bot 69:105–112
Hayat S, Alyemeni M, Hasan S (2012) Foliar spray of brassinosteroid enhances yield and quality of Solanum lycopersicum under cadmium stress. Saudi J Biol Sci 19:325–335
Hu Y, Yu D (2014) BRASSINOSTEROID INSENSITIVE2 Interacts with ABSCISIC ACID INSENSITIVE5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in Arabidopsis. Plant Cell 26:4394–4408
Hu WH, Wu Y, Zeng JZ, He L, Zeng QM (2010) Chill-induced inhibition of photosynthesis was alleviated by 24-epibrassinolide pretreatment in cucumber during chilling and subsequent recovery. Photosynthetica 48:537–544
Hu WH, Yan XH, Xiao YA, Zeng JJ, Qi HJ, Ogweno JO (2013) 24-Epibrassinosteroid alleviate drought-induced inhibition of photosynthesis in Capsicum annuum. Sci Hortic 150:232–237
Huang G, Han M, Yao W, Wang Y (2017) Transcriptome analysis reveals the regulation of brassinosteroids on petal growth in Gerbera hybrida. Peer J 5:e3382. https://doi.org/10.7717/peerj.3382
Iqbal N, Umar S, Khan NA (2015) Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol 178:84–91
Janeczko A, Gullner G, Skoczowski A, Dubert F, Barna B (2007) Effects of brassinosteroid in filtration prior to cold treatment on ion leakage and pigment contents in rape leaves. Biol Plant 51:355–358
Janeczko A, Biesaga-Koscielniak J, Oklestkova J, Filek M, Dziurka M, Szarek-Łukaszewska M, Koscielniak J (2010) Role of 24-epibrassinolide in wheat production: physiological effects and uptake. J Agron Crop Sci 196:311–321
Jatav KS, Agarwal RM, Tomar NS, Tyagi SR (2014) Nitrogen metabolism, growth and yield responses of wheat (Triticum aestivum L.) to restricted water supply and varying potassium treatments. J Indian Bot Soc 93:177–189
Jiang YP, Huang LF, Cheng F, Zhou YH, Xia XJ, Mao WH, Shi K, Yu JQ (2013) Brassinosteroids accelerate recovery of photosynthetic apparatus from cold stress by balancing the electron partitioning, carboxylation and redox homeostasis in cucumber. Physiol Plant 148:133–145
Jin SH, Li XQ, Wang G, Zhu XT (2015) Brassinosteroids alleviate high-temperature injury in Ficus concinna seedlings via maintaining higher antioxidant defence and glyoxalase systems. AoB Plants 7:plv009. https://doi.org/10.1093/aobpla/plv009
Kagale S, Divi UK, Krochko JE, Keller WA, Krishna P (2007) Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225:353–364
Kanwar MK, Poonam Bhardwaj R (2015) Arsenic inducted modulation of antioxidative defense system and brassinosteroids in Brassica juncea. L Ecotox Environ Safe 115:119–125
Kapoor D, Rattan A, Gautam V, Kapoor N, Bhardwaj R (2014) 24-epibrassinolide mediated changes in photosynthetic pigments and antioxidative defence system of radish seedlings under cadmium and mercury stress. J Stress Physiol Biochem 10:110–121
Kaur H, Sirhindi G, Bhardwaj R, Alyemeni MN, Siddique KHM, Ahmad P (2018) 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt- and temperature-induced oxidative stress in Brassica juncea. Sci Rep 8:8735
Kazemi-Shahandashti SS, Maali-Amiri R, Zeinali H, Khazaei M, Talei A, Ramezanpour SS (2014) Effect of short-term cold stress on oxidative damage and transcript accumulation of defense-related genes in chickpea seedlings. J Plant Physiol 171:1106–1116
Kemmerling B, Nurnberger T (2008) Brassinosteroid-independent functions of the BRI1-associated kinase BAK1/SERK3. Plant Sig Beh 3:116–118
Khan M, Rozhon W, Bigeard J, Pflieger D, Husar S, Pitzschke A, Teige M, Jonak C, Hirt H, Poppenberger B (2013) Brassinosteroid-regulated GSK3/Shaggy-like kinases phosphorylate mitogen-activated protein (MAP) kinase kinases, which control stomata development in Arabidopsis thaliana. J Biol Chem 288:7519–7527
Khan MIR, Nazir F, Asgher M, Per TS, Khan NA (2015) Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18
Kim TW, Michniewicz M, Bergmann DC, Wang ZY (2011) Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 482:419–422
Kitanaga Y, Jian C, Hasegawa M, Yazaki J, Kishimoto N, Kikuchi S, Nakamura H, Ichikawa H, Asami H, Yoshida S, Yamaguchi I, Suzuki Y (2006) Sequential regulation of gibberellin, brassinosteroid, and jasmonic acid biosynthesis occurs in rice coleoptiles to control the transcript levels of anti-microbial thionin genes. Biosci Biotech Biochem 70:2410–2419
Kocova M, Rothova O, Hola D, Kvasnica M, Kohout L (2010) The effects of brassinosteroids on photosynthetic parameters in leaves of two field-grown maize inbred lines and their F1 hybrid. Biol Plant 54:785–788
Kozuka T, Kobayashi J, Horiguchi G, Demura T, Sakakibara H, Tsukaya H, Nagatani A (2010) Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol 153:1608–1618
Krumova S, Zhiponova M, Dankov K, Velikova V, Balashev K, Andreeva T, Russinova E, Taneva S (2013) Brassinosteroids regulate the thylakoid membrane architecture and the photosystem II function. J Photochem Photobio B 126:97–104
Li J (2003) Brassinosteroids signal through two receptor-like kinases. Curr Opin Plant Biol 6:494–499
Li J (2005) Brassinosteroid signaling: from receptor kinases to transcription factors. Curr Opin Plant Biol 8:526–531
Li QF, He JX (2013) Mechanisms of signaling crosstalk between brassinosteroids and gibberellins. Plant Sig Behav 8:e24686. https://doi.org/10.4161/psb.24686
Li QF, Wang C, Jiang L, Sun SS, He JX (2012a) An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci Signal 5(244):ra72. https://doi.org/10.1126/scisignal.2002908
Li YH, Liu YJ, Xu XL, Jin M, An LZ, Zhang H (2012b) Effect of 24-epibrassinolide on drought stress-induced changes in Chorispora bungeana. Bio Plant 56:192–196
Li M, Ahammed GJ, Li C, Bao X, Yu J, Huang C, Yin H, Zhou J (2016) Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front Plant Sci 7:615. https://doi.org/10.3389/fpls.2016.00615
Liu YJ, Zhao ZG, Si J, Di CX, Han J, An LZ (2009) Brassinosteroids alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora bungeana. Plant Growth Regul 59:207–214
Liu J, Rowe J, Lindsey K (2014) Hormonal crosstalk for root development: a combined experimental and modeling perspective. Front Plant Sci 5:116. https://doi.org/10.3389/fpls.2014.00116
Liu J, Zhang D, Sun X, Ding T, Lei B, Zhang C (2017) Structure-activity relationship of brassinosteroids and their agricultural practical usages. Steroids 124:1–17
Lu XM, Yang W (2013) Alleviation effects of brassinolide on cucumber seedlings under NaCl stress. Ying Yong Sheng Tai Xue Bao 24:1409–1414
Ma Y, Xue H, Zhang L, Zhang F, Ou C, Wang F, Zhang Z (2016) Involvement of auxin and brassinosteroid in dwarfism of autotetraploid apple (Malus × domestica). Sci Rep 6:26719. https://doi.org/10.1038/srep26719
Machado RMA, Serralheiro RP (2017) Soil salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Hort 3:30. https://doi.org/10.3390/horticulturae3020030
Maharjan PM, Choe S (2011) High temperature stimulates DWARF4 (DWF4) expression to increase hypocotyle elongation in Arabidopsis. J Plant Biol 54:425–429
Maharjan PM, Schulz B, Choe S (2011) BIN2/DWF12 antagonistically transduces brassinosteroid and auxin signals in the roots of Arabidopsis. J Plant Biol 54:126–134
Mahesh K, Balaraj P, Ramakrishna B, Rao SSR (2013) Effect of brassinosteroids on germination and seedling growth of radish (Raphanus sativus L.) under PEG-6000 induced water stress. Am J Plant Sci 4:2305–2313
Maier F, Zwicker S, Huckelhoven A, Meissner M, Funk J, Pfitzner AJ, Pfitzner UM (2011) NONEXPRESSOR OF PATHOGENESIS-RELATED PROTEINS1 (NPR1) and some NPR1-related proteins are sensitive to salicylic acid. Mol Plant Pathol 12:73–91
Mazorra LM, Holton N, Bishop GJ, Nunez M (2011) Heat shock response in tomato brassinosteroid mutants indicates that thermotolerance is independent of brassinosteroid homeostasis. Plant Physiol Biochem 49:1420–1428
Mishra MK, Chaturvedi P, Singh R, Singh G, Sharma LK, Pandey V, Kumari N, Misra P (2013) Overexpression of wssgtl1 gene of Withania somnifera enhances salt tolerance, heat tolerance and cold acclimation ability in transgenic Arabidopsis Plants. PLoS ONE 8:e63064. https://doi.org/10.1371/journal.pone.0063064
Mouchel CF, Osmont KS, Hardtke CS (2006) BRX mediates feedback between brassinosteroid and auxin signaling in root growth. Nature 443:458–461
Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17:181–195
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59(1):651–681
Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogue S (2008) Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27:49–57
Ogweno JO, Hu WH, Song XS, Shi K, Mao WH, Zhou YH, Yu JQ (2010) Photoinhibition-induced reduction in photosynthesis is alleviated by abscisic acid, cytokinin and brassinosteroid in detached tomato leaves. Plant Growth Regul 60:175–182
Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E (2011) Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotech J 9:747–758
Peng Z, Han C, Yuan L, Zhang K, Huang H, Ren C (2011) Brassinosteroid enhances jasmonate-induced anthocyanin accumulation in Arabidopsis seedlings. J Integr Plant Biol 53:632–640
Qu T, Liu R, Wang W, An L, Chen T, Liu G, Zhao Z (2011) Brassinosteroids regulate pectin methylesterase activity and AtPME41 expression in Arabidopsis under chilling stress. Cryobiology 63:111–117
Rady MM (2011) Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci Hortic 129:232–237
Ramakrishna B, Rao SSR (2015) Foliar application of brassinosteroids alleviates adverse effects of zinc toxicity in radish (Raphanus sativus L.) plants. Protoplasma 252:665–677
Ren C, Han C, Peng W, Huang Y, Peng Z, Xiong X, Zhu Q, Gao B, Xie D (2009) A leaky mutation in DWARF4 reveals an antagonistic role of brassinosteroid in the inhibition of root growth by jasmonate in Arabidopsis. Plant Physiol 151:1412–1420
Sahni S, Prasad BD, Liu Q, Grbic V, Sharpe A, Singh SP, Krishna P (2016) Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci Rep 6:28298. https://doi.org/10.1038/srep28298
Saini S, Sharma I, Kaur N, Pati PK (2013) Auxin: a master regulator in plant root development. Plant Cell Rep 32:741–757
Saini S, Sharma I, Pati PK (2015) Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Front Plant Sci 6:950. https://doi.org/10.3389/fpls.2015.00950
Shahana T, Rao PA, Ram SS, Sujatha E (2015) Mitigation of drought stress by 24-epibarassinolide and 28-homobrassinolide in pigeon pea seedlings. Int J Multi Curr Res 3:905–911
Shahbaz M, Ashraf M (2007) Influence of exogenous application of brassinosteroid on growth and mineral nutrients of wheat (Triticum aestivum L.) under saline conditions. Pak J Bot 39:513–522
Shakirova FM, Bezrukova M (1998) Effect of 24-epibrassinolide and salinity on the levels of ABA and lectin. Russ J Plant Physiol 45:388–391
Shakirova FM, Bezrukova MV, Avalbaev AM, Gimalov FR (2002) Stimulation of wheat germ agglutinin gene expression in root seedlings by 24-epibrassinolide. Russ J Plant Physiol 49:225–228
Sharma P, Bhardwaj R, Arora N, Arora HK (2007) Effect of 28-homobrassinolide on growth, zinc metal uptake and antioxidative enzyme activities in Brassica juncea L. seedlings. Braz J Plant Physiol 19:203–210
Sharma I, Pati PK, Bhardwaj R (2010) Regulation of growth and antioxidant enzyme activities by 28-homobrassinolide in seedlings of Raphanus sativus L. under cadmium stress. Indian J Biochem Biophys 47:172–177
Sharma I, Ching E, Saini S, Bhardwaj R, Pati PK (2013) Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol Biochem 69:17–26
Sharma P, Kumar A, Bhardwaj R (2016) Plant steroidal hormone epibrassinolide regulate – heavy metal stress tolerance in Oryza sativa L. by modulating antioxidant defense expression. Environ Exp Bot 122:1–9
Shin R, Jez JM, Basra A, Zhang B, Schachtman DP (2011) 14-3-3 Proteins fine-tune plant nutrient metabolism. FEBS Lett 585:143–147
Siboza XI, Bertling I, Odindo AO (2014) Salicylic acid and methyl jasmonate improve chilling tolerance in cold-stored lemon fruit (Citrus limon). J Plant Physiol 171:1722–1731
Singh I, Shono M (2005) Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid on thermotolerance of tomato. Plant Growth Regul 47:111–119
Singh I, Kumar U, Singh SK, Gupta C, Singh M, Kushwaha SR (2012) Physiological and biochemical effect of 24-epibrassinoslide on cold tolerance in maize seedlings. Physiol Mol Biol Plants 18:229–236
Soares C, de Sousa A, Pinto A, Azenha M, Teixeira J, Azevedo RA, Fidalgo F (2016) Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ Exp Bot 122:115–125
Song S, Liu W, Guo S, Shang Q, Zhang Z (2006) Salt resistance and its mechanism of cucumber under effects of exogenous chemical activator. Yingyong Sheng Xuebao 17:1871–1876
Song YL, Dong YJ, Tian XY, Kong J, Bai XY, Xu LL, He ZL (2016) Role of foliar application of 24-epibrassinolide in response of peanut seedlings to iron deficiency. Biol Plant 60:329–342
Talaat NB, Shawky BT (2016) Dual application of 24-epibrassinolide and spermine confers drought stress tolerance in maize (Zea mays L.) by modulating polyamine and protein metabolism. J Plant Growth Regul 35:518–533
Talaat NB, Shawky BT, Ibrahim AS (2015) Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ Exp Bot 113:47–58
Tanaka K, Asami T, Yoshida S, Nakamura Y, Matsuo T, Okamoto S (2005) Brassinosteroid homeostasis in Arabidopsis is ensured by feedback expressions of multiple genes involved in its metabolism. Plant Physiol 138:1117–1125
Thussagunpanit J, Jutamanee K, Sonjaroon W, Kaveeta L, Chaiarree W, Pankean P, Suksamrarn A (2015) Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynthetica 53:312–320
Tong H, Xiao Y, Liu D, Gao S, Liu L, Yin Y, Jin Y, Qian Q, Chu C (2014) Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 26:4376–4393
Turkan I, Demiral T (2009) Recent developments in understanding salinity tolerance. Environ Exp Bot 67:2–9
Unterholzner SJ, Rozhon W, Papacek M, Ciomas J, Lange T, Kugler KG, Mayer KF, Sieberer T, Poppenberger B (2015) Brassinosteroids are master regulators of gibberellin biosynthesis in Arabidopsis. Plant Cell 27:2261–2272
Upreti KK, Murti GSR (2004) Effects of brassinosteroids on growth, nodulation, phytohormone content and nitrogenase activity in French bean under water stress. Biol Plant 48:407–411
Vandenbussche F, Callebert P, Zadnikova P, Benkova E, VanDer Straeten D (2013) Brassinosteroid control of shoot gravitropism interacts with ethylene and depends on auxin signaling components. Am J Bot 100:215–225
Vercruyssen L, Gonzalez N, Werner T, Schmülling T, Inze D (2011) Combining enhanced root and shoot growth reveals crosstalk between pathways that control plant organ size in Arabidopsis. Plant Physiol 155:1339–1352
Wang Q, Ding T, Gao L, Pang J, Yang N (2012) Effect of brassinolide on chilling injury of green bell pepper in storage. Sci Hortic 144:195–200
Xi Z, Wang Z, Fang Y, Hu Z, Hu Y, Deng M, Zhang Z (2013) Effects of 24-epibrassinolide on antioxidantion defense and osmoregulation systems of young grapevines (V. vinifera L.) under chilling stress. Plant Growth Regul 71:57–65
Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ (2009) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150:801–814
Yadava P, Kaushal J, Gautam A, Parmar H, Singh I (2016) Physiological and biochemical effects of 24-epibrassinolide on heat-stress adaptation in maize (Zea mays L.). Nat Sci 8:171–179
Yang GX, Jan A, Shen SH, Ishikawa JYM, Shimatani Z, Kishimoto N, Matsumoto SKH, Komatsu S (2004) Microarray analysis of brassinosteroids- and gibberellin-regulated gene expression in rice seedlings. Mol Genet Genom 271:468–478
Yang CJ, Zhang C, Lu YN, Jin JQ, Wang XL (2011) The mechanisms of brassinosteroids’ action: from signal transduction to plant development. Mol Plant 4:588–600
Yang X, Bai Y, Shang J, Xin R, Tang W (2016) The antagonistic regulation of abscisic acid-inhibited root growth by brassinosteroids is partially mediated via direct suppression of ABSCISIC ACID INSENSITIVE 5 expression by BRASSINAZOLE RESISTANT 1. Plant Cell Environ 39:1994–2003
Ye H, Liu S, Tang B, Chen J, Xie Z, Nolan TM, Jiang H, Guo H, Lin HY, Li L, Wang Y, Tong H, Zhang M, Chu C, Li Z, Aluru M, Aluru S, Schnable PS, Yin Y (2017) RD26 mediates crosstalk between drought and brassinosteroid signalling pathways. Nat Comm 8:14573. https://doi.org/10.1038/ncomms14573
Yuan L, Zhua S, Shu S, Sun J, Guo S (2015a) Regulation of 2, 4-epibrassinolide on mineral nutrient uptake and ion distribution in Ca(NO3)2 stressed cucumber plants. J Plant Physiol 188:29–36
Yuan LB, Peng ZH, Zhi TT, Zho Z, Liu Y, Zhu Q, Xiong XY, Ren CM (2015b) Brassinosteroid enhances cytokinin-induced anthocyanin biosynthesis in Arabidopsis seedlings. Biol Plant 59:99–105
Yuldashev R, Avalbaev A, Bezrukova M, Vysotskaya L, Khripach V, Shakirova F (2012) Cytokinin oxidase is involved in the regulation of cytokinin content by 24-epibrassinolide in wheat seedlings. Plant Physiol Biochem 55:1–6
Yusuf M, Fariduddin Q, Ahmad A (2012) 24-Epibrassinolide modulates growth, nodulation, antioxidant system, and osmolyte in tolerant and sensitive varieties of Vigna radiata under different levels of nickel: a shot-gun approach. Plant Physiol Biochem 57:143–153
Yusuf M, Fariduddin Q, Ahmad I, Ahmad A (2014) Brassinosteroid-mediated evaluation of antioxidant system and nitrogen metabolism in two contrasting cultivars of Vigna radiata under different levels of nickel. Physiol Mol Biol Plant 20:449–460
Zhang S, Hu J, Zhang Y, Xie XJ, Knapp A (2007) Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust J Agric Res 58:811–815
Zhang S, Cai Z, Wang X (2009) The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc Natl Acad Sci USA 106:4543–4548
Zhang S, Wang S, Xu Y, Yu C, Shen C, Qian Q, Geisler M, Jiang de A, Qi Y (2014) The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell Environ 38:638–654
Zhao BT, Zhu XF, Jung JH, Xuan YH (2016) Effect of brassinosteroids on ammonium uptake via regulation of ammonium transporter and N-metabolism genes in Arabidopsis. Biol Plant 60:563–571
Zhou A, Wang H, Walker JC, Li J (2004) BRL1, a leucine-rich repeat receptor-like protein kinase, is functionally redundant with BRI1 in regulating Arabidopsis brassinosteroid signaling. Plant J 40:399–409
Zhou J, Wang J, Li X, Xia XJ, Zhou YH, Shi K, Chen Y, Yu JQ (2014) H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J Exp Bot 65:4371–4383
Zhu W, Wang H, Fujioka S, Zhou T, Tian H, Tian W, Wang X (2013) Homeostasis of brassinosteroids regulated by DRL1, a putative acyltransferase in Arabidopsis. Mol Plant 6:546–558
Zhu T, Deng X, Zhou X, Zhu L, Zou L, Li P, Zhang D, Lin H (2016) Ethylene and hydrogen peroxide are involved in brassinosteroid induced salt tolerance in tomato. Sci Rep 6:35392. https://doi.org/10.1038/srep35392
Zhu J, Li Y, Cao DM, Yang H, Oh E, Bi Y, Zhu S, Wang ZY (2017) The F-box protein KIB1 mediates brassinosteroid-induced inactivation and degradation of GSK3-like kinases in Arabidopsis. Mol Cell 66:648–657
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict exists among the authors.
Rights and permissions
About this article
Cite this article
Ahanger, M.A., Ashraf, M., Bajguz, A. et al. Brassinosteroids Regulate Growth in Plants Under Stressful Environments and Crosstalk with Other Potential Phytohormones. J Plant Growth Regul 37, 1007–1024 (2018). https://doi.org/10.1007/s00344-018-9855-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9855-2