Abstract
Main conclusion
Solar ultraviolet irradiation regulates anthocyanin synthesis in apple peel by modulating the production of reactive oxygen species via plasma membrane NADPH oxidase instead of other pathways.
The synthesis of anthocyanin in apple peels is dependent upon solar irradiation. Using 3-mm commercial glass to attenuate solar UV-A and UV-B light, we confirmed that solar UV irradiation regulated anthocyanin synthesis in apple peels after exposing previously bagged fruit to sunlight. During sunlight exposure, UV attenuation did not affect the expression of MdHY5, MdCOP1, or MdCRY2, but significantly lowered plasma membrane NADPH oxidase activity and superoxide anion concentrations. UV attenuation also reduced the expression levels of MdMYB10, MdPAL, MdCHS, MdF3H, MdDFR, MdANS and MdUFGT1, UDP-glycose:flavonoid 3-O-glycosyltransferase (UFGT) activity, and local concentrations of anthocyanin and quercetin-3-glycoside. In contrast, exogenous application of hydrogen peroxide could enhance anthocyanin and quercetin-3-glycoside synthesis. Xanthophyll cycle pool size on a chlorophyll basis was higher but its de-epoxidation was lower under direct sunlight irradiation than that under UV-attenuating conditions. This suggests that reactive oxygen species (ROS) produced in chloroplast are not major contributors to anthocyanin synthesis regulation. Inhibition of plasma membrane NADPH oxidase activity lowered the production of ROS through this mechanism, significantly inhibited the synthesis of anthocyanin, and increased the total production of ROS in apple peel under direct sunlight irradiation, suggesting that ROS produced via plasma membrane NADPH oxidase regulates anthocyanin synthesis. In summary, solar UV irradiation regulated anthocyanin synthesis in apple peels by modulating the production of ROS via plasma membrane NADPH oxidase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthocyanins belong to a group of water-soluble pigments responsible for the red, purple, and blue colors of many fruits, vegetables, flowers and other plant tissues. This essential pigment plays a photoprotective role in plants under high levels of light or other stress conditions (Williams et al. 2003; Hughes et al. 2005, 2012). The photoprotective function of anthocyanin is generally attributed to two mechanisms: shielding the chloroplast from excess light by absorbing blue–green light (Smillie and Hetherington 1999; Merzlyak et al. 2008) and directly scavenging reactive oxygen species (ROS; Gould et al. 2002; Neill and Gould 2003; Bi et al. 2014). Anthocyanin has also been shown to extend the shelf life of tomato fruit (Bassolino et al. 2013), and act as an antioxidant to reduce oxidative DNA damage, as observed in rats and humans (Ramirez-Tortosa et al. 2001; Weisel et al. 2006).
The synthesis of anthocyanin has been studied extensively in plants. It is suggested that anthocyanin synthesis in fruit peel is mainly controlled by MYB transcription factors including MYB1, MYBA, and MYB10 (Allan et al. 2008; Lin-Wang et al. 2010). Among their allelic homologs, MYB1 and MYBA share identical sequences (Ban et al. 2007), while MYB10 and MYB1 genes are located at very similar positions on linkage group 9 of the apple genetic map (Chagné et al. 2008). In apple fruit, it was reported that MdMYB10 might control both skin color and anthocyanin accumulation in fruit flesh in certain apple genotypes, whereas MdMYB1 and MdMYBA were expressed in red sections of fruit skin in other genotypes (Takos et al. 2006; Ban et al. 2007; Espley et al. 2007, 2009). Compared to that of non-red fruits, red apple and pear fruit peels showed higher levels of MYB transcription (Takos et al. 2006; Ban et al. 2007; Feng et al. 2010; Lin-Wang et al. 2011; Li et al. 2014). In these fruits, the regulation of anthocyanin synthesis by MYB was mainly correlated with UDP-glycose:flavonoid 3-O-glycosyltransferase (UFGT), an enzyme which catalyzes the final step of anthocyanin synthesis. Other enzymes such as phenylalanine ammonia lyase (PAL), chalcone synthase (CHS), chalcone-flavanone isomerase (CHI), flavanone-3-hydroxylase (F3H), dihydroflavonol 4-reductase (DFR), and anthocyanidin synthase (ANS) are also involved in the synthesis of anthocyanin in apple or pear peels (Li et al. 2013a, 2014). MYB (MdMYB1, PyMYB10, and MdMYBA) and UFGT levels, UFGT activity, and anthocyanin concentration can be enhanced by sunlight (Takos et al. 2006; Li et al. 2008, 2012, 2013a, 2014; Yu et al. 2012) or UV irradiation (Ubi et al. 2006; Ban et al. 2007; Xie et al. 2012) in apple or pear peels, indicating that anthocyanin synthesis is regulated by light. However, the mechanisms underlying sunlight-mediated MYB and anthocyanin synthesis are unknown.
As the regulatory effects of both UV-A and UV-B on anthocyanin synthesis have been observed in leaves (Tossi et al. 2011; Wang et al. 2012), fruit peels (Ubi et al. 2006; Hagen et al. 2007; Xie et al. 2012), and cell cultures (Hirner et al. 2001), it is possible that sunlight regulates MYB expression through UV-A and/or UV-B rays. To date, two classes of blue/UV-A light photoreceptors (phototropins and cryptochromes, Lin 2000; Briggs and Christie 2002), and one UV-B light receptor (UV RESISTANCE LOCUS 8, UVR8, Brown et al. 2005; Rizzini et al. 2011; Christie et al. 2012) have been identified. Meanwhile, two key regulators of UV-B signal transduction have also been identified: the bZIP transcription factor ELONGATED HYPOGOTYL 5 (HY5) and the E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1, Brown and Jenkins 2008; Favory et al. 2009; Jenkins 2009; Jaakola 2013). In Arabidopsis, Rizzini et al. (2011) demonstrated that UVR8 monomerizes in a UV-B-dependent manner before interaction with COP1. Li et al. (2012) found that MYB1 protein in apple peel accumulated in light conditions but degraded via a ubiquitin-dependent pathway in the dark, which was regulated by COP1. Another study of apple peel suggested that UV-B could induce the accumulation of anthocyanin via COP1-mediated signaling, leading to activation and binding of HY5 to the promoter regions of MYB genes (Peng et al. 2013). In addition, a blue/UV-A light receptor gene MdCRY2 from apple was shown to regulate anthocyanin synthesis in Arabidopsis flower (Li et al. 2013b). These studies indicate that UV-A and/or UV-B light receptors might be involved in the regulation of MYB transcription factor and anthocyanin synthesis in plants. However, a UVR8-independent UV-B signaling pathway has also been reported in plants (Brown and Jenkins 2008; González Besteiro et al. 2011). It is suggested that narrow-band UV-B treatment could lead to the up-regulation of the UVR8 signaling pathway, whereas broad-band higher fluence UV-B treatment may be more likely to activate UVR8-independent signaling pathways such as ROS, DNA damage, phytohormones, and mitogen-activated protein kinases (MAPKs, Wargent and Jordan 2013).
Recent studies found that ROS might be involved in the regulation of anthocyanin synthesis in apple peels under sunlight (Chen et al. 2013a; Bi et al. 2014). In plants, the production of ROS is governed by photosynthetic electron transport, photorespiration, respiration, plasma membrane NADPH oxidase, and other production systems (Asada 1999; Foyer and Noctor 2000; Apel and Hirt 2004; Sagi and Fluhr 2006). Among these systems, plasma membrane NADPH oxidase plays a key role. ROS produced by plasma membrane NADPH oxidase can serve as signal molecules to regulate plant senescence (Chen et al. 2013b), nodule functioning (Marino et al. 2011), root hair elongation (Foreman et al. 2003), and biotic or abiotic stress defense responses (Orozco-Cárdenas et al. 2001; Sagi and Fluhr 2001; Torres et al. 2002; Xia et al. 2009). Moreover, the activity of plasma membrane NADPH oxidase can be enhanced by UV-B irradiation (Tossi et al. 2009). Because the photosynthetic capacity of fruit peels is very low (Chen and Cheng 2007; Li and Cheng 2008; Li et al. 2014), ROS may be largely produced in peel chloroplasts. Mubarakshina et al. (2010) suggest that ROS produced in chloroplasts, such as hydrogen peroxide, can also serve as signaling molecules. To date, however, the mechanism by which ROS production may regulate anthocyanin synthesis in apple peels is unknown. A better understanding of this process may help growers improve or control fruit color in the future. In this study, the ROS-mediated regulation of anthocyanin synthesis in apple peels was investigated under sunlight irradiation to further illustrate the light-dependent mechanisms of anthocyanin synthesis in fruit peels.
Materials and methods
Plant materials
Nineteen-year-old apple (Malus domestica Borkh. ‘Golden Delicious’) trees on M111 rootstocks were used in this study. The trees were grown at a spacing of 3 × 4 m in north–south rows in Luochuan (35.765N, 109.442E; elevation 1,033 m), Shaanxi, China. The trees were approximately 4 m in height with a central leader, and were grown under standard horticultural practices with disease and pest control. Crop load was controlled at 8 fruits per trunk cross-sectional area (cm2). On May 10 2012, fruits were bagged with light impermeable double layer paper bags. To reinforce protection from light, the outer layer of each bag is colored yellow on the outside and black on the inside. The inner layer of each bag is red. During sunny afternoon periods, the peel temperature of bagged fruits was 3–6 °C lower than that of unbagged fruits. On August 31 2012, approximately 130 days after full bloom, five replicates of the bagged fruits were sampled (three trees per replicate, fifteen trees total) without removing the bags, to avoid exposure to light before chemical and short-term sunlight exposure treatments.
Chemical and short-term sunlight exposure treatments
Three experiments were carried out on the fruit: time course, hydrogen peroxide treatment, and diphenyleneiodonium chloride (DPI, from Sigma-Aldrich, St. Louis, MO, USA) treatment. For hydrogen peroxide and DPI treatments, fruits were immersed in 0, 2.5, 10, 50, and 150 mM hydrogen peroxide or 0, 10, and 50 μM DPI solutions overnight before sunlight exposure.
Before sunrise, fruits were removed from their bags or respective solutions and placed onto four layers of wet gauze in an open space. For UV alleviation treatment, 3-mm commercial glass (Na2O·CaO·6SiO2) was used to filter solar UV-A and UV-B irradiation, with 20 cm ground clearance. The glass did not noticeably change the visible range of sunlight, but transmitted light had an absorption spectrum from 300–400 nm at the UV range (Online Resource 1). For time course treatment, peels from the sun-exposed part of apples were collected approximately 1, 3, 5, and 7 days after sunlight exposure. For hydrogen peroxide treatment, sun-exposed peels were collected after 3 days exposure. After DPI treatment, sun-exposed peels were collected after 5 days exposure. During the experiment, maximal sunlight intensity was 1,200 μmol m−2 s−1 on the first day due to cloudy conditions, and 1,800 μmol m−2 s−1 on the remaining days. Peel temperature and air humidity were similar between the two different light treatments, with a maximal peel temperature of 35 ± 1 °C and minimal relative air humidity of 45 % at midday. For each of the three experiments, samples at day 0 (i.e. before sunlight exposure) were also taken. Fruit peel samples (~1 mm thick) were collected with a peeler. For each treatment at each sampling date, 20 fruits per replicate were sampled. The samples were immediately frozen in liquid nitrogen, ground into powder and mixed in liquid nitrogen with an A11 grinder from IKA® Works (VWR, Radnor, PA, USA), and stored at −80 °C until analysis.
Pigment and phenolic compound analyses
Chlorophyll compounds in fruit peels were extracted with 80 % acetone and analyzed using a UV-2450 spectrophotometer (Shimadzu, Kyoto, Japan), according to Arnon (1949). The extraction and analysis of xanthophyll cycle pigments were carried out as described by Li and Cheng (2008). Concentrations of the xanthophyll cycle pigments violaxanthin (V), antheraxanthin (A), and zeaxanthin (Z) were determined based on peak area and a calibration curve derived from an authentic standard of Z from Sigma-Aldrich.
Phenolic compounds were assayed as described by Chen et al. (2013a). The concentration of individual phenolic compounds was determined based on peak area and calibration curves derived from corresponding authentic phenolic compounds.
Enzyme activity, antioxidant metabolite, and ROS analyses
Plasma membranes were prepared, according to Sagi and Fluhr (2001), with some modifications. Briefly, fruit peel samples were ground using a mortar and pestle in buffer (5 ml g−1 fresh weight) containing 100 mM Tris-Mes (pH 7.8), 0.33 M sucrose, 3 mM ethylenediaminetetraacetic acid, 5 mM dithiothreitol (DTT), 10 mM ascorbate, 0.1 mM MgCl2, 2 mM phenylmethylsulfonyl fluoride, 10 % glycerol, 0.4 % casein, 0.5 % bovine serum albumin, and 0.2 % (w/v) polyvinylpyrrolidone. The homogenate was filtered through four layers of cheese cloth, and the filtrate was centrifuged at 10,000g for 20 min. Microsomes were pelleted from the supernatant by centrifugation at 140,000g for 45 min and re-suspended in 5 mM potassium phosphate buffer (pH 7.8) containing 0.33 M sucrose and 3 mM KCl. The suspension was then fractionated by the aqueous two-phase partitioning method (Larsson et al. 1987). Phase separations were carried out in a series of 10-g phase systems with a final composition of 6.2 % (w/w) dextran T500, 6.2 % (w/w) polyethylene glycol 3,350, 0.33 M sucrose, 5 mM potassium phosphate (pH 7.8), and 3 mM KCl. Three successive rounds of partitioning yielded the final upper phases (U3 and U3′) and lower phase (L3). The combined upper phase was enriched with plasma membrane vesicles, and the lower phase contained intracellular membranes. Distribution of specific activity of the different membrane marker enzymes in the microsomes after two-phase partition is shown in Online Resource 1. The final upper phase was diluted fivefold in ice cold Tris–HCl dilution buffer (10 mM, pH 7.4) containing 0.33 M sucrose. The fraction was centrifuged at 140,000g for 60 min. The resultant pellets were then re-suspended in Tris–HCl dilution buffer and used immediately for enzyme activity analysis. All procedures were carried out at 4 °C. The activity of plasma membrane NADPH oxidase was assayed based on the reduction of 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilideinner salt (XTT) by O2 − radicals as described by Sagi and Fluhr (2001). Each 1 ml reaction mixture contained 100 mM Tris–HCl (pH 7.5), 2 mM CaCl2, 0.5 mM XTT, 0.1 mM NADPH, and 10 μg upper phase proteins. XTT reduction was monitored at 470 nm in the presence and absence of 50 units SOD.
Superoxide dismutase (SOD, EC 1.15.1.1), ascorbate peroxidase (APX, EC 1.11.1.11), monodehydroascorbate reductase (MDHAR, EC 1.6.5.4), dehydroascorbate reductase (DHAR, EC 1.8.5.1), and glutathione reductase (GR, EC 1.6.4.2) were extracted and assayed according to the protocols of Li and Cheng (2008).
UDP-glycose:flavonoid 3-O-glycosyltransferase (UFGT, EC 2.4.1.91) was extracted as described by Li et al. (2013a). In a final assay volume of 200 μl, reaction mixture contained 100 mM buffer (Hepes–KOH, pH 8.0), 250 mM MgCl2, 2 mM DTT, 9 mM UDP-galactose, 0.3 mM cyanidin, and 10 μl enzyme extract. The reaction mixture was incubated at 37 °C for 10 min and terminated by the addition of 50 μl 35 % trichloroacetic acid. The reaction mixture was extracted twice with ethylacetate, which was then evaporated completely using N2 gas. The final reaction product (cyanidin-3-galactoside) was dissolved with 80 % methanol and analyzed using HPLC at 525 nm.
Total ascorbate and total glutathione were extracted and analyzed as described by Li and Cheng (2008). Superoxide anion and hydrogen peroxide were extracted and analyzed as described by Chen et al. (2013a).
Real-time qPCR expression analysis
Total RNA was isolated using the SDS-phenol method according to Malnoy et al. (2001). First-strand cDNA was synthesized using the PrimeScript™ RT reagent Kit (Takara, Dalian, China), according to the manufacturer’s protocol. Real-Time PCR was performed with the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) and SYBR Green MasterMix (SYBR Premix EX Taq™, Dalian, China). MdActin was used to standardize the cDNA samples for different genes. The primers for MdActin, MdCRY2, MdHY5, MdCOP1, MdMYB10, MdPAL, MdCHS, MdF3H, MdDFR, MdANS, MdUFGT1, and MdUFGT2 are shown in Online Resource 1.
Statistical analysis
All data were statistically analyzed by t test and Least Significant Difference (LSD) using SPSS 16.0 (SPSS Inc. Chicago, IL, USA).
Results
Time course changes of anthocyanin synthesis after sunlight exposure
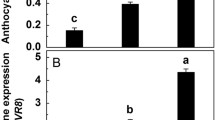
After the bagged fruits were directly exposed to sunlight, transcript levels of MdCRY2 reached maximum levels at day 5 and then decreased (Online Resource 1). In contrast, expression levels of MdHY5, MdCOP1, and MdF3H peaked at day 3 and then decreased slightly (Fig. 1a, b, f). Transcript levels of MdMYB10, MdCHS, and MdUFGT2 peaked at day 1 (Fig. 1c, e, j). MdMYB10 expression changed slightly over the course of the exposure period, whereas that of MdCHS and MdUFGT2 decreased sharply 7 days after sunlight exposure. Transcript levels of MdPAL, MdDFR, MdANS, and MdUFGT1 initially increased, and then remained unchanged after sunlight exposure (Fig. 1d, g, h, i). UFGT activity and the concentration of cyanidin-3-galactoside (which accounts for more than 99 % of anthocyanin in apple peels, data not shown) all increased gradually after sunlight exposure (Fig. 1k, i). The concentrations of quercetin-3-galactoside, quercetin-3-glucoside, and quercetin-3-rutinoside changed in similar patterns to that of cyandin-3-galactoside, whereas that of other phenolic compounds showed little or no response to sunlight exposure (Online Resource 1). UV attenuation, after the bagged fruits were exposed to sunlight, did not affect the expression levels of MdCRY2, MdHY5, and MdCOP1, but significantly inhibited that of eight other genes and the activity of UFGT. The synthesis of cyanidin-3-galactoside was completely inhibited by UV attenuation (Fig. 1). For other phenolic compounds, the synthesis of quercetin-3-glycosides such as quercetin-3-galactoside, quercetin-3-glucoside, and quercetin-3-rutinoside were significantly inhibited. Synthesis rates of other phenolic compounds were hardly changed under UV-attenuating conditions (Online Resource 1).
Transcription levels of MdHY5 (a), MdCOP1 (b), MdMYB10 (c), MdPAL (d), MdCHS (e), MdF3H (f), MdDFR (g), MdANS (h), MdUFGT1 (i) and MdUFGT2 (j), activities of UDP-glycose:flavonoid 3-O-glycosyltransferase (UFGT, k), and concentrations of anthocyanin (l) in ‘Golden Delicious’ apple fruit peels after directly exposing bagged fruits to sunlight (CK) or with UV attenuation by 3-mm glass (+Glass). The asterisk indicates a significant difference between “CK” and “+Glass” at P < 0.05, t test. Each data point represents mean ± SE (n = 5)
Time course changes of ROS production and the antioxidant system after sunlight exposure
Concentrations of superoxide anion and hydrogen peroxide both increased after exposing bagged fruits to sunlight (Fig. 2a, b). UV attenuation lowered the concentration of superoxide anion but left hydrogen peroxide concentration unaffected. The activity of plasma membrane NADPH oxidase increased gradually and then remained unchanged at 7 days after direct sunlight exposure. However, NADPH oxidase activity did not markedly change under UV-attenuating conditions (Fig. 2c).
Concentrations of superoxide anion (a) and hydrogen peroxide (b), activities of plasma membrane NADPH oxidase (c), superoxide dismutase (SOD, d), ascorbate peroxidase (APX, e), monodehydroascorbate reductase (MDHAR, f), dehydroascorbate reductase (DHAR, g) and glutathione reductase (GR, h), and concentrations of total ascorbate (i) and glutathione (j) in ‘Golden Delicious’ apple fruit peels after directly exposing bagged fruits to sunlight (CK) or with UV attenuation by 3-mm glass (+Glass). The asterisk indicates a significant difference between “CK” and “+Glass” at P < 0.05, t test. Each data point represents mean ± SE (n = 5)
After sunlight exposure, SOD activity remained unchanged at first and then increased, with similar values observed with and without UV attenuation (Fig. 2d). APX activity decreased slightly and then increased, with similar values observed with and without UV attenuation (Fig. 2e). MDHAR, DHAR, and GR activities varied in similar patterns; they decreased at first and then increased, with lower values observed under UV attenuation (Fig. 2f–h). Concentrations of total ascorbate and glutathione increased gradually after sunlight exposure (Fig. 2i, j), but UV attenuation did not change the concentrations of these two metabolites.
Time course changes of chlorophyll and the xanthophyll cycle after sunlight exposure
Chlorophyll concentration decreased at day 3 and remained unchanged under direct sunlight exposure, but increased and then plateaued under UV-attenuating conditions. As a result, chlorophyll concentrations significantly increased with UV attenuation after 3 days of sunlight exposure (Fig. 3a). The xanthophyll cycle pigment pool size (V + A + Z) on a peel fresh weight basis increased gradually after sunlight exposure, with similar values observed with and without UV attenuation (Fig. 3b). However, pool size on a chlorophyll basis changed in different patterns (Fig. 3c), increasing under direct sunlight exposure but decreasing with UV attenuation at day 3. As a result, the chlorophyll-based pool size was significantly lower under UV-attenuating conditions. De-epoxidation of xanthophyll cycle pigments increased sharply at day 1 and then increased slowly during the following days (Fig. 3d). However, de-epoxidation was higher under UV-attenuating conditions after 3 days of sunlight exposure.
Concentrations of chlorophyll (a), the xanthophyll cycle pool size (V + A + Z) on the basis of peel fresh weight (b) or chlorophyll content (c), and the de-epoxidation of xanthophyll pigments [(0.5A + Z)/(V + A + Z), d] in ‘Golden Delicious’ apple fruit peels after directly exposing bagged fruits to sunlight (CK) or with UV attenuation by 3-mm glass (+Glass). The asterisk indicates a significant difference between “CK” and “+Glass” at P < 0.05, t test. Each data point represents mean ± SE (n = 5)
Hydrogen peroxide treatments
Exogenous hydrogen peroxide treatment did not change the expression levels of MdMYB10, MdPAL, MdCHS, MdF3H, MdDFR, MdANS, MdUFGT1 and MdUFGT2, UFGT activity, or the concentrations of cyandin-3-galactoside and other phenolic compounds before sunlight exposure treatment (Fig. 4, Online Resource 1). However, 3 days after direct sunlight exposure, levels of MdMYB10, MdPAL, MdCHS, MdDFR, and MdANS increased gradually and then plateaued with increasing hydrogen peroxide concentrations. Meanwhile, levels of MdF3H and MdUFGT1 expression, UFGT activity, and cyanidin-3-galactoside concentration remained unchanged when the concentration of hydrogen peroxide was less than 10 mM. However, all of these parameters increased markedly with further increase in hydrogen peroxide concentration. Levels of MdUFGT2 were not affected by exogenous hydrogen peroxide treatment (Fig. 4). Concentrations of quercetin-3-galactoside, quercetin-3-glucoside, and quercetin-3-rutinoside changed in similar patterns to that of cyanidin-3-galactoside (Online Resource 1). Concentrations of chlorogenic acid and procyanidin B2 remained unchanged until the exogenous hydrogen peroxide concentration increased to 50 mM, whereas that of other phenolic compounds did not significantly change with hydrogen peroxide treatment. Under UV-attenuating conditions, the expression levels of these eight genes, UFGT activity, and the concentrations of cyandin-3-galactoside, quercetin-3-galactoside, quercetin-3-glucoside and quercetin-3-rutinoside were significantly lower compared to that without UV attenuation. The concentration of other phenolic compounds showed slight or no variation with and without UV attenuation.
Transcription levels of MdMYB10 (a), MdPAL (b), MdCHS (c), MdF3H (d), MdDFR (e), MdANS (f), MdUFGT1 (g) and MdUFGT2 (h), activities of UDP-glycose:flavonoid 3-O-glycosyltransferase (UFGT, i), and concentrations of anthocyanin (j) in ‘Golden Delicious’ apple fruit peels after bagged fruits were treated with different concentrations of hydrogen peroxide and directly exposed to sunlight (CK) or with UV attenuation by 3-mm glass (Glass) for 0 or 3 days. Each data point represents mean ± SE (n = 5)
DPI treatments
Before and after sunlight exposure treatments, DPI significantly inhibited plasma membrane NADPH oxidase activity in fruit peels (Fig. 5a). When studying ROS production, DPI treatment did not affect the concentrations of superoxide anion and hydrogen peroxide before sunlight exposure (Fig. 5b, c). However, 5 days after sunlight exposure, concentrations of superoxide anion and hydrogen peroxide in DPI-treated fruit peels were significantly higher under direct sunlight exposure, but significantly lower under UV-attenuating conditions.
Activities of plasma membrane NADPH oxidase (a) and concentrations of superoxide anion (b) and hydrogen peroxide (c) in ‘Golden Delicious’ apple fruit peels after bagged fruits were treated with different concentrations of diphenyleneiodonium chloride (DPI) and directly exposed to sunlight (CK) or with UV attenuation by 3-mm glass (Glass) for 0 or 5 days. Different letters above the bars indicate significant differences at P < 0.05, least significant difference (LSD). Each data point represents mean ± SE (n = 5)
DPI treatment did not affect the gene expression levels, UFGT activity, or concentrations of cyanidin-3-galactoside and other phenolic compounds before sunlight exposure (Fig. 6, Online Resource 1). Levels of MdMYB10 and MdUFGT1 were significantly lower, whereas that of MdPAL, MdCHS, MdF3H, and MdANS did not change in DPI-treated fruit peels under direct sunlight exposure. Lower DPI concentration (10 μM) did not change the levels of MdDFR and MdUFGT2, whereas the higher concentration treatment (50 μM) slightly lowered their levels under direct sunlight exposure (Fig. 6e, h). The expression levels of all eight genes were similar in peels with and without DPI treatment under UV-attenuating conditions. The activity of UFGT and the concentrations of cyanidin-3-galactoside, quercetin-3-galactoside, quercetin-3-glucoside, and quercetin-3-rutinoside changed in similar patterns to that of MdMYB10 and MdUFGT1. Concentrations of other phenolic compounds were not changed by DPI treatments.
Transcription levels of MdMYB10 (a), MdPAL (b), MdCHS (c), MdF3H (d), MdDFR (e), MdANS (f), MdUFGT1 (g) and MdUFGT2 (h), activities of UDP-glycose:flavonoid 3-O-glycosyltransferase (UFGT, i), and concentrations of anthocyanin (j) in ‘Golden Delicious’ apple fruit peels after bagged fruits were treated with different concentrations of diphenyleneiodonium chloride (DPI) and directly exposed to sunlight (CK) or with UV attenuation by 3-mm glass (Glass) for 0 or 5 days. Different letters above the bars indicate significant differences at P < 0.05, least significant difference (LSD). Each data point represents mean ± SE (n = 5)
Discussion
After directly exposing bagged ‘Golden Delicious’ apple fruits to sunlight, this traditional non-red apple cultivar synthesized abundant levels of anthocyanin in fruit peels (Fig. 1, Online Resource 1), consistent with our previous studies (Chen et al. 2013a; Bi et al. 2014). Solar UV-A and UV-B attenuation with 3-mm glass significantly down-regulated the expression levels of MdMYB10 and downstream structural genes (MdPAL, MdCHS, MdF3H, MdDFR, MdANS, and MdUFGT1) as well as the activity of UFGT, and completely inhibited anthocyanin synthesis (Fig. 1). These results indicate that the synthesis of anthocyanin in apple peel was regulated by UV irradiation alone, instead the visible sunlight.
In plants, UV-A and UV-B light photoreceptors have been identified and demonstrated to be involved in UV signal transduction pathways (Brown and Jenkins 2008; Favory et al. 2009; Jenkins 2009; Li et al. 2012, 2013b; Jaakola 2013; Peng et al. 2013). During this study, expression levels of MdHY5, MdCOP1, and MdCRY2 were not affected by UV attenuation, suggesting that non-specific UV signaling regulated MYB expression and anthocyanin synthesis in apple peel. Hideg et al. (2012) and Wargent and Jordan (2013) suggested that UV-B-specific perception and UVR8/COP1/HY5 signaling pathways comprise the main regulatory mechanism under narrow-band, low UV-B conditions, whereas ROS-mediated signaling, DNA damage response signals, phytohormones, and MAPK signaling appear to be restricted to broad-band, high UV-B conditions. In the present study, bagged fruits used were grown in dark conditions prior to sunlight exposure, indicating that solar UV-B irradiation might have a relatively strong effect on the fruits.
Our previous studies indicate that ROS might be involved in the regulation of anthocyanin synthesis in apple peel after exposing bagged fruits to natural light conditions (Chen et al. 2013a; Bi et al. 2014). Along with the inhibition of anthocyanin synthesis, UV attenuation did not change the activities of SOD and APX (Fig. 2), the two key enzymes used for ROS scavenging in apple peel (Li and Cheng 2008). However, UV attenuation significantly lowered the accumulation of superoxide anion (Fig. 2), indicating that UV light might regulate anthocyanin synthesis through controlling ROS production. Indeed, the fact that exogenous application of hydrogen peroxide enhanced anthocyanin synthesis (Fig. 4) demonstrates that the accumulation of ROS could up-regulate anthocyanin synthesis in fruit peel. Compared with direct sunlight irradiation, the reduction of anthocyanin synthesis in hydrogen peroxide-treated peels under UV-attenuating conditions indicates that endogenous ROS production still plays a key role in regulating anthocyanin synthesis in apple peels.
Under high-light conditions (Li and Cheng 2008; Li et al. 2008) or conditions where UV-B impairs photosynthetic electron transport (Jansen et al. 1998), the production of ROS was shown to increase in the chloroplast of fruit peel (Jenkins 2009). Meanwhile, UV-B light was shown to stimulate plasma membrane NADPH oxidase and then increase ROS production in the extracellular space (Fig. 2; Tossi et al. 2009, 2011). In the present study, inhibition of NADPH oxidase activities by the unique inhibitor DPI markedly down-regulated anthocyanin synthesis in apple peel under direct sunlight irradiation conditions (Figs. 5, 6), suggesting that the ROS produced via plasma membrane NADPH oxidase controlled anthocyanin synthesis in apple peel. The similar patterns of change in NADPH oxidase activity and anthocyanin concentration in fruit peels also support this hypothesis (Figs. 1, 2).
The xanthophyll cycle, in which V is converted to Z via the intermediate A under light conditions, can thermally dissipate excess excitation energy before such energy triggers the formation of ROS in chloroplasts (Demmig-Adams and Adams 1992). Under high light, the xanthophyll cycle usually operates with high efficiency in fruit peels (Cheng and Ma 2004; Li and Cheng 2008; Li et al. 2008). Compared to UV attenuation, the relatively higher observed xanthophyll cycle pool size on a chlorophyll basis combined with lower de-epoxidation under direct sunlight irradiation (Fig. 3) suggests that excess excitation energy was relatively lower under this condition. Correspondingly, ROS levels produced in chloroplast should be lower. This indicates that ROS produced in chloroplast was not a major contributor to anthocyanin synthesis regulation. Under UV-attenuating conditions it was shown that DPI treatment could inhibit plasma membrane NADPH oxidase and the subsequent production of ROS through this mechanism (Fig. 5). Since plasma membrane NADPH oxidase activity was already too low to produce enough ROS to trigger anthocyanin synthesis under UV-attenuating conditions (Fig. 2), the further observed decrease in ROS production by DPI treatment did not significantly change the rate of anthocyanin synthesis (Fig. 6). Under direct sunlight irradiation, increased ROS concentrations in DPI-treated fruit peels (Fig. 5) should come from other processes. This further demonstrates that with the exception of the plasma membrane NADPH oxidase pathway, other ROS production processes contributed little to the regulation of anthocyanin synthesis. As anthocyanin may lower ROS production by light attenuation (Smillie and Hetherington 1999; Merzlyak et al. 2008), it is reasonable to surmise that reduction of anthocyanin in DPI-treated fruit peels may increase ROS production via other processes such as chloroplastic photosynthetic electron transport (Li et al. 2008). Recently, it was found that the anthocyanin synthesis pathway was redox sensitive, and ascorbate was involved in the regulation of anthocyanin synthesis in Arabidopsis thaliana under high light (Page et al. 2012). This may explain how activities of enzymes associated with the ascorbate–glutathione cycle became faster under direct sunlight conditions, compared to that with UV attenuation (Fig. 2).
In addition, analysis of the apple genome (Velasco et al. 2010) revealed the existence of two MdUFGT genes (Online Resource 1). It was clear that the synthesis of anthocyanin was related to MdUFGT1, and not MdUFGT2 (Figs. 1, 4, 6), indicating that the two MdUFGTs play different roles in plants. The regulation of anthocyanin synthesis by MdMYB10 should occur through control of MdUFGT1 instead of other up-stream structural genes involved in anthocyanin synthesis. This was supported by the fact that the major difference in phenolic compounds under the three treatments was in the terms of anthocyanin and quercetin-3-glycoside (Online Resource 1), although the expression levels of other structural genes (MdPAL, MdCHS, MdF3H, MdDFR and MdANS) were also enhanced by UV irradiation and H2O2 treatment (Figs. 1, 4). Given the fact that MYB10 up-regulated the whole phenolic pathway in apple peels, the synthesis of most phenolic compounds should be enhanced. More importantly, of the structural genes in the anthocyanin synthesis pathway, it was shown that only MdUFGT1 responded to DPI treatment in similar patterns to MdMYB10 and anthocyanin concentrations (Fig. 6). The enhancement of other structural gene expression levels by UV or H2O2 might be due to the enhanced synthesis of cyanidin-3-galactoside and quercetin-3-glycoside. However, it should be noted that the enhanced expression of these genes may also contribute to the synthesis of these compounds in apple peel.
In general, solar UV irradiation regulated anthocyanin synthesis in apple peel. This regulation occurred through control of the production of ROS via plasma membrane NADPH oxidase. MdMYB10 regulated anthocyanin synthesis through MdUFGT1 in apple peel.
Author Contribution
PL conceived and designed research. JZ, CC and DZ conducted experiments. JZ, CC and HL analyzed data. JZ wrote the manuscript. PL and FM revised the manuscript. All authors read and approved the manuscript.
Abbreviations
- A:
-
Antheraxanthin
- ANS:
-
Anthocyanidin synthase
- APX:
-
Ascorbate peroxidase
- CHS:
-
Chalcone synthase
- COP1:
-
The E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC1
- CRY2:
-
Cryptochrome2
- DFR:
-
Dihydroflavonol 4-reductase
- DHAR:
-
Dehydroascorbate reductase
- DPI:
-
Diphenyleneiodonium chloride
- F3H:
-
Flavanone-3-hydroxylase
- GR:
-
Glutathione reductase
- HY5:
-
The bZIP transcription factor ELONGATED HYPOGOTYL 5
- MAPK:
-
Mitogen-activated protein kinase
- MDHAR:
-
Monodehydroascorbate reductase
- PAL:
-
Phenylalanine ammonia lyase
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- UFGT:
-
UDP-glycose:flavonoid 3-O-glycosyltransferase
- UVR8:
-
UV RESISTANCE LOCUS 8
- V:
-
Violaxanthin
- Z:
-
Zeaxanthin
References
Allan AC, Hellens RP, Laing WA (2008) MYB transcription factors that colour our fruit. Trends Plant Sci 13:99–102
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arnon DL (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris L. Plant Physiol 24:1–15
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T (2007) Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol 48:958–970
Bassolino L, Zhang Y, Schoonbeek H, Kiferle C, Perata P, Martin C (2013) Accumulation of anthocyanins in tomato skin extends shelf life. New Phytol 200:650–655
Bi X, Zhang J, Chen C, Zhang D, Li P, Ma F (2014) Anthocyanin contributes more to hydrogen peroxide scavenging than other phenolics in apple peel. Food Chem 152:205–209
Briggs WR, Christie JM (2002) Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci 7:204–210
Brown BA, Jenkins GI (2008) UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol 146:576–588
Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, Jenkins GI (2005) A UV-B-specific signaling component orchestrates plant UV protection. Proc Natl Acad Sci USA 102:18225–18230
Chagné D, Gasic K, Crowhurst RN, Han Y, Bassett HC, Bowatte DR, Lawrence TJ, Rikkerink EH, Gardiner SE, Korban SS (2008) Development of a set of SNP markers present in expressed genes of the apple. Genomics 92:353–358
Chen LS, Cheng L (2007) The sun-exposed peel of apple fruit has a higher photosynthetic capacity than the shaded peel. Funct Plant Biol 34:1038–1048
Chen C, Li H, Zhang D, Li P, Ma F (2013a) The role of anthocyanin in photoprotection and its relationship with the xanthophyll cycle and the antioxidant system in apple peel depends on the light conditions. Physiol Plant 149:354–366
Chen HJ, Huang CS, Huang GJ, Chow TJ, Lin YH (2013b) NADPH oxidase inhibitor diphenyleneiodonium and reduced glutathione mitigate ethephon-mediated leaf senescence, H2O2 elevation and senescence-associated gene expression in sweet potato (Ipomoea batatas). J Plant Physiol 170:1471–1483
Cheng L, Ma F (2004) Diurnal operation of the xanthophyll cycle and the antioxidant system in the peel of apple fruit. J Am Soc Hortic Sci 129:313–320
Christie JM, Arvai AS, Baxter KJ, Heilmann M, Pratt AJ, O’Hara A, Kelly SM, Hothorn M, Smith BO, Hitomi K, Jenkins GI, Getzoff ED (2012) Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 335:1492–1496
Demmig-Adams B, Adams IIIWW (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43:599–626
Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC (2007) Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J 49:414–427
Espley RV, Brendolise C, Chagné D, Kutty-Amma S, Green S, Volz R, Putterill J, Schouten HJ, Gardiner SE, Hellens RP, Allan AC (2009) Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell 21:168–183
Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, Seidlitz HK, Nagy F, Ulm R (2009) Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J 28:591–601
Feng S, Wang Y, Yang S, Xu Y, Chen X (2010) Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta 232:245–255
Foreman J, Demidchik V, Bothwell JH et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signaling. New Phytol 146:359–388
González Besteiro MA, Bartels S, Albert A, Ulm R (2011) Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UVB stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J 68:727–737
Gould KS, Mckelvie J, Markham KR (2002) Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant, Cell Environ 25:1261–1269
Hagen SF, Borge GIA, Bengtsson GB, Bilger W, Berge A, Haffner K, Solhaug KA (2007) Phenolic contents and other health and sensory related properties of apple fruit (Malus domestica Borkh., cv. Aroma): effect of postharvest UV-B irradiation. Postharvest Biol Tech 45:1–10
Hideg É, Jansen MAK, Strid Å (2012) UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends Plant Sci 18:107–114
Hirner AA, Veit S, Seitz HU (2001) Regulation of anthocyanin biosynthesis in UV-A-irradiated cell cultures of carrot and in organs of intact carrot plants. Plant Sci 161:315–322
Hughes NM, Neufeld HS, Burkey KO (2005) Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol 168:575–587
Hughes NM, Burkey KO, Cavender-Bares J, Smith WK (2012) Xanthophyll cycle pigment and antioxidant profiles of winter-red (anthocyanic) and winter-green (acyanic) angiosperm evergreen species. J Exp Bot 63:1895–1905
Jaakola L (2013) New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci 18:477–483
Jansen MAK, Gaba V, Greenberg BM (1998) Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci 3:131–135
Jenkins GI (2009) Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol 60:407–431
Larsson C, Widell S, Kjellbom P (1987) Preparation of high-purity plasma membranes. Method Enzymol 148:558–568
Li P, Cheng L (2008) The shaded side of apple fruit becomes more sensitive to high light damage with fruit development. Physiol Plant 134:282–292
Li P, Castagnoli S, Cheng L (2008) Red ‘Anjou’ pear has a higher photoprotective capacity than green ‘Anjou’. Physiol Plant 134:486–498
Li YY, Mao K, Zhao C, Zhao XY, Zhang HL, Shu HR, Hao YJ (2012) MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple. Plant Physiol 60:1011–1022
Li P, Ma F, Cheng L (2013a) Primary and secondary metabolism in the sun-exposed peel and the shaded peel of apple fruit. Physiol Plant 148:9–24
Li YY, Mao K, Zhao C, Zhao XY, Zhang RF, Zhang HL, Shu HR, Hao YJ (2013b) Molecular cloning and functional analysis of a blue light receptor gene MdCRY2 from apple (Malus domestica). Plant Cell Rep 32:555–566
Li P, Zhang Y, Einhorn TC, Cheng L (2014) Comparison of phenolic metabolism and primary metabolism between green ‘Anjou’ pear and its bud mutation, red ‘Anjou’. Physiol Plant 150:339–354
Lin C (2000) Plant blue-light receptors. Trends Plant Sci 5:337–342
Lin-Wang K, Bolitho K, Grafton K, Kortstee A, Karunairetnam S, McGhie TK, Espley RV, Hellens RP, Allan AC (2010) An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol 10:50
Lin-Wang K, Micheletti D, Palmer J, Volz R, Lozano L, Espley R, Hellens RP, Chagnè D, Rowan DD, Troggio M, Iglesias I, Allan AC (2011) High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant, Cell Environ 34:1176–1190
Malnoy M, Reynoird JP, Mourgues F (2001) A method for isolating total RNA from pear leaves. Plant Mol Biol Rep 19:69–74
Marino D, Andrio E, Danchin EGJ, Oger E, Gucciardo S, Lambert A, Puppo A, Pauly N (2011) A Medicago truncatula NADPH oxidase is involved in symbiotic nodule functioning. New Phytol 189:580–592
Merzlyak MN, Chivkunova OB, Solovchenko AE, Naqvi KR (2008) Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J Exp Bot 59:3903–3911
Mubarakshina MM, Ivanov BN, Naydov IA, Hillier W, Badger MR, Krieger-Liszkay A (2010) Production and diffusion of chloroplastic H2O2 and its implication to signaling. J Exp Bot 61:3577–3587
Neill SO, Gould KS (2003) Anthocyanins in leaves: light attenuators or antioxidants? Funct Plant Biol 30:865–873
Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13:179–191
Page M, Sultana N, Paszkiewicz K, Florance H, Smirnoff N (2012) The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: further evidence for redox control of anthocyanin synthesis. Plant, Cell Environ 35:388–404
Peng T, Saito T, Honda C, Ban Y, Kondo S, Liu JH, Hatsuyama Y, Moriguchi T (2013) Screening of UV-B-induced genes from apple peels by SSH: possible involvement of MdCOP1-mediated signaling cascade genes in anthocyanin accumulation. Physiol Plant 148:432–444
Ramirez-Tortosa C, Andersen ØM, Gardner PT, Morrice PC, Wood SG, Duthie SJ, Collins AR, Duthie GG (2001) Anthocyanin-rich extract decreases indices of lipid peroxidation and DNA damage in vitamin E-depleted rats. Free Rad Biol Med 31:1033–1037
Rizzini L, Favory JJ, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, Ulm R (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332:103–106
Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126:1281–1290
Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141:336–340
Smillie RM, Hetherington SE (1999) Photoabatement by anthocyanins shields photosynthetic systems from light stress. Photosynthetica 36:451–463
Takos AM, Jaffe FW, Jacob SR, Bogs J, Robinson SP, Walker AR (2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142:1216–1232
Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99:517–522
Tossi V, Lamattina L, Cassia R (2009) An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol 181:871–879
Tossi V, Amenta M, Lamattina L, Cassia R (2011) Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant, Cell Environ 34:909–921
Ubi BE, Honda C, Bessho H, Kondo S, Wada M, Kobayashi S, Moriguchi T (2006) Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature. Plant Sci 170:571–578
Velasco R, Zharkikh A, Affourtit J et al (2010) The genome of the domesticated apple (Malus × domestica Borkh.). Nat Genet 42:833–841
Wang Y, Zhou B, Sun M, Li Y, Kawabata S (2012) UV-A light induces anthocyanin biosynthesis in a manner distinct from synergistic blue + UV-B light and UV-A/blue light responses in different parts of the hypocotyls in turnip seedlings. Plant Cell Physiol 53:1470–1480
Wargent JJ, Jordan BR (2013) From ozone depletion to agriculture: understanding the role of UV radiation in sustainable crop production. New Phytol 197:1058–1076
Weisel T, Baum M, Eisenbrand G, Dietrich H, Will F, Stockis JP, Kulling S, Rüfer C, Johannes C, Janzowski C (2006) An anthocyanin/polyphenolic-rich fruit juice reduces oxidative DNA damage and increase glutathione level in healthy probands. Biotech J 1:388–397
Williams EL, Hovenden MJ, Colse DC (2003) Strategies of light energy utilization, dissipation and attenuation in six co-occurring alpine heath species in Tasmania. Funct Plant Biol 30:1205–1218
Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ (2009) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150:801–814
Xie XB, Li S, Zhang RF, Zhao J, Chen YC, Zhao Q, Yao YX, You CX, Zhang XS, Hao YJ (2012) The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant, Cell Environ 35:1884–1897
Yu B, Zhang D, Huang C, Qian M, Zheng X, Teng Y, Su J, Shu Q (2012) Isolation of anthocyanin biosynthetic genes in red Chinese sand pear (Pyrus pyrifolia Nakai) and their expression as affected by organ/tissue, cultivar, bagging and fruit side. Sci Hortic 136:29–37
Acknowledgments
This work was supported by the National Natural Science Foundation of China [31000890, 31171915], the Program for New Century Excellent Talents in University, the Project Sponsored by the Returned Overseas Chinese Scholars, State Education Ministry, and the Science and Technology Research and Development Program of Shaanxi Province, China [2013KJXX-15]. We thank Dr. Suping Gao and Dr. Ting Lei at Sichuan Agricultural University for their help with the sunlight spectrum measurement.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Zhang and C. Chen have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Chen, C., Zhang, D. et al. Reactive oxygen species produced via plasma membrane NADPH oxidase regulate anthocyanin synthesis in apple peel. Planta 240, 1023–1035 (2014). https://doi.org/10.1007/s00425-014-2120-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-014-2120-4