Abstract
Citrus fruits are an important source of ascorbic acid (AsA) for human nutrition, but the main pathways involved in its biosynthesis and their regulation are still not fully characterized. To study the transcriptional regulation of AsA accumulation, expression levels of 13 genes involved in AsA biosynthesis, 5 in recycling and 5 in degradation were analyzed in peel and pulp of fruit of two varieties with different AsA concentration: Navel orange (Citrus sinensis) and Satsuma mandarin (Citrus unshiu). AsA accumulation in peel and pulp correlated with the transcriptional profiling of the l-galactose pathway genes, and the myo-inositol pathway appeared to be also relevant in the peel of immature-green orange. Differences in AsA content between varieties were associated with differential gene expression of GDP-mannose pyrophosphorylase (GMP), GDP-l-galactose phosphorylase (GGP) and l-galactose-1-phosphate phosphatase (GPP), myo-inositol oxygenase in peel, and GGP and GPP in pulp. Relative expressions of monodehydroascorbate reductase 3 (MDHAR3) and dehydroascorbate reductase1 (DHAR1) correlated with AsA accumulation during development and ripening in peel and pulp, respectively, and were more highly expressed in the variety with higher AsA contents. Collectively, results indicated a differential regulation of AsA concentration in peel and pulp of citrus fruits that may change during the different stages of fruit development. The l-galactose pathway appears to be predominant in both tissues, but AsA concentration is regulated by complex mechanisms in which degradation and recycling also play important roles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ascorbic acid (AsA, l-ascorbate, vitamin C) is synthesized by many vertebrates, however, the biosynthetic capacity has been lost in a number of species, such as birds, guinea pigs, and some primates including humans, for whom AsA has become a vitamin (Linster and Van Schaftingen 2007). Since humans cannot synthesize AsA by themselves, they must obtain it through the diet; therefore, the consumption of vitamin C-rich foods, such as fruits and vegetables, is highly recommended (Block 1991).
AsA is the most abundant antioxidant present in plant cells and plays important roles in a number of processes, including defense mechanisms, photosynthesis, cell division, growth regulation and senescence. In addition, AsA is a cofactor for the activity of many enzymes, and affects the expression of genes involved in defense and hormone signaling pathways in plants (reviewed in Smirnoff 2011).
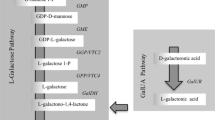
Despite the relevance of AsA in plants, its biosynthesis has remained elusive until recently. The first significant milestone on the knowledge of AsA biosynthesis in plants was the discovery of the l-galactose pathway (Wheeler et al. 1998). Since then, many studies have addressed the molecular mechanisms involved in AsA biosynthesis in plants and there is a general consensus that four AsA biosynthetic pathways are operative in plants. According to the molecules that act as precursors, the pathways have been named as: l-galactose, l-gulose, the myo-inositol and d-galacturonic acid pathways. The l-galactose pathway, which is considered the major AsA biosynthetic route in several plant species, consists of a series of reactions starting from d-mannose-1-P (Fig. 1; Wheeler et al. 1998). The observation that GDP-d-mannose-3′, 5′-epimerase (GME) can produce GDP-l-gulose, in addition to formation of GDP-l-galactose, led to the proposal for an alternative route (l-gulose pathway) in which l-gulose and l-gulono-1, 4-lactone are key intermediates (Fig. 1; Wolucka and Van Montagu 2003, 2007). On the other hand, overexpression of an Arabidopsis gene encoding myo-inositol oxygenase, which catalyzes the oxidation of myo-inositol into d-glucuronate, increased AsA levels, suggesting an alternative biogenesis for l-gulono-1, 4-lactone (myo-inositol pathway). Moreover, a fourth pathway in which d-galacturonic acid is the main intermediate has also been reported in plants [Fig. 1; Loewus et al. 1958; Agius et al. 2003; reviewed in Lorence and Nessler (2007)].
Schematic representation of the biosynthetic, degrading and recycling pathways of AsA in plants. The enzymes catalyzing the reactions are: GMP GDP-mannose pyrophosphorylase, GME GDP-mannose-3′-5′-epimerase, GGP GDP-l-galactose phosphorylase, GPP l-galactose-1-phosphate phosphatase, GalDH l-galactose dehydrogenase, GalLDH l-galactono-1,4-lactone dehydrogenase, GalUR d-galacturonic acid reductase, MIOX myo-inositol oxygenase, GLOase l-gulono-1,4-lactone oxidase, MDHAR monodehydroascorbate reductase, DHAR dehydroascorbate reductase, AO ascorbate oxidase, APX ascorbate peroxidase. Adapted from Smirnoff (2011)
In plants, the involvement of the l-gulose and myo-inositol pathways in the biosynthesis of AsA is still not fully supported by experimental evidence. The last step of the l-gulose pathway that is the oxidation of l-gulono-1,4-lactone (Fig. 1) by GLOase activity, is not confirmed as much as GalLDH. In fact, the GalLDH purified or recombinant proteins from sweet potato, cauliflower and tobacco exhibited only trace amounts of GLOase activity (Oba et al. 1995; Ostergaard et al. 1997; Imai et al. 1998; Yabuta et al. 2000). On the other hand, experimental evidence argued against the contribution of the MIOX pathway to AsA content. In this sense, Endres and Tenhaken (2009) did not found increases in AsA content in leaf tissue of Arabidopsis plants overexpressing the MIOX4 gene. Moreover, they reported that feeding with myo-inositol did not enhance AsA concentration in leaves of transgenic plants. These results suggest that the expression of MIOX gene control the level of myo-inositol, but does not contribute to regulate AsA contents, at least in Arabidopsis plants.
Most of the experiments on AsA biosynthesis were initially carried out on the model plant Arabidopsis thaliana, and subsequently, studies on agronomical important crops with fleshy fruits have been also addressed. Thus, in tomato, kiwifruit and immature peach fruits the l-galactose pathway predominates (Bulley et al. 2009; Imai et al. 2009; Ioannidi et al. 2009; Mellidou et al. 2012b). In contrast, the d-galacturonic acid and the myo-inositol pathways seem to prevail in strawberry fruits, since expression of GalUR and MIOX positively correlates with AsA contents (Agius et al. 2003; Cruz-Rus et al. 2011). In grape berries, GalUR gene expression and protein levels were up-regulated during fruit ripening and also upon high light exposure in parallel to the increment in AsA content (Cruz-Rus et al. 2010).
In tomato, Ioannidi et al. (2009) found that most of the genes involved in the l-galactose pathway followed a temporal pattern of expression opposite to that of AsA accumulation. Interestingly, GPP closely correlated with AsA levels, suggesting a regulatory role of this gene in AsA biosynthesis. Other studies indicated that processes, such as AsA translocation from source leaves and the biosynthesis via the l-galactose pathway in immature fruits, and the transition to the d-galacturonic acid pathway in ripening fruits also contribute to AsA accumulation in tomato fruit (Badejo et al. 2012). Moreover, experiments carried out on recombinant inbred lines (RILs) from a cross of Solanum lycopersicum and its wild relative Solanum pimpinefollium revealed that AsA contents in red ripe fruits did not correlate with the expression of genes involved in its biosynthesis (Lima-Silva et al. 2012).
On the other hand, feeding experiments with AsA precursors demonstrated that the peel of apple fruits was capable of the de novo biosynthesis of AsA via the l-galactose and the d-galacturonic acid pathways while the flesh and seed were only able to synthesize AsA via the l-galactose pathway (Li et al. 2008). In addition, the AsA contents decreased exponentially from the peel/exocarp to the fruit core (Davey et al. 2004). Quantitative trait loci analysis of a F1 progeny derived from a cross between the apple cultivars ‘Telamon’ and ‘Braeburn’ revealed that allelic variation in paralogs of MdGGP is consistently linked to fruit AsA concentrations, clearly indicating a major role for these genes (Mellidou et al. 2012a). Taken together, these studies revealed that AsA content in fruits is highly regulated by a complex metabolic network in which the four AsA biosynthetic pathways act in concert in a species- and tissue-specific manner that may also vary depending on the stage of fruit development.
In addition to the de novo AsA biosynthesis, other mechanisms such as degradation and recycling can significantly contribute to the regulation of AsA concentrations in plant tissues (Fig. 1). Hence, AsA can be transformed into monodehydroascorbate (MDHA) by the enzymes ascorbate oxidase (AO) and ascorbate peroxidase (APX). Then, the short-lived MDHA radical can either be recycled into AsA by monodehydroascorbate reductase (MDHAR) or undergo disproportionation into dehydroascorbate (DHA) and AsA. Moreover, DHA can be recycled into AsA by dehydroascorbate reductase (DHAR) before being irreversibly hydrolyzed [Fig. 1; reviewed in Gallie (2013)].
Experiments with transgenic plants have demonstrated that overexpression of genes encoding AsA biosynthetic (Bulley et al. 2012) and recycling enzymes (Chen et al. 2003; Haroldsen et al. 2011) or the suppression of degrading enzymes (Zhang et al. 2011) can lead to the enhancement of AsA concentrations. Nevertheless, there are exceptions to the general observations, since SlGalLDH silencing by RNAi was accompanied by significant changes in mitochondrial function and altered AsA redox state despite the fact that total AsA content remained unchanged (Alhagdow et al. 2007). These results highlight the relevance of the balance between the biosynthesis and degrading/recycling AsA pathways and the complexity of the regulation of the AsA concentration.
Citrus fruits are widely known by the high consumption of both fresh fruits and juice. In addition, citrus constitute a major source of AsA for humans’ diet as they are among the top ten AsA-accumulating commercial fruits (Cruz-Rus et al. 2012). The concentration of AsA in the different tissues of the citrus fruit is uneven, varies during fruit development, and differs among species and varieties. Analysis of the AsA contents conducted over the years in fruit tissues of different species and varieties demonstrated that AsA contents are generally higher in the peel than in the pulp. Thus, AsA content in the flavedo, the colored layer of the peel, ranges between 130 and 374 mg/100 g fresh weight (FW), whereas in the albedo, the white layer of the peel, is between 50 and 190 mg/100 g FW, and the content in the pulp is around 20–70 mg/100 g FW [reviewed in Martí et al. (2009)]. Citrus fruit juices provide an important source of vitamin C for human nutrition, even though only about one-fourth of the total vitamin C content of a fruit is found in the juice. Early studies already established that the distribution of vitamin C in the components of a fruit was: 34 % flavedo, 19 % albedo, 21 % pulp vesicles and 26 % juice (Atkins et al. 1945). Thus, the edible pulp of the fruit has higher vitamin C content than its corresponding juice, in accordance with the observation that higher vitamin C contents are usually found in commercial citrus juice prepared from fruits with richest concentration in the pulp (Abeysinghe et al. 2007).
Quantitative differences in AsA concentration in fruit tissues appear to be related to the genotypes and/or to the stage of development. In general, oranges contain the highest AsA levels among the citrus genus, followed by lemons, grapefruits and mandarins (reviewed in Martí et al. 2009). Vitamin C content in orange juices ranges between 29 and 82 mg/100 mL, being Navel and Pineapple richer than Valencia Late oranges (Nagy 1980). In fruits of different lemon varieties, vitamin C contents fluctuate from 30 to 50 mg/100 mL, a range slightly higher than that of grapefruits. High variability in vitamin C has been observed among the mandarin genotypes, with the concentrations in Satsuma varieties being lower than those of Clementine (Martí et al. 2009).
Although AsA levels in citrus fruits have been extensively studied, there is very little information about the molecular regulation of AsA metabolism in these fruits. A recent study in the pulp of two varieties of oranges (Egan No.2 and Newhall) suggested that differences in AsA concentration were associated with differences in the expression of genes of the l-galactose pathway as well as in the activity of enzymes involved in AsA degradation (Yang et al. 2011). Moreover, the comparison of transcript levels of genes involved in AsA metabolism in leaves, callus, flowers and fruits of sweet orange, suggested that the d-galacturonic acid pathway could be important in fruits since some members of the GalUR gene family were significantly up-regulated in fruits as compared to other tissues (Xu et al. 2013). However, a global view of the changes in the expression of the multiple genes and isoforms involved in the main metabolic pathways controlling AsA concentration (biosynthesis, degradation and recycling), during the development and ripening of citrus fruit, is still lacking, and constituted the objective of the present work. To that end, we have analyzed the expression levels of 13 genes involved in the AsA biosynthetic pathways and ten genes encoding for enzymes participating in the recycling and degradation mechanisms. To establish a potential relationship between AsA concentrations and transcript levels, AsA content and gene expression were assessed in both peel and pulp, since these tissues accumulate disparate amounts of AsA, and in fruits of two citrus genotypes displaying significant differences in AsA content.
Materials and methods
Plant material and growth conditions
A preliminary screening of fruits from varieties accumulating different concentrations of AsA was performed in fruits of the following genotypes: two sweet oranges (C. sinensis L. Osbeck) cv. Washington Navel and Valencia late; two mandarins, Satsuma (C. unshiu Marc) cv. Owari and Clementine (C. clementine Hort. ex Tan) cv. Clemenules and two grapefruits (C. paradisi Macf.) cv. Marsh and Star Ruby. Fruits were harvested at monthly intervals, from immature-green fruit to full maturity stages, from June until December, from adult trees grafted on Citrange carrizo rootstocks grown at the Citrus Germplasm Bank of the Instituto Valenciano de Investigaciones Agrarias (+39° 35′ 22″, −0° 23′ 40″, Moncada, Valencia, Spain).
Immediately after harvest, fruits were delivered to the laboratory and selected for uniformity and the absence of any lesion or injury. Then, the fruit equatorial diameter was measured in 20 fruits from each variety. Peel color of the same fruits was measured using a Minolta CR-330 colorimeter on three locations around the equatorial plane of the fruit. Color index was expressed as the a/b Hunter ratio (Stewart and Wheaton 1971; Rodrigo et al. 2004) which is negative for green fruit, around zero for yellow fruit at color break and is positive for orange colored fruit. Flavedo (the outer colored part of the fruit peel) and pulp were separated with a scalpel, immediately frozen in liquid nitrogen, ground to a fine powder and stored at −80 °C until analysis.
Maturity index
Juice from fruits was extracted with a household electric hand reamer, filtered through a metal sieve with a pore size of 0.8 mm and analyzed immediately. The acidity (TA) of the juice was determined by titration with 0.1 N NaOH and is expressed as mg of citric acid per 100 mL and the total soluble solid content (TSS, °Brix) by refractometry, using an Atago model PR32. The maturity index (MI), expressed as the ratio between TSS and TA of the juice, was measured in fruits harvested after September because before that time they were too small and the pulp was too dry to extract the juice content.
Ascorbic acid extraction and analysis by HPLC
Ascorbic acid was extracted and determined essentially as described in Alós et al. (2013). Briefly, flavedo and pulp tissue (0.5 g) were homogenized for 1 min using a Polytron homogenizer with 0.1 % metaphosphoric acid (4 mL). The homogenate was centrifuged for 10 min at 4,000g at 4 °C. The supernatant was filtered through a C18 cartridge (SepPak, Waters, Spain), previously activated with 4 mL of methanol, 4 mL of water and 4 mL of 2 % metaphosphoric acid. The extract was subsequently filtered through a 0.45 μm nylon filter (25 mm diameter, Análisis Vínicos, Spain). The filtrate was injected in the HPLC for AsA determination.
Dehydroascorbic acid (DHA) content was calculated from the difference between total vitamin C and the AsA contents. To determine total vitamin C we adapted the protocol described by Davey et al. (2003). Thus, a 200 μL aliquot of the above-mentioned filtrate was incubated for 15 min at room temperature with 100 μL 200 mM DTT in 400 mM Tris-base which generated a final pH of 6 to 6.8. Then, the reaction was stopped by acidification with 100 μL of 8.5 % ortho-phosphoric acid.
Sample analysis by HPLC was carried out using a Dionex HPLC system with a photodiode array detector and Chromeleon software (Dionex), an Ultrabase C18 column (100 × 4.6 mm, 2.5 μm) and a mobile phase of methanol: water pH 2.5 (adjusted with metaphosphoric acid, 15:85, v/v), 0.2 mL/min flux and injection volume 10 μL. The temperature of the column was set at 35 °C. The method was calibrated with a curve of an ascorbic acid standard solution in 2 % metaphosphoric acid, with concentrations between 1 and 100 μg/mL.
RNA extraction and cDNA synthesis
Total RNA was isolated from the fruit tissues using RNeasy Plant Mini Kit (Qiagen) and subsequently treated with DNase I (DNA free, DNase treatment and removal, Ambion). The amount of RNA was measured by spectrophotometric analysis (Nanodrop) and its quality was verified by agarose gel electrophoresis and ethidium bromide staining. The absence of DNA contamination was checked by performing a no-reverse transcription assay which consisted of a PCR with each RNA sample using the ACTIN primers (Supplementary Table S1). No amplified products were detected which confirmed the purity of the RNA extracts. The transcripts present in 5 μg of total RNA were reverse-transcribed using the superscript III reverse transcriptase (invitrogen) in a total volume of 20 μL. One μL of a tenfold diluted first-strand cDNA was used for each amplification reaction.
Gene expression analysis by real-time PCR
Gene expression studies were performed following the MIQE guidelines (Bustin et al. 2009). Quantitative real-time PCR was carried out on a LightCycler 480 instrument (Roche), using the LightCycler 480 SYBRGreen I Master kit (Roche). Reaction mix and conditions followed the manufacturer’s instructions with some modifications. The PCR mix contained 1 μL of diluted cDNA, 5 μL of SYBR Green I Master Mix, 1 μL of 3 μM primer F and 1 μL of 3 μM primer R, being the final volume of 10 μL. The primers (PSF purified, Isogen) used for the amplification of each gene are listed in Supplementary Table S1. The cycling protocol, for all genes, consisted of 10 min at 95 °C for pre-incubation, then 40 cycles of 10 s at 95 °C for denaturation, 10 s at 59 °C for annealing and 10 s at 72 °C for extension. Fluorescent intensity data were acquired during the extension time with the LightCycler 480 Software release 1.5.0, version 1.5.0.39 (Roche) and were transformed into mRNA levels using specific standard curves for all analyzed genes. Amplification efficiency (E) and correlation coefficient (R 2) of each primer were calculated using the standard curve method and the formula E = 10(−1/slope) (Bustin et al. 2009).
The specificity of the PCR reaction was assessed by the presence of a single peak in the dissociation curve performed after the amplification steps followed by the sequencing of the amplicon. Three potential housekeeping genes were tested in this experiment based on previously published primer sequences of citrus genes: ACTIN, ELONGATION FACTOR 1 (EF1), β-TUBULIN (β-TUB) (Romero et al. 2012; Rodrigo et al. 2013) To test the stability of these genes, the BestKeeper software was used (Pfaffl et al. 2004). In this analysis, the most stably expressed genes are the ones having the lowest of SD [± Crossing Point (CP)], and any studied gene with the SD higher than 1 can be considered inconsistent. The SD [± CP] were: 0.38, 0.52 and 0.62 for ACTIN, β-TUB and EF1, respectively. Therefore, the ACTIN gene which had the lowest SD [± CP] value was the best housekeeping gene for our analysis and this is the reason for the normalization against it (Supplementary Table S1; Pfaffl et al. 2004; Romero et al. 2012; Rodrigo et al. 2013). The expression levels relative to values of a reference sample were calculated using the Relative Expression Software Tool (REST Multiple Condition Solver REST-MCS v2, http://rest.gene-quantification.info; Pfaffl et al. 2002). The reference sample was the expression value obtained for each gene on the flavedo of immature-green Satsuma Owari fruits (collected on the 15th of June), which was arbitrarily given the expression value of 1. Results were the mean of at least three independent replicates. The Relative Expression Software Tool REST 2009 software (http://www.gene-quantification.de/rest-2009.html) was used to determine statistical significance between varieties (P < 0.05). In addition, to compare absolute gene expression values the protocol described by Romero et al. (2012) was followed.
Results
Changes in AsA concentration during citrus fruit development and ripening
To select citrus fruits with contrasting AsA concentration in both peel and pulp, a screening of six varieties, representing the four major cultivated species: mandarin (cv. Satsuma Owari), clementine (cv. Clemenules), orange (cvs. Washington Navel and Valencia late) and grapefruit (cvs. Marsh and Star Ruby) was carried out. AsA concentrations were quantified in flavedo and pulp during at least six stages of fruit development and ripening. In fruit of all varieties, AsA content was always higher in the peel than in the pulp, and in the pulp decreased during fruit development. In pulp of full colored fruits, the highest AsA concentrations were registered in oranges (50 mg/100 g FW) followed by clementine (43 mg/100 g FW), both grapefruits (around 35 mg/100 g FW), and Satsuma mandarin (20 mg/100 g FW).
AsA contents in the flavedo followed a different pattern of accumulation than that of the pulp and also displayed differences among genotypes. Thus, in oranges AsA concentrations were rather stable for the first stages of development and increased after color break, to reach 200 and 240 mg/100 g FW in full colored fruits of Washington Navel and Valencia late, respectively. In grapefruits, mandarin and clementine cultivars AsA concentrations increased gradually during the whole studied period. In both varieties of grapefruits AsA levels increased from 130 to 150 mg/100 g FW to 200 mg/100 g FW, from June to December, with minor differences between varieties. Mandarin and clementine fruits followed a similar pattern of AsA accumulation, but the concentrations were significantly lower than in oranges and grapefruits. Dehydroascorbic acid (DHA) was less abundant than AsA in all the varieties studied and in general higher in the peel than in pulp. Moreover, DHA content was higher in the peel and pulp of fruits at immature-green stages (June–July) and declined later in development. In summary, flavedo and pulp of Washington Navel oranges contained the highest AsA contents while tissues of Satsuma mandarin were the lowest among the six genotypes and, therefore, these two varieties were selected to perform further studies on the molecular regulation of AsA metabolism.
Analysis of fruit diameter, external fruit color and internal maturity index in fruits of Satsuma mandarin and Washington Navel orange revealed that fruit maturation proceeded in a similar fashion in both genotypes and, therefore, allowed a reliable and timely comparison of the AsA content (Supplementary Fig. S1). It is remarkable that the AsA contents in the peel of immature-green fruits of mandarin were extremely low (5 mg/100 g FW) in comparison with the corresponding tissue of Washington Navel oranges (Fig. 2). In oranges, AsA remained fairly constant until the mature green stage (early October) and then it experienced an increase to reach maximum levels (240 mg/100 g FW). In the peel of Satsuma mandarin, the AsA concentration increased progressively throughout development and maturation, being between 20 and 40 % lower than in Navel oranges. In the pulp, the AsA contents declined during fruit development in both genotypes, being in oranges about twofold higher than in mandarins (Fig. 2). The highest levels of DHA were detected in the flavedo and pulp of immature fruits of Washington Navel while at the same stage it was not detectable or a very low level in the tissues of Satsuma mandarin. In later stages of fruit development, DHA remained at low levels and without substantial differences between the two varieties (Fig. 1e, f).
External and internal appearance of Satsuma Owari mandarin (a) and Washington Navel orange fruits (b). Evolution of AsA concentration in flavedo (c) and pulp (d) of Satsuma Owari mandarin (white circles) and Washington Navel orange (black circles) during fruit development and ripening. Evolution of DHA concentration in flavedo (e) and pulp (f) of Satsuma Owari mandarin (white circles) and Washington Navel orange (black circles) during fruit development and ripening. Data are mean ± SD of three replicates. JN June, JL July, A August, S September, O October, N November, D December, respectively
Selection of genes involved in AsA metabolism for analysis by qRT-PCR
To understand the transcriptional regulation of the main pathways involved in AsA biosynthesis, degradation and recycling in the flavedo and the pulp of citrus fruits, an important number of genes corresponding to key metabolic steps were selected to get a wide perspective of AsA metabolism during fruit development and ripening (Fig. 1, Supplementary Table S1). Gene sequences were obtained from the draft genome of the double haploid sweet orange (C. sinensis) genome project from the Huazhong Agricultural University (http://citrus.hzau.edu.cn/orange; Xu et al. 2013). A total of 23 genes were selected for further expression analysis corresponding to 13 genes from biosynthetic steps: GDP-mannose pyrophosphorylase (GMP), GDP-mannose-3′-5′-epimerase (GME), GDP-l-galactose phosphorylase (GGP), l-galactose-1-phosphate phosphatase (GPP), l-galactose dehydrogenase (GalDH), l-galactono-1,4-lactone dehydrogenase (GalLDH), myo-inositol oxygenase (MIOX) and six isoforms of galacturonic acid reductase (GalUR); five genes of degradation steps including ascorbate oxidase (AO) and four isoforms of ascorbate peroxidase (APX); and five genes form recycling steps: two isoforms of dehydroascorbate reductase (DHAR) and three isoforms of monodehydroascorbate reductase (MDHAR).
In some cases, the alignment of the nucleotidic sequences of the putative genes contained in the draft double haploid sweet orange genome project (DHSO) (Xu et al. 2013) with the corresponding sweet orange genome project (SOGP) database (2010) (Phytozome; http://www.phytozome.net/citrus.php) and known genes of the AsA metabolism from other plants species revealed that further curation is needed in the annotation of these sequences. The existence of three genes of GME (Cs8g02600.1, Cs8g02610.1 and Cs3g10840.1) has been described in the DHSO (Xu et al. 2013), however, the alignment of these sequences with other known GME proteins from plants showed that only Cs3g10840.1 was encoding for the full-length protein. Thus, Cs3g10840.1 was the gene considered for primer design and subsequent expression analysis. Similarly, two putative isoforms of GalLDH were identified in the DHSO project, but only the one that coincided with the unique GalLDH gene described in the SOGP database, orange1.1t00763.1, encoded for the full-length protein and, consequently, this was the gene selected for analysis. Furthermore, it has also been proposed that there are two isoforms of GGP in citrus (Xu et al. 2013), however, the expression analysis of both genes in fruit tissues of Washington Navel orange and Satsuma mandarin revealed that the isoform encoded by Cs6g17580.1 was not expressed. Therefore, expression levels of the GGP gene presented in this work correspond to the Cs4g09870.1 isoform (Supplementary Table S1).
Expression of genes involved in AsA biosynthesis in flavedo during development and maturation of Washington Navel orange and Satsuma mandarin
The expression levels of 13 genes involved in AsA biosynthesis were analyzed in flavedo of Washington Navel orange and Satsuma mandarin at six developmental stages. The mRNA abundance of each gene was normalized to the values registered in the flavedo of immature-green mandarin (June), to obtain a reliable comparison of the differences in gene expression between the two tissues and the two genotypes (Figs. 3, 4).
Relative expression of genes involved in AsA biosynthetic pathways in flavedo of Satsuma Owari mandarin (white circles) and Washington Navel orange (black circles) during fruit development and ripening. The genes measured were: GMP, GME, GGP, GPP, GalDH, GalLDH, GalUR5, GalUR8, GalUR10, GalUR12, GalUR14, GalUR17 and MIOX. An expression value of 1 was arbitrarily assigned to the values obtained in the flavedo of Satsuma Owari fruits harvested in June. The data are mean ± S.E of at least three replicates. The asterisk indicates statistical significance (p < 0.05) between the expression of Washington Navel orange and Satsuma mandarin. JN June, JL July, A August, S September, O October, N November, D December, respectively
Relative expression of genes involved in AsA biosynthetic pathways in pulp of Satsuma Owari mandarin (white circles) and Washington Navel orange (black circles) during fruit development and ripening. The genes measured were: GMP, GME, GGP, GPP, GalDH, GalLDH, GalUR5, GalUR8, GalUR10, GalUR12, GalUR14, GalUR17 and MIOX. An expression value of 1 was arbitrarily assigned to the values obtained in the flavedo of Satsuma Owari fruits harvested in June. The data are mean ± S.E of at least three replicates. The asterisk indicates statistical significance (p < 0.05) between the expression of Washington Navel orange and Satsuma mandarin. JN June, JL July, A August, S September, O October, N November, D December, respectively
In the flavedo, expression of the genes involved in the L-galactose pathway followed different patterns. GME, GPP, GalDH and GalLDH expression profiles displayed only minor differences between the two varieties. GME, GalDH and GalLDH transcript abundance was rather stable with slight fluctuations during the whole period (Fig. 3). By contrast, accumulation of GPP mRNA in both varieties experienced an important increment at latter stages of fruit maturation when transcript abundance was lower in mandarin than in orange. Other genes of this pathway, such as GMP and GGP, displayed different profiling in the peel of each variety. GMP transcript levels were almost constant until September and then experienced a twofold induction in oranges but not in mandarins, to decline later on. GGP transcript abundance increased gradually in the peel of both varieties until October, declined later on in mandarin but was further induced in oranges. Abundance of GGP transcripts in full mature fruit was about fivefold higher in oranges than in mandarin peel (Fig. 3).
Analysis of the expression of six GalUR isoforms from the D-galacturonic pathway in flavedo of Washington Navel orange and Satsuma mandarin revealed different expression patterns and, interestingly, none of them appeared to be positively correlated with AsA concentration (Figs. 2, 3). GalUR5 and GalUR8 were expressed at higher levels in Satsuma mandarin than in Washington Navel from July until October or December, respectively. In contrast, GalUR10, GalUR12 and GalUR17 mRNA levels were more abundant in Washington Navel orange, especially GalUR12 that was up to 17-fold higher than in Satsuma mandarin. GalUR10 and GalUR17 displayed a maximum in July and thereafter decreased (Fig. 3). GalUR14 was expressed at constant levels during the whole ripening process without significant differences between varieties (Fig. 3). It is worth to mention that the highest levels of absolute gene expression were recorded for GalUR12, followed by GalUR8 and GalUR14 (Supplementary Table S2).
MIOX levels were strikingly higher (45-35-fold) in the flavedo of immature Washington Navel orange fruits (June and July) than in Satsuma mandarin, declining thereafter to very low and similar levels in both genotypes (Fig. 3).
Expression of genes involved in AsA biosynthesis in the pulp during development and maturation of Washington Navel orange and Satsuma mandarin
In general, mRNA levels of genes involved in AsA biosynthesis were lower in the pulp than in the flavedo (up to tenfold), in agreement with AsA concentrations (Figs. 2, 3, 4). It is worth to note that the pattern of expression of most of these genes was distinct in peel and pulp, suggesting a differential regulation in these tissues. Expression of GMP, GME, GalDH and GalLDH genes followed a similar pattern in both varieties. With the exception of GME which reached a peak in November, the other genes declined or did not experience important differences. GGP and GPP transcript abundance was substantially higher in the pulp of Washington Navel orange than in mandarin (up to fivefold in September) throughout the whole period (Fig. 4).
Expression of the genes involved in the alternative pathways (D-galacturonic and myo-inositol) in the pulp of both genotypes is also shown in Fig. 4. Changes in the mRNA abundance of most of these genes did not correlate with the pattern of accumulation of AsA in the pulp, except for GalUR10 which declined gradually during development and maturation. GalUR5 and GalUR17 were the only mRNAs whose levels were higher in the pulp of oranges than in mandarins at late maturation stages. The relative abundance of transcripts corresponding to GalUR8, GalUR12, GalUR14 and MIOX was very similar between the two genotypes, although the MIOX mRNAs were very scarce or undetectable (Fig. 4). In the pulp, GalUR12, GalUR8 and GalUR14 were the most abundant isoforms in terms of absolute abundance (Supplementary Table S2).
Expression of genes involved in AsA recycling and degradation in flavedo during development and maturation of Washington Navel orange and Satsuma mandarin
AsA recycling and degradation mechanisms significantly contribute to determine AsA levels in plants (reviewed in Gallie 2013; Fig. 1). Therefore, to understand the transcriptional regulation of these processes the expression levels of genes involved in the recycling pathways (three MDHAR and two DHAR isoforms) and also in the degradation pathways (AO, and four APX isoforms; Supplementary Tables S1, S3, S4; Figs. 5, 6) were analyzed.
Relative expression of genes involved in AsA recycling and degradation pathways in flavedo of Satsuma Owari mandarin (white circles) and Washington Navel orange (black circles) during fruit development and ripening. The genes measured were: MDHAR1, MDHAR2, MDHAR3, DHAR1, DHAR2, AO, APX1, APX2, APX3 and APX4. An expression value of 1 was arbitrarily assigned to the values obtained in the flavedo of Satsuma Owari fruits harvested in June. The data are mean ± S.E of at least three replicates. The asterisk indicates statistical significance (p < 0.05) between the expression of Washington Navel orange and Satsuma mandarin. JN June, JL July, A August, S September, O October, N November, D December, respectively
Relative expression of genes involved in AsA recycling and degradation pathways in pulp of Satsuma Owari mandarin (white circles) and Washington Navel orange (black circles) during fruit development and ripening. The genes measured were: monodehydroascorbate reductase1 (MDHAR1), MDHAR2, MDHAR3, DHAR1, DHAR2, AO, APX1, APX2, APX3 and APX4. An expression value of 1 was arbitrarily assigned to the values obtained in the flavedo of Satsuma Owari fruits harvested in June. The data are mean ± S.E of at least three replicates. The asterisk indicates statistical significance (p < 0.05) between the expression of Washington Navel orange and Satsuma mandarin. JN June, JL July, A August, S September, O October, N November, D December, respectively
As stated before for genes involved in AsA biosynthesis, genes related to the recycling and degradation pathways were more highly expressed in the flavedo than in the pulp, with the exception of APX2 (Figs. 5, 6; Supplementary Tables S3, S4). MDHAR1, MDHAR2 and MDHAR3 displayed parallel expression patterns in both genotypes. In some stages of fruit development, mRNA levels of MDHAR1 and MDHAR2 were higher in Satsuma mandarin than in Washington Navel orange, whereas abundance of MDHAR3 was always higher in orange. It is interesting to note that MDHAR2 and MDHAR3, which were the most abundant isoforms (Supplementary Table S3), had expression patterns that resembled the evolution of AsA concentration (Figs. 2, 5). On the other hand, DHAR1 transcripts decreased gradually during fruit development and ripening in both varieties, while DHAR2 mRNA levels remained nearly constant in oranges or experienced fluctuations in the flavedo of Satsuma mandarin (Fig. 5).
Genes involved in the degradation pathways followed different expression profiles in the flavedo during development and maturation. AO mRNA levels sharply declined after the immature-green stage to remain at very low levels from September onwards. Other genes like APX1, which was the most highly expressed isoform, and APX3 showed a rather flat profile, whereas APX2 mRNA levels increased from June until September, then transiently decreased and in December experienced a slight increase (Fig. 5 and Supplementary Table S4). APX3 and APX2 were significantly more expressed in Satsuma mandarin than in Washington. In Satsuma mandarin, APX4 displayed a remarkable induction between June and October, while in Washington Navel its expression levels were quite constant and showed a more striking increase in November and December (Fig. 5).
Expression of genes involved in AsA recycling and degradation in the pulp during development and maturation of Navel orange and Satsuma mandarin
With the exception of DHAR1, the genes related to AsA recycling did not parallel the evolution of AsA concentration in the pulp over the whole period of fruit development and ripening. Profiling of the three MDHAR isoforms was similar and experienced an increase with fruit maturation that was slightly higher in oranges than in mandarin. Accumulation of DHAR1 mRNA was higher in Washington Navel oranges than in Satsuma mandarins and declined in parallel with the reduction of AsA concentration and interestingly DHAR1 was the most abundant DHAR isoform (Supplementary Table S3). In contrast, DHAR2 mRNAs were more abundant in Satsuma mandarin than in Washington Navel orange at the early stages of fruit development (Fig. 6).
AO transcript profiling in the pulp was similar to that of flavedo displaying a drastic reduction at the first stages of development to remain at extremely low levels for the rest of the ripening process. Two patterns of expression could be distinguished for the four APX genes, hence APX1 mRNA abundance was rather constant during the whole ripening process while APX2, APX3 and APX4 increased (Fig. 6). Considering the absolute transcript quantification, APX2 and APX4 were more abundant than the APX1 and APX3 isoforms (Supplementary Table S4).
Discussion
Citrus fruits have been classically recognized as one of the main sources of vitamin C for human nutrition and health. Concentrations of AsA in pulp and juice of fruits of the different citrus species and varieties have been long studied and changes in AsA levels related to temperature treatments, processing methods, and storage conditions have been also extensively analyzed (Nagy 1980; Martí et al. 2009). Although environmental and cultural conditions may substantially affect AsA content in citrus fruits, variability appears to be mainly genetically determined (Dhuique-Mayer et al. 2005; Bermejo et al. 2011). Thus, higher AsA contents are usually found in oranges and lemons followed by grapefruit and mandarins (Martí et al. 2009), and similarly to other bioactive compounds, the variability observed is more interspecific than intraspecific (Dhuique-Mayer et al. 2005). In the present work, the genetic diversity of the citrus genus has been exploited to select varieties with contrasting concentration of AsA to identify the predominant metabolic pathways and potential rate limiting steps of AsA metabolism in peel and pulp of citrus fruits. The analysis of AsA concentrations in peel and pulp of fruits of six citrus varieties including two sweet oranges, two grapefruits, a mandarin and a clementine revealed that Washington Navel oranges and Satsuma mandarin fruits contain the highest and lowest AsA concentrations, respectively (Fig. 2). These results are in agreement with previously reported AsA contents in fruits of other citrus varieties and reinforce the observation that Japanese-type mandarins like Satsuma, contain lower AsA concentrations than the Mediterranean-type mandarins, like Clementine (Dhuique-Mayer et al. 2005; Bermejo et al. 2011).
It is noticeable that AsA concentration in fruits of all the varieties analyzed was always higher in peel than in pulp (Fig. 2). Similar results have been also found in other citrus fruits and, in general, AsA content in the pulp is usually one-third to one-fourth that of the peel (Martí et al. 2009; Bermejo et al. 2011). Differences in AsA contents among fruit tissues have also been detected in other fruits, such as apple (Li et al. 2009). The reasons for these differences are not fully known, but it has been speculated that the peel is the first barrier of the fruit and the interphase with the environment, directly exposed to radiation, wounding, wind, etc., and probably requires a higher and more efficient antioxidant system to cope with these biotic and abiotic stresses (Tausz et al. 2004). Moreover, light has also been shown to stimulate AsA content and the expression of AsA biosynthetic and recycling genes in the peel of apple fruits (Li et al. 2009) and in vegetative tissues of rice and Arabidopsis (Yabuta et al. 2007; Fukunaga et al. 2010), where AsA plays a role in photosynthesis and in photoprotection (Smirnoff 2011). Thus, it is likely that the differences in AsA content between peel and pulp of citrus fruits may be at least partially mediated by the direct exposure to light.
The concentration of AsA in peel and pulp of citrus was not only quantitatively different, but also followed a different pattern of accumulation (Fig. 2). In the peel, the AsA concentration increased progressively during development and maturation, and although the profiling was different between oranges and mandarins, AsA content was higher in mature fruits of both varieties. By contrast, AsA concentration declined progressively in the pulp, and differences between genotypes were maintained throughout the whole development and maturation (Fig. 2). These discrepancies in AsA accumulation envisage that different mechanisms of AsA biosynthesis, recycling and degradation may operate or be preponderant in each tissue and also that the regulation of the pathways could be distinct between tissues. Moreover, our data indicated that changes in AsA content during maturation did not correlate with changes in peel color or internal maturation (Supplementary Fig. S1). These results are consistent with other observations, in which other maturation-related processes, such as carotenoid content and composition, flavonoids or essential oils, did not parallel the evolution of AsA content (Dhuique-Mayer et al. 2005; Bermejo et al. 2011). Yang et al. (2013) also observed that the increase in total sugars in the peel of Satsuma mandarin induced by branch girdling was not related to the increase of AsA concentrations. Together, changes in AsA content in both peel and pulp of citrus fruits appeared not to be linked to other modifications of primary metabolism occurring during maturation and should not be considered as a maturation-related process. In fruit of other species, changes in AsA contents during fruit development follow different patterns, for example AsA contents increase during tomato fruit ripening after the turning stage (Ioannidi et al. 2009) or decrease steadily during the development of peach fruit (Imai et al. 2009).
A relevant conclusion from the present work arises from the comparison of AsA levels during development and ripening with the expression of AsA biosynthetic genes in peel and pulp of orange and mandarin fruits. Results indicated that AsA content appears to be differentially regulated between peel and pulp tissues, and that the relative contribution of the different biosynthetic pathways to the AsA pool changes in each tissue during the different stages of fruit development and maturation. In the flavedo of Washington Navel oranges at the first stages of fruit development, high levels of MIOX transcripts were associated with high AsA concentrations. Interestingly, at the same stage of development, AsA content in the peel of Satsuma mandarin was substantially lower (about 50-fold) and the relative expression of MIOX was also 45-fold lower, suggesting that this step may be a limiting factor for the differences in AsA content in the peel between both varieties at early stages of fruit development (Figs. 2, 3). In tomato fruit it has also been described that MIOX transcripts are highly expressed at the early stages of fruit development and not detectable during maturation (Ioannidi et al. 2009). Subsequently in development (September), MIOX mRNA abundance decayed coinciding with the stimulation of genes of the L-galactose pathway and an important increment of AsA in the peel (Figs. 2, 3). Then, expression of GMP experienced a transient increment only in the peel of oranges whereas GGP gene was stimulated in orange fruit, but declined in mandarin. By contrast, evolution of the expression of GPP gene was similar in the flavedo of both mandarin and orange (Fig. 3). These results suggest that during fruit maturation, the L-galactose pathway may predominate in the biosynthesis of AsA in the flavedo and that the selective stimulation of GMP and mainly GGP would contribute to a higher flow of metabolic precursors in the peel of oranges than in mandarins. The stimulation of the expression of GPP could be important for the general activation of this branch, but it appears not to be a limiting factor for the differences observed in AsA contents between genotypes. Together, these results suggest a transition in the biosynthetic pathways of AsA in the flavedo of citrus fruits that may partly explain the differences between the peel of Washington Navel oranges and Satsuma mandarin. In the peel of immature orange at early stages of development it is possible that AsA would be derived from both the myo-inositol and the L-galactose pathways, while in the peel of mandarin fruit, the myo-inositol pathway would not be operative or only at very reduced levels. At latter stages of fruit development and maturation, the L-galactose pathway would be stimulated and become predominant for the synthesis of AsA, being likely that GMP and GGP genes could act as limiting factors that may account for the differences in AsA content in the peel of orange and mandarin fruits (Figs. 2, 3). The transition from one AsA biosynthetic pathway to another at different stages of fruit development has been also described in fruit of other species. For example, in tomato and grape the L-galactose pathway seemed to be the main source of AsA in immature fruits, while the D-galacturonic acid pathway predominated in ripe fruits (Melino et al. 2009; Cruz-Rus et al. 2010; Badejo et al. 2012). High expression level of the MIOX gene in immature orange flavedo may suggest a crucial role for this gene in AsA accumulation in that tissue. Thus, further investigation on GLOase activity would be interesting to support the role of the myo-inositol pathway (Fig. 1).
The involvement of the D-galacturonic acid biosynthetic pathway was also examined by analysis of the expression of six isoforms of GalUR, since previous studies described a differential expression of the isoforms of this gene family in different citrus tissues, but not a clear correlation with AsA content has been demonstrated yet (Xu et al. 2013). Results suggest a complex role of this pathway in the regulation of AsA concentration in the flavedo of Navel oranges and Satsuma mandarin. Transcriptional activation of most of the genes of this pathway did not show a correlation with AsA content. Interestingly, GalUR12 was the most highly expressed gene encoding GalUR isoforms (Supplementary Table S2) and its levels were substantially higher (about 20-fold) in oranges than in mandarins from early stages of development (Figs. 2, 3). Comparative RNAseq experiments in different organs of citrus plants revealed that GalUR12 is an abundant isoform in fruits, although its expression during ripening was not analyzed (Xu et al. 2013). It is then reasonable to assume that the D-galacturonic acid pathway, especially GalUR12, may also contribute and play a role in the regulation of AsA concentration in the flavedo of citrus fruits.
AsA levels in plant cells are tightly regulated not only by its synthesis but also by recycling and degradation processes [reviewed in Lorence and Nessler (2007) and Smirnoff (2011)]. In fact, silencing or overexpression of genes involved in AsA degradation or recycling, respectively, increased AsA concentrations in tomato and potato (Zhang et al. 2011; Haroldsen et al. 2011; Qin et al. 2011). Thus, the pattern of expression of five genes involved in AsA recycling (three MDHAR and two DHAR isoforms) and five genes of the degradation pathways was studied in flavedo and pulp to get a global perspective of the metabolic regulation of AsA in these tissues (Figs. 5, 6). Interestingly, MDHAR3 mRNA levels seemed to contribute to the differences in AsA content between genotypes since this gene was more highly expressed in the peel of Washington Navel orange than in Satsuma mandarin. This could enhance the AsA turnover and contribute to maintain higher AsA concentrations in the former genotype. Expression of genes involved in degradation of AsA displayed various profiles. It is worth to mention that the expression levels of APX1, and to a lesser extent APX4, which were the most abundantly expressed isoforms (Supplementary Table S4) were higher in oranges flavedo than in mandarins, indicating a higher ability of oranges to degrade AsA (Fig. 5). Moreover, the differences in gene expression levels of AO and, APX1 and APX4 together with the higher DHA levels observed in flavedo of immature Washington Navel oranges, suggest an important contribution of AsA degradation in AsA metabolism in the flavedo of these fruits. In tomato fruits, as in citrus, AO transcripts were high during early fruit development, which could be related with the involvement of this enzyme in cell division and expansion since AO has been proposed to participate in cell growth (Sanmartin et al. 2007). Hence, recycling and degradation steps, in particular, MDHAR3, APX1 and APX4, appear to be important factors for the regulation of AsA contents in the flavedo of citrus fruits.
Evolution of AsA content in the pulp during development and ripening of oranges and mandarins was different to that of flavedo, suggesting independent metabolic regulation, as has been demonstrated for other physiological and metabolic processes (Tadeo et al. 2008). The disparity in AsA contents between pulp of oranges and mandarins was associated with minor differences in GalDH and GalLDH transcript abundance, located downstream in the L-galactose pathway, and major changes in the expression of two genes located further upstream in the pathway: GGP and GPP (Figs. 1, 2, 4). These results suggest that the availability of precursors, coming from the steps catalyzed by GGP and GPP, could restrict the capacity to synthesize AsA in the pulp of citrus fruits. Therefore, this biosynthetic route and, particularly, these two genes could be crucial for the regulation of AsA contents in the pulp of developing fruits. Yang et al. (2011) studying AsA biosynthesis in other citrus varieties also indicated that changes in GGP, GalDH and GalLDH gene expression are associated with AsA levels, which are in partial agreement with our results. Likewise, the L-galactose pathway seemed to be pivotal for AsA biosynthesis in fruits of other species, as kiwifruit, acerola, peach and apple (Badejo et al. 2009; Bulley et al. 2009; Imai et al. 2009). The contribution of other biosynthetic pathways to AsA concentration in the pulp was not clearly supported by the analysis of the expression of six GalUR isoforms, and the MIOX gene (Fig. 4). GalUR isoforms have been suggested to be involved in AsA biosynthesis in citrus fruits, based on the abundance of transcripts of some members of this gene family in these tissues (Xu et al. 2013). However, expression levels of different GalUR isoforms did not correlate with the pattern of AsA accumulation in the pulp and with differences between varieties, with the exception of GalUR10 in Satsuma mandarins (Fig. 4). Thus, the d-galacturonic acid pathway appeared not to be rate limiting for AsA biosynthesis in the pulp of citrus fruits.
In addition to biosynthesis, recycling and degradation pathways may also contribute to the regulation of AsA concentration in the pulp of citrus fruits, although in a different fashion than in flavedo. It is expected that stimulation of the recycling gene expression and the corresponding enzymatic activity would contribute to maintain or enhance AsA concentration in the tissue. With the exception of DHAR1 gene whose expression declined in parallel with the evolution of AsA content, the expression levels of three isoforms of MDHAR increased, suggesting an enhancement of the turnover of AsA in the pulp of oranges and mandarins during fruit ripening. Moreover, at early stages of fruit development, expression of MDHAR3 was higher in oranges than in mandarins whereas MDHAR1 and MDHAR2 were higher during fruit maturation (Fig. 6). Thus, it may be assumed that coordinated expression of the different members of the MDHAR family may contribute to maintain a higher recycling ability in the pulp of the variety accumulating the largest AsA concentration. Analysis of the transcriptional changes of AsA degradation genes indicated that the degradation via APX could play a prominent role in the general regulation of AsA concentration in the pulp during development and maturation, but not accounting for the differences between oranges and mandarins (Figs. 2, 6). Moreover, the extremely high induction observed in the expression of the APX2 isoform during the whole development and ripening might indicate that this enzyme could be crucial in the degradation of AsA in the pulp (Fig. 6; Supplementary Table S3). Differences in APX enzymatic activity have been observed in the pulp of two citrus species with contrasting AsA content (Yang et al. 2011), reinforcing our assumption that AsA degradation by APX is key in the regulation of the AsA content in the pulp of citrus fruits.
In summary, the L-galactose pathway appears to be crucial in the biosynthesis of AsA in flavedo and pulp of citrus fruits. Also, the myo-inositol pathway seems to play an important role in the regulation of the AsA contents in flavedo of immature fruits of Washington Navel orange. The differences in AsA levels observed in the flavedo of Satsuma mandarin and Washington Navel oranges seemed to be due to differences in the transcriptional regulation of MIOX, GMP, GGP and GPP genes. In the pulp, the decline in AsA observed in these two genotypes was mainly associated with a differential transcript abundance of genes of the L-galactose pathway, especially GalDH and GalLDH, while the differences between genotypes appeared to be related to GGP and GPP levels. The existence of several isoforms of GalUR whose expression profiles did not correlate with the pattern of AsA accumulation in both flavedo and pulp precluded to assign a significant participation of the D-galacturonic acid pathway in the biosynthesis of AsA in citrus fruits. The involvement of the recycling pathways in the regulation of AsA was also evidenced, but differential and complex transcriptional regulation operates in each tissue. Taken together, the results emphasize and reinforce the idea that complex and imbricated mechanisms operate in the regulation of AsA content in citrus fruit, as have been postulated in other fruits (Ioannidi et al. 2009; Bulley et al. 2009; Cruz-Rus et al. 2011). It seems that peel and pulp tissues of the fruit have developed specific and differential mechanisms that change during development and maturation, including not only transcriptional regulation of genes expression but also post-transcriptional and post-translational modifications and substrate availability.
The results presented in this paper contribute to the identification of potential candidates for future biotechnological approaches and plant breeding programs to increase AsA levels in the fruits. Further biochemical and molecular approaches such as enzymatic activities, precursor feeding and gene promoter analysis might lead to the identification of key regulators of AsA metabolic pathways in citrus fruits.
Abbreviations
- AsA:
-
Ascorbic acid
- AO:
-
Ascorbate oxidase
- APX:
-
Ascorbate peroxidase
- CP:
-
Crossing point
- DHAR:
-
Dehydroascorbate reductase
- GalLDH:
-
l-Galactono-1,4-lactone dehydrogenase
- GalDH:
-
l-Galactose dehydrogenase
- GalUR:
-
d-Galacturonic acid reductase
- GGP:
-
GDP-l-Galactose phosphorylase
- GLOase:
-
l-Gulono-1,4-lactone oxidase
- GME:
-
GDP-Mannose-3′,5′-epimerase
- GMP:
-
GDP-Mannose pyrophosphorylase
- GPP:
-
l-Galactose-1-phosphate phosphatase
- MDHAR:
-
Monodehydroascorbate reductase
- MIOX:
-
Myo-inositol oxygenase
References
Abeysinghe DC, Li X, Sun CD, Zhang WS, Zhou CH, Chen KS (2007) Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem 104:1338–1344
Agius F, González-Lamothe R, Caballero JL, Muñoz-Blanco J, Botella MA, Valpuesta V (2003) Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nature Biotechnol 21:177–181
Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, Baldet P (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme l-galactono-1, 4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol 145:1408–1422
Alós E, Rodrigo MJ, Zacarías L (2013) Transcriptomic analysis of genes involved in the biosynthesis, recycling and degradation of l-ascorbic acid in pepper fruits (Capsicum annuum L). Plant Sci 207:2–11
Atkins CD, Wiederhold E, Moore EL (1945) Vitamin C content of processing residue from Florida citrus waste. Fruit Prod J 24:260–262
Badejo AA, Fujikawa Y, Esaka M (2009) Gene expression of ascorbic acid biosynthesis related enzymes of the Smirnoff–Wheeler pathway in acerola (Malpighia glabra). J Plant Physiol 166:652–660
Badejo AA, Wada K, Gao Y, Maruta T, Sawa Y, Shigeoka S, Ishikawa T (2012) Translocation and the alternative d-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the d-mannose/l-galactose pathway. J Exp Bot 63:229–329
Bermejo A, Llosa MJ, Cano A (2011) Analysis of bioactive compounds in seven citrus cultivars. Food Sci Technol Int 17:55–62
Block G (1991) Dietary guidelines and the results of food consumption surveys. Am J Clin Nutr 53:3565–3575
Bulley SM, Rassam M, Hoser D, Otto W, Schünemann N, Wright M, MacRae E, Gleave A, Laing W (2009) Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-l-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J Exp Bot 60:765–778
Bulley S, Wright M, Rommens C, Yan H, Rassam M, Lin-Wang K, Andre C, Brewster D, Karunairetnam S, Allan AC, Laing WA (2012) Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene GDP-l-galactose phosphorylase. Plant Biotechnol J 10:390–397
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Chen Z, Young TE, Ling J, Chang S, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100:3525–3530
Cruz-Rus E, Botella MA, Valpuesta V, Gomez-Jimenez MC (2010) Analysis of genes involved in l-ascorbic acid biosynthesis during growth and ripening of grape berries. J Plant Physiol 167:739–748
Cruz-Rus E, Amaya I, Sánchez-Sevilla JF, Botella MA, Valpuesta V (2011) Regulation of l-ascorbic acid content in strawberry fruits. J Exp Bot 62:4191–4201
Cruz-Rus E, Amaya I, Valpuesta V (2012) The challenge of increasing vitamin C content in plant foods. Biotechnol J 7:1110–1121
Davey MW, Dekempeener E, Keulemans J (2003) Rocket-powered high-performance liquid chromatographic analysis of plant ascorbate and glutathione. Anal Biochem 316:74–81
Davey MW, Franck C, Keulemans J (2004) Distribution, developmental and stress responses of antioxidant metabolism in malus. Plant Cell Environ 27:1309–1320
Dhuique-Mayer C, Caris-Veyrat C, Ollitrault P, Curk F, Amiot MJ (2005) Varietal and interspecific influence on micronutrient contents in citrus from the Mediterranean area. J Agric Food Chem 52:2140–2145
Endres S, Tenhaken R (2009) Myoinositol oxigenase controls the level of myoinositol in Arabidopsis, but not increase ascorbic acid. Plant Physiol 149:1042–1049
Fukunaga K, Fujikawa Y, Esaka M (2010) Light regulation of ascorbic acid biosynthesis in rice via light responsive cis-elements in genes encoding ascorbic acid biosynthetic enzymes. Biosci Biotechnol Biochem 74:888–891
Gallie DR (2013) The role of l-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Exp Bot 64:433–443
Haroldsen VM, Chi-Ham CL, Kulkarni S, Lorence A, Bennett AB (2011) Constitutively expressed DHAR and MDHAR influence fruit, but not foliar ascorbate levels in tomato. Plant Physiol Biochem 49:1244–1249
Imai T, Karita S, Shiratori G, Hattori M, Nunome T, Oba K, Hirai M (1998) l-Galactono-γ-lactone dehydrogenase from sweet potato: purification and cDNA sequence analysis. Plant Cell Physiol 39:1350–1358
Imai T, Ban Y, Terakami S, Yamamoto T, Moriguchi T (2009) l-ascorbate biosynthesis in peach: cloning of six l-galactose pathway-related genes and their expression during peach fruit development. Physiol Plant 136:139–149
Ioannidi E, Kalamaki MS, Engineer C, Pateraki I, Alexandrou D, Mellidou I, Giovannonni J, Kanellis AK (2009) Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J Exp Bot 60:663–678
Li MJ, Ma FW, Zhang M, Pu F (2008) Distribution and metabolism of ascorbic acid in apple fruits (Malus domestica Borkh cv. Gala). Plant Sci 174:606–612
Li M, Ma F, Shang P, Zhang M, Hou C, Liang D (2009) Influence of light on ascorbate formation and metabolism in apple fruits. Planta 230:39–51
Lima-Silva V, Rosado A, Amorim-Silva V et al (2012) Genetic and genome-wide transcriptomic analyses identify co-regulation of oxidative response and hormone transcript abundance with vitamin C content in tomato fruit. BMC Genomics 13:187
Linster CL, Van Schaftingen E (2007) Vitamin C: biosynthesis, recycling and degradation in mammals. FEBS J 274:1–22
Loewus FA, Jang R, Seegmiller CG (1958) The conversion of C14-labeled sugars to l-ascorbic acid in ripening strawberries. IV. A comparative study of d-galacturonic acid and l-ascorbic acid formation. J Biol Chem 232:533–541
Lorence A, Nessler CL (2007) Pathway engineering of the plant vitamin C metabolic network. In: Verpoorte R, Alfermann AW, Johnson TS (eds) Applications of plant metabolic engineering. Springer, Dordrecht, pp 189–209
Martí N, Mena P, Cánovas JA, Micol V, Saura D (2009) Vitamin C and the role of citrus juices as functional food. Nat Prod Commun 4:677–700
Melino VJ, Soole KL, Ford CM (2009) Ascorbate metabolism and the developmental demand for tartaric and oxalic acids in ripening grape berries. BMC Plant Biol 9:145
Mellidou I, Chagné D, Laing WA, Keulemans J, Davey MW (2012a) Allelic variation in paralogs of GDP-l-galactose phosphorylase is a major determinant of vitamin C concentrations in apple fruit. Plant Physiol 160:1613–1629
Mellidou I, Keulemans J, Kanellis AK, Davey MW (2012b) Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant Biol 17:239
Nagy S (1980) Vitamin C contents of citrus fruit and their products: a review. J Agric Food Chem 28:8–18
Oba K, Ishikawa S, Nishikawa M, Mizuno H, Yamamoto T (1995) Purification and properties of l-galactono-1,4-lactone dehydrogenase, a key enzyme for ascorbic acid biosynthesis, from sweet potato roots. J Biochem 117:120–124
Ostergaard J, Persiau G, Davey MW, Van Montagu M (1997) Isolation of a cDNA coding for l-galactono-γ-lactone dehydrogenase, an enzyme involved in the biosynthesis of ascorbic acid in plants. J Biol Chem 272:30009–30016
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acid Res 30:e36
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: bestkeeper-excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Qin A, Shi Q, Yu X (2011) Ascorbic acid contents in transgenic potato plants overexpressing two dehydroascorbate reductase genes. Mol Biol Rep 38:1557–1566
Rodrigo MJ, Marcos JF, Zacarías L (2004) Biochemical and molecular analysis of carotenoid biosynthesis in flavedo of orange (Citrus sinensis L.) during fruit development and maturation. J Agric Food Chem 52:6724–6731
Rodrigo MJ, Alquezar B, Alós E, Medina V, Carmona L, Bruno M, Al-Babili S, Zacarías L (2013) A novel carotenoid cleavage activity involved in the biosynthesis of citrus fruit-specific apocarotenoid pigments. J Exp Bot. doi:10.1093/jxb/ert260
Romero P, Lafuente MT, Rodrigo MJ (2012) The Citrus ABA signalosome: identification and transcriptional regulation during sweet orange fruit ripening and leaf dehydration. J Exp Bot 63:4931–4945
Sanmartin M, Pateraki I, Chatzopoulou F, Kanellis AK (2007) Differential expression of the ascorbate oxidase multigene family during fruit development and in response to stress. Planta 225:873–885
Smirnoff N (2011) Vitamin C: the metabolism and functions of ascorbic acid in plants. Adv Bot Res 59:109–177
Stewart I, Wheaton TA (1971) Continuous flow separation of carotenoids by liquid chromatography. J Chromatogr 55:325–336
Tadeo FR, Cercós M, Colmenero-Flores JM et al (2008) Molecular physiology of development and quality of citrus. Adv Bot Res 47:147–223
Tausz M, Sircelj H, Grill D (2004) The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid? J Exp Bot 55:1955–1962
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393:365–369
Wolucka BA, Van Montagu M (2003) GDP-mannose 3′, 5′-epimerase forms GDP-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J Biol Chem 278:47483–47490
Wolucka BA, Van Montagu M (2007) The VTC2 cycle and the de novo biosynthesis pathways for vitamin C in plants: an opinion. Phytochemistry 68:2602–2613
Xu Q, Chen LL, Ruan X et al (2013) The draft genome of sweet orange (Citrus sinensis). Nat Genet 45:59–66
Yabuta Y, Yoshimura K, Takeda T, Shigeoka S (2000) Molecular characterization of tobacco mitochondrial l-galactono-γ-lactone dehydrogenase and its expression in Escherichia coli. Plant Cell Physiol 41:666–675
Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S (2007) Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot 58:2661–2671
Yang X, Xie J, Wang F, Zhong J, Liu Y, Li G, Peng S (2011) Comparison of ascorbate metabolism in fruits of two citrus species with obvious difference in ascorbate content in pulp. J Plant Physiol 168:2196–2205
Yang X-Y, Wang F–F, Teixeira da Silva JA, Zhong J, Liu Y-Z, Peng S-A (2013) Branch girdling at fruit green mature stage affects fruit ascorbic acid contents and expression of genes involved in L-galactose pathway in citrus. N Z J Crop Hort Sci. doi:10.1080/01140671.2012.724429
Zhang Y, Li H, Shu W, Zhang C, Zhang W, Ye Z (2011) Suppressed expression of ascorbate oxidase gene promotes ascorbic acid accumulation in tomato fruit. Plant Mol Biol Rep 29:638–645
Acknowledgments
This work was supported by research grants FP7-PEOPLE-2011-CIG-2011-303652 (Marie Curie Actions, European Union), Proyectos de I + D para Grupos de Investigación Emergentes GV/2012/036 (Generalitat Valenciana, Spain). Enriqueta Alós was recipient of a JAE-Doc (CSIC) post-doctoral contract which is co-funded by Fondo Social Europeo de Desarrollo Regional (FEDER). Financial support by PROMETEO2010/010 (Generalitat Valenciana, Spain), Fun-C-Food (CSD2007-0063C, Ministerio de Ciencia e Innovación, Spain) and AGL-2009-11558 and AGL-2012-34573 (Ministerio Economía y Competitividad, Spain) are also acknowledged. We thank Dr. Luis Navarro (IVIA, Moncada, Valencia) for the use of the Citrus Germplasm Bank and Dr. Jose Vicente Gil (Universitat de Valencia; IATA-CSIC) for allowing the use of the HPLC equipment. We also gratefully acknowledge the assistance of Arancha Gurrea, Joanna Lado and Amparo Beneyto.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2014_2044_MOESM2_ESM.pptx
Supplementary Figure S1 Changes in fruit diameter, external color (a/b ratio) and maturity index of the juice (Total soluble solids/total acidity) in fruits of Satsuma Owari (white circles) and Washington Navel (black circles) during fruit development and ripening. Data of fruit diameter and external color are mean ± SD of 20 replicates, and those of juice maturity index are mean ± SD of 3 replicates. JN, JL, A, S, O, N, D indicate June, July, August, September, October, November and December, respectively. (PPTX 72 kb)
Rights and permissions
About this article
Cite this article
Alós, E., Rodrigo, M.J. & Zacarías, L. Differential transcriptional regulation of l-ascorbic acid content in peel and pulp of citrus fruits during development and maturation. Planta 239, 1113–1128 (2014). https://doi.org/10.1007/s00425-014-2044-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-014-2044-z