Abstract
Effects of zinc (Zn) deficiency on shoot metabolites were investigated in contrasting rice (Oryza sativa L.) genotypes with special focus on ascorbic acid (AsA) biosynthesis, recycling, and catabolism. The genotypes IR74 (sensitive) and RIL46 (tolerant) were subjected to −Zn and control treatments for 3 weeks, and samples were taken at three different stages representing the pre-stress phase, emergence of visible stress, and severe visible stress. The emergence of visible symptoms was paralleled by an increase in lipid peroxidation and a decrease in AsA concentration in the sensitive, but not in the tolerant genotype. The tolerant RIL46 showed enhanced transcript levels of several genes involved in the mannose/l-galactose pathway to AsA biosynthesis, and significant up-regulation of a gene involved in the putative alternative myo-inositol pathway under low Zn stress. The level of most AsA precursors was negatively affected by Zn deficiency, but RIL46 had a constitutively higher level of non-phosphorylated precursors. Products of AsA catabolism such as oxalate and threonate did not accumulate in either genotype, suggesting that AsA degradation did not contribute to the stress-induced decline of the AsA pool in IR74. Further factors possibly contributing to tolerance in RIL46 included an almost fivefold higher proline level under −Zn stress and significantly higher trehalose content. The implications of these compounds in AsA metabolism and Zn efficiency thus deserve further attention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) deficiency is one of the most abundant abiotic stress factors worldwide and affects growth and yield of rice (Oryza sativa L.) and other crops on approximately 35 million hectares of agricultural land (Alloway 2004). Zn is essential for plants and has numerous functions in the cell, such as involvement in protein folding, or as a catalytic or co-catalytic factor of thousands of proteins (Broadley et al. 2007). Similar to other abiotic stress factors, Zn deficiency can induce an imbalance in the production and removal of reactive oxygen species (ROS), thereby causing oxidative stress in plants (Cakmak and Marschner 1988). Although ROS are constantly produced in plant cells and have important signaling functions, their excessive production causes damage of lipids, proteins and DNA (Apel and Hirt 2004).
The breeding of rice varieties tolerant to Zn deficiency (also termed as ‘zinc efficient’) is of great importance to global agriculture. Genetic evidence shows that Zn efficiency is caused by multiple tolerance factors in rice, where several quantitative trait loci (QTL) with complex interactions influence plant mortality and leaf bronzing of plants grown on low Zn soil (Wissuwa et al. 2006). In addition, the scientific literature suggests that Zn efficiency relies on at least two components: (1) the ability to acquire sufficient Zn despite low availability in the soil, and (2) efficient utilization of Zn within the plant, also termed as “shoot tolerance” (Hacisalihoglu and Kochian 2003). Both tolerance factors have been investigated in previous studies using the Zn-efficient recombinant inbred line RIL46 selected from a rice mapping population, which showed enhanced growth, lower mortality and fewer stress symptoms when grown in low Zn soil compared to its intolerant parent IR74 (Wissuwa et al. 2006; Frei et al. 2010b; Rose et al. 2012). For instance, it was demonstrated that rhizosphere processes contribute to Zn efficiency, especially the ability to take up Zn chelated by phytosiderophores or organic acids (Hoffland et al. 2006; Wissuwa et al. 2006; Arnold et al. 2010). However, the tolerant line RIL46 showed an even lower Zn concentration in the shoot than its intolerant parent IR74. This observation is in agreement with findings from other crops, where Zn efficient and inefficient genotypes often do not differ in Zn concentration (Hacisalihoglu and Kochian 2003), and highlights the importance of an efficient biochemical utilization of Zn within the shoot. Indeed it was reported that RIL46 was able to protect cells from excess ROS more efficiently than IR74 (Frei et al. 2010b; Rose et al. 2011). Screening of the activity and shoot concentrations of enzymatic and non-enzymatic antioxidants led to the conclusion that tolerance to Zn deficiency was associated with an enhanced ascorbate (AsA) level (Frei et al. 2010b). Unlike most antioxidant enzymes (such as superoxide dismutase, peroxidases, etc.,), the level of AsA decreased when plants encountered low Zn stress, but remained higher in the tolerant genotype RIL46.
Ascorbic acid is the most abundant antioxidant in plants and its metabolism can be divided into three sections (Fig. 1): (i) biosynthesis, (ii) recycling and (iii) catabolism (or degradation).
(i) Biosynthesis: the predominant biosynthesis pathway of AsA starts from hexose sugars and proceeds via GDP-d-mannose and l-galactose (mannose/l-galactose pathway) (Wheeler et al. 1998; Smirnoff and Wheeler 2000; Conklin 2001), but evidence for other pathways via uronic acid intermediates exists (Agius et al. 2003; Wolucka and Van Montagu 2003) (Fig. 1). One alternative pathway involving the conversion of d-galacturonic acid to l-galactono-1,4-lactone was verified in strawberry fruit (Loewus and Kelly 1961; Agius et al. 2003) and Arabidopsis thaliana cell suspension culture (Davey et al. 1999), but not in rice (Jo and Hyun 2011). Another alternative pathway uses myo-inositol as a substrate (Lorence et al. 2004) and is mediated by the enzyme myo-inositol oxygenase (MIOX). The contribution of the myo-inositol pathway to AsA in plants is under debate, as contradictory results have been reported (Lorence et al. 2004; Endres and Tenhaken 2009).
(ii) Recycling: the enzyme ascorbate peroxidase (APX) uses two molecules of AsA to reduce H2O2 to water, leading to the generation of the unstable molecule monodehydroascorbate (MDHA), which disproportionates to dehydroascorbate (DHA) if not reduced back to AsA by the action of monodehydroascorbate reductase (MDHAR). DHA is recycled to AsA using reduced glutathione (GSH) as a reducing substrate, which is catalyzed by DHA reductase (DHAR) (Noctor and Foyer 1998; Smirnoff 1996).
(iii) Degradation: if DHA is not reduced quickly, it decomposes due to irreversible hydrolysis of its lactone ring, leading to the formation of diketogulonic acid (Green and Fry 2005). Additionally, DHA can be cleaved to form oxalate (C-1 and C-2) and l-threonate (C-3 to C-6), which is often oxidized to l-threarate (DeBolt et al. 2004). Green and Fry (2005) proposed that two interchangeable intermediates are formed (cyclic -2,3-O-oxalyl-l-threonate and cyclic -3,4-O-oxalyl-l-threonate) and subsequently, the latter is hydrolyzed to 4-O-oxalyl-l-threonate. It was suggested that this pathway is at least partially catalyzed by yet uncharacterized enzymes.
As our previous results using the contrasting genotypes IR74 and RIL46 suggested that Zn efficiency was associated with the ability to maintain an enhanced AsA pool, the present study focused on genotypic differences in AsA metabolism by targeted metabolite and transcript profiling. More specifically, we investigated two hypotheses: (1) Zn deficiency inhibits AsA biosynthesis in the intolerant genotype, but not in the tolerant one. To test this hypothesis, we focused on transcriptional and metabolic regulation of the different biochemical routes affecting AsA levels. (2) Alternatively, insufficient AsA recycling leads to degradation of AsA in the intolerant genotype, but not in the tolerant one. More specifically, excess production of ROS due to stress may exhaust the enzymatic AsA recycling capacity and consequently lead to irreversible degradation of AsA. Testing these hypotheses was part of a broader metabolomic approach, which included the amino acid and carbohydrate profile to identify further factors interacting with the AsA metabolism and contributing to shoot tolerance under Zn deficiency.
Materials and methods
Plant culture
The experiment was conducted using a Zn-efficient recombinant inbred rice (Oryza sativa L.) line RIL46, which carries positive alleles of QTLs for tolerance to low Zn (Wissuwa et al. 2006), and its Zn-inefficient parent IR74. Seeds were obtained from the Japan International Research Institute for Agricultural Sciences (JIRCAS, Tsukuba, Japan). They were germinated and placed on styrofoam sheets floating on 0.5 mM CaCl2 and 10 μM FeCl3. After 10 days, seedlings were transferred to 60-l hydroponic tanks containing half strength nutrient solution without Zn. After 1 week, plants were transferred to full nutrient solution containing 1 mM NaHCO3 (Rose et al. 2011) without (−Zn) or with 1 μM Zn (+Zn). The composition of the nutrient solution was 1.42 mM NH4NO3, 0.32 mM NaH2PO4, 0.51 mM K2O4, 1 mM CaCl2, 1 mM MgSO4, 9 μM MnCl2, 0.07 μM (NH4)6 × Mo7O24, 18.5 μM H3BO3, 0.16 μM CuSO4, 35.6 μM FeCl3. The pH of the solution was adjusted to 5.5 twice weekly and nutrient solutions were completely replaced after 10 days. Plants were grown in a temperature-controlled glasshouse with minimum night/day temperatures of 22 °C/28 °C. Average relative humidity was 50 %. Plants were grown under supplementary artificial lighting to ensure a minimum photosynthetic photon flux density (PPFD) of 400 μmol m−2 s−1 from 7 am to 8 pm.
Samples of whole shoots were harvested on three different sampling days: firstly 3 days after the start of −Zn treatments, secondly when stress symptoms first emerged in the susceptible genotype after 16 days of −Zn treatment, and finally when IR74 had severe stress after 24 days of −Zn treatment. A leaf bronzing score ranging from 0 (healthy leaf) to 10 (dead leaf) was assigned to the three youngest fully expanded leaves of each plant (Wissuwa et al. 2006).
Gene expression analyses
Samples (ten replicates per genotype/treatment combination per sampling day) were immediately frozen in liquid N2 and stored at −80 °C. RNA for quantitative RT-PCR was extracted from four replicate samples using the RNeasy Plant Minikit (Qiagen). DNA was removed from RNA samples using an RNase-free DNase (Promega, Mannheim, Germany). RNA concentration was determined using a Nanodrop2000C spectrometer (Thermo Scientific) and the integrity of RNA was checked by denaturing formaldehyde agarose gel electrophoresis. One microgram of total RNA was reverse transcribed with the GoScript™ Reverse Transcription System (Promega) and quantitative real-time polymerase chain reaction (qPCR) was performed with 100 ng cDNA using the GoTaq® qPCR Master Mix (Promega) under the following conditions—an initial denaturation step (20 min, 95 °C), followed by 45 cycles of denaturation (5 s, 95 °C) and annealing/extension (40 s, 60 °C). Gene specific primers (Supplementary Table S1) were used for analysis of the different isoforms of all annotated biosynthesis genes of the mannose/l-galactose pathway and the myo-inositol pathway. Expression data were quantified using the comparative δδCT method (Frei et al. 2010a) using the expression level of IR74 in the control on the first sampling day as calibrator and the gene Os05g0564200 (RAP-ID, annotated as U2 snRNP) as endogenous reference to normalize gene expression levels of the targeted genes. The endogenous reference gene was identified using the database “Plant reference gene server” (Patel and Jain 2011) (http://www.nipgr.res.in/PlantRGS/), as it showed more stable expression across genotypes under the experimental conditions as compared to commonly used references such as actin or 18S rRNA.
Biochemical analyses
Zn concentration was determined in dried leaf material by atomic absorption spectrometry (AAS).
To measure malondialdehyde (MDA) (Hodges et al. 1999), leaf material was ground in liquid nitrogen and extracted twice by adding 1 ml of 0.1 % trichloro acetic acid (TCA) to 100 mg of plant material and centrifuged (14,000g, 4 °C, 15 min). The combined supernatants were divided into two 750 μl samples and 750 μl of 0.01 % (v/v) 2,6-di-tert-butyl-4-methylphenol (BHT) in 20 % TCA (v/v) was added to one sample. 750 μl of 0.01 % (v/v) BHT and 0.65 % 2-thiobarbituric acid (TBA) in 20 % TCA (v/v) was added to the other sample. Samples were incubated at 95 °C for 30 min and the reaction was stopped on ice. Samples were centrifuged (8,000g, 4 °C, 10 min) and absorbance was read at 440, 532 and 600 nm.
Total and reduced AsA were measured immediately after plant harvest according to Ueda et al. (2013). Flash-frozen leaves were ground in liquid nitrogen and 1.5 ml of 6 % TCA was added to approximately 80 mg of leaf material. Samples were incubated in a chilled ultrasonic water bath for 15 min and centrifuged for 5 min at 4 °C and 13,000g. 10 μl of the supernatant was used to determine total and reduced AsA. 10 μl of 75-mM phosphate buffer was added to all samples, while 10 μl of 10 mM dithiothreitol (DTT) was added only to the total AsA samples and incubated for 15 min at room temperature. Then, 10 μl of 0.5 % N-ethylmaleimide (NEM) was added to the total AsA samples to remove excess DTT and incubated for at least 30 s. 20 μl of water was added to the reduced AsA samples to account for the amount of DTT and NEM. Thereafter, 50 μl of 10 % TCA, 40 μl of 43 % H3PO4, 40 μl of 4 % α-α-bipyridil and 20 μl of 3 % FeCl3 were added to all tubes. After incubation at 37 °C for 1 h, 100 μl of each sample was transferred to a 96-well microplates and absorbance was read at 525 nm using a microplate reader (Powerwave XSII, BioTec). The amount of DHA was calculated as the difference between the total and reduced AsA concentration.
Metabolomic analyses
Amino acids and sugars were analyzed using either a reverse phase chromatography or an enzyme-coupled photometric assay according to Ahkami et al. (2009). Samples were incubated for 60 min at 80 °C in 80 % ethanol and centrifuged for 10 min at 18,000g. The supernatants were evaporated to dryness, and samples resuspended in milliQ water. Prior to HPLC analysis, samples were derivatized using a fluorescing reagent AQC (6-aminoquinolyl-N-hydroxysuccinimidylcarbamat). Three mg of self-made AQC was dissolved in 1-ml acetonitrile and incubated for 10 min at 55 °C. The reagent was stored at 4 °C and used up to 4 weeks. For derivatization of primary and secondary amino acids, 0.16 ml of a buffer containing 0.2 M boric acid, pH 8.8, was used. An aliquot of 0.02 ml of each sample was added to the buffer, followed by 0.02 ml of prepared AQC reagent solution. The whole mixture was incubated for 10 min at 55 °C and used for chromatographic separation.
The concentrations of amino acids were determined using a reversed phase Alliance HPLC system (Waters Company) connected to a fluorescence detector (300 nm excitation wavelength, and 400 nm emission wavelength). Chromatograms were recorded using the software program Empower 2.1. The gradient was accomplished with a buffer A containing 140-mM sodium acetate, pH 5.8 (Suprapur, Merck) and 7-mM triethanolamine (Sigma). Acetonitrile (Roti C Solv HPLC, Roth) and milliQ water were used as eluents B and C. To separate the amino acids, a reversed phase column (AccQTag, 3.9 mm × 150 mm) was used, which consists of silica as matrix modified by an apolar C18 group. The column was equilibrated with buffer A at a flow rate of 0.6 ml per minute and heated at 37 °C during the whole measurement. The gradient was produced by the following concentration changes: start with 100 % eluent A, 1 % at 0.5 min, 5 % at 27 min, 9 % at 28.5 min, 18 % at 44.5 min, 60 % at 47.5 min and 0 % at 50.5 min of eluent B. Reconditioning of the column was carried out for 10 min using buffer A.
To measure soluble sugars, the same extracts as described above for amino acid preparation were used. The measurement was carried out with a microplate reader (Synergy HT, Tecan, Crailsheim, Germany). Auxiliary enzymes glucose-6-phosphate dehydrogenase (Leuconostoc mesenteroides, 1 U), hexokinase (1 U), phosphoglucose isomerase (0.3 U) and 1 μl of ß-fructosidase (10 mg/100 μl buffer without NAD and ATP) were successively added to a buffer containing 100-mM imidazole-HCl, pH 6.9, 5-mM MgCl2, 2-mM NAD and 1-mM ATP to detect glucose, fructose and sucrose. The difference in absorbance was proportional to the concentration of the individual sugars.
Sugar alcohols were detected using an ion chromatography system (Dionex, Idstein, Germany) which consisted of a gradient pump (GP50), a degasser module, an autosampler (AS50) and a pulsed amperometric detector (EG50). Separation of the sugar alcohols was carried out using a CarboPack MA1 column (4 × 250 mm) connected to a guard column of the same material (4 x 10 mm) and an ATC-1 anion trap column which was placed between the eluents and separation columns to remove the anionic contaminants present in the eluents. A linear gradient was accomplished with purest water as buffer A (Millipore) and 620-mM sodium hydroxide as buffer B (Baker, 50 % solution). The column was equilibrated at a flow rate of 0.4 ml per minute. The duration of the run was 60 min. The calibration and quantitative calculation of sugar alcohols was carried out using the Chromeleon software 6.6 and authentic standards.
All other metabolites were measured using a Dionex ICS 5,000 (Dionex) coupled to a mass spectrometer, Agilent 6,490 triple Quad. The ICS system consisted of a pump (DC), an eluent generator (EG) and an autosampler (AS-AP) module. Separation of the metabolites was carried out using AS11-HC column (2 x 250 mm) connected to a guard column of the same material AG11-HC (2 x 50 mm) and an ATC anion trap column, which was placed between the eluents and separation columns to remove the anionic contaminants present in the eluents. A gradient was accomplished with purest water (eluent A, Millipore 18.2 microOhm) and increasing concentration of potassium hydroxide from a concentrated EluGen Cartridge EGC-KOH (Dionex). The column was equilibrated at a flow rate of 0.32 ml per minute with 4 % KOH. The gradient was set as following: 4 min to 4 %, 4–10 min to 15 %, 10–14 min to 25 %, 14–24 min to 80 %, 24–30 min back to 4 % and 30–40 min equilibration at 4 % KOH. The duration of the run was 40 min. The calibration and quantitative calculation of organic acids was carried out using the Chromeleon software 7.1.
Detection of accurate masses for different compounds was carried out on an Agilent triple Quad MS 6,490 equipped with an easy Jet Spray. Data were acquired and evaluated via Mass Hunter (version B.04.00). The MS was operated in the negative ion and multiple reactions monitoring (MRM) mode. Single analyte standards were infused at a flow rate of 0.4 ml/min for tuning compound dependent MS parameters. The major MS/MS fragment patterns of each analyte were determined. Declustering potential (DP) and collision energy (CE) of each transition was optimized. The optimized parameters were as following: nitrogen gas flow, 12 l per min; ion spray voltage, −3,500 V; the auxiliary gas temperature, 350 °C. The curtain and collision gas was nitrogen generated from pressurized air in a nitrogen generator (SF4, Atlas Copco, Magdeburg, Germany). Dwell time for each transition was 20 ms.
Enzyme assays
Dehydroascorbate reductase activity was measured according to Hossain and Asada (1984). Approximately 100 mg of flash-frozen leaf material was crushed in liquid nitrogen and 1.5-ml extraction buffer containing 50-mM Tris–HCl, 100-mM NaCl, 2-mM ethylenediaminetetraacetic acid (EDTA) and 1-mM MgCl2 was added. Samples were centrifuged at 13,000g for 5 min at 4 °C and a reaction mix with 70 μl of 50-mM KH2PO4/K2HPO4 buffer, pH 6.5, 10 μl of 5-mM dehydroascorbate (DHA), 10 μl of 50-mM reduced glutathione (GSH) was added to 10 μl of the enzyme extract. Absorbance was followed in a 96-well microplate at 265 nm for 3 min (ε = 14 mM−1 cm−1). Non-enzymatic reduction of DHA was measured using a blank sample without enzyme, and the value was deducted from all samples.
Phosphomannose isomerase (PMI) activity was determined according to Gracy and Noltmann (1968a). Plant leaf material was crushed in liquid nitrogen and 1 ml of extraction buffer (100-mM triethanolamine HCl buffer, pH 7.6 at 25 °C, 0.1 % 2-mercaptoethanol) was added. Samples were centrifuged at 13,000g at 4 °C for 15 min. The reaction mix contained 87-mM triethanolamine HCl buffer, pH 7.6, 5.5-mM d-mannose-6-phosphate, 0.45-mM nicotinamide adenine dinucleotide phosphate (NADP), two units phosphoglucoisomerase, and 0.1 unit glucose-6-phosphate dehydrogenase. Absorption was monitored in a 96-well microplate at 340 nm until stable, and 100 μl of the enzyme extract was added to monitor NADP reduction for 10 min (ε = 6.2 mM−1 cm−1).
Statistical analyses
Data were analyzed by a two-way ANOVA with the factors treatment, genotype, sampling date, treatment × genotype interaction and treatment × genotype × day interaction (IBM SPSS Statistics 20). Pairwise genotypic differences in gene expression were determined by post hoc comparison using the LSD test, and Tukey’s adjustment was used for multiple comparison of means if appropriate.
Results
Stress responses of contrasting genotypes
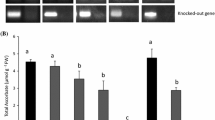
Leaf samples were taken on three different sampling days representing different stages of stress. First samples were taken 2 days after the start of treatments, when the plants showed no symptoms of Zn deficiency. After 16 days (second sampling day), symptoms of Zn deficiency first appeared in IR74, and on the third sampling day after 24 days of treatment, IR74 showed severe stress symptoms as indicated by high leaf bronzing scores (Fig. 2). Shoot Zn concentrations decreased significantly under Zn deficiency and were even lower in the tolerant genotype RIL46 (Fig. 2). MDA concentrations in leaves increased gradually in IR74 under Zn deficiency, but not in RIL46 (Fig. 2). Together, these data confirmed enhanced Zn deficiency tolerance in RIL46 despite even lower Zn concentration in leaves.
Influence of Zn deficiency on stress indicators in contrasting rice genotypes. The susceptible (IR74) and the tolerant (RIL46) rice line were grown hydroponically, and samples were taken after 2, 16, and 24 days of + and −Zn treatments. a Shoot Zn concentration of the two genotypes (n = 4). b Leaf bronzing score (LBS) (n = 10). c Malondialdehyde (MDA) equivalent (n = 4). T treatment, G genotype, D sampling day. ANOVA significance levels: *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant. Data bars not sharing the same superscript letter are statistically different at P < 0.05 by Tukey’s test. Error bars indicate standard errors
RIL46 showed significantly higher average AsA concentrations in leaves than IR74 (Fig. 3). Total and reduced AsA concentrations declined in IR74 under Zn deficiency starting from day 16 after the beginning of treatments, while the opposite trend was seen in RIL46. IR74 had a 24 % lower total AsA concentration than RIL46 under Zn deficiency after 24 days of treatment. Our further analyses aimed at explaining the contrasting trends in AsA levels in the two genotypes by monitoring AsA biosynthesis, as well as AsA recycling and degradation.
Influence of Zn deficiency on total (a) and reduced (b) ascorbic acid concentrations in shoots of contrasting rice genotypes. The susceptible (IR74) and the tolerant (RIL46) rice line were grown hydroponically, and samples were taken after 2, 16, and 24 days of + and −Zn treatments. T treatment, G genotype, D sampling day. ANOVA significance levels: *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant. Data bars not sharing the same superscript letter are statistically different at P < 0.05 by Tukey’s test; lack of superscript letters in b indicates that differences were not statistically significant. Error bars indicate standard errors (n = 6)
AsA biosynthesis
Ascorbic acid biosynthesis was analyzed by monitoring the expression of AsA biosynthesis genes (Table 1), selected enzyme activities (Fig. 4), and the concentrations of AsA precursors (Table 2). Pairwise comparison of gene expression levels showed that 8 out of 12 genes of the mannose/l-galactose pathway had higher expression levels in RIL46 as compared to IR74 on at least one sampling day. No consistent trend was seen in gene regulation in either genotype in response to Zn deficiency. Thus, the decline of the AsA pool in the sensitive line cannot be explained by stress-induced down-regulation of AsA biosynthetic genes. The activity of PMI was determined, because it is a Zn-dependent enzyme of the mannose/l-galactose pathway. The activity was lower under Zn deficiency in both genotypes (Fig. 4), especially on the second sampling day, which was different from PMI gene expression levels (Table 1). In the case of MIOX, the major gene in putative alternative myo-inositol pathway, significant differences were observed between the genotypes (Table 1). RIL46 showed significantly higher expression on all the sampling days in the −Zn treatment.

Influence of Zn deficiency on phosphomannose isomerase (PMI) activity in shoots of contrasting rice genotypes. The susceptible (IR74) and the tolerant (RIL46) rice line were grown hydroponically, and samples were taken after 16 and 24 days of + and −Zn treatments. T treatment, G genotype, D sampling day. ANOVA significance levels: *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant. Data bars not sharing the same superscript letter are statistically different at P < 0.05 by Tukey’s test. Error bars indicate standard errors (n = 4)

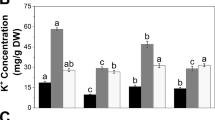
Furthermore, the concentrations of AsA precursors were analyzed (Table 2). Glucose concentration decreased due to Zn deficiency in both genotypes, but was significantly higher in the tolerant RIL46 averaged over all treatment and sampling days. The concentration of phosphorylated intermediates of the mannose/l-galactose pathway was 2–3 orders of magnitude lower than that of glucose, perhaps indicating their high turnover rates. The concentrations of all measured compounds tended to decrease after 16 days of −Zn treatment, although the treatment effect was significant only for glucose-6-phosphate and mannose-1-phosphate. Significant genotype effects were seen in fructose-6-phosphate and GDP-mannose, where the average concentrations were higher in the intolerant IR74. Additionally, we measured galactose concentration (Supplementary Table S2), but it was analytically not possible to separate d-galactose from l-galactose. The mannose/l-galactose pathway uses l-galactose as a precursor, while d-galactose serves as a component of the cell wall and of various sugars (French 1954). Thus the increase in total galactose in response to low Zn, especially in the sensitive IR74 (Supplementary Table S2), cannot be ascribed to AsA biosynthesis.
Concentrations of intermediates of the putative alternative myo-inositol pathway were also measured (Table 2). No significant differences were seen in glucuronic acid, which showed generally very low levels similar to those of phosphorylated intermediates of the mannose/l-galactose pathway (Table 2). In contrast, myo-inositol exhibited significantly higher average concentrations in the tolerant RIL46. Genotypic differences were particularly pronounced on the third sampling day, when IR74 but not RIL46 showed a decline of myo-inositol concentration in the −Zn treatment.
AsA recycling and degradation
We tested DHAR enzyme activity, which showed a slight but significant increase under low Zn stress, but no significant genotypic differences (Supplementary Table S2). Other AsA recycling enzymes were not tested as they had already been measured previously in a series of experiments using the same genotypes (Frei et al. 2010b). The glutathione concentration was measured because it is the redox partner in the enzymatic recycling of AsA. The average glutathione concentration was significantly higher in IR74, where it was strongly induced after 16 days of Zn deficiency and decreased again after 24 days of treatment (Fig. 5). RIL46 did not show a similar induction of glutathione, indicating that an elevated glutathione concentration represented an early sensitive response to stress. The amino acid glycine, which constitutes a building block for glutathione biosynthesis, showed a very similar response pattern as glutathione (Fig. 5c).
Influence of Zn deficiency on the concentrations total and reduced glutathione (a, b) and glycine (c) in shoots of contrasting rice genotypes. The susceptible (IR74) and the tolerant (RIL46) rice line were grown hydroponically, and samples were taken after 2, 16, and 24 days of + and −Zn treatments. T treatment, G genotype, D sampling day. ANOVA significance levels: *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant. Data bars not sharing the same superscript letter are statistically different at P < 0.05 by Tukey’s test. Error bars indicate standard errors (n = 5)
To test whether low Zn stress stimulated AsA degradation, we measured the concentrations of two products of AsA catabolism (Table 2). Unexpectedly, both components tended to decrease under −Zn stress, although the effect was not significant for oxalate. In the case of threonate, the decreases in concentration on days 16 and 24 were more pronounced and highly significant. Genotypic differences did not occur in AsA degradation products.
Further metabolites
Further metabolites were measured that are not directly associated with AsA metabolism, but have been characterized as stress response or tolerance factors in plants. Those compounds may provide additional explanations for genotypic differences in tolerance to Zn deficiency. The antioxidant disaccharide trehalose, which is also involved in sugar signaling/sensing, showed a contrasting response to Zn deficiency in the two genotypes, leading to a significantly higher level in the tolerant RIL46 (Table 2).
The concentration of the stress responsive amino acid proline was increased under low Zn stress on the second and third sampling day, but the increase was much more pronounced in the tolerant RIL46 (Table 2). In fact, RIL46 had a more than fourfold higher proline concentration after 24 days of −Zn treatment compared to IR74. Thus, the response of proline differed from that of most other amino acids: out of 17 amino acids that showed a significant genotypic difference after 24 days of −Zn treatment, 13 exhibited higher concentration in the intolerant IR74 (Table 2, Supplementary Table S2).
Discussion
The stress responses of contrasting genotypes observed in this nutrient solution experiment confirm the results of previous experiments in low Zn soil in the Philippines (Wissuwa et al. 2006; Frei et al. 2010b). RIL46 was more tolerant than IR74, although shoot Zn concentrations of both genotypes had to be considered as Zn deficient (Dobermann and Fairhurst 2000). The present study went one step further and detailed the involvement of AsA in preventing oxidative stress under Zn deficiency. By addressing two hypotheses targeted to AsA metabolism, we explored the metabolic basis underlying genotypic differences in AsA metabolism and its involvement in tolerance to Zn deficiency.
Hypothesis 1: Zn deficiency inhibits AsA biosynthesis in the intolerant genotype, but not in the tolerant one
The mannose/l-galactose pathway (Fig. 1) is considered as the predominant AsA biosynthetic pathway in plants (Wheeler et al. 1998; Linster and Clarke 2008). Most genes of this pathway were more highly expressed in the tolerant RIL46 on at least one sampling day, in particular the genes at the beginning of the pathway and at the terminal steps, converting l-galactose to AsA. Consistent with these results, a global transcript study by Widodo et al. (2010) reported enhanced expression of three AsA biosynthesis genes (GDP-mannose-3,5-epimerase, l-galacose-1,4-lactone dehydrogenase, l-galactose dehydrogenase) in RIL46, when plants were grown in low Zn soil and samples taken on only one sampling day. Given the complexity of the mannose/l-galactose pathway, it is likely that higher expression levels of single genes in the pathway may not have increased AsA tissue levels. Accordingly, various studies with plants overexpressing only single genes of the mannose/l-galactose pathway did not report any effect on AsA pool size (Bartoli et al. 2005). However, we observed higher expression levels of several genes of the AsA pathway in RIL46, which is indicative for an activation of the whole pathway.
RIL46 had a constitutively higher concentration of glucose (RIL46), which may be due to the ability of RIL46 to maintain enhanced photosynthesis under low Zn stress compared to IR74 (Rose et al. 2012). Besides its the role as an initial precursor of AsA biosynthesis and many other plant metabolites, glucose is also involved in a complex sugar signaling network, modulating growth, development and photosynthesis (Rolland et al. 2006) and it may provide a higher energy level for RIL46. Thus, a higher glucose concentration of RIL46 under Zn deficiency may represent an important tolerance factor.
On the other hand, phosphorylated intermediates of AsA biosynthesis showed no significant effects or even higher concentration in IR74, and their availability was generally lower in the −Zn treatments. However, it was suggested that the amount of phosphorylated mannose intermediates is not a limiting factor for AsA biosynthesis (Hancock and Viola 2005). Moreover, the first part of the mannose/l-galactose pathway is also responsible for producing cell wall polysaccharides, while the second part, subsequent to GDP-l-galactose, is committed only to AsA biosynthesis (Ishikawa et al. 2006). Thus, the early intermediates cannot be interpreted as AsA precursors only.
Compared to the genes of the mannose/l-galactose pathway, an even more pronounced up-regulation in RIL46 was seen for the expression levels of MIOX (Table 1). Additionally, RIL46 exhibited a higher concentration of myo-inositol (Table 2), the substrate for this enzyme. However, the contribution of the myo-inositol pathway to AsA biosynthesis in plants has been discussed controversially. One study reported a 2–3 fold increase of AsA in MIOX overexpressing A. thaliana plants (Lorence et al. 2004), while another study could not confirm these results using the same transgenic lines (Endres and Tenhaken 2009). In a recent study, overexpression of MIOX improved proline concentrations and drought tolerance in rice, although it did not significantly influence AsA pool size (Duan et al. 2012). Thus, the myo-inositol pathway is apparently not a major contributor to the AsA pool under normal conditions. Nevertheless, it may provide a backup in the tolerant RIL46 for the mannose/l-galactose pathway, which contains the Zn-dependent enzyme PMI (Gracy and Noltmann 1968b; Maruta et al. 2008). While gene expression of PMI was slightly increased in the −Zn treatment in both genotypes on the second sampling day (Table 1), the enzyme activity was decreased (Fig. 4). This discrepancy may occur because the protein requires Zn to develop its structure and catalytic activity. Whether MIOX is able to contribute to AsA biosynthesis in rice under Zn deficiency is a hypothesis which remains to be tested using MIOX gene mutants.
In summary, the analysis of the AsA biosynthetic pathways suggests that factors preventing degradation of the AsA level in RIL46 could be higher substrate availability, enhanced expression of some of the biosynthetic genes, and AsA synthesis via the Zn-independent myo-inositol pathway. Limited substrate availability, reduced enzyme activity (especially PMI) and the lack of induction of the myo-inositol pathway may be the cause for the decline of the AsA pool in the sensitive line IR74.
Hypothesis 2: insufficient AsA recycling leads to degradation of AsA in the intolerant genotype, but not in the tolerant one
This hypothesis implies that Zn deficiency causes excessive production of ROS exhausting the recycling capacity of the AsA–glutathione cycle, and consequently leads to catabolism of DHA and accumulation of products such as oxalate and threonate. The recycling enzyme DHAR influenced the AsA poolsize in transgenic gain-of-function mutants of tobacco and maize (Chen et al. 2003; Naqvi et al. 2009). In our study, the activity of this enzyme was slightly increased by Zn deficiency, but no genotypic differences were observed (Supplementary Table S2). This is in agreement with a previous study, in which all enzymes of the AsA–glutathione cycle were measured in the same genotypes as used in this experiment, and none of them showed enhanced activity in RIL46 (Frei et al. 2010b). Glutathione forms the redox partner of AsA in the AsA–glutathione cycle (Noctor and Foyer 1998). Its concentration and its precursor glycine (Noctor and Foyer 1998) were strongly induced by Zn deficiency on the second sampling day in IR74, but were almost unaffected in RIL46 (Fig. 5). This pattern is congruent with the stress response concept proposed by Tausz et al. (2004), in which a first initial response of the glutathione system is followed by an acclimation step, leading to a new steady state. This acclimation step may be represented in the present study by the second sampling day in IR74 (Fig. 5), because glutathione concentration increased in parallel with the stress level. A continuously high level of stress subsequently may have caused degradation of the glutathione-dependent redox system, which was represented by decreased glutathione concentrations on the third sampling day. Therefore, we suggest that high glutathione concentrations reflect a typical early stress indicator, but do not significantly contribute to tolerance.
If DHA is not recycled to AsA by enzymatic reduction, it is catabolized to compounds such as oxalate and threonate (Parsons et al. 2011). Some evidence exists that these catabolic pathways also exist in rice (Guo et al. 2005). As opposed to the above-mentioned hypothesis, a decrease instead of an increase in these products of AsA catabolism was found in the −Zn treatment. In other words, plants (especially the sensitive genotype) stimulated AsA recycling, but there was no indication of DHA catabolism due to exhaustion of the recycling capacity. As we do not find evidence for our second hypothesis, we conclude that AsA catabolism is unlikely to contribute to the decline of the AsA pool under low Zn stress.
Other tolerance factors
Ascorbic acid is the most abundant antioxidant in plants (Noctor and Foyer 1998). The genotypic differences in AsA metabolism (especially biosynthesis) elaborated above can, therefore, partly explain the differences in oxidative stress tolerance under Zn deficiency. However, our metabolic profiling revealed that other compounds involved in plant stress tolerance also showed genotypic differences, which may have contributed to tolerance in RIL46.
RIL46 maintained a high trehalose level under Zn deficiency, while IR74 showed a constantly lower level under Zn deficiency (Table 2). The antioxidant disaccharide trehalose protected membranes and macromolecules under drought stress as a compatible solute (Rontein et al. 2002), and conferred tolerance to multiple stresses in rice (Garg et al. 2002). Plants with higher trehalose content were also able to maintain a higher photosynthesis level, which is consistent with the proposed role of trehalose as a sugar-sensing and carbohydrate metabolism-modulating metabolite (Goddijn and van Dun 1999). There is also an evidence that trehalose can scavenge ROS directly under heat stress (Luo et al. 2008). However, it is still under debate if trehalose concentration in higher plants is sufficient to be considered as an active metabolite in stress protection (Wingler and Wingler 2002). Alternatively, the role of trehalose (or rather trehalose-6-P) in sugar signaling may be involved in stress reactions and contribute to tolerance (Avonce et al. 2004). Thus, the role of trehalose in stress tolerance should be further explored.
Proline has been well characterized as a stress tolerance factor under salt and drought stress (Ashraf and Foolad 2007). The fivefold higher concentration in RIL46 under low Zn stress (Table 2) indicates that it may also be involved in tolerance to Zn deficiency. Apart from its function as an osmo-protectant, previous studies demonstrated a ROS scavenging function of proline (Matysik et al. 2002), and a stabilizing effect on key enzymes of the AsA–glutathione cycle (Szekely et al. 2008). A number of studies suggested important functions of proline in the antioxidant system rather than as a factor in osmotic adjustment (Tripathi and Gaur 2004; Molinari et al. 2007). For example, proline reduced lipid peroxidation in transgenic sugarcane plants overexpressing the Δ1-pyrroline-5-carboxylate synthetase gene, which catalyzes the first part of the proline biosynthesis (Molinari et al. 2007). Moreover, overexpression of MIOX in rice induced a higher proline concentration and conferred enhanced tolerance to drought stress (Duan et al. 2012). Thus, an additional explanation for the tolerance of RIL46 could be a higher MIOX expression leading to an increased proline concentration.
With the exception of proline, alanine and methionine, all amino acids showed either higher accumulation in IR74 under Zn deficiency or no significant difference between genotypes (Table 2). For example, asparagine concentrations were substantially higher in IR74 under Zn deficiency, which may indicate inhibited protein synthesis. It was shown earlier that Zn plays a role in regulating protein synthesis (Hossain et al. 1997) and Zn-deficient plants accumulated free amino acids, especially asparagine due to impaired protein biosynthesis (Possingham 1956; Kitagishi and Obata 1986; Cakmak et al. 1989). Thus, besides enhanced AsA metabolism, the ability to maintain protein synthesis could be an additional tolerance factor of RIL46 under Zn deficiency.
In conclusion, our data suggest that the ability to maintain a high AsA pool is linked to tolerance to zinc efficiency in rice. Further experiments with AsA mutants will be necessary to determined whether differences in AsA metabolism are a cause or an effect of zinc efficiency. Moreover, the role of other putative tolerance factors such as sugar signaling (trehalose) or proline and their interaction with the AsA metabolism need to be further explored.
Abbreviations
- AsA:
-
Ascorbic acid
- DHA:
-
Dehydroascorbic acid
- DHAR:
-
Dehydroascorbic acid reductase
- MDA:
-
Malondialdehyde
- MDHA:
-
Monodehydroascorbic acid
- MDHAR:
-
Monodehydroascorbic acid reductase
- MIOX:
-
Myo-inositol oxygenase
- PMI:
-
Phosphomannose isomerase
- ROS:
-
Reactive oxygen species
- QTL:
-
Quantitative trait locus
- Zn:
-
Zinc
References
Agius F, Gonzalez-Lamothe R, Caballero JL, Munoz-Blanco J, Botella MA, Valpuesta V (2003) Engineering increased vitamin C levels in plants by overexpression of a d-galacturonic acid reductase. Nat Biotechnol 21:177–181
Ahkami AH, Lischewski S, Haensch K-T, Porfirova S, Hofmann J, Rolletschek H, Melzer M, Franken P, Hause B, Druege U, Hajirezaei MR (2009) Molecular physiology of adventitious root formation in Petunia hybrida cuttings: involvement of wound response and primary metabolism. New Phytol 181:613–625
Alloway BJ (2004) Zinc in soils and crop nutrition. International Fertilizer Industry Association, Paris
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arnold T, Kirk GJD, Wissuwa M, Frei M, Zhao F-J, Mason TFD, Weiss DJ (2010) Evidence for the mechanisms of zinc uptake by rice using isotope fractionation. Plant Cell Environ 33:370–381
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Avonce N, Leyman B, Mascorro-Gallardo JO, Van Dijck P, Thevelein JM, Iturriaga G (2004) The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol 136:3649–3659
Bartoli CG, Guiamet JJ, Kiddle G, Pastori GM, Di Cagno R, Theodoulou FL, Foyer CH (2005) Ascorbate content of wheat leaves is not determined by maximal l-galactono-1,4-lactone dehydrogenase (GalLDH) activity under drought stress. Plant Cell Environ 28:1073–1081
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Cakmak I, Marschner H (1988) Enhanced superoxide radical production in roots of zinc deficient plants. J Exp Bot 39:1449–1460
Cakmak I, Marschner H, Bangerth F (1989) Effect of zinc nutritional status on growth, protein metabolism and levels of indole-3-acetic acid and other phytohormones in bean (Phaseolus vulgaris L.). J Exp Bot 40:405–412
Chen Z, Young TE, Ling J, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100:3525–3530
Conklin PL (2001) Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ 24:383–394
Davey MW, Gilot C, Persiau G, Ostergaard J, Han Y, Bauw GC, Van Montagu MC (1999) Ascorbate biosynthesis in Arabidopsis cell suspension culture. Plant Physiol 121:535–543
DeBolt S, Hardie J, Tyerman S, Ford CM (2004) Composition and synthesis of raphide crystals and druse crystals in berries of Vitis vinifera L. cv. Cabernet Sauvignon: ascorbic acid as precursor for both oxalic and tartaric acids as revealed by radiolabelling studies. Aust J Grape Wine R 10:134–142
Dobermann A, Fairhurst T (2000) Rice: nutrient disorders & nutrient management. International Rice Research Institute, Los Banos
Duan J, Zhang M, Zhang H, Xiong H, Liu P, Ali J, Li J, Li Z (2012) OsMIOX, a myo-inositol oxygenase gene, improves drought tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). Plant Sci 196:143–151
Endres S, Tenhaken R (2009) Myoinositol oxygenase controls the level of myoinositol in Arabidopsis, but does not increase ascorbic acid. Plant Physiol 149:1042–1049
Frei M, Tanaka JP, Chen CP, Wissuwa M (2010a) Mechanisms of ozone tolerance in rice: characterization of two QTLs affecting leaf bronzing by gene expression profiling and biochemical analyses. J Exp Bot 61:1405–1417
Frei M, Wang Y, Ismail AM, Wissuwa M (2010b) Biochemical factors conferring shoot tolerance to oxidative stress in rice grown in low zinc soil. Funct Plant Biol 37:74–84
French D (1954) The raffinose family of oligosaccharides. Adv Carbohyd Chem Bi 9:149–184
Garg AK, Kim JK, Owens TG, Ranwala AP, Do Choi Y, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99:15898–15903
Goddijn OJM, van Dun K (1999) Trehalose metabolism in plants. Trends Plant Sci 4:315–319
Gracy RW, Noltmann EA (1968a) Studies on phosphomannose isomerase. I. Isolation homogeneity measurements and determination of some physical properties. J Biol Chem 243:3161–3168
Gracy RW, Noltmann EA (1968b) Studies on phosphomannose isomerase: II. Characterization as a zinc metalloenzyme. J Biol Chem 243:4109–4116
Green MA, Fry SC (2005) Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-l-threonate. Nature 433:83–87
Guo ZF, Tan HQ, Zhu ZH, Lu SY, Zhou BY (2005) Effect of intermediates on ascorbic acid and oxalate biosynthesis of rice and in relation to its stress resistance. Plant Physiol Bioch 43:955–962
Hacisalihoglu G, Kochian LV (2003) How do some plants tolerate low levels of soil zinc? Mechanisms of zinc efficiency in crop plants. New Phytol 159:341–350
Hancock RD, Viola R (2005) Biosynthesis and catabolism of l-ascorbic acid in plants. Crit Rev Plant Sci 24:167–188
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hoffland E, Wei C, Wissuwa M (2006) Organic anion exudation by lowland rice (Oryza sativa L.) at zinc and phosphorus deficiency. Plant Soil 283:155–162
Hossain MA, Asada K (1984) Purification of dehydroascorbate reductase from spinch and its characterization as a thiol enzyme. Plant Cell Physiol 25:85–92
Hossain B, Hirata N, Nagatomo Y, Akashi R, Takaki H (1997) Internal zinc accumulation is correlated with increased growth in rice suspension culture. J Plant Growth Regul 16:239–243
Ishikawa T, Dowdle J, Smirnoff N (2006) Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol Plant 126:343–355
Jo Y, Hyun TK (2011) Genome-wide identification of antioxidant component biosynthetic enzymes: comprehensive analysis of ascorbic acid and tocochromanols biosynthetic genes in rice. Comput Biol Chem 35:261–268
Kitagishi K, Obata H (1986) Effects of zinc deficiency on the nitrogen-metabolism of meristematic tissue of rice plants with reference to protein synthesis. Soil Sci Plant Nutr 32:397–405
Linster CL, Clarke SG (2008) l-Ascorbate biosynthesis in higher plants: the role of VTC2. Trends Plant Sci 13:567–573
Loewus FA, Kelly S (1961) Identity of l-ascorbic acid formed by d-glucose by strawberry (Fragaria). Nature 191:1059–1061
Lorence A, Chevone BI, Mendes P, Nessler CL (2004) myo-Inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol 134:1200–1205
Luo Y, Li W-M, Wang W (2008) Trehalose: protector of antioxidant enzymes or reactive oxygen species scavenger under heat stress? Environ Exp Bot 63:378–384
Maruta T, Yonemitsu M, Yabuta Y, Tamoi M, Ishikawa T, Shigeoka S (2008) Arabidopsis phosphomannose isomerase 1, but not phosphomannose isomerase 2 is essential for ascorbic acid biosynthesis. J Biol Chem 283:28842–28851
Matysik J, Alia, Bhalu B, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci India 82:525–532
Molinari HBC, Marur CJ, Daros E, De Campos MKF, De Carvalho JFRP, Pereira LFP, Vieira LGE (2007) Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol Plant 130:218–229
Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J, Perez Conesa D, Ros G, Sandmann G, Capell T, Christou P (2009) Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci USA 106:7762–7767
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Parsons HT, Yasmin T, Fry SC (2011) Alternative pathways of dehydroascorbic acid degradation in vitro and in plant cell cultures: novel insights into vitamin C catabolism. Biochem J 440:375–383
Patel RK, Jain M (2011) PlantRGS: a web server for the identification of most suitable candidate reference genes for quantitative gene expression studies in plants. DNA Res 18:463–470
Possingham JV (1956) The effect of mineral nutrition on the content of free amino acids and amides in tomato plants. Aust J Biol Sci 9:539–551
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Rontein D, Basset G, Hanson AD (2002) Metabolic engineering of osmoprotectant accumulation in plants. Metab Eng 4:49–56
Rose MT, Rose TJ, Pariasca-Tanaka J, Widodo, Wissuwa M (2011) Revisiting the role of organic acids in the bicarbonate tolerance of zinc-efficient rice genotypes. Funct Plant Biol 38:493–504
Rose MT, Rose TJ, Pariasca-Tanaka J, Yoshihashi T, Neuweger H, Goesmann A, Frei M, Wissuwa M (2012) Root metabolic response of rice (Oryza sativa L.) genotypes with contrasting tolerance to zinc deficiency and bicarbonate excess. Planta 236:959–973
Smirnoff N (1996) Botanical briefing: the function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Plant Sci 19:267–290
Szekely G, Abraham E, Cseplo A, Rigo G, Zsigmond L, Csiszar J, Ayaydin F, Strizhov N, Jasik J, Schmelzer E, Koncz C, Szabados L (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53:11–28
Tausz M, Sircelj H, Grill D (2004) The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid? J Exp Bot 55:1955–1962
Tripathi B, Gaur J (2004) Relationship between copper-and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 219:397–404
Ueda Y, Wu LB, Frei M (2013) A critical comparison of two high throughput ascorbate analyses methods for plant samples. Plant Physiol Bioch 70:418–423
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393:365–369
Widodo B, Broadley MR, Rose T, Frei M, Pariasca-Tanaka J, Yoshihashi T, Thomson M, Hammond JP, Aprile A, Close TJ, Ismail AM, Wissuwa M (2010) Response to zinc deficiency of two rice lines with contrasting tolerance is determined by root growth maintenance and organic acid exudation rates, and not by zinc-transporter activity. New Phytol 186:400–414
Wingler A, Wingler (2002) The function of trehalose biosynthesis in plants. Phytochemistry 60:437–440
Wissuwa M, Ismail AM, Yanagihara S (2006) Effects of zinc deficiency on rice growth and genetic factors contributing to tolerance. Plant Physiol 142:731–741
Wolucka BA, Van Montagu M (2003) GDP-mannose 3′, 5′-epimerase forms GDP-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J Biol Chem 278:47483–47490
Acknowledgments
This study was partly funded by Deutsche Forschungsgemeinschaft (FR2952/1-1).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Höller, S., Hajirezaei, MR., von Wirén, N. et al. Ascorbate metabolism in rice genotypes differing in zinc efficiency. Planta 239, 367–379 (2014). https://doi.org/10.1007/s00425-013-1978-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-013-1978-x