Abstract
Tomato fruit-weight 2.2 (FW2.2) was reported to control up to 30 % fruit weight. Recent studies demonstrated that FW2.2-like (FWL) genes also play important roles in plant growth and development. For instance, a maize homolog of FW2.2, named cell number regulator 1 (CNR1), negatively regulates plant and organ size. However, FWL genes in rice have not been characterized yet. In this study, eight FWL genes were identified in rice genome and designated as OsFWL1-8. The chromosome location, gene structure, protein motif, and phylogenetic relationship of OsFWL genes were analyzed. RT-PCR result and microarray data revealed that OsFWL genes exhibited diverse expression patterns and the detailed expression patterns of OsFWL5, 6, and 7 negatively correlated with leaf growth activity. Rice protoplast transient transformation experiment showed that most OsFWL proteins locate at cell membrane but OsFWL8 is present in the nucleus. In addition, the functions of OsFWL genes were investigated by analyzing two T-DNA insertion lines for OsFWL3 and 5. Compared with wild type, the grain weight of osfwl3 mutant and the plant height of osfwl5 mutant were increased by 5.3 and 12.5 %, respectively. We also found that the increase in grain length of osfwl3 mutant was due chiefly to incremental cell number, not cell size and the expression of OsFWL3 negatively correlated with glume growth activity. These results provide a comprehensive foundation for further study of OsFWL functions in rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato fruit-weight 2.2 (fw2.2) is a major quantitative trait locus which is known to contribute up to 30 % of the fruit weight variation and is a key to the evolution of fruit size (Alpert et al. 1995). A gene previously named ORFX, which is responsible for the fw2.2 effect, was identified by map-based cloning and was renamed FW2.2. The gene FW2.2, which is a negative regulator of cell proliferation, regulates the tomato fruit size through controlling carpel cell number, relying on the changes in the expression level and expression timing rather than coding sequence (Cong et al. 2002; Frary et al. 2000; Liu et al. 2003). It was also reported to cause other phenotypes on fruit number and photosynthate distribution (Nesbitt and Tanksley 2001). The results of yeast two-hybrid and in vitro binding assays showed that FW2.2 directly interacts with β subunit of casein kinase II (Cong and Tanksley 2006). Casein kinase II (CKII) was validated to take part in cell cycle-related signaling transduction pathway in plants (Espunya et al. 1999; Moreno-Romero et al. 2008). These results indicate that FW2.2 may participate in cell cycle regulation via CKII-mediated pathways. However, the hypothesis still lacks direct evidence so far.
Previous studies provided strong evidence that FW2.2 has many homologs in plant, animal and fungus (Frary et al. 2000; Guo et al. 2010; Libault et al. 2010). It represents an ancient eukaryotic family of cysteine-rich proteins containing a PLAC8 domain, originally identified in mammalian placental protein with unknown function. The FW2.2-like (FWL) proteins have a highly conserved core motif: one or two transmembrane motifs locating between two cysteine/proline-rich domains (Libault and Stacey 2010). Numerous findings imply that FWL genes play important roles in plant development. In Arabidopsis, a FWL gene named AtPCR1 was reported to be involved in cadmium resistance (Song et al. 2004) and two FWL genes (MCA1 and MCA2) were found to mediate Ca2+ uptake (Nakagawa et al. 2007; Nakano et al. 2011; Yamanaka et al. 2010). A soybean homolog of FW2.2 named GmFWL1 was found to affect the nodule organogenesis when the plant was infected by the nitrogen-fixing symbiotic bacterium, Bradyrhizobium japonicum (Libault et al. 2010). The functions of two FW2.2 homologs in maize were reported. Cell number regulator 1 (CNR1) was revealed to control plant and organ size by altering cell number. The expression of CNR2 was found to be negatively interrelated with plant growth activity and hybrid seedling vigor (Guo et al. 2010). A FWL gene in avocado named Pafw2.2-like showed much higher expression in small fruit species than in normal ones at whole fruit growth stage (Dahan et al. 2010). With more FWL genes identified in other plant species, more evidences validated the hypothesis that FWL genes act as a general regulator of plant cell number and organ size (Guo and Simmons 2011).
Rice grain weight is a significant quantitative trait which is determined by length, width, thickness, and filling of rice (Xing and Zhang 2010). Now a series of grain weight-related genes were identified in rice, such as GS3, GW2, and GS5. GS3, a vital gene for rice grain length and weight, encodes a membrane protein composed of four different functional domains and acts as a negative regulator for grain size (Fan et al. 2006; Mao et al. 2010; Takano-Kai et al. 2009). GW2 encodes an atypical RING-type E3 ubiquitin ligase and negatively modulates cell division through the ubiquitin–proteasome pathway (Song et al. 2007). GS5 encodes a putative serine carboxypeptidase and acts as a positive regulator of grain width and grain weight (Li et al. 2011).
Although the FWL genes were reported to be general regulators of plant fruit and organ size (Guo and Simmons 2011), the molecular characteristics of FWL gene family in rice is still unclear. To provide a global glimpse of FW2.2/CNR1 homologs in rice, we investigated their gene family members, chromosomal locations, gene structures, protein motifs, phylogenetic relationships, expression patterns, and subcellular localizations in this study. Moreover, the phenotypic test of osfwl3 and osfwl5 mutants indicated that OsFWLs may regulate seed size or plant height in rice.
Materials and methods
Identification and analysis of OsFWL genes
The amino acid (aa) sequence of FW2.2 was used as a query to blast The Rice Annotation Project Database (RAP-DB, http://rapdb.dna.affrc.go.jp/) with BLASTP. Then, domain search was executed against Rice Genome Annotation Project database (RGAP, http://rice.plantbiology.msu.edu/domain_search.shtml) (Kawahara et al. 2013). The full length cDNA sequences of OsFWL were searched in Knowledge-Based Oryza Molecular Biological Encyclopedia database (KOME, http://cdna01.dna.affrc.go.jp/cDNA) (Kikuchi et al. 2003). Co-expression analysis was executed on Rice Oligonucleotide Array Database (ROAD, http://www.ricearray.org/index.shtml) (Cao et al. 2012).
The analysis of chromosomal localization, gene structure, protein motif, and phylogenetic relationship
The chromosomal localization data of OsFWLs were obtained from RGAP and mapped by MapChart 2.2 software. Gene structures of OsFWLs were analyzed by comparing the genomic sequences with their corresponding full length cDNA sequences. Then, the exon–intron organizations were mapped by Gene Structure Display Server (GSDS, http://gsds.cbi.pku.edu.cn/) (Guo et al. 2007). Multiple sequence alignment was performed by ClustalW (Larkin et al. 2007). “DAS” Transmembrane Prediction Server (http://www.sbc.su.se/~miklos/DAS/maindas.html) (Cserzo et al. 1997) was used for membrane topology analysis. The phylogenetic tree was constructed using MEGA4.0 (Tamura et al. 2007) by the neighbor-joining method with 1,000 replicates bootstrap analysis.
Expression pattern analysis of OsFWL gene family
Total RNA of various tissues from Zhonghua 11 (Oryza. sativa L. ssp. japonica) was extracted using Trizol reagent in accordance with manufacturer’s instructions (Invitrogen). First-strand cDNA was reverse transcribed from 1 μg RNA using PrimeScript RT reagent Kit (TaKaRa). The GAPDH gene was used to normalize the cDNA concentration. RT-PCR was performed on ABI 9700 PCR instrument in 20 μl reaction volume including 1 μl cDNA sample, 0.2 mM dNTPs, 0.25 μM gene-specific primers, 1× PCR buffer and 1 U rTaq polymerase (Takara). The reaction profiles were the following: 94 °C for 4 min, 94 °C for 30 s, 55–65 °C for 30 s, 72 °C for 1 min (28–40 cycles) and 72 °C for 7 min. All of the primers are listed in Supplemental Table S1. The expression profiles were obtained from Collection of Rice Expression Profiles (CREP, http://crep.ncpgr.cn/crep-cgi/home.pl) (Wang et al. 2010) and Rice Expression Profile Database (RiceXPro, http://ricexpro.dna.affrc.go.jp/) (Sato et al. 2013).
Subcellular localization analysis
Subcellular localization was predicted with Plant-mPLoc software (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) (Chou and Shen 2010) and further confirmed through rice protoplast transient transformation. The protoplast preparation and plasmid transformation were performed as described (Bart et al. 2006). Each target protein was fused with green fluorescent protein (GFP). A reported rice copper transporter COPT1 (Yuan et al. 2010) was fused with red fluorescent protein (RFP) and used as an indicator for cell membrane localization. Another reported rice transcription factor Ghd7 (Xue et al. 2008) was fused with cyan fluorescent protein (CFP) and used as a reference for nuclear localization. The fusion proteins of OsFWLs were introduced into rice protoplasts with COPT1 or Ghd7. Then, the protoplasts were maintained at 28 °C for 18–24 h in dark. At last, the images were captured with a confocal laser scanning microscope (Leica, Germany).
Analysis of T-DNA insertion mutants of OsFWLs
The insertion mutant lines were searched against Rice Functional Genomic Express Database (RiceGE, http://signal.salk.edu/cgi-bin/RiceGE). The mutant lines we received were collected from Rice Mutant Database (RMD) (Zhang et al. 2006) and Pohang University of Science and Technology (POSTECH) (Jeong et al. 2006). For genotyping analysis, the gene-specific PCR primers (P1 and P2) flanking the T-DNA insertion site and a vector border primer (P3) were designed. The primers for OsFWL3 and OsFWL5 are listed in Supplemental Table S1. The details about the two sets of PCR were as described (Dai et al. 2009). Grain length, grain width, and 1,000-grain weight were measured three times for each plant. The yield traits including panicle length, panicle number per plant, primary branch number per panicle, secondary branch number per panicle, and plant height were contrasted between wild type and mutant following the described method (Thomson et al. 2003). The cell length of glume inner epidermis was analyzed according to a previously reported method (Li et al. 2012).
Histochemical analysis of glucuronidase (GUS) activity
The promoter of OsFWL3 (approximately 2 kb upstream of the translation start site) was fused to GUS gene and the construct was transformed into rice Zhonghua 11.
Expression of GUS was assayed following previous description (Li et al. 2012).
Results
Identification of FW2.2-like gene family in rice
To identify homologs of FW2.2 in rice, the aa sequence of FW2.2 was used as a query to search against RAP-DB with BLASTP. Twelve putative proteins were identified. Then using each putative protein sequence as a query we searched PLAC8 domain against RGAP database. Eventually, we identified 8 putative FW2.2-like genes in rice genome and named them from OsFWL1 to OsFWL8 (Table 1). The full length cDNAs of OsFWL1, 2, 3, 5, and 7 were found in KOME database. The functions of OsFWL genes were unknown except for OsFWL5, which was reported as OsPcr1 (Plant cadmium resistance) and validated to increase cadmium resistance when expressed in yeast (Song et al. 2004).
Chromosomal locations, gene structures, protein motifs, and phylogenetic relationships of OsFWL genes
The eight OsFWL genes are distributed on three rice chromosomes: OsFWL1, 2, and 3 are located on chromosome 2, where OsFWL2 and 3 are located in adjacent gene locus; OsFWL4, 6, 7, and 8 form a gene cluster on chromosome 3; OsFWL5 is located on chromosome 10 (Fig. 1a). For further study of OsFWLs, the genomic and coding sequences of all the genes were cloned and sequenced. Then, we identified the intron and exon structures of OsFWL genes (Fig. 1b). The coding sequences of OsFWL genes are all short and similar, from 411 to 561 bp. Based on the number of introns, OsFWL genes can be classified into three groups: Group 1 (OsFWL4, 6, 7, and 8, have 1 intron); Group 2 (OsFWL2 and 3 contain 2 introns); Group 3 (OsFWL1 and 5 harbor 3 introns).
The aa sequences of deduced OsFWL proteins range from 136 to 186 aa. The contents of cysteine residues in these proteins range from 10 to 20, which are higher than those in general proteins. Topology prediction of membrane protein using “DAS” software showed that the OsFWL proteins contained a predicted transmembrane (TM) domain (Fig. 2a). For example, OsFWL1 harbors two potential TM segments, aa 58–70 and aa 86–101, which may function as a TM domain (Fig. 2b). Most OsFWLs (OsFWL2, 4, 5, 6, 7, and 8) include the CCXXXXCPC motif in this predicted TM domain (Fig. 2a). This motif was found to be the cadmium resistance-conferring motif of Pcr family (Song et al. 2004).
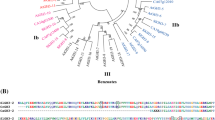
Multiple sequence alignment and phylogenetic relationship of OsFWLs, CNR1, and FW2.2. a Multiple sequence alignment of OsFWLs, CNR1, and FW2.2. Identical and similar amino acids were showed by dark and gray shadow, respectively. The predicted transmembrane domain was indicated by underline. The conserved motif (CXXXXXCPC) was mentioned by asterisks. b Hydrophobic profile of OsFWL1. Hydrophobicity was determined by “DAS”—Transmembrane Prediction Server. c Phylogenetic relationships of OsFWLs, CNR1, and FW2.2. Phylogenetic tree was created by MEGA 4.0 using the neighbor-joining method. The numbers at the clades are percentages of bootstrap using 1,000 replicates. Only values >50 % are given
According to the phylogenetic tree (Fig. 2c) of OsFWL, CNR1, and FW2.2 proteins constructed using the Neighbor-Joining method, with 1,000 replicates bootstrap, OsFWL proteins can be divided broadly into two groups (OsFWL1, 2, 3, 4, and 5 are in Group I; OsFWL6, 7, and 8 constitute Group II).
Expression patterns of OsFWL genes
To analyze the expression patterns of OsFWL genes, RT-PCR of seven tissue materials, i.e. calli, seedling, leaf blade at the tillering stage, four tissues (stem, root, flag leaf, panicle) at the heading stage was performed. The result showed that the expression patterns of OsFWL genes are various in different organs (Fig. 3a). The OsFWL1 is expressed in root, stem, panicle, and seedling. The OsFWL2 is mostly expressed in root, flag leaf, leaf blade, and calli. The OsFWL3 has the strongest expression in panicle and low expressions in calli and seedling. The OsFWL4 has a low expression in all seven materials. The OsFWL5 is mainly expressed in root, seedling, flag leaf, leaf blade, and panicle. The OsFWL6 is expressed in root, leaf blade, and flag leaf. The OsFWL7 has a relatively high expression in root but faint expression in leaf blade and panicle. The OsFWL8 is expressed in root, seedling, and leaf blade.
The expression patterns of OsFWL genes. a RT-PCR analysis of OsFWL genes in seven tissues: calli (C); seedling (S); leaf blade (LB) at the tillering stage; stem (St), root (R), flag leaf (FL), and panicle (P) at the heading stage. GAPDH gene was used as an internal control. The results of RT-PCR were similar by three biological replicates. b Hierarchical cluster display of expression profile for 8 OsFWL genes with corresponding probes in rice variety Minghui 63. Twenty-five samples used for expression analysis are mentioned at the top of each column. Color key shows log2 expression values: green indicates low expression, black represents medium expression, red represents high expression. c The detailed expression patterns of specific OsFWL genes in relation to leaf growth activity in RiceXPro database. The x axis is the day after transplant (DAT). From left to right, the growth activity is decreasing and maturation is increasing. The y axis is the probe signal value which is the results of three biological replicates and is shown with SEM. d RT-PCR analysis of the three OsFWL genes in leaf at seedling (S), tillering (T), and heading (H) stage. GAPDH gene was also used as the internal control
To reveal the expression patterns of OsFWL genes in the whole life cycle, we have also searched CREP and RiceXPro expression profile databases. All 8 OsFWL genes can be found in CREP and 7 genes in RiceXPro. We extracted the signal values of the probes corresponding to the 8 OsFWL genes in CREP database. The information of the 8 OsFWL genes in 25 samples was normalized and subjected to Hierarchical cluster analysis (Fig. 3b). The expression patterns of OsFWL2, 5, 6, 7, and 8 are relatively similar. They are mostly detected in seed after imbibition, root at 2-tillers stage, and leaf, flag leaf, sheath at the whole life cycle. The OsFWL1 holds a high expression in root, stem and young panicle. The OsFWL4 has an absolutely low expression at the whole life cycle. The expression pattern of OsFWL3 is special. It possesses a low expression level in most tissues/organs except for panicle at heading stage. As OsFWL genes were expected to be negative regulators in rice cell number, we analyzed whether their expression patterns related to tissue growth activity. According to microarray data in RiceXPro database, the expressions of OsFWL5, 6, and 7 negatively correlated with leaf growth activity (Fig. 3c). RT-PCR results of the three OsFWL genes in leaf at different development stage confirmed the microarray data (Fig. 3d).
Subcellular localization of OsFWL proteins
To understand the protein properties of OsFWLs, we investigated their subcellular localization. Firstly, Plant-mPLoc software was used to predict their subcellular localization. The results suggested that seven OsFWLs (OsFWL1-7) are cell membrane proteins. This result is consistent with that of FW2.2 which was localized on the plasma membrane (Cong and Tanksley 2006). Only OsFWL8 is predicted in the nucleus. To provide experimental evidences for the above analysis, we carried out a transient gene expression assay in rice protoplast. Since the subcellular localization of CNR1 was only predicted by computer analysis but not confirmed by experiment, we included CNR1 in our study. CNR1 and OsFWL1, 2, 4, 6, and 8 were fused to GFP. The GFP empty vector was used as a negative control. The reported cell membrane protein COPT1 and nuclear protein Ghd7 were tagged with RFP and CFP, respectively to be used as positive controls. The OsFWLs and CNR1 were used to co-transform rice protoplasts with COPT1 or Ghd7. The result showed that the fusion proteins of OsFWL1, 2, 4, 6 and CNR1 were co-located with COPT1 on the cell membrane (Fig. 4a–e), while OsFWL8 was co-located with Ghd7 in the nucleus (Fig. 4f). OsFWL8 is the lowest homolog of OsFWL protein family and its subcellular localization is possibly different from other OsFWL proteins. GFP alone was distributed in cell membrane, cytoplasm, and nucleus (Fig. 4g).
The subcellular localizations of CNR1 and OsFWL1, 2, 4, 6, 8 in rice protoplast cells. a GFP-CNR1 and RFP-COPT1 were co-localized on the rice protoplast cell membrane. b GFP-OsFWL1 and RFP-COPT1 were co-localized on cell membrane. c GFP-OsFWL2 and RFP-COPT1 were co-localized on cell membrane. d GFP-OsFWL4 and RFP-COPT1 were co-localized on cell membrane. e GFP-OsFWL6 and RFP-COPT1 were co-localized on cell membrane. f GFP-OsFWL8 and CFP-Ghd7 were co-localized in the nucleus. g GFP vector was expressed in cell membrane, cytoplasm, and nucleus
Functional analysis of OsFWL3 and OsFWL5
For studying the biological function of OsFWL genes, T-DNA or Tos17 insertion mutants were searched against RiceGE. In total, we found 8 putative T-DNA mutant lines with insertions in 5 OsFWL genes (Supplemental Table S2). Through two sets of PCR for genotyping, only the insertion sites of two mutant lines for OsFWL3 (03Z11HZ85) and 5 (PFG_3A-50652) were confirmed. The other 6 lines we obtained were not confirmed due to <10 seeds or no specific flanking sequences.
The T-DNA mutant line 03Z11HZ85 contains an insertion in the 5′-UTR region of OsFWL3 (Fig. 5a). PCR genotyping resulted in expected band pattern (Fig. 5b). RT-PCR result showed that OsFWL3 transcript was absent in osfwl3 homozygous mutant (Fig. 5c). We tested the yield-related traits of 14 homozygous plants and 12 wild-type plants, and found that panicle length, panicles, primary branches, second branches, and plant height of mutants were not significantly different from that of control (data not shown). Neither the grain width nor thickness was affected. However, the 10-seed grain length of mutant was about 7.70 ± 0.06 cm, which was approximately 5.8 % longer than that of wild type (7.28 ± 0.04 cm; Fig. 5d, f). So the 1,000-grain weight of mutant (25.56 ± 0.28 g) was increased by 5.3 % compared with wild type (24.27 ± 0.35 g; Fig. 5e). The size of the rice glume is an important determinant of rice grain size (Hong et al. 1996). To investigate whether cell number or cell size was affected in osfwl3 mutant, the length of glume inner epidermal cells was measured by scanning electron microscopy (Fig. 5g). The result showed that cell length of osfwl3 is not significantly longer than those of wild type (Fig. 5h). The data indicated that the increase in grain length of osfwl3 is due chiefly to incremental cell number, not cell size. To further understand the role of OsFWL3 in grain length control, we examined the expression pattern of OsFWL3 in glume development via histological analysis of GUS activity. The promoter of OsFWL3 (approximately 2 kb upstream of the translation start site) was fused to GUS and the construct was transformed into rice Zhonghua 11. The GUS expression level of mature glume was higher than that of developing glume (Fig. 5i). The result revealed that OsFWL3 expression negatively correlated with glume growth activity.
The functional analysis of OsFWL3. a OsFWL3 gene structure and T-DNA insertion site. T-DNA was inserted into the 5′-UTR region. White boxes, thin lines and gray boxes represent the exons, introns and UTRs, respectively. LB and RB represent the left and right border of T-DNA, respectively. P1 and P2 represent the gene-specific PCR primers flanking the T-DNA insertion site and P3 represents the vector border primer. b PCR result for genotyping. c RT-PCR result of OsFWL3 in homozygous mutant and wild type. GAPDH gene was used as the control. In part b and c W, H, and M indicate the wild type, heterozygous mutant, and homozygous mutant, respectively. d Grain length of 10 seeds in wild type and osfwl3 mutant. e 1,000-grain weight in wild type and osfwl3 mutant. In part d and e, t test was generated between wild types and osfwl3 mutants. The columns are present as mean ± standard deviation (n ≥ 12). Three asterisks indicate P < 0.001, two asterisks represent 0.001 ≤ P < 0.01. f Grain phenotype of wild type and osfwl3 mutant. g An example image of glume inner epidermal cell in wild type by scanning electron microscope. h The analysis of glume inner epidermal cell length between wild type and osfwl3 mutant. The columns are present as mean ± standard deviation (n = 50). P value = 0.37 was generated by t test. i The expression pattern of OsFWL3 in relation to glume growth activity by GUS activity test
The T-DNA mutant line PFG_3A-50652 contains an insertion in the first intron of OsFWL5 (Fig. 6a). Figure 6b showed the genotypes. The expression of OsFWL5 was suppressed in homozygous mutant (Fig. 6c). The plant height of homozygous mutant (9 plants; 94.27 ± 0.96 cm) was increased by 12.5 % compared with that of wild type (11 plants; 83.78 ± 0.89 cm; Fig. 6d, e). The primary contribution to this phenotype is that the first internode of mutant was increased (Fig. 6f). The other yield traits were not distinctly different from that of control (data not shown). As mentioned above, the detailed expression of OsFWL5 negatively correlated with leaf growth activity (Fig. 3c, d).
The T-DNA insertion mutant analysis of OsFWL5. a OsFWL5 gene structure and T-DNA insertion site. White boxes, thin lines and gray boxes represent the exons, introns and UTRs, respectively. LB and RB represent the left and right border of T-DNA, respectively. P1 and P2 represent the gene-specific PCR primers flanking the T-DNA insertion site and P3 represents the vector border primer. b PCR result for genotyping. c RT-PCR result of OsFWL5 in homozygous mutant and wild type. GAPDH gene was used as the control. In part b and c W, H, and M indicate the wild type, heterozygous mutant, and homozygous mutant, respectively. d Plant height of wild type and osfwl5 mutant. t test was generated between wild types and mutants. The columns are present as mean ± standard deviation (n ≥ 9). Three asterisks indicate P < 0.001. e Adult plants of wild type and osfwl5 mutant. f Main culms of wild type and osfwl5 mutant
Discussion
Studies showed that gene families were produced by four main mechanisms: segmental duplication, tandem duplication, transpositional duplication and genome duplication (Cannon et al. 2004; Freeling 2009). It was reported that there were 18 distinct pairs of duplicated segments which cover 65.7 % genome sequences in rice (Yu et al. 2005). After large-scale genome duplications, approximately 30–65 % duplicated genes were lost because of chromosomal deletions and rearrangements (Wang et al. 2005). OsFWL1 and 5 were found to be located in the duplicated segments on chromosome 2 and 10, respectively. But their duplicated counterparts at their corresponding duplicated regions are absent. The chromosomal deletions and rearrangements may be responsible for it. OsFWL2 and 3 are located in tandem on chromosome 2. OsFWL6, 7, and 8 are located in tandem on chromosome 3 (LOC_Os03g61490 which located between OsFWL7 and OsFWL8 is identified as a pseudogene). The data indicate that tandem duplication may contribute to the OsFWL family expansion.
The aa sequence comparison between OsFWL1 and CNR1 exhibited 82 % similarity and 73 % identity. The aa sequence comparisons between other OsFWLs and CNR1 showed <51 % similarity and 41 % identity. FW2.2 and CNR1 were reported to possess a specific CLXXXXCPC domain (Guo et al. 2010). Only OsFWL1 owns a CLITCVCPC domain, whereas in other OsFWL proteins the leucine residue was substituted by cysteine or phenylalanine (Fig. 2a). It is reported that there are numerous collinear regions between rice and maize genomes (Salse et al. 2004). For example ZmGS3, a homolog of rice GS3, regulates maize kernel development with a similar role of GS3 in rice. The homologs of genes surrounding the rice GS3 region are present in the corresponding ZmGS3 region (Li et al. 2010). We chose 16 putative maize genes in the surrounding CNR1 region to Blast against the rice genome (RGAP) and found ten genes with high homologies in the rice region surrounding OsFWL1 (Supplemental Fig. S1). The result showed a collinear relationship between maize CNR1 and rice OsFWL1 regions. The protein similarity and regional collinear relationship suggested that OsFWL1 is an ortholog to CNR1. We received two T-DNA mutant lines (PFG_3D-01373 and PFG_1A-08235) for OsFWL1 but the inserted sites were not confirmed. Thereby, we are performing the over-expressing and RNAi silencing experiments of OsFWL1.
The expression patterns of OsFWL genes (Fig. 3a–d) can offer valuable clues to the function of the corresponding gene. OsFWL1 and 2 widely expressed in multiple organs indicated that they might have pleiotropic functions at different development stages. OsFWL3 specifically expressed in panicle at heading stage suggested that it might be associated with pollen and grain development. OsFWL4 had a very low expression in the whole life cycle and its transcripts were even absent in RiceXPro database. OsFWL5, 6, and 7 showed similar expression patterns and may play redundant roles in rice development. The detailed expression patterns of OsFWL5, 6, and 7 negatively correlated with leaf growth activity (Fig. 3c, d), but other OsFWL did not show such characteristic. OsFWL8 might be involved in different biological processes in contrast to other OsFWLs, as it was located in the nucleus. Co-expression analysis was executed on Rice Oligonucleotide Array Database (ROAD). The results showed that OsFWL1, 2, and 5 were co-expressed with a series of zinc finger proteins. OsFWL1, 2 and 5 were also co-expressed with the ubiquitination-related proteins, such as C3HC4 type domain-containing proteins, RING-H2 finger proteins, and ubiquitin-conjugating enzymes, suggesting that the three OsFWL proteins may be involved in ubiquitination pathway (Supplemental Table S3). The zinc finger and ubiquitination-related proteins were not found in co-expression analysis of other OsFWLs.
We found that OsFWL3 negatively regulated grain length (Fig. 5d–f), and the increase in grain length of osfwl3 mutant was due chiefly to incremental cell number, not cell size (Fig. 5h). The data suggested that OsFWL3 might have similar functional mechanisms to tomato FW2.2 and maize CNR1. The expression of OsFWL3 negatively correlated with glume growth activity (Fig. 5i). We also found that OsFWL5 negatively regulated plant height (Fig. 6d, e) and its expression level negatively correlated with leaf growth activity (Fig. 3c, d). These results suggested that OsFWL3 and OsFWL5 might act as negative regulators in rice growth and development. The impacts of the two genes are not strong, possibly because functional redundancy exists among OsFWL genes. It was reported that both AtPcr1 and OsPcr1/OsFWL5 can increase cadmium resistance when expressed in yeast and the Arabidopsis plants of reduced AtPcr1 expression are more sensitive to cadmium. (Song et al. 2004). These data suggested that compared with wild-type ospcr1/osfwl5 mutant may be more sensitive to cadmium, too. CCXXXXCPC is the cadmium resistance-conferring domain of Pcr family. All OsFWL proteins contain this domain except for OsFWL1 and 3 (Fig. 2a). It indicated that most OsFWL genes may have the similar function as AtPcr1. In Arabidopsis, MCA1 and MCA2 which mediate Ca2+ uptake (Nakagawa et al. 2007; Yamanaka et al. 2010) were considered as FWL genes, as they include a PLAC8 motif in the C-terminal half. However, the N-terminal half containing an EF hand-like region and a coiled-coil motif was found to be responsible for Ca2+ uptake activity (Nakano et al. 2011). Because OsFWLs do not contain the two functional regions, they may not be involved in Ca2+ uptake.
The FWL genes are ubiquitous in plant, animal and fungal genomes. A total of 136 FWL genes were identified based on similarity to the tomato FW2.2 gene (Guo et al. 2010; Libault and Stacey 2010). FWL genes were known to play important roles in plant development. In this study, we identified OsFWL gene family and focused on functional analysis of OsFWL3 and OsFWL5. Based on the differences in protein domain, expression pattern and T-DNA insertion mutant impact, OsFWLs may play diverse roles in rice growth and development.
Abbreviations
- aa:
-
Amino acid(s)
- CNR1:
-
Cell Number Regulator 1
- FW2.2:
-
Fruit-weight 2.2
- FWL:
-
FW2.2-like
- OsFWL:
-
Oryza sativa FW2.2-like
- RT-PCR:
-
Reverse transcription polymerase chain reaction
References
Alpert KB, Grandillo S, Tanksley SD (1995) fw 2.2: a major QTL controlling fruit weight is common to both red- and green-fruited tomato species. Theor Appl Genet 91:994–1000
Bart R, Chern M, Park CJ, Bartley L, Ronald PC (2006) A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts. Plant Methods 2:13
Cannon SB, Mitra A, Baumgarten A, Young ND, May G (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol 4:10
Cao P, Jung K-H, Choi D, Hwang D, Zhu J, Ronald P (2012) The rice oligonucleotide array database: an atlas of rice gene expression. Rice 5:1–9
Chou KC, Shen HB (2010) Plant-mPLoc: a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 5:e11335
Cong B, Tanksley SD (2006) FW2.2 and cell cycle control in developing tomato fruit: a possible example of gene co-option in the evolution of a novel organ. Plant Mol Biol 62:867–880
Cong B, Liu J, Tanksley SD (2002) Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. Proc Natl Acad Sci USA 99:13606–13611
Cserzo M, Wallin E, Simon I, von Heijne G, Elofsson A (1997) Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng 10:673–676
Dahan Y, Rosenfeld R, Zadiranov V, Irihimovitch V (2010) A proposed conserved role for an avocado FW2.2-like gene as a negative regulator of fruit cell division. Planta 232:663–676
Dai X, You C, Wang L, Chen G, Zhang Q, Wu C (2009) Molecular characterization, expression pattern, and function analysis of the OsBC1L family in rice. Plant Mol Biol 71:469–481
Espunya MC, Combettes B, Dot J, Chaubet-Gigot N, Martinez MC (1999) Cell-cycle modulation of CK2 activity in tobacco BY-2 cells. Plant J 19:655–666
Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, Li X, Zhang Q (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet 112:1164–1171
Frary A, Nesbitt TC, Grandillo S, Knaap E, Cong B, Liu J, Meller J, Elber R, Alpert KB, Tanksley SD (2000) fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science 289:85–88
Freeling M (2009) Bias in plant gene content following different sorts of duplication: tandem, whole-genome, segmental, or by transposition. Annu Rev Plant Biol 60:433–453
Guo M, Simmons CR (2011) Cell number counts—the fw2.2 and CNR genes and implications for controlling plant fruit and organ size. Plant Sci 181:1–7
Guo AY, Zhu QH, Chen X, Luo JC (2007) GSDS: a gene structure display server. Yi Chuan 29:1023–1026
Guo M, Rupe MA, Dieter JA, Zou J, Spielbauer D, Duncan KE, Howard RJ, Hou Z, Simmons CR (2010) Cell Number Regulator1 affects plant and organ size in maize: implications for crop yield enhancement and heterosis. Plant Cell 22:1057–1073
Hong SK, Kitano H, Satoh H, Nagato Y (1996) How is embryo size genetically regulated in rice? Development 122:2051–2058
Jeong DH, An S, Park S, Kang HG, Park GG, Kim SR, Sim J, Kim YO, Kim MK, Kim J, Shin M, Jung M, An G (2006) Generation of a flanking sequence-tag database for activation-tagging lines in japonica rice. Plant J 45:123–132
Kawahara Y, de la Bastide M, Hamilton J, Kanamori H, McCombie W, Ouyang S, Schwartz D, Tanaka T, Wu J, Zhou S, Childs K, Davidson R, Lin H, Quesada-Ocampo L, Vaillancourt B, Sakai H, Lee S, Kim J, Numa H, Itoh T, Buell C, Matsumoto T (2013) Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 6:1–10
Kikuchi S, Satoh K, Nagata T, Kawagashira N, Doi K, Kishimoto N, Yazaki J, Ishikawa M, Yamada H, Ooka H, Hotta I, Kojima K, Namiki T, Ohneda E, Yahagi W, Suzuki K, Li CJ, Ohtsuki K, Shishiki T, Otomo Y, Murakami K, Iida Y, Sugano S, Fujimura T, Suzuki Y, Tsunoda Y, Kurosaki T, Kodama T, Masuda H, Kobayashi M, Xie Q, Lu M, Narikawa R, Sugiyama A, Mizuno K, Yokomizo S, Niikura J, Ikeda R, Ishibiki J, Kawamata M, Yoshimura A, Miura J, Kusumegi T, Oka M, Ryu R, Ueda M, Matsubara K, Kawai J, Carninci P, Adachi J, Aizawa K, Arakawa T, Fukuda S, Hara A, Hashizume W, Hayatsu N, Imotani K, Ishii Y, Itoh M, Kagawa I, Kondo S, Konno H, Miyazaki A, Osato N, Ota Y, Saito R, Sasaki D, Sato K, Shibata K, Shinagawa A, Shiraki T, Yoshino M, Hayashizaki Y, Yasunishi A (2003) Collection, mapping, and annotation of over 28,000 cDNA clones from japonica rice. Science 301:376–379
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Li Q, Yang X, Bai G, Warburton ML, Mahuku G, Gore M, Dai J, Li J, Yan J (2010) Cloning and characterization of a putative GS3 ortholog involved in maize kernel development. Theor Appl Genet 120:753–763
Li Y, Fan C, Xing Y, Jiang Y, Luo L, Sun L, Shao D, Xu C, Li X, Xiao J, He Y, Zhang Q (2011) Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat Genet 43:1266–1269
Li J, Chu H, Zhang Y, Mou T, Wu C, Zhang Q, Xu J (2012) The rice HGW gene encodes a ubiquitin-associated (UBA) domain protein that regulates heading date and grain weight. PLoS ONE 7:e34231
Libault M, Stacey G (2010) Evolution of FW2.2-like (FWL) and PLAC8 genes in eukaryotes. Plant Signal Behav 5:1226–1228
Libault M, Zhang XC, Govindarajulu M, Qiu J, Ong YT, Brechenmacher L, Berg RH, Hurley-Sommer A, Taylor CG, Stacey G (2010) A member of the highly conserved FWL (tomato FW2.2-like) gene family is essential for soybean nodule organogenesis. Plant J 62:852–864
Liu J, Cong B, Tanksley SD (2003) Generation and analysis of an artificial gene dosage series in tomato to study the mechanisms by which the cloned quantitative trait locus fw2.2 controls fruit size. Plant Physiol 132:292–299
Mao H, Sun S, Yao J, Wang C, Yu S, Xu C, Li X, Zhang Q (2010) Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc Natl Acad Sci USA 107:19579–19584
Moreno-Romero J, Espunya MC, Platara M, Arino J, Martinez MC (2008) A role for protein kinase CK2 in plant development: evidence obtained using a dominant-negative mutant. Plant J 55:118–130
Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, Kishigami A, Sokabe M, Kojima I, Sato S, Kato T, Tabata S, Iida K, Terashima A, Nakano M, Ikeda M, Yamanaka T, Iida H (2007) Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA 104:3639–3644
Nakano M, Iida K, Nyunoya H, Iida H (2011) Determination of structural regions important for Ca(2+) uptake activity in Arabidopsis MCA1 and MCA2 expressed in yeast. Plant Cell Physiol 52:1915–1930
Nesbitt TC, Tanksley SD (2001) fw2.2 directly affects the size of developing tomato fruit, with secondary effects on fruit number and photosynthate distribution. Plant Physiol 127:575–583
Salse J, Piegu B, Cooke R, Delseny M (2004) New in silico insight into the synteny between rice (Oryza sativa L.) and maize (Zea mays L.) highlights reshuffling and identifies new duplications in the rice genome. Plant J 38:396–409
Sato Y, Takehisa H, Kamatsuki K, Minami H, Namiki N, Ikawa H, Ohyanagi H, Sugimoto K, Antonio BA, Nagamura Y (2013) RiceXPro Version 3.0: expanding the informatics resource for rice transcriptome. Nucleic Acids Res 41:D1206–D1213
Song WY, Martinoia E, Lee J, Kim D, Kim DY, Vogt E, Shim D, Choi KS, Hwang I, Lee Y (2004) A novel family of cys-rich membrane proteins mediates cadmium resistance in Arabidopsis. Plant Physiol 135:1027–1039
Song XJ, Huang W, Shi M, Zhu MZ, Lin HX (2007) A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 39:623–630
Takano-Kai N, Jiang H, Kubo T, Sweeney M, Matsumoto T, Kanamori H, Padhukasahasram B, Bustamante C, Yoshimura A, Doi K, McCouch S (2009) Evolutionary history of GS3, a gene conferring grain length in rice. Genetics 182:1323–1334
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thomson MJ, Tai TH, McClung AM, Lai XH, Hinga ME, Lobos KB, Xu Y, Martinez CP, McCouch SR (2003) Mapping quantitative trait loci for yield, yield components and morphological traits in an advanced backcross population between Oryza rufipogon and the Oryza sativa cultivar Jefferson. Theor Appl Genet 107:479–493
Wang X, Shi X, Hao B, Ge S, Luo J (2005) Duplication and DNA segmental loss in the rice genome: implications for diploidization. New Phytol 165:937–946
Wang L, Xie W, Chen Y, Tang W, Yang J, Ye R, Liu L, Lin Y, Xu C, Xiao J, Zhang Q (2010) A dynamic gene expression atlas covering the entire life cycle of rice. Plant J 61:752–766
Xing Y, Zhang Q (2010) Genetic and molecular bases of rice yield. Annu Rev Plant Biol 61:421–442
Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, Zhang Q (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40:761–767
Yamanaka T, Nakagawa Y, Mori K, Nakano M, Imamura T, Kataoka H, Terashima A, Iida K, Kojima I, Katagiri T, Shinozaki K, Iida H (2010) MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol 152:1284–1296
Yu J, Wang J, Lin W, Li S, Li H, Zhou J, Ni P, Dong W, Hu S, Zeng C, Zhang J, Zhang Y, Li R, Xu Z, Li X, Zheng H, Cong L, Lin L, Yin J, Geng J, Li G, Shi J, Liu J, Lv H, Li J, Deng Y, Ran L, Shi X, Wang X, Wu Q, Li C, Ren X, Li D, Liu D, Zhang X, Ji Z, Zhao W, Sun Y, Zhang Z, Bao J, Han Y, Dong L, Ji J, Chen P, Wu S, Xiao Y, Bu D, Tan J, Yang L, Ye C, Xu J, Zhou Y, Yu Y, Zhang B, Zhuang S, Wei H, Liu B, Lei M, Yu H, Li Y, Xu H, Wei S, He X, Fang L, Huang X, Su Z, Tong W, Tong Z, Ye J, Wang L, Lei T, Chen C, Chen H, Huang H, Zhang F, Li N, Zhao C, Huang Y, Li L, Xi Y, Qi Q, Li W, Hu W, Tian X, Jiao Y, Liang X, Jin J, Gao L, Zheng W, Hao B, Liu S, Wang W, Yuan L, Cao M, McDermott J, Samudrala R, Wong GK, Yang H (2005) The genomes of Oryza sativa: a history of duplications. PLoS Biol 3:e38
Yuan M, Chu Z, Li X, Xu C, Wang S (2010) The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell 22:3164–3176
Zhang J, Li C, Wu C, Xiong L, Chen G, Zhang Q, Wang S (2006) RMD: a rice mutant database for functional analysis of the rice genome. Nucleic Acids Res 34:D745–D748
Acknowledgments
We thank Professor Guo-liang Wang for the gift of subcellular localization vector. We also thank RMD and POSTECH for the offer of T-DNA insertion mutants and public laboratory of electron microscopy in Huazhong Agricultural University for technical assistance with scanning electron microscope. This work was supported by the National Natural Science Foundation of China (Grant No. 30971748).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, J., Xiong, W., Cao, B. et al. Molecular characterization and functional analysis of “fruit-weight2.2-like” gene family in rice. Planta 238, 643–655 (2013). https://doi.org/10.1007/s00425-013-1916-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-013-1916-y