Abstract
Ethylene and light act through specific signal transduction mechanisms to coordinate the development of higher plants. Application of 1-aminocyclopropane-1-carboxylic acid (ACC, an ethylene precursor) suppresses the hypocotyl elongation of Arabidopsis seedlings in dark, but stimulates it in light. However, the mechanisms of opposite effects of ethylene on hypocotyl elongation in light and dark remain unclear. In the present study, we investigated the key factors involved in the opposite effects of ethylene on hypocotyl elongation in Arabidopsis seedlings. The effects of ACC on hypocotyl elongation of IAA-insensitive mutants including tir1-1, axr1-3, and axr1-12 seedlings were reduced in light but not in dark. The DR5 promoter, a synthetic auxin-response promoter, was used to quantify the level of IAA responses. There was a marked increase in DR5-GFP signals in response to ACC treatment in hypocotyls of DR5-GFP seedlings in light, but not in dark. CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) is an important downstream component of light signaling. ETHYLENE-INSENSITIVE3 (EIN3, an ethylene-stabilized transcription factor) directly regulates ETHYLENE-RESPONSE-FACTOR1 (ERF1). The cop1-4 mutant treated with ACC and cop1-4/EIN3ox plants developed long hypocotyls in darkness. Expression of ERF1 in the cop1-4 mutant was induced by ACC treatment in dark, but the expression of ERF1 in the wild type was not affected. Taken together, ethylene-promoting hypocotyl via IAA is mediated by light, and COP1 has a significant impact on the transcription of some genes downstream of EIN3. Thus, COP1 plays a crucial role in the opposite effects of ethylene on hypocotyl elongation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light, as one of the most influential environmental factors, not only provides the source of energy for plant life, but also acts as a signal that affects plant growth and development throughout the entire life cycle (Smith 2000; Sullivan et al. 2003; Lee et al. 2007). A microarray analysis using Arabidopsis thaliana indicated that photomorphogenesis involves a regulation change in the expression of up to 30 % of the genes in its genome (Ma et al. 2001). Light signals are perceived by multiple photoreceptors and are transduced to downstream regulators, where they regulate the photomorphogenic development in a quantitative manner. Two key downstream components, CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) and LONG HYPOCOTYL 5 (HY5), act antagonistically in Arabidopsis seedling development (Osterlund et al. 2000). In darkness, COP1-regulated genes were estimated to account for 20 % of the gene content (Ma et al. 2002). The light repression of COP1 involves a quantitative reduction of the COP1 abundance in the nucleus depending on light intensity (von Arnim and Deng 1994). Multiple photoreceptors negatively regulate COP1 activity (Osterlund et al. 1999). COP1 may act as a putative E3 ubiquitin ligase within the nucleus, interacting directly with the transcription factor HY5 and targeting its degradation via the 26S proteasome (Osterlund et al. 2000; Schwechheimer and Deng 2000). Therefore, it is hypothesized that COP1 modulates gene expression by targeting key transcription factors for degradation in the nucleus (Yamamoto et al. 1998). The gene expression profile of dark-grown cop1 mutants is very similar to that of light-grown wild-type seedlings (Ma et al. 2002). Light can release COP1 from the nucleus, but the total amount of cellular COP1 does not change. Exclusion of COP1 prevents the degradation of the positive signaling factors, such as HY5 and HYH, and allows photomorphogenesis to occur (Osterlund et al. 2000; Holm et al. 2002). Thus, HY5 is believed to be one of the central modulators for the coordination of light signals and the regulation of appropriate gene expression (Lee et al. 2007). The phenotype of hy5 seedlings includes defects in light inhibition of hypocotyl elongation and light-induced chlorophyll accumulation. The copl-4 seedlings develop short hypocotyls and expanded cotyledons in dark, whereas their hypocotyls are of the same length as the wild type in light (Ang and Deng 1994).
The phytohormone ethylene plays significant roles in physiological processes throughout the life cycle of plants. Molecular and genetic analyses have revealed many details of ethylene signaling pathways, from perception to transcriptional regulation (Li and Guo 2007). Ethylene is perceived by proteins encoded by a gene family containing five membrane-bound receptors, which can be divided into two types (ETR1, ERS1, and ETR2, ERS2, EIN4) (Chen et al. 2005). CTR1 inhibits various downstream signaling components including EIN2 and EIN3/EIL1. Once ethylene inactivates the receptor CTR1, ethylene signaling is activated (Li and Guo 2007; Zhu and Guo 2008). The abundance of the EIN3 protein rapidly increases in ethylene treatment. In the absence of ethylene, EIN3 is targeted by SCFEBF1/EBF2 complexes and degraded by the 26S proteasome (Potuschak et al. 2003; Guo and Ecker 2003; An et al. 2010). By binding to specific promoter elements (EBS, EIN3 binding sites), EIN3 regulates the expression of primary target genes, such as ERF1, which bind to secondary target genes and lead to changes in the morphological phenotype. Overexpression of ERF1 can rescue only a subset of ein3 phenotypes, suggesting that EIN3 regulates other target genes to mediate ethylene responses (Solano et al. 1998). Ethylene-responsive element binding factors (ERFs) are members of a novel family of plant-specific transcription factors. Five different ERF proteins have been described (gene products of AtERF1 to AtERF5). The AtERF genes are differentially regulated by ethylene (Fujimoto et al. 2000).
Light and ethylene signals can be coordinated to affect the formation of the apical hook in Arabidopsis seedlings (Lehman et al. 1996). A well-known example of light and ethylene interaction is hookless1. The hookless1 (hls1) mutant was initially isolated as one that could not form an exaggerated hook under ethylene treatment (Lehman et al. 1996). The level of HLS1 protein is increased in response to ethylene treatment in etiolated seedlings of the wild type and decreased upon light exposure, even in the presence of the ethylene precursor, ACC (Li et al. 2004). Both light and ethylene pathways function in this interaction, but the individual roles and the cross talk between them need to be further studied. The mechanisms of ethylene and light interaction are still a mystery. The most typical and research-focused ethylene response is the triple response, in which dark-grown seedlings under ethylene treatment exhibit shortened hypocotyls and roots, thickened hypocotyls, and an exaggerated apical hook. However in light, ethylene induces marked elongation of the hypocotyl of Arabidopsis seedlings (Smalle et al. 1997). It is still unknown how and why ethylene has opposite effects on the elongation of Arabidopsis hypocotyls in dark and light. In this study, the interaction of light and ethylene signals on the elongation of seedling hypocotyls was investigated. We found that ethylene affected hypocotyl elongation by two distinct pathways in the light and in dark. Ethylene-promoting IAA pathway to elongate the hypocotyl is mediated by light. EIN3 elevates the expression of YUCCA1 and YUCCA5, which are necessary for hypocotyl development (Cheng et al. 2007), and up-regulates IAA in promoting hypocotyl elongation in light. COP1 serves as a critical nuclear regulator to inhibit hypocotyl elongation induced by ACC. Here, we also provide evidence that EIN3 cannot induce high levels of ERF1 transcripts in dark, probably because of COP1 in the nucleus.
Materials and methods
Plant materials and growth conditions
The Arabidopsis thaliana ecotype Columbia (Col-0) and ethylene-insensitive mutants, etr1-3 and ein2-1, were provided by Prof. Ning Li (The Hong Kong University of Science and Technology). Arabidopsis seeds of cop1-4/EIN3ox, EIN3ox and COP1ox were provided by Prof. Hongwei Guo (Peking University). Arabidopsis seeds of cop1-4, hy5, hy5hyh, and hyh mutants were provided by Prof. Xingwang Deng (Peking University). DR5::GUS seeds were provided by Prof. Ben Scheres (Utrecht University). Arabidopsis seeds of tir1-1, axr1-3, and axr1-12 mutants were purchased from the Arabidopsis Biological Resource Center (Columbus, OH, USA). DR5::GFP seeds were purchased from the European Arabidopsis Stock Centre. Seeds were surface sterilized for 20 min in 20 % (v/v) commercial bleach and 0.1 % Triton X-100, rinsed four times with autoclaved distilled water, chilled for 2 days at 4 °C, and then sown on agar medium in Petri dishes. Seeds on agar plates were transferred to a growth chamber and maintained at 23 °C under continuous white light (approximately, 60–70 μmol m−2 s−1) or in darkness for 7 days. All experiments were carried out using low nutrient medium (LMN) (Smalle et al. 1997), alone or supplemented with 1-aminocyclopropane-1-carboxylic acid (Sigma).
Hypocotyl length measurement
At indicated growth and treatment time points, at least 20 seedlings were laid horizontally on an agar plate and digital images were acquired. The hypocotyl length was measured using a standard 5-mm scaled ruler with the ImageJ software. The statistical significance of differences among mean values was analyzed using the SPSS 18.0 software.
Cell length measurement
Cells from the middle part of hypocotyls were photographed under a digital microscope. Cell length was measured using the ImageJ software with a 100-μm scaled ruler. A total of ten plants were measured for each treatment, and 20 cells were measured per plant.
Localization of DR5-GFP reporter proteins and quantitative analysis
Confocal laser-scanning micrographs were obtained with a Leica TCS SP2 AOBS under a 488-nm argon laser line for excitation of green fluorescent protein (GFP) fluorescence. Emissions were detected between 505 and 580 nm. Using a 20× water immersion objective (NA = 1.25, pinhole), confocal scans were performed with the pinhole at 1 Airy unit. Each image represents either a single focal plane or a projection of individual images taken as a z-series. The fluorescence intensity of the membrane DR5-GFP signal was quantified with the ImageJ software. At least ten seedlings were analyzed per treatment.
Histochemical GUS staining and quantitative fluorometric GUS assays
Whole seedlings (n = 50–100 seedlings), roots, and shoots were homogenized in extraction buffer as described elsewhere (Oono et al. 1998). After centrifugation to remove cell debris, the GUS activity in the supernatant was measured with 1 mM 4-methyl umbelliferyl β-d-glucuronide as the substrate at 37 °C. For the histochemical GUS assay, seedlings were washed three times with buffer A (100 mM sodium phosphate, pH 7.0, 10 mM EDTA, 0.5 mM K4Fe(CN)6, 0.5 mM K3Fe(CN)6, and 0.1 % Triton X-100) and then incubated in the staining buffer (buffer A containing 1 mM 5-bromo-4-chloro-3-indolyl β-d-glucuronide (X-gluc), the substrate for histochemical staining) at 37 °C until sufficient staining was developed.
Quantitative RT-PCR analysis
Total RNA was extracted from fresh tissues of 100 plants using Trizol reagent. The complementary DNA (cDNA) was then synthesized using the Takara Primescript RT Reagent kit (Takara) reverse transcriptase at 37 °C for 15 min. Then, quantitative real-time RT-PCR analysis (SYBR Premix, Takara) was carried out to analyze gene expression. The sequences of the PCR primers used in this study are listed in Table S1.
Statistical analysis
Each experiment was repeated at least three times. Values were expressed as mean ± SE. All comparisons were done using Student’s t test for independent samples. In all cases, the confidence coefficient was set at 0.01.
Results
Opposite effects of ethylene on hypocotyl elongation
To confirm the effects of ethylene on the light-mediated hypocotyl elongation, we measured the hypocotyl length of Arabidopsis seedlings treated with different ACC concentrations under light and darkness. In the presence of 100 μM ACC, the hypocotyl length of Col-0 seedlings in light was approximately twice as long as those of the control (no ACC treatment). In contrast, in the presence of 10 μM ACC, the hypocotyl length of Col-0 seedlings in dark was approximately half of the control (Fig. 1). These results showed that, depending on the light condition, ethylene can induce opposite effects on the elongation of Arabidopsis hypocotyls. The sensitivity of ethylene in the hypocotyl elongation is different in light and dark.
Ethylene stimulates hypocotyl elongation in light, but suppresses it in dark. Col-0 seedlings were treated with different concentrations of ACC (0, 10, 20, 50, 100, or 200 μM) in continuous white light or darkness for 7 days. The hypocotyl lengths were measured as previously described. Error bars represent SE of the mean (n = 20)
It has been reported that high concentration of indole-3-acetic acid (IAA) promotes hypocotyl elongation in light (Smalle et al. 1997; Gray et al. 1998; Boerjan et al. 1995). To test whether ethylene affects the action of IAA in Arabidopsis hypocotyl elongation, we examined the response of IAA-insensitive mutants [transport inhibitor response 1-1 (tir1-1), auxin resistant 1-3 (axr1-3), and auxin resistant 1-12 (axr1-12)] and ethylene-insensitive mutants [(ethylene-response 1-3 (etr1-3) and ethylene-insensitive 2-1 (ein2-1)]. The results showed that the hypocotyl elongation of etr1-3 and ein2-1 was not affected by ACC treatment in both light and dark (Fig. 2). This result demonstrated that ethylene signaling pathway is involved in this process. Meanwhile, the hypocotyl elongation of tir1-1, axr1-3, and axr1-12 mutants in light was almost not affected by ACC treatment. However, the hypocotyl elongation of tir1-1, axr1-3, and axr1-12 in dark was inhibited by ACC (Fig. 2). Taken together, these results suggest that ethylene affects the hypocotyl elongation via IAA in light, but not in dark.
IAA is required for the effects of ethylene on hypocotyl elongation in light. Untreated seedlings and those treated with 100 μM ACC (Col-0, tir1-1, axr1-3, axr1-12, etr1-3, and etr2-1) were grown for 7 days in constant white light at 22 °C or in darkness. Hypocotyl lengths were measured as previously described. Error bars represent SE of the mean (n = 20). Within each set of experiments, bars with different letters were significantly different at the 0.01 level
Effect of ethylene on IAA in hypocotyl elongation is mediated by light
It has been reported that ethylene up-regulates IAA in roots (Ruzicka et al. 2007; Swarup et al. 2007). How ethylene mediates IAA in hypocotyl elongation is not clear. The DR5 promoter, which is composed of tandem elements taken from the primary auxin-responsive GH3 promoter, can quantify the level of IAA responses (Ulmasov et al. 1997). As shown in Fig. 3a, b, ethylene induced DR5:GFP expression in the stele and epidermis in hypocotyls of light-grown DR5:GFP seedlings, but not in dark-grown seedlings. In particular, DR5-GFP was also induced by ACC in shoot meristems in light, but not in dark (Fig. 3a–c). Although a strong DR5:GFP signal was observed on the convex side of the apical hook, the GFP signals in the stele, epidermis, and shoot meristems of dark-grown seedlings remained weak. In light, the DR5:GUS staining in ACC-treated seedlings was virtually strong in shoots, especially in petioles, cotyledon tips, and shoot apex (Fig. 3d, e). In contrast, the staining pattern of DR5:GUS in dark indicates an auxin maximum in the middle of the hypocotyls (Fig. 3d). However, in the presence of ACC in dark, DR5:GUS exhibited obvious staining in the apical hook, but showed only a very faint signal in hypocotyls (Fig. 3d, e). In whole plants and roots, ACC up-regulated DR5:GUS, no matter the seedlings were light grown or dark grown (Fig. 3e). These results showed that the effect of ethylene on IAA in hypocotyl elongation is mediated by light.
Effects of ACC on IAA accumulation in light or in darkness. a DR5-GFP seedlings treated or untreated with 100 μM ACC were grown for 7 days in constant white light or darkness at 23 °C. Bar 100 μm. b Relative fluorescence intensity of DR5-GFP in the seedlings treated as described in a. The fluorescence intensity of DR5-GFP seedlings in light was adjusted to 1. Error bars represent SE of the mean (n = 10). c DR5-GFP expression patterns in the shoot meristems of seedlings treated as shown in a. The image is a projection of individual images taken as a z-series of meristems. Bar 50 μm. d DR5::GUS seedlings treated as described in a. Bar 1 mm. e Quantitative determination of GUS activity in DR5::GUS lines treated as described in a. The GUS activity was determined fluorometrically by the fluorogenic substrate, 4-methyl umbelliferyl β-d-glucuronide. GUS activity in ACC-treated seedlings in dark was adjusted to 100 % value. f Col-0 seedlings treated as described in a, and shoots were collected from each treatment for RNA analyses by qRT-PCR
To understand how ethylene mediates IAA, some key genes of main auxin biosynthesis pathways were investigated. TAA1, YUCCA, and CYP79B2/B3 are the key genes of the IPA pathway, the YUC pathway, and the IAOx and glucosinolate pathway, respectively (Zhao 2010). Ethylene exerts opposite effects on the expression in some of the tested genes (Fig. 3f). Among them, the expression of TAA1, YUCCA1, and YUCCA5 was induced by ethylene in light. In dark, the expression of TAA1 was up-regulated but the expression of YUCCA1 and YUCCA5 was down-regulated by ethylene (Fig. 3f). The expression of CYP79B2 and CYP79B3 markedly decreased in response to ACC treatment and a very low level of expression in dark was observed. Ethylene increased the level of IAA response in hypocotyls in light, but decreased it in dark (Fig. 3b, d). These results indicated that the changes in expression levels of YUCCA1 and YUCCA5 were consistent with the IAA level in hypocotyls.
To test whether ethylene also affects the IAA transport in hypocotyl elongation, we used the IAA polar transport inhibitor 1-N-naphthylphthalamic acid (NPA). NPA reduced the ethylene-induced elongation of hypocotyls in light, but had almost no effects in dark (Fig. 4a). NPA had a significant impact on DR5-GFP signals in the stele and shoot meristems in light-grown seedlings. The result showed that NPA affected the IAA distribution in light. But in dark-grown seedlings, IAA distribution was not affected by NPA (Fig. 4b). NPA also inhibited IAA accumulation induced by ACC on the convex side of the apical hook in dark-grown seedlings. These results suggested that IAA transport might contribute to ethylene-induced hypocotyl elongation in light. We also analyzed the expression of some genes related to IAA transport. Ethylene had almost no effects on the expression of PIN1, PIN2, or PIN4. PIN1 encodes the most important IAA transport protein. PIN1 is almost not expressed in dark. The expression of PIN7, AUX1, and PGP19 was induced by ACC in light, but weakened in dark. The expression of PIN3 and PGP1 was strongly stimulated by ACC only in light-grown seedlings (Fig. 4c). These results indicated that the effect of ethylene on IAA transport is mediated by light in hypocotyls.
Effects of ACC on IAA transport in darkness or in light. Col-0 seedlings (7-days-old) were grown under constant white light or in darkness on LNM or LNM supplemented with 100 μM ACC, 100 μM ACC + 1 μM NPA, or 1 μM NPA. a Hypocotyl lengths were measured as previously described. Error bars represent SE of the mean (n = 20). Within each set of experiments, bars with different letters were significantly different at the 0.01 level. b DR5-GFP expression pattern in plants treated as described above. Bar 100 μm. c Shoots were collected from each treatment for RNA analyses by qRT-PCR
Taken together, these results demonstrated that the effect of ethylene on IAA biosynthesis, transport, and distribution in the hypocotyl was also mediated by light.
COP1 plays a key role in the opposite effects of ethylene on hypocotyl elongation
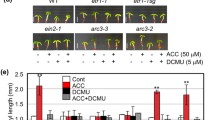
The Arabidopsis HY5 gene has been defined as a positive regulator of photomorphogenesis. The HY5 gene product is a basic leucine zipper-type transcription factor that is involved in a wide range of light-regulated gene expressions (Chattopadhyay et al. 1998; Lee et al. 2007). To test whether the opposite effects of ethylene on hypocotyl elongation depend on light signaling, we used some mutants defective in light signaling. COP1 acts as a light-inactive repressor of photomorphogenic development and negatively regulates HY5. It has been shown that hy5-215 exhibits a skotomorphogenic (etiolated) feature in light and COP1ox partly suppresses the photomorphogenic development (McNellis et al. 1994). On the contrary, the morphology of cop1 seedlings in dark is the same as that of light-grown wild-type seedlings. In our study, ACC still promoted hypocotyl elongation of hy5-215, hyh, hy5hyh, and COP1ox in light (Fig. 5), but the extent of ACC-induced elongation decreased in hy5-215, hy5hyh, and COP1ox. The ACC-induced hypocotyl elongation in Col-0, hy5-215, hy5hyh, hyh, and COP1ox were 2.0-, 1.6-, 1.6-, 2.0-, and 1.3-fold, respectively.
HY5 has no effect on ethylene-induced hypocoytl elongation. Col-0, Ws, hy5-215, hyh, hy5hyh, and COP1ox seedlings treated or not treated with 100 μM ACC were grown for 7 days under constant white light at 22 °C. Hypocotyl lengths were measured as previously described. Error bars represent SE of the mean (n = 20). Asterisks denote significant differences (P < 0.01, according to Student’s t test) between indicated ACC treatment and controls
The hypocotyl length of cop1-4 mutant seedlings under ACC treatment was 2.0 times longer than that of the control in dark. In wild-type seedlings treated with ACC, the hypocotyl length was just half of the control in dark. Hypocotyl elongation appears to be caused by longitudinal cell expansion instead of cell division (Smalle et al. 1997; Gray et al. 1998). Therefore, we measured the cell length of mutant and wild-type seedlings under various treatments to confirm that cell expansion, and not cell division, is responsible for hypocotyl elongation. The length of longitudinal cell expansion and hypocotyls was consistent (Fig. 6k). These results indicated that COP1 is a key factor in ethylene-induced opposite effects on hypocotyl elongation of Arabidopsis.
COP1 may be a key factor in ethylene-induced opposite effects on hypocotyl elongation of Arabidopsis. a–c Col-0, Col-0 treated with 100 μM ACC, and EIN3ox in light. d–f Col-0, Col-0 treated with 100 μM ACC, and EIN3ox in dark. g–i cop1-4, cop1-4 treated with 100 μM ACC, and cop1-4/EINox in dark. j Hypocotyl lengths were measured as previously described. Error bars represent SE of the mean (n = 20). Asterisks denote significant differences (P < 0.01, according to Student’s t test) between indicated ACC treatment (or EIN3ox) and controls (23 °C). k Cell length was measured as previously described. Error bars represent SE of the mean (n = 50). Asterisks denote significant differences (P < 0.01, according to Student’s t test) between indicated ACC treatment (or EIN3ox) and controls (23 °C). Images were captured under a digital microscope. l cop1-4 (7-days-old) were grown in darkness on LNM, or on LNM supplemented with 100 μM ACC, 100 μM ACC + 1 μM NPA, or 1 μM NPA. Hypocotyl lengths were measured as previously described. Error bars represent SE of the mean (n = 20). Within each set of experiments, bars with different letters were significantly different at the 0.01 level
Interaction of COP1 and EIN3 in hypocotyl elongation
COP1 regulates genes by directly interacting with transcription factors, such as HY5 and PIF (Feng et al. 2008). EIN3 is the most important transcription factor in the ethylene signaling pathway (Zhong et al. 2009). To elucidate whether COP1 interacts with EIN3, we first examined the phenotype of EIN3-overexpressing plants in Col-0 (EIN3ox) and cop1-4 (cop1-4/EIN3ox) backgrounds. The hypocotyl length of EIN3ox plants was about 1.6-fold longer than that of the wild type in light, and about 0.5-fold shorter than that of the wild type in dark. The hypocotyl length of cop1-4/EIN3ox plants was still significantly longer than those of the cop1-4 mutant in dark (Fig. 6j). These results showed that EIN3 plays a key role in the ethylene-affected hypocotyl elongation.
To know whether auxins are necessary in ethylene-triggered cop1-4 hypocotyl elongation in dark, we studied whether NPA affects cop1-4 mutant in dark. NPA significantly inhibited the hypocotyl elongation induced by ACC (Fig. 6l). The result indicated that ACC induced hypocotyl elongation in cop1-4 dark-grown seedlings via IAA, and that IAA transport plays a role in this process. It is worth noting that NPA did not completely inhibit the ACC-induced hypocotyl elongation, but there was also a slight elongation of NPA–ACC-treated Col-0 in light (Fig. 4a). Thus, there are apparently some factors independent of auxins effective in the ACC-induced hypocotyl elongation.
Next, we analyzed the expression of YUCCA1 and YUCCA5, two genes that promote IAA biosynthesis to affect hypocotyl elongation. As shown in Fig. 7, the expression of both YUCCA1 and YUCCA5 was enhanced in all ACC-treated seedlings and in EIN3ox plants that were not treated with ACC in light, but not induced in dark. Maybe due to the feedback inhibition, YUCCA1 was inhibited in EIN3ox plants. These two genes were still induced in cop1-4 plants treated with ACC or cop1-4/EIN3ox mutant seedlings untreated with ACC in dark. These results indicated that COP1 may regulate the transcriptional activity of EIN3. To confirm the role of COP1 in regulating the transcriptional activity of EIN3, we examined the expression of some direct downstream components of EIN3, including the positively regulated ERF1 (Solano et al. 1998) and the negatively regulated SALICYLIC ACID INDUCTION DEFICIENT2 (SID2) (Chen et al. 2009). The expression of ERF1 was elevated in all ACC-treated seedlings and in EIN3ox without ACC treatment in light, but was inhibited in dark (Fig. 7). Same as in Col-0 in light, ERF1 was also induced in cop1-4 by ACC and in the cop1-4/EIN3ox without ACC treatment in dark. The opposite expression pattern was observed in the expression of SID2. Its expression level in ACC-treated wild-type and EIN3ox seedlings was increased in dark, but decreased in light. These results suggest that COP1 regulates the transcriptional activity of EIN3. To further test this hypothesis, we determined the time course of ERF1 expression. The expression of ERF1 in Col-0 treated with 100 μM ACC was significantly increased and reached a maximum at 1 h of treatment (to approximately 3.6-fold of the control) and then decreased in light (Fig. 8). However, in dark, the expression level of ERF1 in Col-0 treated with 100 μM ACC decreased at 12 h of treatment (to approximately half of its expression level at 0 h). The expression pattern of ERF1 in cop1-4 in dark was similar to that of Col-0 in light, and a marked increase in the ERF1 expression was observed at 3 h of treatment (Fig. 8). These results showed that COP1 also affects the transcription of EIN3 downstream genes.
Discussion
Hypocotyl elongation is a useful model for investigating the regulation of plant growth. Hypocotyl elongation is mainly controlled by environmental cues and hormones (Gray et al. 1998). This elongation is caused by cell expansion and is correlated with IAA levels (Davies 1995). Ethylene inhibits hypocotyl elongation in dark-grown Arabidopsis seedlings. In light, however, the ethylene precursor ACC markedly induces hypocotyl elongation in Arabidopsis (Smalle et al. 1997). In the present study, we investigated the mechanism of the antagonistic effects of ethylene on hypocotyl elongation in light and dark, and provided evidence for the involvement of COP1 in this process.
The roles of auxin in hypocotyl development are different in light and dark. Auxin-overproducing mutant sur1 and 35S::iaaM transgenic seedlings have long hypocotyls when grown in light, yet exhibit normal hypocotyl length in dark (Boerjan et al. 1995; Romano et al. 1995). Conversely, auxin-underproducing iaaL plants exhibit short hypocotyls in light, yet have normal hypocotyl length in dark (Romano et al. 1995). Together, IAA affects hypocotyl elongation in light, but has less effect in dark.
Ethylene influences hypocotyl growth in the respective pathways in light and dark. Under normal light conditions, such as 60–70 μmol m−2 s−1, ACC promotes hypocotyl elongation (Saibo et al. 2003; Vandenbussche et al. 2007), but inhibits hypocotyl elongation under low light conditions, such as 40 μmol m−2 s−1 (Collett et al. 2000). In continuous normal light and darkness, our results demonstrated similar patterns (Fig. 1). These observations indicate that the role of ethylene on hypocotyl elongation is dependent on light intensity. ACC-related long hypocotyl 1 (alh1) mutant has long hypocotyls in light, but normal length yet thicker hypocotyls in dark with an exaggerated apical hook (Vandenbussche et al. 2003). This proves that the effects of ethylene on hypocotyl elongation may be through two respective pathways in light and dark. In light, ethylene increases the level of IAA responsiveness and eventually results in hypocotyl elongation. However, ethylene reduces the level of IAA responsiveness in hypocotyls in dark. On the other hand, ethylene does not affect the hypocotyl length via IAA (Fig. 2), and the ACC inhibition of hypocotyl length may be due to ethylene responses. Because auxin transport is required for hypocotyl elongation in light-grown seedlings but not in dark-grown seedlings (Jensen et al. 1998), ethylene may induce hypocotyl elongation by promoting IAA transport in light. But in darkness, ethylene reduces the auxin transport to maintain the apical hook (Vandenbussche et al. 2010; Zadnikova et al. 2010). More detailed mechanism of ethylene function in hypocotyl elongation in light and dark needs to be studied.
YUCCA flavin monooxygenases are key enzymes in auxin biosynthesis. Overexpression of each YUCCA gene leads to auxin overproduction and long hypocotyls and cotyledons in light. Although disruption of a single YUCCA gene causes no obvious developmental defects, all of the triple and quadruple mutants of the four YUCCA genes (YUCCA1, YUCCA2, YUCCA4, and YUCCA6) show dramatically decreased expression of the auxin reporter DR5-GUS in tissues where YUCCA genes are expressed (Cheng et al. 2006). OsYUCCA knockdown plants showed severe dwarfism, resulting from inhibited shoot elongation and inhibited root formation/elongation (Yamamoto et al. 2007). In the present study, we found that the high level of DR5:GFP expression and the long hypocotyl were associated with high levels of YUCCA expression (Figs. 3, 6, 7). Seedlings of the yuc1yuc4yuc10yuc11 quadruple mutants lack hypocotyls (Cheng et al. 2007). YUCCA is expressed in the shoot apex and vascular tissues (Cheng et al. 2006, 2007). Perhaps due to the fact that the YUC pathway may be downstream of TAA1 (Stepanova et al. 2011), higher TAA1 but lower YUCCAs were not associated with higher level of IAA responses in hypocotyls. TAA1 plays a more important role in response to ethylene in roots (Stepanova et al. 2008). However, CYP79B was not induced by ACC (Fig. 3f). Therefore, YUCCA may play a crucial role in ethylene-affected hypocotyl elongation.
EIN3 regulates the expression of ethylene-responsive genes. Upon ethylene treatment, the EIN3 protein is quickly stabilized and accumulates in the nucleus (An et al. 2010). In estradiol-inducible EIN3-FLAG transgenic plants, the accumulation of EIN3-FLAG fusion proteins was induced by estradiol in a dose-dependent manner. The expression level of ERF1 (a direct target of EIN3) was similarly induced by estradiol in a concentration-dependent manner in light-grown seedlings (An et al. 2010). In the present study, the accumulation of EIN3 indeed activated the ERF1 transcription in light. However, we observed that in wild-type plants treated with ACC and in seedlings overexpressing EIN3, the level of ERF1 did not increase in dark (Figs. 7, 8). Furthermore, we also observed that the expression of YUCCA1 and YUCCA5 was not increased in ACC-treated wild-type and EIN3ox plants in dark (Fig. 7). These results demonstrated that the regulation of EIN3 was altered by light.
COP1, an important component of the light signaling pathway, regulates the expression of EIN3. In dark, COP1-regulated genes are estimated to account for more than 20 % of the genome. The genes that are regulated by HY5 in light largely overlap with those regulated by COP1 in dark (Ma et al. 2002). The hy5-215 mutant and COP1ox show a skotomorphogenic feature, and normal development of its hypocotyls could be promoted by ethylene in light (Fig. 5). However, COP1ox inhibited most of the elongation induced by ACC in light, and cop1-4 was induced by ACC in dark. This shows that COP1 play an important role in this process and that other factors may take part in this process. Overexpression of EIN3 resulted in the elongation of the cop1-4 mutant hypocotyls in dark (Fig. 6). Likewise, YUCCA1 and YUCCA5 were elevated on cop1-4 treated with ACC and cop1-4/EIN3ox. These results indicate that the effects of light on ethylene responses may be due to the interaction between COP1 and EIN3. COP1 can constantly degrade a number of transcription factors that are required for development in light, such as the bZIP transcription factors HY5 and HYH, but it allows accumulation of others that promote etiolated growth, such as PHYTOCHROME INTERACTING FACTOR 1 (PIF1), PIF3, and PIF4 (Alabadi et al. 2008). Similarly, COP1 positively regulates the accumulation of EIN3 protein, although the exact regulatory mechanism is yet to be identified (Zhong et al. 2009). COP1 may inhibit the transcription of ERF1, YUCCA1, and YUCCA5 via its effects on EIN3 (Fig. 7). However, it is yet to be investigated whether the effects of COP1 on genes downstream of EIN3 are direct or indirect. In addition, the effects of ethylene on hypocotyl elongation and the quantitative reduction of the COP1 abundance in the nucleus are dependent on light intensity. COP1 not only regulates the amount of EIN3, but also significantly affects the transcription of EIN3 downstream genes.
Figure 9 shows a hypothetical model based on our results. In light, ethylene enhances the amount of EIN3 protein and induces the transcription of YUCCA1 and YUCCA5, resulting in hypocotyl elongation via IAA. In dark, COP1 inhibits some genes downstream of EIN3, such as ERF1, YUCCA1, and YUCCA5.
In conclusion, the roles of ethylene in hypocotyl elongation are affected by light. In light, ethylene promotes the accumulation of IAA in hypocotyls, thus promoting hypocotyl elongation. In dark, ethylene inhibits hypocotyl elongation due to ethylene responses. EIN3 and COP1, as important components in the ethylene-signaling and light-signaling pathways, play important roles in the cross talk between these pathways. COP1 may modulate EIN3 and affect the transcription of its downstream genes such as ERF1, YUCCA1, and YUCCA5. Thus, COP1 may be the key factor which determines the opposite effects of ethylene on hypocotyl elongation in dark and light.
Abbreviations
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- COP1:
-
CONSTITUTIVELY PHOTOMORPHOGENIC 1
- HY5:
-
LONG HYPOCOTYL 5
- LMN:
-
Low nutrient medium
- EIN3:
-
ETHYLENE-INSENSITIVE3
- ERF1:
-
ETHYLENE-RESPONSE-FACTOR 1
- IAA:
-
Indole-3-acetic acid
- NPA:
-
1-N-naphthylphthalamic acid
- PIF1:
-
PHYTOCHROME INTERACTING FACTOR 1
References
Alabadi D, Gallego-Bartolome J, Orlando L, Garcia-Carcel L, Rubio V, Martinez C, Frigerio M, Iglesias-Pedraz JM, Espinosa A, Deng XW (2008) Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J 53:324–335
An F, Zhao Q, Ji Y, Li W, Jiang Z, Yu X, Zhang C, Han Y, He W, Liu Y (2010) Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 22:2384–2401
Ang LH, Deng XW (1994) Regulatory hierarchy of photomorphogenic loci: allele-specific and light-dependent interaction between the HY5 and COP1 loci. Plant Cell 6:613–628
Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Onckelen HV, Montagu MV, Inzé D (1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7:1405–1419
Chattopadhyay S, Ang LH, Puente P, Deng XW, Wei N (1998) Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 10:673–684
Chen YF, Etheridge N, Schaller G (2005) Ethylene signal transduction. Ann Bot 95:901–915
Chen H, Xue L, Chintamanani S, Germain H, Lin H, Cui H, Cai R, Zuo J, Tang X, Li X (2009) ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 21:2527–2540
Cheng Y, Dai X, Zhao Y (2006) Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20:1790–1799
Cheng Y, Dai X, Zhao Y (2007) Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19:2430–2439
Collett CE, Harberd NP, Leyser O (2000) Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiol 124:553–562
Davies PJ (1995) Plant hormones: Physiology, biochemistry and molecular biology. Kluwer Academic Publishers, New York
Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451:475–479
Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M (2000) Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 12:393–404
Gray WM, Östin A, Sandberg G, Romano CP, Estelle M (1998) High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA 95:7197–7202
Guo H, Ecker JR (2003) Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell 115:667–677
Holm M, Ma LG, Qu LJ, Deng XW (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16:1247–1259
Jensen PJ, Hangarter RP, Estelle M (1998) Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol 116:455–462
Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19:731–749
Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85:183–194
Li H, Guo H (2007) Molecular basis of the ethylene signaling and response pathway in Arabidopsis. J Plant Growth Regul 26:106–117
Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR (2004) Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev Cell 7:193–204
Ma L, Li J, Qu L, Hager J, Chen Z, Zhao H, Deng XW (2001) Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13:2589–2607
Ma L, Gao Y, Qu L, Chen Z, Li J, Zhao H, Deng XW (2002) Genomic evidence for COP1 as a repressor of light-regulated gene expression and development in Arabidopsis. Plant Cell 14:2383–2398
McNellis TW, Arnim AG, Deng XW (1994) Overexpression of Arabidopsis COP1 results in partial suppression of light-mediated development: evidence for a light-inactivable repressor of photomorphogenesis. Plant Cell 6:1391–1400
Oono Y, Chen QG, Overvoorde PJ, Köhler C, Theologis A (1998) Age mutants of Arabidopsis exhibit altered auxin-regulated gene expression. Plant Cell 10:1649–1662
Osterlund MT, Ang LH, Deng XW (1999) The role of COP1 in repression of Arabidopsis photomorphogenic development. Trends Cell Biol 9:113–118
Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405:462–466
Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115:679–689
Romano CP, Robson PRH, Smith H, Estelle M, Klee H (1995) Transgene-mediated auxin overproduction in Arabidopsis: hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol Biol 27:1071–1083
Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19:2197–2212
Saibo NJM, Vriezen WH, Beemster GTS, Van Der Straeten D (2003) Growth and stomata development of Arabidopsis hypocotyls are controlled by gibberellins and modulated by ethylene and auxins. Plant J 33:989–1000
Schwechheimer C, Deng XW (2000) The COP/DET/FUS proteins—regulators of eukaryotic growth and development. Semin Cell Dev Biol 11:495–503
Smalle J, Haegman M, Kurepa J, Van Montagu M, Straeten DVD (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94:2756–2761
Smith H (2000) Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407:585–591
Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12:3703–3714
Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133:177–191
Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM (2011) The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 23:3961–3973
Sullivan JA, Shirasu K, Deng XW (2003) The diverse roles of ubiquitin and the 26S proteasome in the life of plants. Nat Rev Genet 4:948–958
Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19:2186–2196
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Vandenbussche F, Smalle J, Le J, Saibo NJM, De Paepe A, Chaerle L, Tietz O, Smets R, Laarhoven LJJ, Harren FJM (2003) The Arabidopsis mutant alh1 illustrates a cross talk between ethylene and auxin. Plant Physiol 131:1228–1238
Vandenbussche F, Vancompernolle B, Rieu I, Ahmad M, Phillips A, Moritz T, Hedden P, Van Der Straeten D (2007) Ethylene-induced Arabidopsis hypocotyl elongation is dependent on but not mediated by gibberellins. J Exp Bot 58:4269–4281
Vandenbussche F, Petrasek J, Zadnikova P, Hoyerova K, Pesek B, Raz V, Swarup R, Bennett M, Zazimalova E, Benkova E, Van Der Straeten D (2010) The auxin influx carriers AUX1 and LAX3 are involved in auxin–ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 137:597–606
von Arnim AG, Deng XW (1994) Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell 79:1035–1045
Yamamoto YY, Matsui M, Ang LH, Deng XW (1998) Role of a COP1 interactive protein in mediating light-regulated gene expression in Arabidopsis. Plant Cell 10:1083–1094
Yamamoto Y, Kamiya N, Morinaka Y, Matsuoka M, Sazuka T (2007) Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol 143:1362–1371
Zadnikova P, Petrasek J, Marhavy P, Raz V, Vandenbussche F, Ding Z, Schwarzerova K, Morita MT, Tasaka M, Hejatko J, Van Der Straeten D, Friml J, Benkova E (2010) Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137:607–617
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61:49–64
Zhong S, Zhao M, Shi T, Shi H, An F, Zhao Q, Guo H (2009) EIN3/EIL1 cooperate with PIF1 to prevent photo-oxidation and to promote greening of Arabidopsis seedlings. Proc Natl Acad Sci USA 106:21431–21436
Zhu Z, Guo H (2008) Genetic basis of ethylene perception and signal transduction in Arabidopsis. J Integr Plant Biol 50:808–815
Acknowledgments
We would like to thank Professor Hongwei Guo (Peking University) for providing Arabidopsis seeds of cop1-4/EIN3ox and EIN3ox mutants, and Professor Xingwang Deng (Peking University) for providing Arabidopsis seeds of cop1-4, hy5, hy5hyh, and hyh mutants used in this study. This work was supported by the National Natural Science Foundation of China (31170225), the Doctoral Program of Higher Education of China (ratification number: 20100211110009), Foundation of Science and Technology Program of Gansu Province (1107RJYA005), and the Fundamental Research Funds for the Central Universities (lzujbky-2012-104).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, X., Wang, H., Mao, L. et al. Involvement of COP1 in ethylene- and light-regulated hypocotyl elongation. Planta 236, 1791–1802 (2012). https://doi.org/10.1007/s00425-012-1730-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-012-1730-y