Abstract
To understand the complex drought response mechanism in crop plants, a systematic root proteomics approach was adopted to identify and analyze the expression patterns of differentially expressed major root proteins of Vigna radiata during short-term (3 days) and consecutive long-term water-deficit (6 days) as well as during recovery (6 days after re-watering). Photosynthetic gas exchange parameters of the plant were measured simultaneously during the stress treatment and recovery period. A total of 26 major protein spots were successfully identified by mass spectrometry, which were grouped according to their expression pattern during short-term stress as significantly up-regulated (9), down-regulated (10), highly down-regulated, beyond detection level of the software (2) and unchanged (5). The subsequent changes in the expression patterns of these proteins during long-term stress treatment and recovery period was analyzed to focus on the dynamic regulation of these functionally important proteins during progressive drought and recovery period. Cytoskeleton-related proteins were down-regulated initially (3d) but regained their expression levels during subsequent water-deficit (6d) while glycoprotein like lectins, which were primarily known to be involved in legume–rhizobia symbiosis, maintained their enhanced expression levels during both short and long-term drought treatment indicating their possible role in drought stress response of legumes. Oxidative stress-related proteins including Cu/Zn superoxide dismutase, oxidoreductase and aldehyde reductase were also up-regulated. The analyses of the dynamic regulation of these root proteins during short- and long-term water-deficit as well as recovery period may prove crucial for further understanding of drought response mechanisms in food legumes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought is one of the major abiotic stress factors, limiting productivity of crop plants worldwide. Moreover, in the current scenario of global climate change, there are possibilities of long-lasting droughts across the globe in the near future (Overpeck and Cole 2006; Hashiguchi et al. 2010). Food legumes such as Vigna radiata (L.) Wilczek (mungbean) serves as a major source of proteins in most of the Asian countries including India and also many other parts of the world in general. It is also important agriculturally as a renowned rotation crop, as it can fix atmospheric nitrogen in association with symbiotic soil bacteria (Rhizobia) and thus enhances soil fertility. However, the crop is highly susceptible to various environmental cues, especially drought, as it is usually grown in marginal areas under rain-fed conditions leading to a considerable yield loss (Lawn and Ahn 1985). As plant adaptation to drought is the result of a diverse array of inter-connected physiological, biochemical and molecular mechanisms, the quest to understand the various responses of food legumes under drought has been the high priority for stress biologists since decades and various aspects, including physiological, biochemical and growth responses of legumes under different abiotic stress conditions have been extensively studied (Hafeez et al. 1988; Zayed and Zeid 1998; Pinheiro et al. 2004; Rashid et al. 2004; Sumithra et al. 2006; Saleh et al. 2007; Jaleel et al. 2009; Manavalan et al. 2009).
During water-deficit conditions, declining water-potential of the soil is primarily perceived by the plant root system, owing to its direct proximity with the drying soil. Thus, analysis of the root responses under water-deficit conditions is essential to understand drought response mechanisms of crop plants. Several earlier studies have demonstrated the stress defense mechanisms operative inside the plant roots during abiotic stress conditions and the dynamic modulation of root architecture under drought conditions (Sharp et al. 2004; Asch et al. 2005; Malamy 2005). In fact, a plant’s susceptibility or tolerance capacity under various abiotic stress conditions is highly correlated to its root development modulation and to the corresponding stress response mechanisms (Smucker 1993).
Since legume roots are able to form symbiotic association with nitrogen-fixing soil bacteria (rhizobia), study on legume root responses under drought becomes an interesting as well as complex area of stress biology research. Earlier reports have shown interesting data on legume root responses to drought with respect to antioxidants and osmolytes synthesis (Porcel et al. 2003; Jain et al. 2006; Akcay et al. 2010). However, studies on legume roots under drought at protein level are quite rare and some studies focused mainly on root nodules proteomes (Pedersen et al. 1996; Larrainzar et al. 2007). Also, as evident in natural conditions (rain-fed systems), plants often experience cycles of water-deficit rather than continuous drought. In order to have a proper understanding of stress response mechanisms of crop plants, analyses should be focused at different levels of stress intensity as well as stress recovery rather than at a single stress treatment period. Hence, a systematic analysis of the root protein expression patterns during progressive drought stress and recovery is quite important for a comprehensive understanding of the dynamic regulatory mechanisms of the plant under water-deficit conditions.
For such studies, comparative proteomics proves to be the best approach as it has emerged as a promising tool for global analyses of protein expression levels in the recent past (Cánovas et al. 2004). Different aspects of biological processes including protein identification, post-translational modifications (PTMs), protein expression profiles under stress conditions during plant development and protein–protein interaction could be successfully analyzed using proteomics-based approaches (Hashiguchi et al. 2010). Proteomics-based dissection of differentially expressed proteins under different environmental factors including drought in various plants have been investigated (Salekdeh et al. 2002; Hajheidari et al. 2005; Yan et al. 2005; Gazanchian et al. 2007; Yoshimura et al. 2008; Yamaguchi et al. 2010). However, to our knowledge, only one report on comparative proteomic analysis in mungbean exists till date, which focused on involvement of brassinosteroid during chilling stress (Huang et al. 2006). Moreover, majority of the current understanding of drought responses of legumes at the molecular level were based on foliar proteins only and a focused analysis, involving root protein expression patterns at different levels of stress intensity and recovery period, were not yet reported. A recent review on plant proteomics emphasized the importance of undertaking root proteomics-based analyses under various stress conditions (Mehta et al. 2008).
In the present study, we aim to analyze the root responses of mature V. radiata with respect to its root protein expression dynamics during progressive drought stress as well as recovery period employing a comparative proteomics approach.
Materials and methods
Plant material, growth conditions and stress treatment
Seeds of V. radiata (L.) Wilczek cv. Vamban (Gg) 2 were procured from Tamil Nadu Agricultural University (TNAU), Coimbatore, India. Seeds were surface sterilized using 0.1% sodium hypochlorite (15 min) and subsequently washed in running tap water for 5–6 times. Germination was carried out in pots (20 L cement pots filled with mixture of red soil and sand) inside glasshouse, with an average of 15 seeds per pot. The pots were arranged in a completely randomized block design (CRBD) with eight replications. Seedlings were grown for 30 days at pot water-holding capacity (PC) of 80–90% (measured according to Ennahli and Earl 2005) till well-developed root system is established in all the plants. After 30 days, plants were subjected to two different watering treatments: well-watered (control) and water-stressed (drought). Control plants were maintained at PC of 80–90% throughout the experiment and the stressed plants were subjected to progressive water stress by withholding water for a period of 3 days (short-term) and 6 days (long-term) and then re-watered for the next 6 days for recovery. As Vigna is a nitrogen-fixing plant, any substantial dose of nitrogenous fertilizer was not applied. However, a meager quantity (8 g) of standard slow release fertilizer (10% N, 10% P, 14% K) was added to each pot in both the treatments only once during 15th day of the plant’s vegetative growth. For all experiments, samples (whole roots) were collected from plants having similar morphology and leaf gas exchange characteristics during 3 days after onset of stress treatment (DAS), 6 DAS and 6 days after re-watering (DAR). All readings for the root growth and leaf gas exchange studies were done in fifteen replications (n = 15). The photosynthetic photon flux density (PPFD) inside the glasshouse ranged from 900 to 1,200 μmol m−2 s−1, air temperature 23 ± 1(early morning) to 34 ± 4°C (early afternoon) and relative humidity 36 ± 5 to 48 ± 2%.
Leaf water status, photosynthetic gas exchange and root growth measurements
Leaf water status was determined by measuring the leaf relative water content (RWC), which was calculated as: RWC (%) = [(FW − DW)/(TW − DW)] × 100, where FW is fresh weight, TW is the turgid weight (mass after rehydration obtained by storing leaf samples for 24 h in distilled water) and DW is oven-dried weight (105°C) of leaves (Castillo 1996). Net photosynthesis (P n), stomatal conductance to CO2 (g s) transpiration rates (E) and internal CO2 concentration (C i) were measured periodically between 10.00 –and 11.00 a.m. under natural PPFD inside the glasshouse on 0 DAS (before stress initiation), 3 DAS, 6 DAS and 6 DAR using portable infrared gas analyzer (LCpro+, ADC Bioscientific Ltd., Great Amwell, Herts, UK). While measuring leaf gas exchange, the cuvette conditions were maintained at 40% air humidity, 25°C temperature, 360 μmol mol−1 CO2 concentration and flow rate of 500 μmol s−1. After keeping single intact leaf inside the leaf chamber, an incubation time of 2 min was given for adaptation of leaf to the microclimate of the leaf chamber and readings were taken thereafter. The instant water-use efficiency (WUEi) was calculated as P n/E. Fully expanded young leaves (third–fourth positions from the apex) were chosen for all photosynthetic measurements. Primary root length was measured using a cm scale and numbers of lateral roots and root nodules/plant were manually counted for control, 3 DAS, 6 DAS as well as 6 DAR groups of plants (n = 15). Nodule dry weight/plant and root dry weight were taken after completely drying the tissue samples inside a hot air oven.
Protein extraction and two-dimensional electrophoresis (2-DE)
Whole roots from both control and stressed plants were collected, washed thoroughly, immediately frozen in liquid nitrogen and stored at −80°C till further experiment. Root proteins were extracted as described by Sarvanan and Rose (2004) with minor modifications. One gram of the frozen root tissue was ground to fine powder in liquid nitrogen and suspended in 4 ml of the extraction buffer [0.5 M Tris–HCl (pH 7.5), 0.7 M sucrose, 0.1 M KCl, 50 mM EDTA, 2% β mercaptoethanol and 1 mM PMSF]. Equal volume of phenol saturated with Tris–HCl (pH 7.5) was added, mixed for 30 min at 4°C and centrifuged at 5,000g for 30 min at 4°C. The upper phenolic phase was collected and an equal volume of extraction buffer was added to it. The above step was repeated and the upper phenolic phase was re-extracted. Four volumes of 0.1 M ammonium acetate in methanol was added to the collected phenolic phase and kept overnight at −20°C for protein precipitation. The samples were then centrifuged at 10,000g at 4°C for 30 min and the precipitate was washed thrice in ice cold methanol and twice in ice cold acetone and air dried for few minutes. The final pellet was solubilized in 200 μL of the rehydration solution [8 M (w/v) urea, 2 M (w/v) thiourea, 4% (w/v) CHAPS, 30 mM DTT, 0.8% (v/v) immobilized pH gradient (IPG) buffer pH range 4–7 (GE Healthcare, Uppsala, Sweden)] and the protein concentration was determined by using RC–DC protein assay kit (Bio-Rad, Hercules, CA, USA) using BSA as standard. Aliquots of 600 μg protein were mixed with rehydration solution (8 M urea, 2 M thiourea, 4% CHAPS, 30 mM DTT, 0.8% IPG buffer pH range 4–7 and 0.004% bromophenol blue) to a final volume of 320 μL and used for 2-DE, which was done according to Rasineni et al. 2010. Active rehydration of protein (600 μg) was done on IPG strips (18 cm, 4–7 pH linear gradient; GE Healthcare) for 12 h at 50 V. Rehydration and focusing was carried out in Ettan IPGphor II (GE Healthcare) at 20°C, using the following program: 30 min at 500 V, 3 h to increase from 500 to 10,000 V and 6 h at 10,000 V (a total of 60,000 Vh). After IEF, strips were equilibrated twice for 30 min with gentle rocking at room temperature (25 ± 2°C) in equilibration buffers. The first equilibration was performed in a solution containing 6 M urea, 50 mM Tris–HCl buffer (pH 8.8), 30% (w/v) glycerol, 2% (w/v) SDS and 2% DTT and the second equilibration was performed by using 2.5% (w/v) iodoacetamide instead of DTT. The proteins were separated in the second dimension SDS–PAGE (12% vertical polyacrylamide slab gels) at 10 mA gel−1 for 1 h and then 38 mA gel−1 for 6 h, using an EttanDalt6 chamber (GE Healthcare). The gels were stained with modified colloidal coomassie staining (Wang et al. 2007a). Protein patterns in the gels were recorded as digitized images using a calibrated densitometric scanner (GE Healthcare) and analyzed (normalization, spot matching, expression analyses, and statistics) using Image Master 2-D Platinum version 6 image analysis software (GE Healthcare).
Proteomic analyses: in-gel digestion and mass spectrometry (MS)
In-gel digestion and matrix-assisted laser desorption/ionization time of flight mass spectrometric (MALDI-TOF MS) analysis was conducted with a MALDI-TOF/TOF mass spectrometer (Bruker Autoflex III smartbeam, Bruker Daltonics, Bremen, Germany) according to the method described by Shevchenko et al. (1996) with slight modifications. Colloidal coomassie-stained protein spots were manually excised from three reproducible gels. The excised gel pieces were destained with 100 μL of 50% acetonitrile (ACN) in 25 mM ammonium bicarbonate (NH4HCO3) for five times. Thereafter, the gel pieces were treated with 10 mM DTT in 25 mM NH4HCO3 and incubated at 56°C for 1 h. This is followed by treatment with 55 mM iodoacetamide in 25 mM NH4HCO3 for 45 min at room temperature (25 ± 2°C), washed with 25 mM NH4HCO3 and ACN, dried in speed vac and rehydrated in 20 μL of 25 mM NH4HCO3 solution containing 12.5 ng μL−1 trypsin (sequencing grade, Promega, Wisconsin, USA). The above mixture was incubated on ice for 10 min and kept overnight for digestion at 37°C. After digestion, a short spin for 10 min was given and the supernatant was collected in a fresh eppendorf tube. The gel pieces were re-extracted with 50 μL of 1% trifluoroacetic acid (TFA) and ACN (1:1) for 15 min with frequent vortexing. The supernatants were pooled together and dried using speed vac and were reconstituted in 5 μL of 1:1 ACN and 1% TFA. 2 μL of the above sample was mixed with 2 μL of freshly prepared α-cyano-4-hydroxycinnamic acid (CHCA) matrix in 50% ACN and 1% TFA (1:1) and 1 μL was spotted on target plate.
Protein identification: peptide mass fingerprinting and MS/MS analysis
Protein identification was performed by database searches (PMF and MS/MS) using MASCOT program (http://www.matrixscience.com) employing Biotools software (Bruker Daltonics).The similarity search for mass values was done with existing digests and sequence information from NCBInr and Swiss Prot database. The taxonomic category was set to Viridiplantae (green plants). The other search parameters were: fixed modification of carbamidomethyl (C), variable modification of oxidation (M), enzyme trypsin, peptide charge of 1+ and monoisotropic. According to the MASCOT probability analysis (P < 0.05), only significant hits were accepted for protein identification.
Statistical analysis
Results of the physiological and root morphological parameters were represented as mean ± standard deviations (n = 15). The significance of the differences between mean values of well watered and water-stressed plants was determined using Student’s t test. All the statistical analyses were performed using the statistical package, Sigma Plot 11.0. For proteomic analysis, three independent experiments with three replications of both control and stressed samples of each time point with each replication comprising of 10–15 pooled plants were considered and the spots were analyzed using Image Master 2-D Platinum image analysis software (GE Healthcare). Statistical one-way factor ANOVA (P < 0.05) was performed considering the values (n = 6) of the best-matched replicate gels from the three independent experiments. The normalized volume (% vol) of each spot was automatically calculated by the software as a ratio of the volume of a particular spot to the total volume of all the spots present on the gel.
Results
Changes in leaf gas exchange physiology and leaf RWC with progressive water-stress and recovery
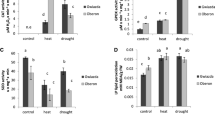
As shown in Fig. 1, with progressive drought stress treatment, the leaf RWC (a), which serves as an indicator of stress intensity, declined gradually from 78% in the control plants to 66 and 56% during short- (3 DAS) and long- (6 DAS) term water-deficit, respectively, and was back to 70% on recovery (6 DAR). The photosynthetic gas exchange parameters were severely affected with declining leaf RWC and recovered only to a certain extent on re-watering. On 3 DAS, P n (Fig. 1b) declined by 54% with a concomitant decrease of 59% in g s (Fig. 1c) when compared with the control plants (0 DAS). While on 6 DAS, there is 74% decline in P n with a subsequent 80% decline in g s. On re-watering (6 DAR), P n values were enhanced by 67% when compared with 6 DAS and it was found to be only 20% less when compared with 0 DAS. A similar trend of recovery was also observed in g s where the stressed plants regained their stomatal conductivity and the g s values were similar to the well-watered counterparts. The reduction in E (Fig. 1d) was 66 and 86% on 3 and 6 DAS, respectively. Upon recovery E values were almost enhanced by 73% when compared with the 6 DAS plants. However, the E value could recover only 50% in comparison with 0 DAS value. Unlike P n, g s and E, the reduction in leaf C i (Fig. 1e) was not apparent, however significant. The C i values were found to be slightly declining on the 3 DAS, which continued on the consecutive days of stress treatments, C i was reduced by 13% on 6 DAS when compared with 0 DAS. Even after re-watering, C i remained 21% <0 DAS. The WUEi (Fig. 1f) in the stressed plants showed 27 and 49% increase on 3 and 6 DAS when compared with the 0 DAS plants, respectively. While on recovery, WUEi showed reduction when compared with the 6 DAS plants; however, WUEi values were larger in relation to the controls.
Effect of progressive drought stress (0, 3, 6 DAS) and recovery (6 DAR) on photosynthetic gas exchange characteristics: leaf relative water content, RWC (a), net photosynthetic rate, P n (b), stomatal conductance, g s (c), transpiration rate, E (d), internal CO2, C i (e) and instantaneous water-use efficiency, WUEi (f) of Vigna radiata. Values are means ± SD (n = 15). Vertical bars represent ±SD, significant difference at *P < 0.05
Root growth parameters under progressive drought stress
The exposure of mature mungbean plants to progressive water-deficit conditions inhibited the growth of primary root and induced lateral root abscission. In the present analysis, the longer lateral roots were abscised and only the short-roots were visible which could possibly be either pre-existing or newly induced (Fig. 4a–d). Primary root exhibited an average growth of 0.35 cm per day under well-watered conditions, whereas the growth rate declined to 0.12 and 0.04 cm per day during 3 and 6 DAS, respectively (Fig. 4e). The alterations in the root dry weight under progressive drought stress were not significant within the stress treatment intensities, but were found to be significant when compared with control plants (Fig. 4f). Number of lateral roots declined considerably under drought stress conditions (Fig. 4g). As expected, we also observed a reduction in root nodule number (Fig. 4h) and nodule dry weight/plant (Fig. 4i) in response to progressive drought stress. Upon re-watering (6 DAR), all the above root growth parameters showed slight recovery trend but did not come back to the control values immediately.
2-DE and protein expression profiling
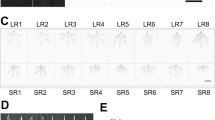
To investigate the root protein expression patterns in response to progressive water-stress treatment (short- and long-term) and recovery (6 DAR), root protein profiles of V. radiata were examined. Triplicate gels were obtained from three independent experiments and the representative gels for control, 3d, 6d and recovery were illustrated in Fig. 2a–d. Enlarged versions of the few identified spots from the triplicate gels of the control sample were illustrated in Suppl. Fig. 1. More than 500 spots were reproducibly detected in the colloidal coomassie-stained gels with Image Master 2D Platinum software and all gels showed highly similar distribution patterns in 2D image. Overall analysis of all the gels revealed that out of the matched spots between control and 3d stress, 4.2% were significantly up-regulated (>1.5-fold) and 6.2% were down-regulated while during the 6d stress treatment 3.8% up-regulated and 3.2% were down-regulated (Fig. 2e, f). A total of 34 major spots which were distinct, well-separated but not in complexes and of considerable intensity were selected for protein identification by MALDI, which showed differential expression during progressive stress treatment and recovery. 26 proteins were successfully identified (Fig. 3a) and the rest eight did not show any significant hits in the database and hence not considered. Few of the identified spots were enlarged (Fig. 3b) to visualize their expression patterns during progressive water-deficit and recovery qualitatively (expression patterns of the rest of the spots showing significant changes were presented in Suppl. Fig. 2). A Venn diagram analysis of the quantitative expression patterns of all the up and down-regulated proteins from V. radiata roots during short- (3 DAS) and long- (6 DAS) term water-deficit and recovery was illustrated in Fig. 2g and h, respectively.
Colloidal Coomassie-stained 2D gels of proteins extracted from roots of Vigna radiata (L.) Wilczek cv. Vamban (Gg)-2 grown under well watered (a), short- (b) and long-term (c) water withdrawal and recovery (d). 600 μg of protein was loaded on 18 cm IPG strip with a linear gradient of pH 4–7. 12% SDS–PAGE gels were used for the second dimension. Diagrammatic representation of Vigna root protein expression patterns during 3 DAS (e) and 6 DAS (f). Venn diagram analysis of the expression patterns of up-regulated (g) and down-regulated (h) root proteins under progressive drought and recovery. The numbers of differentially expressed spots at different time points are shown in different segments
2-DE master gel from the control root sample illustrating 26 identified proteins (a). The spot numbering in the master gel shown corresponds to the spot numbers given in Table 1. Enlarged view of the expression patterns of few spots during short- (3 DAS), long-term (6 DAS) drought stress and recovery period (b)
Identification of differentially expressed proteins during progressive drought stress and recovery
In order to avoid complexity, we first grouped the 26 identified proteins as up-regulated, down-regulated, highly down-regulated beyond detection level of the software and unchanged based on their expression patterns during short-term drought and then the subsequent changes in the expression patterns of these proteins during long-term stress treatment and recovery period was analyzed. The relative spot intensities during progressive drought stress and recovery (0d, 3d, 6d, R), matched peptide sequence, accession number, source organism, sequence coverage, experimental and theoretical molecular weight and pI, and the MS/MS score of each individual proteins were shown in Table 1. In some cases, more than one spot were identified as the same protein. For example, lectin (spot 1 and 2) and actin (spot 15 and 16). Usually, this phenomenon results from the presence of different isoforms, PTM or degradation.
Discussion
The complexity of drought stress response in legume roots has been well documented in the present study through comparative proteomic analysis which showed a dynamic regulation of the root proteins involved in different cellular functions of V. radiata. Identification and analyses of the expression patterns of the proteins, differentially expressed during short- and long-term drought stress and recovery, can contribute significantly to our present understanding of drought response mechanism of legume roots and also help to identify the key regulators of drought tolerance in crop plants.
Regulation of photosynthetic gas exchange parameters during progressive drought stress and recovery
During stress conditions, plants experience a series of metabolic changes in order to adjust to the immediate environmental conditions (Reddy et al. 2004). As the process of photosynthesis is directly related to plant productivity and energy utilization, it is one of the most affected processes during drought stress. As soon as the root hair cells sense the water-deficit conditions, they transmit signal to the shoot system and the immediate response is the closure of stomata to avoid water loss through transpiration and hence, the g s declines (Davies and Zhang 1991; Tardieu and Davies 1993). Also, due to inhibition of root growth as well as abscission of lateral roots in dry soil, the overall hydraulic conductivity of the plant should have been affected. As a result, we observed a significant concomitant decline in the P n and g s with progressive increase in drought stress which recovered to near control levels on re-watering. Both stomatal and non-stomatal factors have been shown to limit photosynthetic efficiency under water-deficit conditions. If stomata impose a significant limitation on P n, an apparent decrease in C i may be expected (Farquhar and Sharkey 1982). However, in our study the reductions in P n and g s were not accompanied by a corresponding acute decline in C i during drought progression, supporting the co-existence of strong non-stomatal inhibition factors. Such non-stomatal factors affecting photosynthetic CO2 fixation might occur due to increased mesophyll resistance to CO2 supply or any metabolic limitations to photosynthetic processes (Flexas and Medrano 2002). Transpiration rate has been known to be inversely proportional to WUEi. In the present study, we observed similar response with respect to E and WUEi of V. radiata under water-deficit conditions and recovery. Reduction in various photosynthetic gas exchange parameters was a direct consequence of the alterations in leaf RWC, which in turn was partly dependent on the root’s architectural alterations. Among the observed gas exchange parameters, P n, g s and E showed positive correlation with RWC levels while the WUEi displayed a negative correlation, clearly indicating the dynamic regulation of overall plant metabolism under different water stress regimes.
Root morphology dynamics in response to increasing water-deficit and recovery
Plant growth and survival rely upon the rooting vigor and root architecture in response to the varied soil environments. Drought stress is known to cause abscission of most lateral roots in dry soil and induction of secondary lateral roots in regions of soil containing higher soil water content (Smucker 1993). Our data on V. radiata, depict abscission of the longer lateral roots while a few short-roots were observed in the drought-stressed populations. Since the same root was not observed during progressive drought stress treatment, it could not be predicted whether these short-roots were pre-existing or newly induced. It was reported earlier that rapid growth of functional roots into unoccupied regions of the soil provides better survival chances to the plant due to their greater acquisition rates of the biogeochemical resources (Eissenstat and Caldwell 1989). In our study, the primary root growth was inhibited gradually during the progressive drought stress treatment and the short-lateral roots were observed during 3 DAS. However, with increase in stress intensity (6 DAS), the number of lateral roots further declined indicating more abscission of these roots with gradual drying up of soil. The results demonstrate plant’s innate ability to regulate the root morphology towards maximum water up-take strategies depending on the availability of water in the soil. Our data also suggest that V. radiata is able to tolerate medium level drought stress intensity (3 DAS) but gets susceptible under subsequent enhancement of the drought treatment (6 DAS).
But the question remains as what actually happens at the protein level inside the roots? We observed that root morphology-related proteins including actin (spot 14, 15), α-tubulin (spot 13) and β-tubulin (spot 12) were significantly down-regulated during 3d stress but consecutively enhanced their expression under long-term water-deficit, which declines again on re-watering. Actin and tubulins are the subunits of cellular microfilament and microtubule, respectively, whose major function is to impart mechanical strength to the cell and assist in cell wall synthesis and direction of cell expansion, apart from involving in cytokinesis, mitosis and intracellular localization of organelles and vesicles (Niini et al. 1996). Yoshimura et al. (2008) have also found induction of actin and tubulin proteins in roots of wild watermelon during water-deficit conditions, though the induction was at earlier stages of drought stress in their case, which could be due to differences in drought tolerance capacity of the two plants as wild watermelon is a xerophyte. Nevertheless, the result signifies that regulation of cytoskeleton-related proteins is essential during drought stress adaptation in crop plants. The xyloglucan endotransglucosylase (XET) (spot7) expression levels enhanced during 3d stress but subsequently suppressed upon severe water-deficit conditions, when compared to 3 DAS. However, the expression level of XET during 6 DAS remained slightly higher than the control values and enhanced further on re-watering. Xyloglucans form the primary cell walls of most seed plants along with cellulose microfibrils and pectin. The enzyme XET is known to cut and graft these xyloglucan molecules to one another both in vivo and in vitro and thus assists in cell wall extension, which is particularly important in case of roots as they have to maintain their growth and elongation in order to maximize water uptake in drying soil (Van Sandt et al. 2007). Earlier studies demonstrated that over-expression of XET imparts tolerance in transgenic plants under various abiotic stress conditions (Wu and Cosgrove 2000; Cho et al. 2006). The expression patterns of the root growth and morphology-related proteins describes that actin, tubulin expression and XET activity were probably complementing each other at different levels of stress intensity to alter the root growth rate under different stress intensities. However, the exact mechanism of these proteins, affecting the growth rate of the root during progressive drought, needs further investigation.
Role of lectins as stress-induced proteins
Plant lectins are a heterogeneous group of proteins, classified together on the basis of their ability to bind in a reversible way to well-defined simple sugars and/or complex carbohydrates. There is evidence in support of the idea that plants respond to specific biotic or abiotic stimuli through the expression of cytoplasmic and/or nuclear plant lectins, which were involved in specific endogenous protein–carbohydrate interactions, leading to the idea that lectins might be involved in cellular regulation and signaling (Van Damme et al. 2004; Jiang et al. 2010). The role of a cereal lectin, agglutinin, as a stress-induced protein has also been demonstrated (Cammue et al. 1989; Shakirova et al. 1993, 2001). Spadoro et al. (1988) reported that heat shock can also enhance synthesis of lectin-related proteins in Dolichos cell suspension cultures. In our study, we found an interesting expression pattern of the legume lectin (spot 1, 2), which was significantly up-regulated on 3 DAS, maintained the same levels during 6 DAS and continued to increase their expression during the recovery period. The actual role of lectins under drought stress is not yet completely established. Based on our observations, we hypothesize that under drought stress, lectins were over-expressed due to their possible involvement in the endogenous cellular regulation and signaling pathways, which may assist in imparting adaptive advantages to the plant under low water regimes. The lectin precursor levels continued to be enhanced even during recovery treatment which indicates that these proteins play an important role in maintenance of normal growth, development and metabolism of the plant apart from just being a stress-induced protein.
Chalcone isomerase during progressive drought stress and recovery
Flavonoids are a group of plant polyphenolic secondary metabolites, known to be involved in various plant growth and developmental processes, including pollen tube germination, UV light protection and pathogen resistance. An enzyme, involved in the flavonoid biosynthetic pathway, chalcone isomerase (CHI) (spot 11) isomerizes chalcone to form the flavonone naringenin, was highly down-regulated under drought stress conditions and failed to recover completely even on re-watering. Flavanoids are also known to mediate communication between soil bacteria and plant roots and thus assist in the formation of root nodules (Stafford 1997). Drought stress is known to affect nitrogen fixation and root nodule activity through various complex mechanisms (Abdel-Waheb et al. 2002; Marino et al. 2007). The above results indicate that CHI plays an important role in the overall process of nitrogen fixation and any decrease in the activity of this enzyme may interrupt the initial signal communication between the host (legume) and the symbiont (Rhizobium) for establishing a successful association, which is required for proper nodule formation and in turn nitrogen fixation. In the present analysis, we observed a significant reduction in the root nodule number and mass (Fig. 4h, i, respectively) in response to progressive drought stress.
Root growth patterns of control and drought-stressed plants of V. radiata during 0 DAS (a), 3 DAS (b) 6 DAS (c) and 6 DAR (d). Abscission of lateral roots is clearly visible at 3 and 6 DAS. The arrows indicate the short-roots (either pre-existing or newly-induced) visible in the drought stressed populations during 3 and 6 DAS. Root growth parameters before onset (0 DAS), 3 days (3 DAS), 6 days (6 DAS) after onset of drought stress treatment and 6 days after re-watering (6 DAR). Primary root length (cm, e), root dry weight (mg DW, f), number of lateral roots (g), number of root nodules/plant (h) and root nodule dry weight/plant (i). Results are mean ± SD (n = 15). Vertical bars represent ±SD, significant difference at *P < 0.05
Protection against oxidative stress
Drought stress is known to imbalance the cellular redox homeostasis leading to formation of reactive oxygen species (ROS) and reactive aldehydes such as 4-hydroxy nonenal (HNE) and methylglyoxal) causing oxidative damage of cellular structures (membrane lipids and proteins). Plants develop ROS scavenging mechanisms to cope with the oxidative stress. SOD is an essential antioxidative enzyme, which dismutates two superoxide radicals to produce hydrogen peroxide and oxygen. Over-expression of different types of SOD’s was known to confer protection against oxidative stress (Bowler et al. 1992; McKersie et al. 1996). In this study, we observed up-regulation of Cu/Zn SOD (spot 8) during 3 DAS, which slightly declined during further continuation of the stress treatment (6 DAS) and maintained the same expression levels even after re-watering (6 DAR). Another enzyme aldehyde reductase (spot 3) showed up-regulation during the initial stress period (3d) but its expression levels were suppressed during later stages of water withdrawal. Ectopic over-expression of aldehyde reductase is known to detoxify the highly toxic lipid peroxide degradation products, such as HNE in transgenic tobacco plants under low temperature and cadmium stress (Hegedüs et al. 2004). Hideg et al. (2003) have demonstrated the role of aldehyde reductase during drought and UV-B stress in transgenic tobacco. The results indicate that endogenous aldehyde reductase could provide protection against oxidative stress only up-to a certain level of stress intensity but if the protein is over-expressed, it could possibly enhance the drought tolerance level to a significant extent. From the expression patterns of the above enzymes, we can assume that V. radiata’s defense strategy against oxidative stress through over-expression of antioxidative enzymes is limited till moderate drought stress levels (3 DAS). Expression levels were not further enhanced upon severe drought stress (6 DAS) which could be due to the inhibitory effects of the higher oxidant levels.
Role of oxidoreductases in maintaining plant metabolism under progressive drought stress
Oxidoreductases represent a superfamily of enzymes, which primarily catalyze any reaction involving redox changes of the substrates including signal transduction, metabolic pathways as well as stress defense mechanisms (Jacquot et al. 2009). Recently, a specific pepper oxidoreductase CaOXR1 was shown to be involved in salt and osmotic stress tolerance (Lee et al. 2010). Here, we observed that the expression pattern of a particular oxidoreductase which contains two domains namely NADB Rossmann (NADP+ binding dehydrogenase) superfamily and Gfo_IDH_MocA_C (glucose fructose oxidorectase, myo-inositol dehydrogenases superfamily (spot 9) is up-regulated under progressive drought stress. The presence of the above two domains in the identified protein indicates its possible role in the plant metabolic pathways. The expression pattern of this enzyme under progressive drought stress signifies the importance of maintenance of plant’s primary metabolism during stress conditions.
Regulation of protein synthesis, folding and energy metabolism under drought stress
Protein synthesis is one of the major metabolic pathways to be affected by drought stress. We observed that a mitochondrial translational initiation factor (spot 17) is initially down-regulated (3 DAS) but later its expression levels were enhanced on subsequent water withdrawal (6 DAS). However, upon re-watering the expression levels declined again and were almost equivalent to the 3 DAS expression value. Interestingly, similar expression pattern was observed for heat shock protein-90 (spot 16) except that upon re-watering the expression levels became almost equal to the control. HSP-90 is known to be associated with protein folding by acting as a chaperone under different abiotic stress conditions (Wang et al. 2004). The present study reveals that drought susceptible food legumes induce these proteins only at a later stage of drought stress period.
The V-ATPase at the tonoplast of higher plants is a complex multi-subunit enzyme that establishes and maintains an electrochemical proton gradient across the membrane, which is the driving force for various transport processes of electrically charged as well as neutral solutes. Apart from being an essential housekeeping enzyme it also performs special turgor-dependent mechanisms such as extension growth and movements like that of stomatal guard cells and pulvini. It is also known to have dynamic plasticity with respect to its structure under different abiotic stress conditions (Lüttge et al. 2001). In the present study only one subunit, i.e. subunit E, of V-ATPase (spot 4) was highly up-regulated exclusively during short-term water-deficit conditions (3 DAS). Enolase is an essential glycolytic enzyme that catalyzes the dehydration of 2-phosphoglycerate (2-PGA) to phosphoenolpyruvate. This enzyme has been known to have various isoforms and is expressed more in roots than in leaves. It was reported to be up-regulated under hypoxic conditions when the plants shift their carbohydrate metabolism from oxidative pathway to fermentative pathway (Van Der Straeten et al. 1991) as well as under PEG treatment and salt stress in rice roots (Yan et al. 2005; Wang et al. 2007b). However, we recorded down-regulation of enolase (spot 10) during 3 DAS, which enhanced its expression level on 6 DAS when compared with 3 DAS. Enolase quantity remained lower than the control, which might be due to the susceptible nature of V. radiata towards drought or the protein identified here could be a different isoform of enolase enzyme. The nature of the initial down-regulation of this enzyme needs further investigation.
Regulation of sulfur metabolism under progressive drought stress
Methionine is a sulfur-containing amino acid, which serves as the building block of proteins and also as a component of the universal activated methyl donor S-adenosyl methionine. The enzyme methionine synthase (MS) catalyzes the last step of methionine synthesis pathway (Ravanel et al. 2004). Induction of MS under various abiotic stresses in certain plants has been previously reported (Narita et al. 2004). In the present study, there was significant down-regulation of the MS (spot 18) on 3 DAS but the expression levels enhanced upon 6 DAS. Similarly, another spot which corresponds to cobalamine-independent MS (spot 19), which catalyzes the same step but does not require the intermediary methyl donor (vitamin B12), was also highly down-regulated during the 3 DAS but on subsequent water-withdrawal for 6 days the protein’s expression levels enhanced considerably. Enhanced levels of the enzyme might be required for maintaining the methionine biosynthetic rate during severe stress conditions as it serves as a substrate for many essential metabolic reactions during plant growth and development.
In conclusion, the proteomics-based study of the dynamic expression patterns of the root proteins of V. radiata provides an insight into the regulatory mechanisms of the plant under different levels of drought stress intensity and shows that V. radiata expresses different sets of proteins at different stages of drought stress. Our results also demonstrate that under different levels of drought stress intensity, components of both primary and secondary metabolic pathways of the plant, are dynamically regulated and these responses are highly complex and inter-related. Primarily, the root proteins involved in the root architectural changes, energy metabolism, ROS detoxification, primary as well as secondary metabolite biosynthetic pathways and cell signaling were differentially regulated during progressive drought stress. Our data provide a snapshot of the root responses of Vigna under varying water-deficit levels, which could be beneficial for further research to have a comprehensive understanding of highly complex drought stress responses, involving an array of signaling pathways.
Abbreviations
- CRBD:
-
Completely randomized block design
- DAR:
-
Days after re-watering
- DAS:
-
Days after onset of stress treatment
- IEF:
-
Isoelectric focussing
- MALDI-TOF MS:
-
Matrix-assisted laser desorption/ionization time of flight mass spectrometry
- PPFD:
-
Photosynthetic photon flux density
- SOD:
-
Superoxide dismutase
References
Abdel-Waheb AM, Shabeb MSA, Younis MAM (2002) Studies on the effect of salinity drought stress and soil type on nodule activities of Lablab purpureus (L.) sweet (Kashrangeeg). J Arid Environ 51:587–602
Akcay UC, Eercan O, Kavas M, Yildiz L, Yilmaz C, Octem HA, Yucel M (2010) Drought-induced oxidative damage and antioxidant responses in peanut (Arachis hypogaea L.) seedlings. Plant Growth Regul 61:21–28
Asch F, Dingkuhn M, Sow A, Audebert A (2005) Drought-induced changes in rooting patterns and assimilate partitioning between root and shoot in upland rice. Field Crops Res 93:223–236
Bhushan D, Pandey A, Choudhary MK, Datta A, Chakraborty S, Chakraborty N (2007) Comparative proteomics analysis of differentially expressed proteins in chickpea extracellular matrix during dehydration stress. Mol Cell Proteomics 6:1868–1884
Bowler C, Van Montagu M, Inzé D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Cammue BPA, Broekaert WF, Kellens JTC, Raikhel NV, Peumans WJ (1989) Stress-induced accumulation of wheat germ agglutinin and abscisic acid in roots of wheat seedlings. Plant Physiol 91:1432–1435
Cánovas FM, Gaudot ED, Recorbet G, Jorrin J, Mock HP, Rossingol M (2004) Plant proteome analysis. Proteomics 4:285–298
Castillo FJ (1996) Antioxidative protection in the in the inducible CAM plant Sedum album L following the imposition of severe water stress and recovery. Oecologia 107:469–477
Cho SK, Kim JE, Park JA, Eom TJ, Kim WT (2006) Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett 580:3136–3144
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plant in dry soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
Eissenstat DM, Caldwell MM (1989) Invasive root growth into disturbed soil of two tussock grasses that differ in competitive effectiveness. Funct Ecol 3:345–353
Ennahli S, Earl HJ (2005) Physiological limitations to photosynthetic carbon assimilation in cotton under water stress. Crop Sci 45:2374–2382
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Flexas J, Medrano H (2002) Drought inhibition of photosynthesis in C3 plants: stomatal and non stomatal limitations revisited. Ann Bot 89:183–189
Gazanchian A, Hajheidari M, Sima NK, Salekdeh GH (2007) Proteome response of Elymus elongatum to severe water stress and recovery. J Exp Bot 58:291–300
Hafeez FY, Aslam Z, Malik KA (1988) Effect of salinity and inoculation on growth nitrogen fixation and nutrient uptake of Vigna radiata (L.) Wilczek. Plant Soil 106:3–8
Hajheidari M, Abdollahian-Noghabi N, Askari H, Heidari M, Sadeghiyan SY, Ober ES, Salekdeh GH (2005) Proteome analysis of sugar beet leaves under drought stress. Proteomics 5:950–960
Hashiguchi A, Ahsan N, Komatsu S (2010) Proteomics application of crops in the context of climate changes. Food Res Int 43:1803–1813
Hegedüs A, Erdei S, Janda T, Tóth E, Horváth G, Dudits D (2004) Transgenic tobacco plants overproducing alfalfa aldose/aldehyde reductase show higher tolerance to low temperature and cadmium stress. Plant Sci 166:1329–1333
Hideg É, Nagy T, Oberschall A, Dudits D, Vass I (2003) Detoxification function of aldose/aldehyde reductase during drought and ultraviolet-B (280–320 nm) stresses. 26:513–522
Huang B, Chu CH, Chen SL, Juan HF, Chen YM (2006) A proteomics study of the mung bean epicotyl regulated by brassinosteroids under conditions of chilling stress. Cell Mol Biol Lett 11:264–278
Jacquot J-P, Eklund H, Rouhier N, Schürmann P (2009) Structural and evolutionary aspects of thioredoxin reductases in photosynthetic organisms. Trends Plant Sci 14:336–343
Jain M, Nandwal AS, Kundu BS, Kumar B, Sheoran IS, Kumar N, Mann A, Kukreja S (2006) Water relations, activities of antioxidants, ethylene evolution and membrane integrity of pigeonpea roots as affected by soil moisture. Biol Plant 50:303–306
Jaleel CA, Jayakumar K, Chang-Xing Z, Azooz MM (2009) Antioxidant potentials protect Vigna radiata (L.) Wilczek plants from soil cobalt stress and improve growth and pigment composition. Plant Omics J 2:120–126
Jiang SY, Ma Z, Ramachandran S (2010) Evolutionary history and stress regulation of the lectin superfamily in higher plants. BMC Evol Biol 10:79
Kottapalli KR, Rakwal R, Shibato J, Burow G, Tissue D, Burke J, Puppala N, Burow M, Payton P (2009) Physiology and proteomics of the water deficit stress response in three contrasting peanut genotypes. Plant Cell Environ 32:380–407
Larrainzar E, Weinkoop S, Weckwerth W, Ladrera R, Arrese-Igor C, González EM (2007) Medicago truncatula root nodule proteome analysis reveals differential plant and bacteroid responses to drought stress. Plant Physiol 144:1495–1507
Lawn RJ, Ahn CS (1985) Mung bean (Vigna radiata (L.) Wilczek/Vigna mungo (L.) Hepper). In: Summerfield RJ, Roberts EH (eds) Grain legume crops. William Collins Sons & Co. Ltd, London, pp 584–623
Lee SC, Choi DS, Hwang IS, Hwang BK (2010) The pepper oxidoreductase Ca OXR1 interacts with the transcription factor CaRAV1 and is required for salt and osmotic stress tolerance. Plant Mol Biol 73:409–424
Lüttge U, Fischer-Schliebs E, Ratajczak R (2001) The H+-pumping V-ATPase of higher plants: a versatile “eco-enzyme” in response to environmental stress. Cell Biol Mol Lett 6:356–361
Malamy JE (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ 28:67–77
Manavalan LP, Guttikonda SK, Tran LSP, Nguyen HT (2009) Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol 50:1260–1276
Marino D, Frendo P, Ladrera R, Zabalza A, Puppo A, Arrese-Igor C, González EM (2007) Nitrogen fixation control under drought stress: localized or systemic? Plant Physiol 143:1968–1974
Mckersie BD, Bowley SR, Harjanto E, Leprince O (1996) Water-deficit tolerance and field performance of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol 111:1177–1181
Mehta A, Magalhães BS, Souza DSL, Vasconcelos EAR, Silva LP, Grossi-deSa MF, Franco OL, da Costa PHA, Rocha TL (2008) Rooteomics: the challenge of discovering plant defense-related proteins in roots. Curr Protein Pept Sci 9:108–116
Narita Y, Taguchi H, Nakamura T, Ueda A, Shi W, Takabe T (2004) Characterization of the salt-inducible methionine synthase from barley leaves. Plant Sci 167:1009–1016
Niini SS, Tarkka MK, Raudaskoski M (1996) Tubulin and actin protein patterns in scots pine (Pinus sylvestris) roots and developing ectomycorrhiza with Suillas bovines. Physiol Plant 96:186–192
Overpeck JT, Cole JE (2006) Abrupt change in earth’s climate system. Annu Rev Environ Res 31:1–31
Pedersen AL, Feldner HC, Rosendahl L (1996) Effect of proline on nitrogenase activity in symbiosomes from root nodules of soybean (Glycine max L.) subjected to drought stress. J Exp Bot 47:1533–1539
Pinheiro C, Passarinho JA, Ricardo CP (2004) Effect of drought and rewatering on the metabolism of Lupinus albus organs. J Plant Physiol 161:1203–1210
Porcel RS, Barea JM, Ruiz-Lozano JM (2003) Antioxidant activities in mycorrhizal soybean plants under drought stress and their possible relationship to the process of nodule senescense. New Phytol 157:135–143
Rashid P, Karmoker JL, Chakrabortty S, Sarker BC (2004) The effect of salinity on ion accumulation and anatomical attributes in mungbean (Phaseolus radiatus L. cv. BARI-3) seedlings. Int J Agric Biol 6:495–498
Rasineni GK, Chinnaboina M, Reddy AR (2010) Proteomic approach to study leaf proteins in a fast-growing tree species, Gmelina arborea Linn. Roxb. Trees 24:129–138
Ravanel S, Block MA, Rippert P, Jabrin S, Curien G, Rébeillé F, Douce R (2004) Methionine metabolism in plants. J Biol Chem 279:22548–22557
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Saleh AAH, Abdel-Kader DZ, El Elish AM (2007) Role of heat shock and salicylic acid in antioxidant homeostasis in mungbean (Vigna radiata L.) plant subjected to heat stress. Am J Plant Physiol 2:344–355
Salekdeh GH, Siopongco G, Wade LJ, Ghareyazie B, Bennett J (2002) Proteomic analysis of rice leaves during drought stress and recovery. Proteomics 2:1131–1145
Sarvanan RS, Rose JKC (2004) A critical evaluation of sample extraction techniques for enhanced proteomic analysis of recalcitrant plant tissues. Proteomics 4:2522–2532
Shakirova FM, Bezrukova MV, Khairullin RM (1993) The increase in lectin level in wheat shoots under the action of salt stress. Izv Russ Acad Sci 1:142–145
Shakirova FM, Avalbaev AM, Bezrukova MV, Gimalov FR (2001) Induction of wheat germ agglutinin synthesis by abscisic and gibberellic acids in roots of wheat seedlings. Plant Growth Regul 33:111–115
Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT (2004) Root growth maintenance during water deficits: physiology to functional genomics. J Exp Bot 55:2343–2351
Shevchenko A, Wilm A, Vorm O, Mann M (1996) Mass spectrometric sequencing of protein from silver-stained polyacrylamide gels. Anal Chem 68:850–858
Smucker AJM (1993) Soil environmental modifications of root dynamics and measurement. Annu Rev Phytopathol 31:191–216
Spadoro-Tank PJ, Etzler MM (1988) Heath shock enhances the synthesis of a lectin-related protein in Dolichos biflorus cell suspension cultures. Plant Physiol 88:1131–1135
Stafford HA (1997) Role of flavonoids in symbiotic and defense functions in legume roots. Bot Rev 63:27–39
Sumithra K, Jutur PP, Carmel BD, Reddy AR (2006) Salinity-induced changes in two cultivars of Vigna radiate: responses of antioxidative and praline metabolism. Plant Growth Regul 50:11–22
Tardieu F, Davies WJ (1993) Integration of hydraulic and chemical signalling in the control of stomatal conductance and water status of droughted plants. Plant Cell Environ 16:341–349
Van Damme EJM, Barre A, Rougé P, Peumans WJ (2004) Cytoplasmic/nuclear plant lectins: a new story. Trends Plant Sci 9:484–489
Van der Straeten D, Rodrigues-Pousada RA, Goodman HM, van Montagu M (1991) Plant enolase: gene structure, expression, and evolution. Plant Cell 3:719–735
Van Sandt VST, Suslov D, Verbelen JP, Vissenberg K (2007) Xyloglucan endotransglucosylase activity loosens a plant cell wall. Ann Bot 100:1467–1473
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wang H, Zhang H, Li Z (2007a) Analysis of gene expression profile induced by water stress in upland rice (Oryza sativa L. var.IRAT109) seedlings using subtractive expressed sequence tags library. J Integr Plant Biol 49:1455–1463
Wang X, Li X, Li Y (2007b) A modified Commassie Brilliant Blue staining method at nanogram sensitivity compatible with proteomic analysis. Biotechnol Lett 29:1599–1603
Wu Y, Cosgrove DJ (2000) Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J Exp Bot 51:1543–1553
Yamaguchi M, Valliyodan B, Zhang J, Lenoble ME, Yu O, Rogers EE, Nguyen HT, Sharp RE (2010) Regulation of growth response to water stress in the soybean primary root. I. Proteomic analysis reveals region-specific regulation of phenylpropanoid metabolism and control of free iron in the elongation zone. Plant Cell Environ 33:223–243
Yan S, Tang Z, Su W, Sun W (2005) Proteomic analysis of salt stress-responsive proteins in rice root. Proteomics 5:235–244
Yoshimura K, Masuda A, Kuwano M, Yokota A, Akashi K (2008) Programmed proteome response for drought avoidance/tolerance in the root of a C3 xerophyte (wild watermelon) under water deficits. Plant Cell Physiol 49:226–241
Zayed MA, Zeid IM (1998) Effect of water and salt stress on growth, chlorophyll, mineral ions and organic solutes contents and enzyme activities in mungbean seedlings. Biol Plant 40:351–356
Acknowledgments
We are thankful to Mr. Anirban Guha and Mr. Girish Rasineni for their critical reading of our manuscript. We thank Prof. N. Nadarajan, Tamil Nadu Agricultural University (TNAU), Coimbatore, India for providing Vigna radiata seeds. Our thanks are also to the Proteomics Facility (CREBB) of School of Life Sciences, University of Hyderabad and DST-FIST facility of our department. Debashree Sengupta acknowledges the fellowship from Council of Scientific and Industrial Research (CSIR), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1 Enlarged version of the few identified spots from the three triplicate gels of the control sample
Suppl. Fig. 2 Expression patterns of the identified spots showing significant changes during 3 DAS, 6 DAS and 6 DAR
Rights and permissions
About this article
Cite this article
Sengupta, D., Kannan, M. & Reddy, A.R. A root proteomics-based insight reveals dynamic regulation of root proteins under progressive drought stress and recovery in Vigna radiata (L.) Wilczek. Planta 233, 1111–1127 (2011). https://doi.org/10.1007/s00425-011-1365-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1365-4