Abstract
Two barley (Hordeum vulgare L.) β-amylase genes (Bmy1 and Bmy2) were studied during the late maturation phase of grain development in four genotypes. The Bmy1 and Bmy2 DNA and amino acid sequences are extremely similar. The largest sequence differences are in the introns, seventh exon, and 3′ UTR. Accumulation of Bmy2 mRNA was examined in developing grain at 17, 19, and 21 days after anthesis (DAA). One genotype, PI 296897, had significantly higher Bmy2 RNA transcript accumulation than the other three genotypes at all developmental stages. All four genotypes had Bmy2 mRNA levels decrease from 17 to 19 DAA, and remain the same from 19 to 21 DAA. Levels of Bmy1 mRNA were twenty thousand to over one hundred thousand times more than Bmy2 mRNA levels in genotypes Legacy, Harrington, and Ashqelon at all developmental stages and PI 296897 at 19 and 21 DAA. PI 296897 had five thousand times more Bmy1 mRNA than Bmy2 mRNA at 17 DAA. However, Bmy2 protein was not found at 17 DAA in any genotype. The presence of Bmy2 was immunologically detected at 19 DAA and was present in greater amounts at 21 DAA. Also, Bmy2 protein was found to be stored in mature grain and localized in the soluble fraction. However, Bmy1 protein was far more prevalent than Bmy2 at all developmental stages in all genotypes. Thus, the vast majority of β-amylase activity in developing and mature grain can be attributed to endosperm-specific β-amylase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
β-Amylase (3.2.1.2) is a starch degrading enzyme that hydrolytically cleaves α-1,4-d-glucosidic bonds to liberate β-maltose from the non-reducing ends of a variety of polyglucans. In barley grain and malt (i.e., germinated and kilned), β-amylase has the highest activity of all of the amylolytic enzymes (Doehlert and Duke 1983; Duke and Henson 2009a; Henson and Duke 2008). There are two known genes encoding β-amylase in barley (Hordeum vulgare L.); Bmy1 and Bmy2. The Bmy1 gene encodes the 535 amino acid ‘endosperm-specific’ barley β-amylase located in the telomeric region on the long arm of chromosome 4H (Kreis et al. 1987, 1988; Nielsen et al. 1983; Powling et al. 1981; Yoshigi et al. 1994). In the mature grain, ‘endosperm-specific’ β-amylase is present in either a free (soluble) form, which is fully active, or a bound (insoluble) form, which is only fully activated upon conversion to the free form (Hara-Nishimura et al. 1986; Sopanen and Laurière 1989; Guerin et al. 1992). ‘Endosperm-specific’ β-amylase is one of the four barley malt enzymes involved in the fermentable sugar production during mashing, and of these four enzymes β-amylase best correlates with diastatic power which is a measurement of total amylolytic activity and an important determinant of malt quality (Duke and Henson 2009b; Henson and Duke 2008; Sun and Henson 1991). The barley Bmy2 gene encodes a 505 amino acid protein and is located on the short arm of chromosome 2H (Clark et al. 2005; Jung et al. 2001; Kreis et al. 1988). This β-amylase is homologous to wheat and rye ‘ubiquitous’ β-amylase, and in barley this nomenclature has been perpetuated despite never being identified in any tissue other than the grain in early development. The deduced amino acid sequence of barley Bmy2 shares over 90% identities with ‘ubiquitous’ β-amylases from other Triticeae species (Jung et al. 2001). The deduced amino acid sequences of barley Bmy1 and Bmy2 share 76 and 80% identities (Jung et al. 2001 and Clark et al. 2005, respectively).
Spatial and temporal expression of Triticeae ‘ubiquitous’ β-amylase differs from species to species. ‘Ubiquitous’ β-amylase is present in the outer pericarp, roots, leaves, flowers, and both developing and mature grain of wheat (Triticum aestivum L.) (Daussant and Laurière 1990; Wagner et al. 1999). However, in wild-type barley, β-amylase mRNA corresponding to Bmy2 was only found in early grain development at 5 DAA and was not identified in germinating grain, roots, etiolated leaves, or fully grown leaves (Jung et al. 2001). In rye (Secale cereal L.), Bmy2 mRNA and protein accumulation occurred early in grain development (Rorat et al. 1995). In both rye and barley, Bmy2 mRNA accumulated at much lower levels than Bmy1 (Jung et al. 2001; Rorat et al. 1995).
However, a recent study identified the Bmy2 transcript in various tissues during grain development in barley (Radchuk et al. 2009). This study reported levels of Bmy2 mRNA at around half the levels of Bmy1 in the barley endosperm during late grain development. They concluded that the observed β-amylase activity during late grain development was the result of both Bmy1 and Bmy2 gene expression. This was the first report of barley Bmy2 transcript accumulation this late in barley grain development. Radchuk et al. (2009) also showed that Bmy2 mRNA was present in large amounts in the pericarp during early grain development, whereas the Bmy1 transcript was not detected.
The research presented here was conducted in part to clarify the aforementioned conflicting research on barley Bmy2 mRNA accumulation. In this report the Bmy2 gene, from two cultivated and two wild (Hordeum vulgare ssp. spontaneum) barley genotypes, was sequenced and accumulation of Bmy2 mRNA in developing grain at 17, 19, and 21 DAA was determined using relative reverse transcription quantitative real-time PCR (RT-qPCR). Also, the ratio of Bmy1 to Bmy2 RNA transcript accumulation is compared in all genotypes at 17, 19, and 21 DAA. Antibodies specific for Bmy1 and Bmy2 were used to determine the presence or absence of the specific gene products in the developing grain of each genotype at 17, 19, and 21 DAA and to determine whether the proteins were located in the bound or free fraction and to further enable us to determine the significance of any differences in Bmy1 to Bmy2 transcript accumulation.
Materials and methods
Plant material
Grain from Legacy, Harrington, Ashqelon, and PI 296897 were germinated in Petri dishes containing germination paper for 6 weeks at 1–2°C. Seedlings were transferred to pots containing a 1:1 ratio of peat moss and perlite. Plants were randomized and grown under greenhouse conditions with the photoperiod and temperatures of L12(20°C)/D12(15°C) for 4 weeks followed by L16(25°C)/D8(20°C) until grain was harvested at 17, 19, and 21 DAA, and maturity. Anthesis was considered to have occurred when the awn emerged from the boot. Developing grain was harvested between 8 and 11 o’clock in the morning. The top four grains from each spike were removed, dehulled, frozen in liquid nitrogen and stored at −80°C.
Relative RT-qPCR
Total RNA was isolated from the developing grain using the Concert™ Plant RNA Reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s small scale RNA isolation protocol. RNA was purified further using the RNeasy® Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s RNA clean up protocol with on-column DNase I digestion and stored at −80°C. Total RNA integrity was determined using gel electrophoresis. Duplicate cDNA was generated using 1 μg of total RNA with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA).
Quantitative PCR reactions were conducted using either genomic DNA (PCR efficiencies), plasmid DNA (PCR efficiencies), or a 10-fold cDNA dilution in duplicate or triplicate using SYBR® Premix Ex Taq ™ (Takara, Madison, WI, USA) according to the manufacturer’s instructions using primers listed in Table 1. The relative expression ratio (RER) was calculated using the 2−ΔΔCt formula (Livak and Schmitgen 2001; Pfaffl 2001).
Reference gene transcripts were validated across all of the time points from each genotype and across all four genotypes using the BestKeeper program (Pfaffl et al. 2004). Coefficient of correlation to the BestKeeper index was 0.966 > r > 0.748 for all samples at a p value of 0.001. The stable accumulation of transcripts from our reference genes allows for comparison of target gene levels across all genotypes and all time points.
Cloning and sequencing of Bmy2
Amplification of the Bmy2 gene was performed using DNA isolated from mature barley grain and primers found in Table 2 under the following cycling conditions; 95°C for 5 min (1 cycle), 95°C for 45 s, 57°C for 30 s, 72°C for 4 min (35 cycles), with a final extension of 72°C for 7 min (1 cycle). Amplification products were separated using agarose gel electrophoresis, excised, purified, and cloned using the TOPO TA Cloning® Kit for Sequencing (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. Sequencing reactions were conducted with primers found in Table 2 using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystem, Foster City, CA, USA) following the manufacturer’s protocol. Reaction products were sequenced by the DNA Sequencing Facility at the University of Wisconsin Biotechnology Center (Madison, WI, USA).
Sequence chromatograms were viewed and edited using Chromas (Technelysium Pty. Ltd, 1988–2000). Contigs were assembled using the GeneStudio™ Contig Editor (Suwanee, GA, USA).
GenBank numbers for Bmy2 DNA sequences are FJ936154–FJ936157.
DNA and amino acid alignments
DNA and amino acid alignments were created using GeneStudioTM Alignment Editor (Suwanee, GA, USA) using the default parameters in the slow/accurate pairwise alignment method.
Protein extraction, assay, and immunoblot
Total protein was extracted from developing and mature grain in buffer (50 mM Tris-base, 1 mM EDTA, 100 mM Cysteine, pH 8.0) according to Megazyme’s instructions (Betamyl Method, 12/04). Developing grain was initially ground in liquid nitrogen.
Soluble and insoluble proteins were extracted in six successive extractions. Five millilitres of soluble extraction buffer (50 mM Tris-base and 1 mM EDTA) were added to 500 mg of ground barley grain from Legacy, and incubated for 1 h at room temperature with vigorous vortexing every 10 min. Extracts were centrifuged for 10 min at 1,000×g and the supernatant removed. After five soluble extractions, cysteine (100 mM) was added to the extraction buffer and one additional extraction was performed.
Quantification of total protein was accomplished using the Coomassie Plus Assay Reagent following the manufacturer’s instructions (Pierce Biotechnology, Rockford, IL, USA). In extracts containing cysteine, the samples were either diluted 10-fold or dialyzed (MWCO 8,000–10,000 Da) overnight at 4°C in 4 L of soluble extraction buffer (50 mM Tris-base and 1 mM EDTA).
Antibodies for protein immunoblots were raised against short peptide sequences from the C-terminal end of both β-amylases (AnaSpec, Fremont, CA, USA). Peptide sequence used for the production of ‘endosperm-specific’ β-amylase antibodies was QVKGPTGGMGGQAEDPTS. Peptide sequence used for the production of antibodies from the β-amylase encoded by the Bmy2 gene was PVKDHTDVVDEALLAP. Protein immunoblots were conducted using a 1:20,000 dilution of Bmy1 polyclonal anti-sera or 1:10,000 dilution of Bmy2 polyclonal anti-sera and probed with an Immun-StarTM GAR-HRP Conjugate dilution of 1:50,000 (Bio-Rad, Hercules, CA, USA). The Immun-StarTM WesternCTM Chemiluminescent Kit was used to detect Bmy1 and Bmy2 (Bio-Rad, Hercules, CA, USA). The chemiluminescent signal was captured using the UVP AutoChemiTM System (Upland, CA, USA).
Multiple immunoblots were conducted for each genotype and time point. Figures 5 and 6 depict a characteristic sample of each genotype and time point.
Statistical analysis
The Fisher’s least significant difference (LSD) test was conducted in Figs. 2 and 3 using SAS 9.1 (Cary, NC, USA). The alpha level set at 0.05. The LSD analysis was considered significant at the P < 0.05 level.
Results and discussion
DNA and amino sequence analysis
The DNA and deduced amino acid sequences of Bmy1 and Bmy2 from the genotype Legacy is very similar (Fig. 1). The deduced amino acid sequences from the Legacy Bmy1 and Bmy2 genes share 83% identities which is similar to the previous reports of 76 and 80% identities (Jung et al. 2001 and Clark et al. 2005, respectively). However, it is important to note that after amino acid 488, only one amino acid aligns between Bmy1 and Bmy2 (Fig. 1b). This is largely due to the lack of the glycine rich C-terminal end in Bmy2. The introns are the most dissimilar regions between Bmy1 and Bmy2. The seventh exon and 3′ UTR are the only coding regions with sequences sufficiently dissimilar to create gene specific primers (Fig. 1a). Bmy1 qPCR primers span the seventh exon and 3′ UTR, whereas the Bmy2 qPCR primers amplify a region entirely within the 3′ UTR (Red and blue arrows in Fig. 1a).
Sequence alignment of Legacy Bmy1 (FJ161080) and Bmy2 (FJ936156). a DNA sequence alignment from 5′ UTR to 3′ UTR. Transcription start site is denoted by an arrow at 0. Exons and UTRs are denoted by solid black lines above the sequence. Introns are denoted by perforated black lines above the sequence. Exon/intron boundaries are denoted by a vertical black line. Stop codons are in boxes. Yellow bar above sequence denotes where the Bmy2 probe (EST HZ50o15) used by Radchuk et al. (2009) aligns with Bmy1. Red arrows denote location of Bmy1 RT-qPCR primers used in this study. Blue arrows denote location of Bmy2 RT-qPCR primers used in this study. b Deduced amino acid sequence alignment. Identical sequences are shaded gray. Dashes represent gaps
The alignment of full length Bmy2 DNA sequences from genotypes Legacy, Harrington, Ashqelon, and PI 296897 revealed eight polymorphisms (Supplemental Fig. 1). Of the eight polymorphisms, there were six single nucleotide polymorphisms (SNPs), one 1-bp indel, and one region in Harrington that contained eight deletions and three SNPs. Ashqelon and PI 296897 had the exact same sequence and differed from Legacy in four positions. There were only two SNPs located within the exons and both were synonymous. The first synonymous SNP was located in the second exon at nucleotide 281, and the second synonymous SNP was located in the sixth exon at nucleotide 2328. All four genotypes studied here had the exact same-deduced Bmy2 amino acid sequence as the malt cv. Morex (Clark et al. 2005). In contrast, there have been numerous amino acid substitutions reported in the ‘endosperm-specific’ β-amylase, encoded by Bmy1, which also has the highly polymorphic third intron (Clark et al. 2003; Eglinton et al. 1998; Erkkilä et al. 1998; Erkkilä 1999; Erkkilä and Ahokas 2001; Filichkin et al. 2010; Ma et al. 2000, 2001; Sjakste and Zhuk 2006; Vinje et al. 2010, 2011).
Bmy2 mRNA levels during grain development
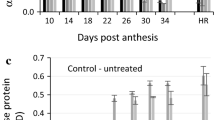
In this work, Bmy2 mRNA levels were measured, using RT-qPCR, during the late maturation stage of grain development in all four genotypes (Fig. 2). Legacy was the calibrator for all three developmental stages and, thus, has a RER of 1.0. Bmy2 mRNA levels in PI 296897 were significantly higher than the Bmy2 mRNA levels in Legacy, Harrington, and Ashqelon at 17, 19, and 21 DAA (Fig. 2). At 17 DAA, PI 296897 has 11.1-, 13.3-, and 3.6-fold more Bmy2 transcript accumulation than Legacy, Harrington, and Ashqelon, respectively. Bmy2 mRNA levels in PI 296897 decrease relative to Legacy at 19 and 21 DAA. In barley, Bmy2 mRNA is present in high levels at 5 DAA and observed as late as 12 DAA (Jung et al. 2001; Shewry et al. 1988). In rye, Bmy2 mRNA was detected in very small amounts at 10, 15, 20, and 25 days after pollination (Rorat et al. 1995). In a rye mutant, deficient in ‘endosperm-specific’ β-amylase, the Bmy2 mRNA levels were 3.8- and 7.6-fold higher than the wild-type line, at 10 and 15 days after pollination, respectively (Rorat et al. 1995). However, the β-amylase deficient mutant did not have any observable Bmy1 transcript present. In wheat, one of the two ‘ubiquitous’ β-amylase transcripts was detected in small amounts during late grain development and the other ‘ubiquitous’ β-amylase transcript was detected in larger amounts in grain development and germination (Wagner et al. 1999). Jung et al. (2001) did not find any barley Bmy2 transcript after 9 DAA using RNA blots. The data reported here were collected using RT-qPCR which is much more sensitive than RNA blotting, and could be a reason for the detection of Bmy2 mRNA when others had not. However, it is important to note that our data show relative expression ratios between four genotypes as opposed to absolute quantities of each transcript. The raw qPCR data indicate the Bmy2 transcript levels in all four genotypes are very low (data not shown).
Relative expression ratio of Bmy2 in developing grain at 17, 19, and 21 days after anthesis (DAA) from Legacy, Harrington, Ashqelon, and PI 296897. Genotypes are calibrated to Legacy. a 17 DAA, b 19 DAA, c 21 DAA. Bars within the same panel that have different letters are significantly different (P < 0.05). Bars represent standard errors
Bmy2 transcript levels were calibrated to 19 DAA for each genotype to determine the temporal accumulation of Bmy2 mRNA from 17 to 21 DAA (Fig. 3). All four genotypes had a decrease in Bmy2 transcript levels from 17 to 19 DAA with decreases of 3.3-, 4.4-, 4.4-, and 4.2-fold for Legacy, Harrington, Ashqelon, and PI 296897, respectively, although the change in Legacy was not statistically significant. There were no significant changes in Bmy2 mRNA levels from 19 to 21 DAA for any genotype. The decrease in Bmy2 mRNA levels from 17 to 19 DAA supports the previous reports of decreasing Bmy2 mRNA as grain development proceeds (Jung et al. 2001; Rorat et al. 1995).
Relative expression ratio of Bmy2 mRNA in developing grain at 17, 19, and 21 days after anthesis (DAA) from Legacy, Harrington, Ashqelon, and PI 296897. Each genotype is calibrated to 19 DAA. a Legacy, b Harrington, c Ashqelon, d PI 296897. Bars within the same panel that have different letters are significantly different (P < 0.05). Bars represent standard errors
Accumulation of Bmy1 and Bmy2 mRNA
The ratio of Bmy1 to Bmy2 mRNA levels was determined for each genotype at three stages in grain development (Fig. 4). Levels of Bmy1 mRNA were between twenty thousand and over one hundred thousand times higher than Bmy2 mRNA levels in Legacy, Harrington, and Ashqelon at all developmental stages. Legacy is a U.S. six-row cultivar and Harrington is a Canadian two-row cultivar. Both cultivars are widely used in malting for brewing and have high diastatic power and malt extract values (Duke and Henson 2009b). The wild barleys used in this study have high protein content per unit weight (Vinje et al. 2011) making them unsuitable for malting and brewing. PI 296897, which had the highest Bmy2 mRNA levels (Fig. 2), had around five thousand times more Bmy1 than Bmy2 mRNA levels at 17 DAA (Fig. 4). However, Bmy1 mRNA levels of PI 296897 at 19 DAA are about thirteen thousand times more than Bmy2 and at 21 DAA the ratio of Bmy1 to Bmy2 mRNA is over one hundred thousand. This is vastly different than a recent report that found Bmy2 expressed in the endosperm of developing barley grain at about half the rate as Bmy1 (Radchuk et al. 2009) and more in line with the studies of Jung et al. (2001) and Rorat et al. (1995). When studying barley Bmy1 and Bmy2, it is imperative that gene-specific probes or primers be used. Jung et al. (2001) created a Bmy2 probe using 1.3 kb that aligned to Bmy1 with a high degree of identity, and identified β-amylase RNA in early and late grain development. However during later grain development, the hybridization of the 1.3 kb Bmy2 probe was markedly weaker than when a Bmy1-specific probe was used indicating that the Bmy2 probe was hybridizing weakly to the abundant Bmy1 mRNA. The specificity of the qPCR primers used in this research was accomplished by creating the forward Bmy1 primer to align within a region in the seventh exon that does not have any significant similarities with the Bmy2 gene and the Bmy2 primer set was created in the 3′ UTR also in a region that did not share any significant similarities with Bmy1 (Fig. 1a and Table 1). Also, qPCR reactions using the Bmy1 primer set with a plasmid containing the Bmy2 gene and vice versa were conducted that determined specificity (data not shown). Radchuk et al. (2009) used the reference EST HZ50o15 to represent Bmy2 in their macroarray study, which is 424 bp and aligns to the first 700 bp of the Legacy Bmy1 coding region with 83% identities (Yellow bar in Fig. 1a). It is possible that the high levels of Bmy2 transcript reported by Radchuk et al. (2009) in the endosperm during late grain development were caused by partial hybridization of highly abundant Bmy1 mRNA to their Bmy2 sequence used on the macroarray.
Accumulation of Bmy1 and Bmy2 protein
The presence of Bmy2 during grain development was detected using Bmy2-specific antibodies (Fig. 5). This, as far as we know, is the first documentation of barley Bmy2 protein in any tissue. Bmy2 protein was not observed at 17 DAA despite the Bmy2 mRNA levels being at their highest at 17 DAA (Fig. 3). At 19 DAA, Bmy2 protein was observed at very low levels. Similarly, the gene product of the barley sbeIIb gene was not detected until 5 days after the maximal mRNA accumulation was observed (Mutisya et al. 2003). In rye, Rorat et al. (1995) observed an increase in Bmy2 protein levels 20 days after pollination despite a decrease in Bmy2 transcript levels from 10 to 30 days after pollination. Barley Bmy2 mRNA levels decrease from 17 to 19 DAA and remain the same at 21 DAA, whereas the Bmy2 protein levels increase from 19 to 21 DAA (Figs. 3, 5). The amount of Bmy2 increases from 21 DAA to maturity indicates that, perhaps, Bmy2 expression is bi-modal and expression is re-initiated in the developing grain after 21 DAA. Low levels of Bmy2 protein are not unexpected due to the extremely low levels of Bmy2 mRNA (Fig. 5). Low levels of Bmy2 protein are observed in Legacy, Harrington, and Ashqelon at 21 DAA. Radchuk et al. (2009) identified Bmy2 mRNA in the pericarp and endosperm, but did not identify Bmy1 mRNA in the pericarp. Jung et al. (2001) also found Bmy2 mRNA in developing barley grain (5 DAA) that contained tissue predominantly of maternal-origin, including pericarp. It appears that the Bmy2 expression that occurs early in the grain development is caused by maternal tissue-specific gene expression, whereas the Bmy2 that was observed to accumulate in the mature grain could be derived from maternal and/or filial tissue-specific gene expression.
Mature grain was found to contain more Bmy2 protein than all other developmental stages studied (Fig. 5). β-Amylase is stored in mature barley grain as either bound (insoluble) or free (soluble) β-amylase, where free β-amylase is converted to bound β-amylase during the desiccation phase of grain development (Hara-Nishimura et al. 1986). During germination the bound form is released by the reduction of disulphide bonds or via proteolytic cleavage resulting in a completely active enzyme (Guerin et al. 1992; Sopanen and Laurière 1989). In this work, Bmy2 was found to be located only in the soluble fraction of the mature barley grain (Fig. 6). It was previously assumed that Bmy1 was the only β-amylase that was stored, but these data show that Bmy2 as well as Bmy1 is stored in the mature grain.
Bmy1 and Bmy2 protein levels in the soluble and insoluble fractions of mature barley grain. Six successive 1 h extractions with Lanes 1 through 5 depict salt extractions. Lane 6 is an extraction with the addition of 100 mM cysteine. Six micrograms of total protein is loaded in each lane. 500 s exposure time
Low levels of Bmy2 observed in the developing and mature grain likely preclude the Bmy2 protein from having a significant contribution to the overall β-amylase activity in the developing and mature grain. By far most of the β-amylase present in both the developing and mature grain is Bmy1 (Fig. 5). The prediction by Radchuk et al. (2009) is that Bmy2 may be contributing to as much as half of the β-amylase activity in developing and mature grain could only be true, if Bmy2 had an extraordinarily higher Kcat than Bmy1. Our data indicate that Bmy1 is by far the major contributor to β-amylase activity based upon the considerably larger quantities of protein observed. However, the presence of the Bmy2 protein, albeit small, during development and maturity is interesting and requires further examination to determine if the Bmy2 gene has a role during development and/or germination of barley grain.
Abbreviations
- DAA:
-
Days after anthesis
- RT-qPCR:
-
Reverse transcription-quantitative polymerase chain reaction
- SNP:
-
Single nucleotide polymorphism
- RER:
-
Relative expression ratio
References
Burton RA, Shirley NJ, King BJ, Harvey AJ, Fincher GB (2004) The CesA gene family of barley. Quantitative analysis of transcripts reveals two groups of co-expressed genes. Plant Physiol 134:224–236
Clark SE, Hayes PM, Henson CA (2003) Effects of single nucleotide polymorphisms in β-amylase1 alleles from barley on functional properties of the enzymes. Plant Physiol Biochem 41:798–804
Clark SE, Hayes PM, Henson CA (2005) Characterization of barley tissue-ubiquitous β-amylase2 and effects of the single nucleotide polymorphisms on the enzyme’s thermostability. Crop Sci 45:1868–1877
Daussant J, Laurière C (1990) Detection and partial characterization of 2 antigenically distinct β-amylases in developing kernels of wheat. Planta 181:505–511
Doehlert DC, Duke SH (1983) Specific determination of α-amylase activity in crude plant extracts containing β-amylase. Plant Physiol 71:229–234
Duke SH, Henson CA (2009a) A comparison of barley malt amylolytic enzyme activities as indicators of malt sugar concentrations. J Am Soc Brew Chem 67:99–111
Duke SH, Henson CA (2009b) A comparison of barley malt osmolyte concentrations and standard malt quality measurements as indicators of barley malt amylolytic enzyme activities. J Am Soc Brew Chem 67:206–216
Eglinton JK, Langridge P, Evans DE (1998) Thermostability variation in alleles of barley β-amylase. J Cereal Sci 28:301–309
Erkkilä MJ (1999) Intron III-specific markers for screening of β-amylase alleles in barley cultivars. Plant Mol Biol Rep 17:139–147
Erkkilä MJ, Ahokas H (2001) Special barley β-amylase allele in a Finnish landrace line HA52 with high grain enzyme activity. Hereditas 134:91–95
Erkkilä MJ, Leah R, Ahokas H, Cameronmills V (1998) Allele-dependent barley grain β-amylase activity. Plant Physiol 117:679–685
Filichkin TP, Vinje MA, Budde AD, Corey AE, Duke SH, Gallagher L, Helgesson J, Henson CA, Obert DE, Ohm JB, Petrie SE, Ross AS, Hayes PM (2010) Phenotypic variation for diastatic power, β-amylase activity, and β-amylase thermostability vs. allelic variation at the Bmy1 locus in a sample of North American barley germplasm. Crop Sci 50:826–834
Guerin JR, Lance RCM, Wallace W (1992) Release and activation of barley β-amylase by malt endopeptidases. J Cereal Sci 15:5–14
Hara-Nishimura I, Nishimura M, Daussant J (1986) Conversion of free β-amylase to bound β-amylase on starch granules in the barley endosperm during desiccation phase of seed development. Protoplasma 134:149–153
Henson CA, Duke SH (2008) A comparison of standard and nonstandard measures of malt quality. J Am Soc Brew Chem 66:11–19
Jung W, Skadsen RW, Peterson DM (2001) Characterization of a novel barley β-amylase gene expressed only during early grain development. Seed Sci Res 11:325–333
Kreis M, Williamson M, Buxton B, Pywell J, Hejgaard J, Svendsen I (1987) Primary structure and differential expression of β-amylase in normal and mutant barleys. Eur J Biochem 169:517–525
Kreis M, Williamson MS, Shewry PR, Sharp P, Gale M (1988) Identification of a second locus encoding β-amylase on chromosome 2 of barley. Genet Res 51:13–16
Livak KJ, Schmitgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Ma Y, Stewart DC, Eglinton JK, Logue SJ, Langridge P, Evans DE (2000) Comparative enzyme kinetics of two allelic forms of barley (Hordeum vulgare L.) β-amylase. J Cereal Sci 31:335–344
Ma YF, Evans DE, Logue SJ, Langridge P (2001) Mutations of barley β-amylase that improve substrate-binding affinity and thermostability. Mol Genet Genomics 266:345–352
Mutisya J, Sathish P, Sun CX, Andersson L, Ahlandsberg S, Baguma Y, Palmqqvist S, Odhiambo B, Aman P, Jansson C (2003) Starch branching enzymes in sorghum (Sorghum bicolor) and barley (Hordeum vulgare): comparative analyses of enzyme structure and gene expression. J Plant Physiol 160:921–930
Nielsen G, Johansen H, Jensen J, Hejgaard J (1983) Localization on barley chromosome 4 of genes coding for β-amylase (Bmy1) and protein Z (Paz1). Barley Genet Newsl 13:55–57
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Powling A, Islam AKMR, Shepherd KW (1981) Isozymes in wheat-barley hybrid derivative lines. Biochem Genet 19:237–254
Radchuk VV, Borisjuk L, Sreenivasulu N, Merx K, Mock HP, Rolletschek H, Wobus U, Weschke W (2009) Spatiotemporal profiling of starch biosynthesis and degradation in the developing barley grain. Plant Physiol 150:190–204
Rasmussen R (2001) Quantification on the LightCycler instrument. In: Meurer S, Wittwer C, Nakagawara K (eds) Rapid cycle real-time PCR: methods and applications. Springer, Heidelberg, pp 21–34
Rorat T, Sadowski J, Irzykowski W, Ziegler P, Daussant J (1995) Differential expression of two β-amylase genes of rye during seed development. Physiol Plant 94:19–24
Shewry PR, Parmar S, Buxton B, Gale MD, Liu CJ, Hejgaard J, Kreis M (1988) Multiple molecular forms of β-amylase in seeds and vegetative tissues of barley. Planta 176:127–134
Sjakste TG, Zhuk AF (2006) Novel haplotype description and structural background of the eventual functional significance of the barley β-amylase gene intron III rearrangements. Theor Appl Genet 113:1063–1079
Sopanen T, Laurière C (1989) Release and activity of bound β-amylase in germinating barley grain. Plant Physiol 89:244–249
Sun Z, Henson CA (1991) A quantitative assessment of the importance of barley seed α-amylase, β-amylase, debranching enzyme, and α-glucosidase in starch degradation. Arch Biochem Biophys 284:298–305
Vinje MA, Duke SH, Henson CA (2010) Utilization of different Bmy1 intron III alleles for predicting β-amylase activity and thermostability in wild and cultivated barley. Plant Mol Biol Rep 28:491–501
Vinje MA, Willis DK, Duke SH, Henson CA (2011) Differential RNA expression of Bmy1 during seed development and the association with β-amylase accumulation, activity, and total protein. Plant Physiol Biochem 49:39–45
Wagner G, Zemanova L, Hager KP, Ziegler P (1999) The major β-amylase isoforms of wheat leaves correspond to one of two ubiquitously expressed β-amylase genes. Plant Physiol Biochem 37:515–530
Yoshigi N, Okada Y, Sahara H, Koshino S (1994) PCR cloning and sequencing of the β-amylase cDNA from barley. J Biochem 115:47–51
Acknowledgments
We thank Charles Karpelenia for his excellent technical assistance and Dr. Ron Skadsen for the generous donation of antibodies. Research supported by USDA-ARS and USDA-CREES U.S. Barley Genome Project Special Grant. Mention of a proprietary product does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other suitable products.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vinje, M.A., Willis, D.K., Duke, S.H. et al. Differential expression of two β-amylase genes (Bmy1 and Bmy2) in developing and mature barley grain. Planta 233, 1001–1010 (2011). https://doi.org/10.1007/s00425-011-1348-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1348-5