Abstract
Selenium (Se) is an essential micronutrient for animals and humans. Increasing Se content in food crops offers an effective approach to reduce the widespread selenium deficiency problem in many parts of the world. In this study, we evaluated 30 diverse accessions of lettuce (Lactuca sativa L.) for their capacity to accumulate Se and their responses to different forms of Se in terms of plant growth, nutritional characteristics, and gene expression. Lettuce accessions responded differently to selenate and selenite treatment, and selenate is superior to selenite in inducing total Se accumulation. At least over twofold change in total Se levels between cultivars with high and low Se content was found. Synergistic relationship between Se and sulfur accumulation was observed in nearly all accessions at the selenate dosage applied. The change in shoot biomass varied between lettuce accessions and the forms of Se used. The growth-stimulated effect by selenate and the growth-inhibited effect by selenite were found to be correlated with the alteration of antioxidant enzyme activities. The different ability of lettuce accessions to accumulate Se following selenate treatment appeared to be associated with an altered expression of genes involved in Se/S uptake and assimilation. Our results provide important information for the effects of different forms of Se on plant growth and metabolism. They will also be of help in selecting and developing better cultivars for Se biofortification in lettuce.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential micronutrient for humans and animals, as it constitutes the key component of selenoproteins and selenoenzymes with important biological functions (Rayman 2002). The deficiency of Se in diets is considered to be the key factor in the development of serious illnesses in humans, such as Keshan disease, a congestive cardiomyopathy (Yang et al. 1984). Moreover, studies indicate that Se deficiency weakens the immune system, increases viral infections, and promotes cancer (Beck et al. 2003; Diwadkar-Navsariwala et al. 2006). Because of large areas of soil that contains low levels of Se and consequently low levels of Se supply in food, Se deficiency is a widespread problem. Combs (2001) reports that Se level in a population is highly correlated with Se content in agricultural crops. Thus, increasing Se content in food crops can have a positive impact on reducing Se deficiency in the world.
Selenium is obtained from dietary sources, including cereal grains and vegetables. Se biofortification in agricultural crops by means of Se fertilization or selection of crop genotypes with high capacity of accumulation provides a useful approach to increase the consumption of Se by animals and humans (Graham et al. 2007; White and Broadley 2009). Although a number of recent works have demonstrated the effectiveness of Se fertilization in increasing Se content in a number of food crops (Chen et al. 2002; Pedrero et al. 2006; Broadley et al. 2010), only few studies have investigated the genetic variation of crop species in accumulation of Se (Lyons et al. 2005; Zhao et al. 2009).
Given that Se is chemically similar to sulfur (S), plants and other organisms readily take up and metabolize Se via S uptake and assimilation pathway (Terry et al. 2000; Sors et al. 2005; Pilon-Smits and Quinn 2010). Se is acquired by plants from soils mainly as selenite and selenate. Plants uptake selenate through a process of active transport, which is mediated by sulfate transporters (Terry et al. 2000). The selectivity of these transporters for selenate and sulfate varies between plant species and with S nutritional status (White et al. 2004). Selenite is passively taken up into plants, and phosphate transporters are believed to partially act in the process (Hopper and Parker 1999; Li et al. 2008). The reduction in selenate and selenite to form selenoaminoacids involves a number of key enzymes in S assimilation pathway, such as ATP-sulfurylase (APS), APS reductase (APR) and serine acetyltransferase (SAT) in plants.
In contrast to the essentiality of Se in human and animal nutrition, Se is considered not essential for plants (Terry et al. 2000; Sors et al. 2005; Pilon-Smits and Quinn 2010). Nevertheless, several studies report the beneficial effect of Se at low concentrations in stimulating plant growth (Hartikainen et al. 2000; Rios et al. 2010). However, at high concentrations, Se inhibits plant growth. In general, selenate is less toxic to plants than selenite, but the effect of Se application to plants also depends on other factors, such as soil redox conditions, soil pH, and the presence of competing anions, e.g. sulfate and phosphate (Blaylock and James 1994; Dhillon and Dhillon 2003).
Lettuce is one of the most consumed leaf vegetables in many parts of the world. Lettuce provides a good dietary source for various phytochemicals, such as phenolics and vitamin A, C, and E, as well as minerals. Beyond its basic nutrition, lettuce has been the target for Se biofortification in providing daily requirement of Se (Rios et al. 2008b; Ramos et al. 2010). Many different types of cultivated lettuce are known. They contain broad diversity in leaf color, shape, growth habit, and texture (Lebeda et al. 2007). The ability of different genotype of lettuce in accumulating Se, however, is unknown.
Exploiting genotypic variation is likely to be an effective method for Se biofortification in crops. The aim of this study was to evaluate the genotypic variation of lettuce germplasm in response to selenate and selenite treatment for Se biofortification in lettuce. Thirty heirloom and modern lettuce cultivars, plant introductions (PI), and landraces representing a broad range of market types were selected and used in the study. The effect of Se forms on plant growth, total Se and S levels, antioxidant enzyme activities, total amino acid content, and the expression of genes involved in Se/S uptake and assimilation were examined in attempting to provide the information for the selection of lettuce germplasm with substantial Se accumulation without negative effect on plant growth and nutritional characteristics.
Materials and methods
Lettuce germplasm
A population of 30 diverse accessions (twenty-nine named cultivars and PI251246) of lettuce (Lactuca sativa L.) were selected from lettuce database at the USDA, Salinas, CA, and used in this study. The population includes the most widely grown iceberg and romaine-type lettuces, as well as those of green and red leaf, butterhead, and the lesser known types of Batavia, stem, Latin, lolla rossa, and oilseed lettuce (Table 1). Included are commercially important cultivars, historically significant landmark cultivars found in the pedigrees of many modern cultivars, cultivars with unique morphology, and cultivars adapted to northeast production conditions. Additional details on these accessions can be found at http://www.ars-grin.gov/, http://cuke.hort.ncsu.edu/cucurbit/wehner/vegcult/vgclintro.html, and http://compositdb.ucdavis.edu/database/lettcv2/display/. The phenotypes of 24-day-old young plants for these cultivars are shown in Fig. 1.
Phenotypes of 30 lettuce accessions at 24 days of growth. The numbers correspond to the ID numbers in Table 1
Plant growth and treatments
Seeds of each accession were sown in growth medium (Metro-Mix 360, Sun Gro Horticulture) and grown in a greenhouse with a 14-h light and 10-h dark photoperiod at 24°C. The 20-day-old young seedlings were transplanted to 2.2-L pots containing Hoagland nutrient solution (Hoagland and Arnon 1950) with 40% ionic strength and grown hydroponically in the same greenhouse. After 4 days of transplantation, these plants were either grown in the nutrient solution or exposed to the nutrient solution containing 15 μM Na2SeO4 or 15 μM Na2SeO3 (Sigma-Aldrich). This concentration of Se was chosen as it showed to be the most suitable with minimal effects on plant growth in our preliminary studies with a few cultivars. The nutrient solution was changed twice each week. Throughout the experimental period, the nutrient solution underwent constant aeration, and pH was monitored and adjusted to 6.0 ± 0.2 by addition of 0.1 mol L−1 NaOH or HCl. After 2 weeks of Se treatment, a total of 360 plants (30 × 3 × 4; accessions × treatments × repeats) were harvested individually. The fresh weights of aerial part were weighed. The young leaf samples from each plant were immediately frozen in liquid nitrogen and stored at −80°C. For analysis of total Se and S, the leaf samples were dried at 65°C in an oven for 6 days.
Analysis of total Se and S levels
Total Se and S contents in the samples were determined using an inductively coupled plasma (ICP) trace analyzer emission spectrometer (model ICAP 61E trace analyzer, Thermo Electron, San Jose, CA) essentially as described previously (Lyi et al. 2005). The instrument was calibrated with 7% HCIO4 as the low standard and 5.0 μg mL−1 of Se and 40.0 μg mL−1 of S in a multi-element standard as the high standard. The Se and S were determined using the 196.0 and 182.0 nm line, respectively.
Analysis of antioxidant enzyme activities
Antioxidant enzymes were extracted according to Grace and Logan (1996). In brief, 200 mg leaves were ground in 1.5 mL ice-cold extraction buffer containing 50 mM KH2PO4–KOH (pH 7.5), 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.3% (w/v) Triton X-100, and 4% (w/v) insoluble polyvinylpolypyrrolidone (PVPP). The extract was kept on ice for 10 min and then centrifuged at 13,000 rpm for 10 min at 4°C. The supernatant was used immediately for enzyme activity assays.
Ascorbate peroxidase (APX, EC 1.1.11.1) activity was measured by monitoring the decrease in absorbance at 290 nm (Nakano and Asada 1981). The assay mixture (1 mL) contained 50 mM Hepes–KOH (pH 7.6), 0.1 mM EDTA, 0.2 mM H2O2, 0.5 mM reduced ascorbate (AsA), and enzyme extract. The reaction was initiated by adding H2O2. The extinction coefficient of 2.8 mM−1 cm−1 was used for the calculation of enzyme activity.
Catalase (CAT, EC 1.11.1.6) activity was determined by monitoring the decrease of absorbance at 240 nm (Rao et al. 1997). The reaction mixture (1 mL) contained 100 mM potassium phosphate buffer (pH 7.0), 10 mL 10% (w/v) H2O2 and enzyme extract. The reaction was initiated by adding H2O2. The extinction coefficient of 39.4 mM−1 cm−1 was used for the calculation.
Glutathione peroxidase (GSH-Px, EC 1.11.1.9) activity was measured following the method of Flohe and Gunzler (1984) with slight modifications. The enzyme from 200 mg frozen leaf tissue was extracted in a 1.5 mL pre-chilled KNaHPO4 buffer (pH 7.0). Following centrifugation at 12,000 rpm for 5 min, 0.2 mL of the supernatant was placed into a tube and mixed with 0.4 mL of 0.1 mM GSH (reduced glutathione, Sigma) and 0.2 mL of 67 mM KNaHPO4. The reaction mixture without enzyme extract was used as control. After pre-heating, the mixtures in a water bath at 25°C for 5 min, 0.2 mL H2O2 (1.3 mM) was added to initiate the reaction. The reaction was carried out for 10 min, terminated by adding 1 mL 1% trichloric acetic acid, and left on ice for 30 min. The mixture was then centrifuged for 5 min at 12,000 rpm, and 0.48 mL of the supernatant was added into a tube containing 2.2 mL of 0.32 M Na2HPO4 and 0.32 mL of 1.0 mM 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB). Following 5 min of incubation, the absorbance at wavelength 412 nm was measured. The enzyme activity was calculated as a decrease in GSH within the reaction time in comparison with that in the control.
Amino acid analysis by ultra performance liquid chromatography (UPLC)
The procedures for extraction and analysis of amino acids were preformed essentially as described previously (Lyi et al. 2005). The frozen samples (100 mg) were crushed into powder and extracted in 0.5 mL of 20 mM HCl containing 20 μL norleucine (250 μg mL−1) as an internal standard. AccQ.Tag derivatives of extracted amino acids were generated using AccQ.Tag Ultra Derivatization Kit following the manufacturer’s instruction (Waters). For derivatization, 70 μL of AccQ.Tag Ultra borate buffer was added to 10 μL of extract sample, followed by 20 μL of AccQ.Tag reagent solution. Derivatized amino acids were analyzed using an Acquity UPLC system equipped with a binary solvent delivery system and an auto sampler, and separated on an AccQ.Tag Ultra column (100 × 2.1 mm) (Waters). Derivatized sample (5 μL) was eluted with a mixture of tenfold diluted AccQ-Tag Eluent A (Waters) and 100% acetonitrile (eluent B) at a flow rate of 0.7 mL min−1 following a gradient of 99.9%:1–75%:25% eluent A:B. Derivatized amino acids were detected at 260 nm using a photo diode array detector. Amino acids in the samples were identified by co-elution with amino acid standard H (Pierce) and commercially available individual amino acids (Sigma). Concentration of amino acids in each sample was calculated based on the peak areas and calibration curves generated with commercial standards.
RNA extraction, reverse transcription, and quantitative PCR
Total RNA from leaves of lettuce plants was extracted using Trizol reagent according to the manufacturer’s instruction (Invitrogen). RNA (5 μg) was primed with oligo(dT) and reverse-transcribed into cDNA using Superscript III reverse transcriptase (Invitrogen). The synthesized cDNA were diluted ten times in water and their quality was checked based on the amplification of lettuce Actin gene. PCR amplification with gene-specific primers (Suppl. Table S1) and the calculation of the relative transcript levels were performed as described previously (Lyi et al. 2007). The analysis of all gene expression was run in triplicate with two biological repeats.
Results
Lettuce accessions exhibit different growth responses to Se treatment
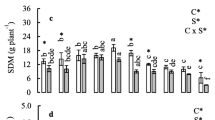
To examine the effect of Se on lettuce growth, 30 lettuce accessions were grown hydroponically in the Hoagland nutrient solution without or with 15 μM Na2SeO4 or Na2SeO3 for 2 weeks and their biomasses were measured. Depending on the form of Se application, different responses of the lettuce accessions in terms of shoot growth were observed and they can be divided into three groups (P < 0.05, Fig. 2). In the first group of seven accessions, plant growth was not affected by either selenate or selenite treatment at the dosage used. No significant difference in shoot fresh weight was observed between Se-treated and non-treated plants. In the second group of 18 accessions, shoot biomasses were similar between non-treated and selenate-treated plants, whereas plant growth was significantly inhibited when selenite was supplied. An average of 20% decrease in shoot biomass was noted, suggesting that these accessions were more sensitive to selenite. For the third group of five accessions, selenate treatment stimulated plant growth. An average of 13% increase in shoot biomass was observed in these accessions. Exposure of them to selenite caused no effect on plant growth. These results indicate that lettuce exhibits genetic variation for biomass production in response to different forms of Se treatment.
Shoot biomass of 38-day-old lettuce accessions subjected to different forms of Se treatment for 2 weeks. The lettuce accessions were divided into three groups based on their different growth in response to selenate and selenite treatment. The accession numbers correspond to the ID numbers in Table 1. Error bars indicate standard error of the mean (SEM) (n = 4)
Lettuce accessions show different capacity in accumulating Se and S
To investigate the capacity of different lettuce accessions in accumulating Se and the effect of different forms of Se on Se and S accumulation, we examined the total Se and S levels in each accession following Na2SeO4 or Na2SeO3 application. Se was not detectable in Se non-treated lettuce. When plants were exposed to selenate and selenite, the lettuce accessions accumulated different levels of Se (Fig. 3a). The total Se levels showed over twofold difference between accessions containing high and low levels of Se. For all lettuce accessions, the total Se accumulation in leaves was higher when plants were treated with selenate than with selenite. Although there was variation in the levels of Se accumulation treated with selenate and selenite among accessions, on an average, the selenate-treated plants contained 41% more Se than the selenite-treated plants.
The different forms of Se also imposed different effect on S accumulation in the lettuce accessions. At the dosage of Se supplemented, selenate dramatically enhanced total S accumulation in nearly all accessions examined except for one accession (i.e. 29) (Fig. 3b). The extent in stimulating S accumulation varied among the lettuce accessions, and about half of them doubled S levels when exposed to selenate in comparison with controls. These results imply a synergism relationship between selenate and S at the dosage. Depending on the lettuce accessions, selenite had different effects on S accumulation (Fig. 3b). In some accessions, selenite exhibited no effect to total S accumulation, while in the others selenite treatment led to decreased levels of total S accumulation. An average of 22% reduction in comparison with the controls was observed. The capacity of different lettuce accessions to accumulate Se and S appears not to be correlated with plant growth.
Effect of Se treatment on the activity of antioxidant enzymes
The effect of different forms of Se on the activities of several antioxidant enzymes, i.e. APX, CAT, and GSH-Px, in each lettuce accession was examined. In general, the activities of these enzymes varied depending on cultivars (Fig. 4). The biggest activity difference among the cultivars was observed with the CAT enzyme activity. When lettuce plants were exposed to Na2SeO4 or Na2SeO3, the activities of APX, CAT and GSH-Px showed no significant difference in response to selenate and selenite treatment in the first group of lettuce accessions (Fig. 4) like the case for plant biomass. In agreement with reduced plant growth, a general decrease in the APX, CAT and GSH-Px activities was observed in the second group of accessions when selenite was added in the nutrient solution. In the case of APX, the decrease was statistically significant for nearly all accessions in this group. A significant increase in CAT and GSH-Px activities in response to selenate treatment was noted in the third group of accessions, while their activities remained similar to the controls when they were exposed to selenite, showing a similar pattern of response as plant growth. APX activity appeared not to be affected by both selenate and selenite treatment in this group. The higher CAT and GSH-Px activities correlated well with higher shoot biomass when lettuce accessions of this group were treated with selenate. These results indicate that alteration of antioxidant enzyme activities following Se treatment may exert direct effect on plant growth.
Se treatment does not dramatically affect amino acid accumulation
To examine the effect of Se treatment on nutritional quality, the free individual and total amino acid levels in these lettuce accessions were examined. Different accession accumulated different level of total amino acids (Fig. 5). In general, the most predominant amino acids in lettuce included glutamic acid, arginine, aspartic acid, serine, and threonine (Suppl. Fig. S1). Their contents varied greatly among accessions to a level of tenfold difference (Suppl. Fig. S1). The total amino acid contents appear not to be dramatically affected by either selenate or selenite treatment except a few accessions in the second group (Fig. 5). Although it is difficult to detect free cysteine due to its instability, the other S-amino acid methionine is readily detectable (Suppl. Fig. S1). When the plants were exposed to Se, the organic form of Se compound, selenomethionine (SeMet), was found (Suppl. Fig. S1). Moreover, SeMet accumulation shared similar pattern as total Se levels when selenate was applied. SeMet has been reported as an effective chemopreventive agent (Combs and Gray 1998).
Total free amino acid content in lettuce accessions subjected to different forms of Se treatment. Total levels of amino acids are the sum of histidine, serine, arginine, glycine, aspartic acid, glutamic acid, threonine, alanine, proline, tyrosine, valine, isoleucine, leucine, and phenylalanine. Error bars indicate standard error of the mean (SEM) (n = 3)
Expression of genes involved in S/Se transport and assimilation
Selenium is known to share the S transport and assimilation pathways. Thus, examination of expression of genes encoding the key steps in the pathways should help us in better understanding of Se metabolism in lettuce. To examine whether Se accumulation was correlated with the expression of genes involved in S/Se transport and assimilation, we investigated the transcript levels of some crucial genes, i.e., Sultr, APS, APR, and SAT in six selected accessions which accumulated either high or low levels of Se following selenate treatment.
Sultr1;1, Sultr2;1 and Sultr3;1 are sulfate transporters with Sultr1;1 representing high-affinity transporter in the plasma membrane, Sultr2;1 being low affinity transporter; and Sultr3;1 with unknown function (Takahashi 2010). As shown in Fig. 6a–c, these sulfate transporters were found to express at high levels in plants with high Se content and at relatively low levels in plants with low Se content. When the lettuce plants were treated with 15 μM NaSeO4, the transcript levels of Sultr1;1 were significantly increased, whereas those of Sultr2;1 and Sultr3;1 remained at similar level as the non-treated plants. The results suggest an increase uptake by roots.
Relative expression of genes involved in S transport and assimilation in lettuce accessions. Transcript levels of genes in leaves of lettuce cultivars with high and low Se content following 15 μM Na2SeO4 treatment were measured by qRT-PCR. The expression of cultivar 13 in non-treated samples was set to 1. Data are means of three technical trials with two biological repeats. Values marked by asterisks and plus indicate significantly different between plants with high and low Se levels and treated and non-treated samples, respectively (P < 0.05; n = 3)
Following translocation into shoots, selenate is either stored in the vacuole of the cells or is further assimilated. ATP-sulfurylase (APS) is the first and rate-limiting enzyme in the sulfate assimilation pathway (Saito 2004) and catalyzes the activation of selenate. APS1 has been predicted to localize to plastids, whereas APS2 may function in the cytosol (Rotte and Leustek 2000). The transcript levels of APS were similar between the accessions examined. However, when exposed to selenate treatment, the transcript levels of APS1 were significantly enhanced in accessions with high level of Se, but remained similar in those with low level of Se (Fig. 6d). No significant difference in the transcript levels of APS2 were observed between accessions containing high and low levels of Se following Se treatment (Fig. 6e).
In plant plastids, APS reductase (APR) catalyzes the reduction of activated selenate to produce sulfite. APR1 was found to express highly in plants with high Se level and show relatively low expression in plants with low Se level (Fig. 6f). Se treatment significantly enhanced APR1 expression only in those accessions with high Se levels. APR2 transcript levels showed no significant difference between Se-treated and non-treated samples in accessions with both high and low Se levels (Fig. 6g).
Serine acetyltransferase (SAT) catalyzes the formation of O-acetyl-l-serine (OAS) from serine and acetyl-CoA, which represents a key metabolic regulatory step in selenoamino acid assimilation (Saito 2004). Examination of the transcript levels of SAT1;1, SAT2;1 and SAT3;1 in these accessions showed no significant difference of expression between Se-treated and non-treated plants (Fig. 6h–j).
Discussion
Lettuce is a widely consumed vegetable in the world, and encompasses broad genetic and morphology diversity. The capacity of lettuce to accumulate Se in the edible part is important in determining the effectiveness of a biofortification program in the crop. Se content varied greatly between the accessions evaluated. We found that there was at least over twofold change in total Se levels between those accessions with low and high levels of Se accumulation. A dozen of accessions showed higher capacity in accumulating more Se than the others.
Selenium accumulation in lettuce was affected by the chemical species of Se. Selenate application was more effective than selenite in inducing Se accumulation in edible part of lettuce plants for all accessions examined. The result is consistent with other studies of different crops in showing that selenate is superior to selenite in inducing Se accumulation in shoots of plants (Hopper and Parker 1999; Chen et al. 2002; Zhang et al. 2003; Cartes et al. 2005; Sharma et al. 2010). Rios et al. (2008b) and Ramos et al. (2010) also showed that for the same concentration of Se applied, selenate promotes higher accumulation of Se in lettuce plants. The different accessions, however, responded differently to the same concentration of selenate and selenite treatment, resulting in different levels and ratio of Se accumulation. While many plants accumulate mainly selenate from selenate supplement and predominantly organic Se from selenite treatment (Whanger 2002), the speciation of Se in lettuce accessions remains to be determined.
Since Se is an analog of S, antagonistic relationship between selenate and sulfate are generally reported in plants when high dosages of selenate or sulfate are used (Zayed et al. 1998; White et al. 2004; Lyi et al. 2005). The results from the present study showed that selenate in nutrient solution increased total S level in nearly all accessions tested in spite with different extent. These results reflect a synergistic instead of antagonistic relationship between the two elements at the concentration of 15 μM selenate. Our data agree with studies showing that selenate application at low levels could promote S accumulation in plants (White et al. 2004; Lyons et al. 2005; Lefsrud et al. 2006). On the other hand, selenite exhibited no effect on or decreased total S levels in the lettuce accessions we tested. Rios et al. (2008a) reported that the application of selenite even at high dosages does not alter total S level in lettuce shoot. The decreased S levels in some lettuce accessions imply diverse genotypic variation of lettuce in response to selenite.
Selenium application is known to have both positive and negative effects on plant growth and development depending on the forms and dosages used. Our results with respect to shoot biomass reveal significant differences based on the Se forms and accessions grown. In general, the results indicate that selenate is less toxic than selenite to plant growth as shown in other studies (Hopper and Parker 1999; Chen et al. 2002; Cartes et al. 2005; Pedrero et al. 2006). A previous study in lettuce reports that the levels of Se accumulation increase with increased amount of Se application and lettuce can tolerance up to 40 μM selenate and 5 μM selenite without negative effects on plant growth and metabolism (Rios et al. 2008b). In agreement with this study, we found that at the Se dosage used the shoot biomasses of the majority accessions showed no visible detrimental change when exposed to selenate. In contrast, the growth of many accessions was reduced following treatment with selenite. The different effect of selenate and selenite on plant growth could be caused by distinct mechanisms of metabolism of different Se forms (Sors et al. 2005). Selenate is translocated to the shoots mainly in the inorganic form, whereas selenite is rapidly converted into selenoamino acids in roots (de Souza et al. 1998; Zayed et al. 1998) and translocated to the shoots in organic forms that are readily incorporated into proteins in replacement of S to cause toxicity to the plant (Hopper and Parker 1999). The group of accessions whose growth was stimulated by selenate exhibited no inhibition of growth when exposed to selenite, indicating a general higher tolerance to Se. While selenite at concentration of over 10 μM is noted to inhibit lettuce growth (Rios et al. 2008b), it is clear from our results that some lettuce accessions can tolerance high selenite levels.
Selenium treatment has been shown to affect antioxidant enzyme activities, which in turn influences plant growth (Hartikainen et al. 2000; Djanaguiraman et al. 2005; Rios et al. 2008b). Our experiments showed that the growth-stimulated effect of selenate in the third group of lettuce accessions was correlated with enhanced activities of CAT and GSH-Px, while the growth-inhibited effect of selenite in the second group of accessions was associated with the general reduction of enzymatic activities of APX, CAT and GSH-Px. The data are consistent with other studies showing that a decline in shoot biomass is associated with a decrease in these enzyme activities and the growth-stimulated effect of Se is related to its antioxidant function (Xue et al. 2001; Rios et al. 2009).
Amino acids have many functions in metabolism and therefore are important nutrients. Although the lettuce accessions exhibited a large variation in total free amino acid content, growing lettuce accessions in nutrient solution containing 15 μM selenate or selenite did not significantly modify the total amino acid levels in nearly all cultivars as in other studies (Frias et al. 2010) except a few showing increased total amino acid contents when treated with selenite. In these few cultivars, the increased amino acid accumulation was associated with significantly inhibited growth. A similar result was reported in a previous study (Rios et al. 2008a). The growth inhibition effect by selenite is suggested to be due to its promotion of the most selenocysteine formed to be used for the formation of non-functional proteins, which gives greater phytotoxicity and therefore lower plant growth (Rios et al. 2008a).
The genotypic variation of lettuce cultivars led to Se accumulation at different levels when exposed to Se. The different ability to accumulate Se in response to selenate treatment was found to be associated with altered expression of Sultr1;1, APS1 and APR1. The higher expression of these genes in the high Se cultivars implies a greater uptake for both selenate and sulfate in roots (Takahashi 2010; Shinmachi et al. 2010) and an increased assimilation in plastids (Rotte and Leustek 2000; Sors et al. 2005).
In summary, by examination of diverse lettuce accessions, we found genotypic variation of lettuce germplasm upon selenate and selenite treatment in terms of Se accumulation as well as plant growth, total antioxidant enzyme activities, and amino acid contents. Our research will be helpful in selecting and developing better cultivars with relatively higher ability to accumulate Se without negative effects on plant growth and other nutritional characteristics. In agreement with a previous report (Rios et al. 2008b), selenate would be the most suitable for lettuce biofortification. Lettuce cultivars in groups 1 and 3 should have higher capacity in tolerance to Se and increased antioxidant levels.
Abbreviations
- Se:
-
Selenium
- S:
-
Sulphur
- Sultr:
-
Sulfate transporter
- APS:
-
Adenosine 5′-phosphosulfate sulfurylase
- APR:
-
Adenosine phosphosulfate reductase
- SAT:
-
Serine acetyl transferase
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- GSH-Px:
-
Glutathione peroxidase
- ROS:
-
Reactive oxygen species
- SeMet:
-
Selenomethionine
- qRT-PCR:
-
Quantitative reverse transcription-PCR
- UPLC:
-
Ultra performance liquid chromatography
References
Beck MA, Levander OA, Handy J (2003) Selenium deficiency and viral infection. J Nutr 133:1463–1467
Blaylock MJ, James BR (1994) Redox transformations and plant uptake of selenium resulting from root–soil interactions. Plant Soil 158:1–12
Broadley MR, Alcock J, Alford J, Cartwright P, Foot I, Fairweather-Tait SJ, Hart DJ, Hurst R, Knott P, McGrath SP, Meacham MC, Norman K, Mowat H, Scott P, Stroud JL, Tovey M, Tucker M, White PJ, Young SD, Zhao FJ (2010) Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 332:5–18
Cartes P, Gianfreda L, Mora ML (2005) Uptake of selenium and its antioxidant activity in ryegrass when applied as selenate and selenite forms. Plant Soil 276:359–367
Chen LC, Yang FM, Xu J, Hu Y, Hu QH, Zhang YL, Pan GX (2002) Determination of selenium concentration of rice in China and effect of fertilization of selenite and selenate on selenium content of rice. J Agric Food Chem 50:5128–5130
Combs GF (2001) Selenium in global food systems. Brit J Nutr 85:517–547
Combs GF, Gray WP (1998) Chemopreventive agents: selenium. Pharmacol Ther 79:179–192
de Souza MP, Pilon-Smits EAH, Lytle CM, Hwang S, Tai J, Honma TSU, Yeh L, Terry N (1998) Rate-limiting steps in selenium assimilation and volatilization by Indian mustard. Plant Physiol 117:1487–1494
Dhillon KS, Dhillon SK (2003) Distribution and management of seleniferous soils. Adv Agron 79:119–184
Diwadkar-Navsariwala V, Prins GS, Swanson SM, Birch LA, Ray VH, Hedayat S, Lantvit DL, Diamond AM (2006) Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc Nat Acad Sci USA 103:8179–8184
Djanaguiraman M, Devi DD, Shanker AK, Sheeba JA, Bangarusamy U (2005) Seleninm—an antioxidative protectant in soybean during senescence. Plant Soil 272:77–86
Flohe L, Gunzler WA (1984) Assays of glutathione-peroxidase. Methods Enzymol 105:114–121
Frias J, Gulewicz P, Martinez-Villaluenga C, Penas E, Piskula MK, Kozlowska H, Ciska E, Gulewicz K, Vidal-Valverde C (2010) Changes in nutritional value and cytotoxicity of garden cress germinated with different selenium solutions. J Agric Food Chem 58:2331–2336
Grace SC, Logan BA (1996) Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol 112:1631–1640
Graham RD, Welch RM, Saunders DA, Ortiz-Monasterio I, Bouis HE, Bonierbale M, de Haan S, Burgos G, Thiele G, Liria R, Meisner CA, Beebe SE, Potts MJ, Kadian M, Hobbs PR, Gupta RK, Twomlow S (2007) Nutritious subsistence food systems. Adv Agron 92:1–74
Hartikainen H, Xue TL, Piironen V (2000) Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 225:193–200
Hoagland DR, Arnon D (1950) The water culture method for growing plants without soil. Calif Agric Expt Sta Circ 374:1–32
Hopper JL, Parker DR (1999) Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant Soil 210:199–207
Lebeda A, Ryder E, Grube R, Dolezalova I, Kristkova E (2007) Lettuce (Asteraceae; Lactuca spp.). In: Singh R (ed) Genetic resources, chromosome engineering, and crop improvement series, vol 3. Vegetable crops. CRC Press, Boca Raton, pp 377–472
Lefsrud MG, Kopsell DA, Kopsell DE, Randle WM (2006) Kale carotenoids are unaffected by, whereas biomass production, elemental concentrations, and selenium accumulation respond to changes in selenium fertility. J Agric Food Chem 54:1764–1771
Li HF, McGrath SP, Zhao FJ (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102
Lyi SM, Heller LI, Rutzke M, Welch RM, Kochian LV, Li L (2005) Molecular and biochemical characterization of the selenocysteine Se-methyltransferase gene and Se-methylselenocysteine synthesis in broccoli. Plant Physiol 138:409–420
Lyi SM, Zhou X, Kochian LV, Li L (2007) Biochemical and molecular characterization of the homocysteine S-methyltransferase from broccoli (Brassica oleracea var. italica). Phytochemistry 68:1112–1119
Lyons G, Ortiz-Monasterio I, Stangoulis J, Graham R (2005) Selenium concentration in wheat grain: is there sufficient genotypic variation to use in breeding? Plant Soil 269:369–380
Nakano Y, Asada K (1981) Hydrogen-peroxide is scavenged by ascorbate-specific peroxidase in spinach-chloroplasts. Plant Cell Physiol 22:867–880
Pedrero Z, Madrid Y, Cámara C (2006) Selenium species bioaccessibility in enriched radish (Raphanus sativus): a potential dietary source of selenium. J Agric Food Chem 54:2412–2417
Pilon-Smits E, Quinn C (2010) Selenium metabolism in plants. In: Hell R, Mendel RR (eds) Cell biology of metal and nutrients. Springer, Berlin, pp 225–241
Ramos SJ, Faquin V, Guilherme LRG, Castro EM, Avila FW, Carvalho GS, Bastos CEA, Oliveira C (2010) Selenium biofortification and antioxidant activity in lettuce plants feed with selenate and selenite. Plant Soil Environ 12:583–587
Rao MV, Paliyath C, Ormrod DP, Murr DP, Watkins CB (1997) Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2 metabolizing enzymes–salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol 115:137–149
Rayman MP (2002) The argument for increasing selenium intake. Proc Nutr Soc 61:203–215
Rios JJ, Blasco B, Cervilla LM, Rubio-Wilhelmi MM, Ruiz JM, Romero L (2008a) Regulation of sulphur assimilation in lettuce plants in the presence of selenium. Plant Growth Regul 56:43–51
Rios JJ, Rosales MA, Blasco B, Cervilla LM, Romero L, Ruiz JM (2008b) Biofortification of Se and induction of the antioxidant capacity in lettuce plants. Sci Horticult 116:248–255
Rios JJ, Blasco B, Cervilla LM, Rosales MA, Sanchez-Rodriguez E, Romero L, Ruiz JM (2009) Production and detoxification of H2O2 in lettuce plants exposed to selenium. Ann Appl Biol 154:107–116
Rios JJ, Blasco B, Rosales MA, Sanchez-Rodriguez E, Leyva R, Cervilla LM, Romero L, Ruiz JM (2010) Response of nitrogen metabolism in lettuce plants subjected to different doses and forms of selenium. J Sci Food Agric 90:1914–1919
Rotte C, Leustek T (2000) Differential subcellular localization and expression of ATP sulfurylase and APS reductase during ontogenesis of Arabidopsis thaliana leaves indicates that cytosolic and plastid forms of ATP sulfurylase may have specialized functions. Plant Physiol 124:715–724
Saito K (2004) Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol 136:2443–2450
Sharma S, Bansal A, Dhillon S, Dhillon K (2010) Comparative effects of selenate and selenite on growth and biochemical composition of rapeseed (Brassica napus L.). Plant Soil 329:339–348
Shinmachi F, Buchner P, Stroud JL, Parmar S, Zhao FJ, McGrath SP, Hawkesford MJ (2010) Influence of sulfur deficiency on the expression of specific sulfate transporters and the distribution of sulfur, selenium, and molybdenum in wheat. Plant Physiol 153:327–336
Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86:373–389
Takahashi H (2010) Regulation of sulfate transport and assimilation in plants. In: Kwang WJ (ed) International review of cell and molecular biology. Academic Press, pp 129–159
Terry N, Zayed AM, de Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51:401–432
Whanger PD (2002) Selenocompounds in plants and animals and their biological significance. J Am Coll Nutr 21:223–232
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182:49–84
White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, Smith BM, Thomas B, Broadley MR (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937
Xue T, Hartikainen H, Piironen V (2001) Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 237:55–61
Yang G, Chen JS, Wen ZM, Ge KY, Zhu LZ, Chen XC, Chen XS (1984) The role of selenium in Keshan disease. Adv Nutr Res 6:203–231
Zayed A, Lytle CM, Terry N (1998) Accumulation and volatilization of different chemical species of selenium by plants. Planta 206:284–292
Zhang YL, Pan GX, Chen J, Hu QH (2003) Uptake and transport of selenite and selenate by soybean seedlings of two genotypes. Plant Soil 253:437–443
Zhao FJ, Su YH, Dunham SJ, Rakszegi M, Bedo Z, McGrath SP, Shewry PR (2009) Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J Cereal Sci 49:290–295
Acknowledgments
We thank Dr. Xiangjun Zhou for help with gene expression analysis, and Mr. Laurence Heller for help with amino acid analysis. S.J.R thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for granting the doctorate scholarships (regular and sandwich program).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramos, S.J., Rutzke, M.A., Hayes, R.J. et al. Selenium accumulation in lettuce germplasm. Planta 233, 649–660 (2011). https://doi.org/10.1007/s00425-010-1323-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1323-6