Abstract
The moss Physcomitrella patens (P. patens) is a useful model to study abiotic stress responses since it is highly tolerant to drought, salt and osmotic stress. However, very little is known about the defense mechanisms activated in this moss after pathogen assault. In this study, we show that P. patens activated multiple and similar responses against Pythium irregulare and Pythium debaryanum, including the reinforcement of the cell wall, induction of the defense genes CHS, LOX and PAL, and accumulation of the signaling molecules jasmonic acid (JA) and its precursor 12-oxo-phytodienoic acid (OPDA). However, theses responses were not sufficient and infection could not be prevented leading to hyphae colonization of moss tissues and plant decay. Pythium infection induced reactive oxygen species production and caused cell death of moss tissues. Taken together, these data indicate that Pythium infection activates in P. patens common responses to those previously characterized in flowering plants. Microscopic analysis also revealed intracellular relocation of chloroplasts in Pythium-infected tissues toward the infection site. In addition, OPDA, JA and its methyl ester methyl jasmonate induced the expression of PAL. Our results show for the first time JA and OPDA accumulation in a moss and suggest that this defense pathway is functional and has been maintained during the evolution of plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants attempt to prevent infection of attacking pathogens by generating a variety of cellular defense responses. Preformed physical and chemical barriers such as cuticles, cell walls, and constitutively produced antimicrobial compounds are effective and prevent most invasions. However, if the pathogen overcomes these barriers, complex signaling pathways are activated leading to cellular reactions that contribute to the overall response. These responses are divided into resistant and susceptible depending on the extent and speed of symptom development and the ability of the host to limit pathogen growth. Common cellular responses are activated in resistant and susceptible hosts, including the reinforcement of the cell wall, changes in gene expression and the synthesis of antimicrobial compounds. The hormones salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) play a role in many host reactions to pathogen infection (Glazebrook 2005; Lorenzo and Solano 2005; van Loon et al.2006; Loake and Grant 2007). SA is generally associated with resistant responses to biotrophs, whereas JA and ET are generally associated with responses to necrotrophs. SA, JA and ET are also involved in the regulation of susceptible responses (Pilloff et al. 2002; O’Donnell et al. 2003). In addition to these defense hormones, recent progress in the field has revealed that modulation of additional plant hormone pathways is a relevant component to define the outcome of a plant–pathogen interaction (López et al. 2008).
Bryophytes, including mosses, are non-vascular plants that have diverged from flowering plants at least 450 million years ago and thus occupy an ideal phylogenetic position to study different biological processes in plants (Lang et al. 2008). The moss Physcomitrella patens (P. patens) has recently emerged as a powerful model system since it has several interesting features including the possibility of targeted gene disruption by homologous recombination (Schaefer 2002). The analysis of the P. patens genome has enabled the reconstruction of evolutionary changes in genomes of mosses, aquatic algae and flowering plants (Rensing et al. 2008). Land colonization by plants has strengthened tolerance to terrestrial stresses such as drought, radiation and extreme temperatures (Rensing et al. 2008). P. patens is highly tolerant to abiotic stresses including drought, salt and osmotic stress and is therefore a valuable source of genes involved in stress adaptation (Frank et al. 2005). The availability of ESTs, annotated transcripts and expression data obtained by macro and microarrays together with the assembled genome, has enabled the identification of genes involved in P. patens abiotic stress tolerance (Nishiyama et al. 2003; Frank et al. 2005; Lang et al. 2005; Cuming et al. 2007; Sun et al. 2007; Rensing et al. 2008). However, only few data exist on biotic stress responses of P. patens, although it possesses many genes with high sequence identity to defense-related genes of flowering plants. Recently, we have shown that the Gram-negative enterobacterium Erwinia carotovora ssp. carotovora (E.c. carotovora) and the ascomycete Botrytis cinerea (B. cinerea) infect P. patens causing browning and maceration of the tissues (Ponce de León et al. 2007). The main virulence factors of E.c. carotovora and B. cinerea are cell wall degrading enzymes, and in case of B. cinerea toxins also play an important role in pathogenesis (Toth et al. 2003; van Kan 2006). Both necrotrophic pathogens induce the hypersensitive response in flowering plants (Pandey et al. 2005; van Kan 2006), and previous results suggest that they could also induce this type of cell death in P. patens (Ponce de León et al. 2007). Similar changes as those occurring in flowering plants after pathogen infection were observed in P. patens cells infected with B. cinerea or treated with E.c. carotovora elicitors, including chloroplasts breakdown, cytoplasmic collapse and accumulation of autofluorescent compounds. In addition, the expression of four genes with high identity to defense genes of flowering plants, including LOX (lipoxygenase), PAL (phenylalanine ammonia-lyase), CHS (chalcone synthase) and PR-1 was induced by B. cinerea and E.c. carotovora elicitors (Ponce de León et al. 2007). It has been demonstrated that the P. patens products of the same LOX and CHS genes possess lipoxygenase and chalcone synthase activities, respectively (Senger et al. 2005; Jiang et al. 2006). LOX is a key enzyme in the synthesis of defense-related compounds including JA, PAL mediates the biosynthesis of phenylpropanoids and SA, and CHS is the first enzyme in the synthesis of flavonoids (Dixon and Paiva 1995; Feussner and Wasternack 2002).
In order to broaden our knowledge on the different defense mechanisms acting in this moss after pathogen challenge, we continued our studies in P. patens using the oomycetes Pythium irregulare (P. irregulare) and Pythium debaryanum (P. debaryanum) as pathogens. Pythium species are non-specific soil-borne vascular pathogens, which under wet, humid conditions cause diseases in many plants including agronomic crops. Infections occur on roots, lower stems and soft plant tissues, which are naturally present in seedlings, causing root rot, stem rot and seedling damping-off respectively. In most mature plants Pythium shows a preference for young, juvenil tissue as compared to older woody tissues with secondary wall thickenings. Tissue degradation is caused by both cell wall degrading enzymes such as pectinases, hemicellulases, cellulases and proteinases and toxins produced by Pythium (Martin 1994; Campion et al. 1997).
The previously characterized P. irregulare isolate was chosen for this study since the process of infection and the plant defense pathways have been determined in the flowering plant Arabidopsis thaliana (A. thaliana) (Adie et al. 2007), and the P. debaryanum uruguayan isolate was included to compare moss defense responses activated by two different Pythium species. In A. thaliana, the infection process of P. irregulare begins with the formation of appresoria, penetration of the first host cell, hyphal ramification giving rise to haustoria-like structures and further hyphal growth which penetrate adjacent cells (Adie et al. 2007). The JA, SA, ET and abscisic acid pathways have been implicated in defense responses of flowering plants against Pythium infection (Staswick et al. 1998; Adie et al. 2007), and instead of involving the hypersensitive response, defense responses are mediated by physical barriers, including reinforcement of the cell wall (Kamoun et al. 1999). In this study, we describe the development of a new pathosystem based on the model moss P. patens and the oomycete Pythium. We have characterized the infection process of P. irregulare and P. debaryanum in P. patens tissues and analyzed the induction of host defense responses.
Materials and methods
Physcomitrella patens and Pythium growth conditions
Physcomitrella patens Gransden WT isolate (Schaefer et al. 1991) was provided by S. Vidal (Facultad de Ciencias, UdelaR, Uruguay), and grown on cellophane overlaid agar BCDAT medium and protonemal cultures and moss colonies were generated as described previously (Saavedra et al. 2006). Plants were grown at 22°C under a photoperiod of 16 h light and 3-week-old colonies were used for all the experiments, except for cell death quantification where 2-week-old colonies were used. Pythium debaryanum (INIA; Las Brujas, Uruguay) and Pythium irregulare (Centro Nacional de Biotecnología-CSIC; Madrid, Spain) were cultivated on 24 g/l potato dextrose agar (PDA) (Difco laboratories, Detroit, Michigan, USA) at 22°C.
Pythium inoculation, Pythium staining and evaluation of disease symptoms
Pythium inoculations were performed as described by Adie et al. (2007). Briefly, 0.5 cm diameter PDA plugs containing P. debaryanum or P. irregulare were taken from the growing edge of 5 days fresh grown cultures. Single agar plugs bearing Pythium mycelium were carefully placed on top of each moss colony without being in contact with the plant culture medium. PDA plugs without mycelium were placed on moss colonies as a control. Development of Pythium inside leaf tissues was monitored either by staining with lactophenol–trypan blue and destaining in saturated chloral hydrate as described (Koch and Slusarenko 1990) or with Coomassie blue according to Mellersh et al. (2002). Pythium tissues were also stained with 0.1% solophenyl flavine 7GFE 500 in water for 10 min, rinsed in water and visualized with epifluorescence (Hoch et al. 2005). Disease symptoms were analyzed in three independent experiments using three Petri dishes, each containing 16 moss colonies. Both Pythium were reisolated from infected tissue and inoculated in new moss colonies, which developed the same disease symptoms. All pictures were taken at 1 day after inoculation unless otherwise indicated and representative pictures are shown.
Visualization of plant cell wall-associated defense responses
Phenolic compounds were visualized by appearance of autofluorescence under epifluorescent microscopy in boiled alcoholic lactophenol and ethanol rinsed leaves as described (Dietrich et al. 1994). To detect cell wall modifications safranin-O staining was performed according to Lucena et al. (2003). Tissues were incubated with 0.01% safranin-O in 50% ethanol for 5 min. For callose detection, tissues were fixed in ethanol, rinsed in water and stained with 0.01% methyl blue in phosphate buffer pH 7.0 for 30 min and observed with epifluorescence. Brigth field and fluorescence microscopy were performed with an Olympus BX61 microscope and images captured with Microsuite software package.
ROS production and cell death detection
Intracellular production of reactive oxygen species (ROS) was analyzed by incubating moss tissues with 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) for 15 min in 0.1 M phosphate buffer pH 7.5 in the dark. Leaves were detached for microscopic examination and visualized with epifluorescence. For detection of cell death, moss colonies were incubated for 30 min in 0.1% Evans blue and washed four times with water to remove unbound dye. For quantification, dye bound to dead cells was solubilized in 50% methanol with 1% SDS for 30 min at 60°C and the absorbance was measured at 600 nm (Levine et al. 1994). Each sample consisted of four colonies incubated in 6 ml of the mixture methanol/SDS. Six samples, corresponding to 24 colonies, were analyzed per experiment. All experiments were repeated thrice and data expressed as OD/mg dry weight. Dry weight was measured after drying plant colonies on cellophane for 18 h at 65°C.
Hormone analysis
Three-week-old moss colonies were inoculated with mycelium of P. irregulare and P. debaryanum taken from the growing edge of a fresh colony. Tissue was collected 2, 4, 8 and 24 h after inoculation, frozen, grinded and weighed until hormone quantification. Eight moss colonies were pooled and 200 mg ground tissue was homogenized, derivatized, vapor phase extracted, and analyzed by isobutane chemical-ionization gas chromatography-mass spectrometry as previously described (Schmelz et al. 2004). This procedure was repeated thrice.
Jasmonic acid (JA), methyl jasmonate (MeJA) and 12-oxo-phytodienoic acid (OPDA) treatments
Jasmonic acid, MeJA and OPDA treatments were performed by spraying moss colonies with a 50 μM solution. Control moss colonies were sprayed with 0.1% ethanol. Tissue was collected 2, 4, 8 and 24 h after treatment and frozen for RNA extraction.
RNA gel blot analysis
Total RNA was isolated from moss colonies inoculated with both Pythium species and treated with MeJA, JA and OPDA using standard procedures based on phenol/chloroform extraction followed by LiCl precipitation. Each sample consisted of 64 colonies. For RNA gel blot analysis, 10 μg of total RNA was separated in denaturing agarose–formaldehyde gels and transferred to nylon membranes (Hybond N) following standard procedures (Sambrook et al. 1989). Membranes were hybridized and washed as described previously by Ponce de León et al. (2007). DNA probes were labelled with [α32P]-dCTP using Rediprime II Random Prime labelling system (GE Healthcare, Amersham, Buckinghamshire, UK). Probes were prepared by PCR using primers M13 forward and reverse. The cDNA clones used as templates were: PR-1 (BJ182301), PAL (BJ201257), CHS (BJ192161) and LOX (BJ159508). Equal loading was visualized by ethidium bromide staining of rRNA. Similar results were obtained from three independent experiments.
Results
Pythium debaryanum and Pythium irregulare infect Physcomitrella patens tissues and cause disease symptoms
Physcomitrella patens tissues were inoculated with P. debaryanum and P. irregulare and disease symptoms were analyzed. P. patens showed clear susceptibility to P. debaryanum and P. irregulare infection 24 h after inoculation, characterized by the appearance of brown stems (Fig. 1b, c), brown midline vein and brown cells at the base of the leaves (Fig. 1e, f). Brownish protonemal tissues could also be observed at the edge of Pythium-inoculated colonies (Fig. 1h, i). In control tissues, no browning was visible (Fig. 1a, d, g). Pythium colonization progressed from the base to the tips of the leaves (Fig. 1e, f). Disease symptoms were similar after inoculation with both Pythium species. After 24 h of inoculation, hyphal tissue grew on top of the moss colonies (Fig. 1h, i), and after 2 days, the mycelium covered the moss colonies, softening and macerating the plant tissues (data not shown).
Disease symptoms in Pythium-infected moss tissues. a PDA-treated gametophore, b P. debaryanum-inoculated gametophore, c P. irregulare-inoculated gametophore, d PDA-treated leaf, e P. debaryanum-inoculated leaf, f P. irregulare-inoculated leaf, g PDA-treated colonies, h P. debaryanum-inoculated colonies, i P. irregulare-inoculated colonies. Disease symptoms were analyzed in three independent experiments using three Petri dishes, each containing 16 moss colonies. Pictures of representative tissues were taken 1 day after inoculation. The scale bar represents 0.5 mm (a–c), 20 μm (d–f) or 5 mm (g–i)
Taking advantage of the fact that most P. patens tissues are formed by a monolayer of cells (leaves, rhizoids and protonemal filaments), we conducted microscopic analysis to study the infection process of P. irregulare and P. debaryanum. Both Pythium species were able to penetrate P. patens tissues and invade all the tissue types including leaves, protonemal filaments, rhizoids and stems by 24 h after inoculation (Fig. 2a–d). Infection process was similar for both P. debaryanum and P. irregulare, and representative pictures at different infection stages are shown in Fig. 2. Appressoria were observed in early stages of infection of P. patens tissues (Fig. 2e). After infection of several cells, multidigital haustoria-like structures were visible in moss-infected tissues (Fig. 2f). Host wall penetration to adjacent cells was achieved by means of constricted hyphae (Fig. 2g). Pythium growth was mainly intracellular, and in heavily infected tissues, hyphae growth outside the cells colonizing new tissues and hyphal swelling were observed (Fig. 2i). Oospores, oogonium and antheridium were detected 1 d after Pythium inoculation, although they were more frequently observed after 2 d (Fig. 2j, h). This is consistent with the fact that after 2 days of Pythium inoculation mycelium colonized heavily all tissues leading to tissue maceration and plant decay.
Invasion of P. patens tissues by Pythium irregulare and Pythium debaryanum. a P. debaryanum-infected leaf (trypan blue staining), b P. debaryanum-infected protonemal filament (trypan blue staining), c P. irregulare-infected rhizoids (trypan blue staining), d P. irregulare-infected stem (solophenyl staining), e formation of appresoria in P. debaryanum-inoculated tissues (indicated with an arrow) (solophenyl staining), f haustorium-like structures in P. debaryanum-infected leaf (trypan blue staining), g constricted hyphae penetrating through a cell wall (solophenyl staining), h P. debaryanum oogonium (oo) and antheridium (an) in a P. patens leaf (Coomassie staining), i P. irregulare hyphal swelling (solophenyl staining), j oospore of P. debaryanum inside a cell (indicated with an arrow). The scale bar represents 20 μm
Chloroplasts are relocated intracellularly toward the Pythium-infected area
Visualization of P. patents leaves during infection with P. irregulare and P. debaryanum allowed to observe a relocalization of the chloroplasts that was clearly evident in cells near the brownish infected tissues (Fig. 1e, f). Closer examination of chloroplasts distribution in Pythium-infected and non-infected leaves revealed that whereas chloroplasts are uniformly distributed throughout the cells of non infected leaves (Fig. 3a, b) these organelles are intracellularly redistributed during infection to be located near the cell walls and preferentially in close vicinity to the infected area (Fig. 3c). In Fig. 3d, we show how the chloroplasts of cells adjacent to an infected-cell containing an oospore, are relocated toward the cell wall. This process was further examined after staining living moss tissues with the fluorescent dye solophenyl flavine 7GFE 500. This dye was recently described as a useful dye to stain Pythium cell walls, although it is not known to which cell wall components it binds (Hoch et al. 2005). We found that relocation of chloroplasts inside the cells was not only observed in infected cells (arrow in Fig. 3e), but also in adjacent non-infected cells where chloroplasts move toward the infection site (white arrow in Fig. 3f). A further observation from these analyses was that the dye not only stained Pythium cell walls, but also cell walls of Pythium-infected cells that showed a brighter fluorescence (black arrow in Fig. 3f) likely indicative of changes in the cell wall of the infected moss tissues.
Intracellular relocation of chloroplasts after Pythium infection. a PDA-treated leaf, b PDA-treated leaf stained with solophenyl, c P irregulare-infected leaf, d P. debaryanum-infected leaf containing an oospore, e P. debaryanum-inoculated leaf where infected cells are indicated with an arrow, f P. debaryanum-inoculated leaf stained with solophenyl. The white arrows point to a Pythium-infected cell (e) and to a non-infected cell (f). The black arrow in f highlights the brighter fluorescence of cell walls in Pythium-infected cells. Pictures of representative tissues were taken 1 day after inoculation. The scale bar represents 20 μm
Pythium infection elicits wall-associated defense responses
During the initial stages of Pythium colonization, single cells were observed to respond by changing the composition of the cell wall and allowing binding of the dye solophenyl flavine (Fig. 4a). With the progress of infection, brighter fluorescence was observed circumscribing the entire cell walls (Fig. 4b). Changes in cell walls of Pythium-infected tissues were further studied by analyzing accumulation of phenolic compounds and callose deposition 1 day after inoculation. Since in non-inoculated leaves differences in cell wall composition were observed in the base and tip compared to the rest of the leaf (data not shown), studies were performed focusing on the middle of the leaf. Safranin-O staining of Pythium-infected cell walls reflects the incorporation of phenolic compounds, which is an indicator of a fortification mechanism (Fig. 4c, d). Methyl blue staining revealed the deposition of callose at the entire cell wall of infected cells (Fig. 4e). Moreover, papillae rich in callose were occasionally observed at sites of attempted penetration and only when an old Pythium inoculum was used (Fig. 4f). Hyphal tissues were also visible after methyl blue staining (Fig. 4e).
Wall-associated defense responses in Pythium-infected tissues. a, b solophenyl staining of P. debaryanum-infected leaf, c P. irregulare-infected leaf stained with safranin-O, d closer view of c, where incorporation of phenolic compounds are indicated with an arrow, e callose deposition in P. debaryanum-infected leaf detected by methyl blue staining, f papillae in leaf inoculated with a 2-week-old P. debaryanum inoculum. Pictures of representative tissues were taken 1 day after inoculation. The scale bar represents 20 μm
Pythium infection cause ROS production and cell death in P. patens tissues
Pathogen infection can induce ROS production and subsequent death of plant cells (Morel and Dangl 1997), a response that we also examined here. Since wounding induced the generation of ROS in P. patens tissues (data not shown), observations were focused on leaf tissue located far from the detached site. As shown in Fig. 5, intracellular ROS production was detected in tissues infected with both Pythium species, while no fluorescence resulting from staining with H2DCFDA could be observed in control tissues (Fig. 5a, b). ROS production was detected in a few cells 4 h after Pythium inoculation (Fig. 5b) spreading at later times simultaneously with the progress of the pathogen (Fig. 5c, d). A faint autofluorescence was also detectable in cells of Pythium-inoculated leaves, suggesting that phenolic compounds were liberated during cell death (Fig. 5e, f). In Fig. 5f, the purple spots probably correspond to phenolic compounds present in Pythium tissues. Cell death in Pythium-infected tissues was further analyzed by staining moss colonies with Evans blue. As shown in Fig. 5g, tissues in contact with mycelium were stained, while no staining could be observed in moss tissues exposed to PDA. Quantification of the cell death caused by Pythium infection 1 day after inoculation showed an approximately four-fold increment in the number of dead cells in comparison to those in PDA-treated moss colonies (Fig. 5h).
ROS production and cell death in Pythium-infected tissues. a ROS production in PDA-treated leaf, b ROS production in P. debaryanum-inoculated leaf (4 h after inoculation), c ROS production in P. debaryanum-inoculated leaf after 1 day of inoculation, d closer view of c where appresoria are indicated with an arrow, e control PDA-treated leaf showing no accumulation of autofluorescent compounds, f accumulation of autofluorescent compounds in a P. debaryanum-inoculated leaf, g Evans blue staining of moss colonies treated with PDA (left), or inoculated with P. debaryanum (middle) or P. irregulare (right), h measurement of cell death by Evans blue staining after 4, 8 and 24 h of inoculation. PDA-treated colonies (white bars), P. debaryanum-inoculated colonies (striped bars) and P. irregulare-inoculated colonies (black bars). Data were expressed as OD 600 nm/mg dry weigth (DW). Values in h are means ± SE of six independent replicate moss samples. Experiments were repeated thrice with similar results. Pictures of representative tissues were taken after 1 day of inoculation, except for b which was taken after 4 h of inoculation. The scale bar represents 20 μm
Pythium colonization induces the accumulation of 12-oxo-phytodienoic acid and jasmonic acid and the expression of genes encoding defense proteins
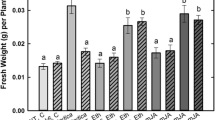
Jasmonic acid and salicylic acid have been shown to play an important role in plant defense signaling (Glazebrook 2005; Lorenzo and Solano 2005; Loake and Grant 2007). To evaluate the involvement of JA and SA in the P. patens defense response against both Pythium species, both hormones and the JA precursor OPDA were measured in treated and control plants. The results show that OPDA and JA synthesis is induced in tissues infected with P. debaryanum and P. irregulare, while no induction of SA was detected (Fig. 6a). After 1 day of inoculation OPDA content increased approximately 25-fold in plants infected with both Pythium species, while JA increased approximately 7- and 5-fold in P. debaryanum and P. irregulare infected plants, respectively. To analyze whether P. debaryanum and P. irregulare infection trigger defense gene expression, we characterized the expression of PAL, CHS, LOX and PR-1. We have previously shown that these genes are induced in P. patens after infection with B. cinerea and treatment with E.c. carotovora elicitors (Ponce de León et al. 2007). The deduced amino acid sequences of P. patens PAL, CHS, LOX and PR-1 cDNAs used in this work exhibited identity scores of 61–77, 65, 43–38 and 45%, respectively, when compared to the homologous A. thaliana sequences. The results show that P. debaryanum and P. irregulare infection trigger the expression of CHS, PAL and LOX genes, and a slight induction of PR-1 expression in P. patens tissues (Fig. 6b). LOX transcript accumulation starts at 2 h increasing up to 6–8 h after inoculation. The level of PAL and CHS expression increased from 2 to 24 h after both Pythium inoculations, reaching a maximum level at 24 h. In the case of CHS, two transcripts with an identical expression pattern were detected. A slight induction in the expression of PR-1 was observed at 2 and 4 h. To further analyze whether OPDA and JA induce the expression of genes involved in defense responses, we treated moss colonies with OPDA, JA and its methyl ester methyl jasmonate (MeJA). As shown in Fig. 6c, the expression of PAL was induced 2 h after treatment with JA, MeJA and OPDA and in case of MeJA treatment PAL induction was also observed at 4 h.
OPDA, JA and SA accumulation and expression analysis of defense-related genes. a Endogenous OPDA, JA and SA levels (ng/g fresh weight (FW)) in PDA-treated tissues and in P. irregulare- and P. debaryanum-inoculated tissues were analyzed at the indicated timepoints. Values are means ± SE of three independent replicate experiments. b Expression of CHS, PAL, LOX and PR-1 genes after P. debaryanum and P. irregulare inoculation. Plants treated with PDA were used as a control. Plant samples were harvested at the indicated times (hours) after treatment. 10 μg of RNA was separated on formaldehyde–agarose gels, transferred to nylon membranes and hybridized to 32P-labeled DNA probes. Ethidium bromide staining of rRNA was used to ensure equal loading of RNA samples. c Expression of PAL was determined by RNA gel blot hybridization after treatment with 50 μM JA, 50 μM methyl jasmonate (MeJA) and 50 μM OPDA. Plants treated with 0.1% ethanol were used as a control
Discussion
In order to gain further information on the evolution of defense mechanisms in plants, we have focused our studies on the interaction between the model moss P. patens and the oomycetes P. irregulare and P. debaryanum. Here, we show that both Pythium species have a similar penetration and colonization process, produce similar disease symptoms and induce the same plant responses in this moss. However, the activation of these defense mechanisms was not sufficient to prevent infection. Like A. thaliana, P. patens is susceptible to P. irregulare infection, and a rapid progression of P. irregulare and P. debaryanum hyphal tissues was observed covering moss colonies within 2 days. Moss tissues were easily macerated by both Pythium species and probably the nutrients were rapidly obtained from the plant tissues enabling a rapid completion of the oomycete life cycle. Pythium oogonium, antheridium and oospores were sometimes detected after 1 day of inoculation, although they were more frequently observed after 2 days. In the A. thaliana–P. irregulare interaction, oogonium, antheridium and oospores were not observed after 1 day of inoculation (Adie et al. 2007). This higher susceptibility of P. patens could be due to different factors. First, the high humidity generated in the Petri dishes during P. patens growth facilitated Pythium infection and proliferation. Pythium colonization of plant tissues was slower when lower humidity conditions were used (data not shown). Second, most P. patens tissues are formed by a monolayer of cells and are more easily macerated. Third, Pythium infection might be facilitated in moss tissues since P. patens lacks the enzyme needed to make the S-lignols required for the accumulation of lignin (Rensing et al. 2008). In A. thaliana, genes involved in the biosynthesis of lignin are induced after P. irregulare infection (Adie et al. 2007), suggesting a role of this metabolite in the defense mechanism against this oomycete.
Modifications of the plant cell wall are important defense responses against oomycetes since they often directly penetrate plant cell wall to gain access to cell contents. Callose deposition plays a role in the defense response of A. thaliana against P. irregulare (Adie et al. 2007). Consistent with this and previous results obtained in Pythium-infected tissues of flowering plants (Benhamou and Bélanger 1998), P. patens responses against P. irregulare and P. debaryanum infection involve accumulation of phenolic compounds and deposition of callose. Binding of the dye solophenyl flavine is likely reflecting additional unknown changes occurring in the cell wall of infected cells since non-infected cells do not show the same intense fluorescence. Wall appositions (papillae) are rich in callose and cellulose and are formed as an early response strengthening the walls of host cells to inhibit pathogen penetration (Jacobs et al. 2003). In P. patens, the entire cell wall of infected cells was positively stained for callose deposition. While in A. thaliana papillae could be observed after inoculation with a fresh Pythium inoculum (Adie et al. 2007), callose-containing wall appositions were only detected in P. patens tissues when an old inoculum was used and infection was not extensive. The absence of papillae in moss tissues inoculated with a fresh Pythium inoculum could be an additional factor for the higher susceptibility observed in P. patens compared to A. thaliana. Taken together, these results indicate that both Pythium elicited wall-associated defense responses in P. patens, although they were not sufficient to stop infection.
Reactive oxygen species production is an important plant defense response to pathogens involved in cell wall modification, defense signaling, induction of the hypersensitive response, or by their direct toxicity to pathogens (Torres et al. 2006). However, necrotrophic pathogens appear to stimulate ROS production to their own advantage by generating cellular damage and subsequent cell death (Govrin and Levine 2000). Consistent with this, cell death induced by Pythium is preceded by accumulation of ROS. After infection, ROS production was observed mainly in the cytoplasm, which could contribute to the cell wall strengthening observed. After 4 h of Pythium inoculation, several cells produced ROS, while no cell death could be detected in P. patens tissues at this time point. After 24 h of inoculation, ROS production was detected in the Pythium-infected spreading area, which correlated with an increase in cell death. This observation shows that Pythium stimulated ROS production in P. patens-infected tissue, which could result in plant cell death, enhancing the susceptibility to this pathogen and facilitating subsequent infection.
Chloroplasts are a source of ROS during plant defense responses to pathogens due to their intensive electron transport activity. Previous studies have shown that some fungal toxins may interfere with electron transport in thylakoids (Albrecht et al. 1998), and E. c. carotovora-derived elicitors treated tissues show a down-regulation of the NADP+ photoreduction activity of the photosystem I, associated to ROS accumulation in chloroplasts (Montesano et al. 2004). In this study, we do not detect ROS generation in chloroplasts after Pythium inoculation, although we do observe a relocation of chloroplasts toward the side of the cell wall close to the infection site. Movement of intracellular organelles is an active process associated to the adaptation of plant cells to various environmental stimuli. In bryophytes, chloroplasts move away from the site of the cell where a mechanical or light stimulation is produced (Sato et al. 2001; Wada et al. 2003). In fungal infected plant cells, rearrangement of the cytoskeleton, translocation of the cytoplasm and the nucleus toward the fungal penetration sites have been observed (Gross et al. 1993). To our knowledge, this is the first report showing intracellular movements of chloroplasts after pathogen infection. The fact that chloroplasts from non-infected cells adjacent to infected cells also show relocation of these organelles suggest that this phenomenon could be regulated by a gradient of some signal generated by the moss-infected cells or by Pythium. Further studies are needed to understand the role of chloroplasts movement in plant–pathogen interactions. We show that P. patens is an interesting experimental host for investigation of plant–pathogen interactions because changes occurring in infected plant cells can be more easily visualized than in flowering plants.
Our results indicate that both Pythium species induced defense-related gene expression in P. patens. Pythium infection increased PAL, CHS and LOX transcript levels significantly, while only a slight increase of PR-1 was detectable. These four genes are also induced in P. patens after E.c carotovora elicitors and B. cinerea spores treatment, although with a different expression pattern (Ponce de León et al. 2007). In A. thaliana, Pythium induce the expression of PAL, LOX, PR-1, CHS and other genes encoding enzymes involved in phenylpropanoid and flavonoid synthesis (Vijayan et al. 1998; Bednarek et al. 2005; Adie et al. 2007). Similar to flowering plants, the biochemical products of LOX, PAL and CHS are likely to also play a role in P. patens response mechanisms against pathogens. The higher number of P. patens members composing the CHS and PAL multigene family compared to flowering plants, together with the existence of oxylipins (produced by LOX) not present in flowering plants (Senger et al. 2005; Jiang et al. 2006), makes this moss an interesting system to identify new defense-related compounds and defense mechanisms that could have been changed or lost during the evolution of plants. The fact that novel oxylipins, absent in flowering plants, were generated from arachidonic acid by the same P. patens LOX gene product induced in this study by Pythium (Senger et al. 2005), supports this idea.
Pythium irregulare and P. debaryanum infection induced the accumulation of OPDA and JA in P. patens, while no significant increase in SA could be detected. In A. thaliana tissues, P. irregulare infection increased OPDA and JA levels as well as SA levels compared to non-infected plants (Adie et al. 2007). In addition, analysis with A. thaliana mutants has established that SA and JA/ET cooperate in signaling to induce defenses against P. irregulare, with a much higher contribution of JA (Staswick et al. 1998; Adie et al. 2007). Our results support a role of the JA signaling pathway in the response of P. patens against the oomycetes P. irregulare and P. debaryanum, although the contribution of SA remains elusive. The generation of P. patens mutants affected in these signaling pathways will contribute to understand the roles of JA and SA in the defense response against Pythium as well as other pathogens. OPDA, JA and its methyl ester MeJA induced the expression of the PAL gene, suggesting that the endogenous rise of OPDA and JA lead to enhanced expression of this defense-related gene. It has been recently reported that all components of the JA pathway are present in P. patens (Rensing et al. 2008). However, oxylipin profiles and analysis of the JA-biosynthetic pathway in P. patens suggested that oxylipins are not further metabolized into JA or its derivatives (Wichard et al. 2004; Senger et al. 2005; Stumpe et al. 2006; Chico et al. 2008). Here, we show that JA is produced in P. patens after Pythium infection and our results suggest that JA signaling pathway is functional in this moss. Interestingly, the components of the JA pathway are not present in the genome of the unicellular aquatic algae Ostreococcus tauri, Ostreococcus lucimarinus and Chlamydomonas reinhardtii (http://genome.jgi-psf.org/). Taken together, our findings suggest that JA pathway is conserved among land plants and probably appeared before bryophytes and flowering plants diverged.
Abbreviations
- P. patens :
-
Physcomitrella patens
- P. irregulare :
-
Pythium irregulare
- P. debaryanum :
-
Pythium debaryanum
- JA:
-
Jasmonic acid
- MeJA:
-
Methyl jasmonate
- OPDA:
-
12-Oxo-phytodienoic acid
- SA:
-
Salicylic acid
- ET:
-
Ethylene
- ROS:
-
Reactive oxygen species
- LOX:
-
Lipoxygenase
- PAL:
-
Phenylalanine ammonia-lyase
- CHS:
-
Chalcone synthase
References
Adie BA, Pérez-Pérez J, Pérez-Pérez MM, Godoy M, Sánchez-Serrano JJ, Schmelz EA, Solano R (2007) ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19:1665–1681
Albrecht A, Heiser I, Baker R, Nemec S, Elstner EF, Osswald W (1998) Effects of the Fusarium solani toxin dihydrofusarubin on tobacco leaves and spinach chloroplasts. J Plant Physiol 153:462–468
Bednarek P, Schneider B, Svatos A, Oldham NJ, Hahlbrock K (2005) Structural complexity, differential response to infection, and tissue specificity of indolic and phenylpropanoid secondary metabolism in Arabidopsis roots. Plant Physiol 138:1058–1070
Benhamou N, Bélanger R (1998) Induction of systemic resistance to Pythium damping-off in cucumber plants by benzothiadiazole: ultrastructure and cytochemistry of the host response. Plant J 14:13–21
Campion C, Massiot P, Rouxel F (1997) Aggressiveness and production of cell-wall degrading enzymes by Pythium violae, Pythium sulcatum and Pythium ultimum, responsible for cavity spot on carrots. Eur J Plant Pathol 103:725–735
Chico JM, Chini A, Fonseca A, Solano R (2008) JAZ repressors set the rhythm in jasmonate signaling. Curr Opin Plant Biol 11:1–9
Cuming AC, Cho SH, Kamisugi Y, Graham H, Quatrano RS (2007) Microarray analysis of transcriptional responses to abscisic acid and osmotic, salt, and drought stress in the moss, Physcomitrella patens. New Phytol 176:275–287
Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL (1994) Arabidopsis mutants simulating disease resistance response. Cell 77:565–577
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Feussner I, Wasternack C (2002) The lipoxygenase pathway. Annu Rev Plant Biol 53:275–297
Frank W, Ratnadewi D, Reski R (2005) Physcomitrella patens is highly tolerant against drought, salt and osmotic stress. Planta 220:384–394
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Govrin E, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10:751–757
Gross P, Julius C, Schmelzer E, Hahlbrock K (1993) Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defence gene activation in infected, cultured parsley cells. EMBO J 12:1735–1744
Hoch HC, Galvani CD, Szarowski DH, Turner JN (2005) Two new fluorescent dyes applicable for visualization of fungal cell walls. Mycologia 97:580–588
Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P, Fincher GB (2003) An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell 15:2503–2513
Jiang C, Schommer CK, Kim SY, Suh DY (2006) Cloning and characterization of chalcone synthase from the moss, Physcomitrella patens. Phytochemistry 67:2531–2540
Kamoun S, Huitema E, Vleeshouwers VG (1999) Resistance to oomycetes: a general role for the hypersensitive response? Trends Plant Sci 4:196–200
Koch E, Slusarenko A (1990) Arabidopsis is susceptible to infection by downy mildew fungus. Plant Cell 2:437–455
Lang D, Eisinger J, Reski R, Rensing SA (2005) Representation and high-quality annotation of the Physcomitrella patens transcriptome demonstrates a high proportion of proteins involved in metabolism in mosses. Plant Biol (Stuttg) 7:238–250
Lang D, Zimmer AD, Rensing SA, Reski R (2008) Exploring plant biodiversity: the Physcomitrella genome and beyond. Trends Plant Sci 13:542–549
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Loake G, Grant M (2007) Salicylic acid in plant defence—the players and protagonists. Curr Opin Plant Biol 10:466–472
López MA, Bannenberg G, Castresana C (2008) Controlling hormone signaling is a plant and pathogen challenge for growth and survival. Curr Opin Plant Biol 11:420–427
Lorenzo O, Solano R (2005) Molecular players regulating the jasmonate signalling network. Curr Opin Plant Biol 8:532–540
Lucena MA, Romero-Aranda R, Mercado JA, Cuartero J, Valpuesta V, Quesada MA (2003) Structural and physiological changes in the roots of tomato plants over-expressing a basic peroxidase. Physiol Plant 118:422–429
Martin F (1994) Pythium. In: Komoto K, Singh US, Singh RP (eds) Pathogenesis and host specificity in plant diseases: histopathological, biochemical, genetic and molecular bases. Pergamon Press, Oxford, pp 17–36
Mellersh DG, Foulds IV, Higgins VJ, Heath MC (2002) H2O2 plays different roles in determining penetration failure in three diverse plant-fungal interactions. Plant J 29:257–268
Montesano M, Scheller HV, Wettstein R, Palva ET (2004) Down-regulation of photosystem I by Erwinia carotovora-derived elicitors correlates with H2O2 accumulation in chloroplasts of potato. Mol Plant Pathol 5:115–123
Morel JB, Dangl JL (1997) The hypersensitive response and the induction of cell death in plants. Cell Death Differ 4:671–683
Nishiyama T, Fujita T, Shin-I T, Seki M, Nishide H, Uchiyama I, Kamiya A, Carninci P, Hayashizaki Y, Shinozaki K, Kohara Y, Hasebe M (2003) Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution. Proc Natl Acad Sci USA 100:8007–8012
O’Donnell PJ, Schmelz E, Block A, Miersch O, Wasternack C, Jones JB, Klee HJ (2003) Multiple hormones act sequentially to mediate a susceptible tomato pathogen defense response. Plant Physiol 133:1181–1189
Pandey AK, Ger MJ, Huang HE, Yip MK, Zeng J, Feng TY (2005) Expression of the hypersensitive response-assisting protein in Arabidopsis results in harpin-dependent hypersensitive cell death in response to Erwinia carotovora. Plant Mol Biol 59:771–780
Pilloff RK, Devadas SK, Enyedi A, Raina R (2002) The Arabidopsis gain-of-function mutant Dll1 spontaneously develops lesions mimicking cell death associated with disease. Plant J 30:61–70
Ponce de León I, Oliver JP, Castro A, Gaggero C, Bentancor M, Vidal S (2007) Erwinia carotovora elicitors and Botrytis cinerea activate defense responses in Physcomitrella patens. BMC Plant Biol 8:52
Rensing SA, Lang D, Zimmer AD et al (2008) The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319:64–69
Saavedra L, Svensson J, Carballo V, Izmendi D, Welin B, Vidal S (2006) A dehydrin gene in Physcomitrella patens is required for salt and osmotic stress tolerance. Plant J 45:237–249
Sambrook J, Fitsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor
Sato Y, Wada M, Kadota A (2001) External Ca(2+) is essential for chloroplast movement induced by mechanical stimulation but not by light stimulation. Plant Physiol 127:497–504
Schaefer DG (2002) A new moss genetics: targeted mutagenesis in Physcomitrella patens. Annu Rev Plant Biol 53:477–501
Schaefer D, Zryd JP, Knight CD, Cove DJ (1991) Stable transformation of the moss Physcomitrella patens. Mol Gen Genet 226:418–424
Schmelz EA, Engelberth J, Tumlinson JH, Block A, Alborn HT (2004) The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J 39:790–808
Senger T, Wichard T, Kunze S, Gobel C, Lerchl J, Pohnert G, Feussner I (2005) A multifunctional lipoxygenase with fatty acid hydroperoxide cleaving activity from the moss Physcomitrella patens. J Biol Chem 280:7588–7596
Staswick PE, Yuen GY, Lehman CC (1998) Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J 15:747–754
Stumpe M, Bode J, Göbel C, Wichard T, Schaaf A, Frank W, Frank M, Reski R, Pohnert G, Feussner I (2006) Biosynthesis of C9-aldehydes in the moss Physcomitrella patens. Biochim Biophys Acta 1761:301–312
Sun MM, Li LH, Xie H, Ma RC, He YK (2007) Differentially expressed genes under cold acclimation in Physcomitrella patens. J Biochem Mol Biol 30:986–1001
Torres MA, Jones JD, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378
Toth IK, Bell KS, Holeva MC, Birch PRJ (2003) Soft rot erwiniae: from genes to genomes. Mol Plant Pathol 4:17–30
van Kan JAL (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci 11:247–253
van Loon LC, Geraats BP, Linthorst HJ (2006) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11:184–191
Vijayan P, Shockey J, Lévesque CA, Cook RJ, Browse J (1998) A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA 9:7209–7214
Wada M, Kagawa T, Sato Y (2003) Chloroplast movement. Annu Rev Plant Biol 54:455–468
Wichard T, Göbel C, Feussner I, Pohnert G (2004) Unprecedented lipoxygenase/hydroperoxide lyase pathways in the moss Physcomitrella patens. Angew Chem Int Ed 44:158–161
Acknowledgments
We gratefully acknowledge Roberto Solano (Centro Nacional de Biotecnología-CSIC, Spain) and Alicia Arias (IIBCE, Uruguay) for their generous gift of Pythium irregulare (Centro Nacional de Biotecnología-CSIC; Madrid, Spain) and Pythium debaryanum (collection INIA; Las Brujas, Uruguay), respectively. We thank Harvey C. Hoch (NYSAES, Cornell University, USA) for supplying us with the dye solophenyl flavine 7GFE500. We also thank Marcos Montesano (Facultad de Ciencias, UdelaR, Uruguay), Roberto Solano and Sabina Vidal (Facultad de Ciencias, UdelaR, Uruguay) for helpful discussion and critical reading of the manuscript. The authors thank DINACYT (Fondo Clemente Estable 9008) Uruguay, ANII (Fondo Clemente Estable FCE2007_376) Uruguay and UdelaR Uruguay/CSIC Spain (Joint project) for financial support. A. Castro was supported by a PEDECIBA fellowship, Uruguay. The Physcomitrella ESTs were obtained from the RIKEN Biological Research Center, Tsukuba, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors Juan Pablo Oliver and Alexandra Castro contributed equally to this work.
Rights and permissions
About this article
Cite this article
Oliver, J.P., Castro, A., Gaggero, C. et al. Pythium infection activates conserved plant defense responses in mosses. Planta 230, 569–579 (2009). https://doi.org/10.1007/s00425-009-0969-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-0969-4