Abstract
Arbuscular mycorrhizal fungi (AMF) reduce disease incidence of host plants through the competition of carbon sources and direct inhibition of pathogens, as well as through induction of biochemical and molecular responses. However, it is not known whether AMF enhance the resistance to Phytophthora parasitica-induced root rot in citrus and what the underlying mechanisms are. This study was carried out to analyze roles of Funneliformis mosseae (a mycorrhizal fungus) in plant defence responses of Poncirus trifoliata infected by P. parasitica. A week after the pathogen infection, mycorrhizal seedlings possessed higher expression of root mitogen-activated protein kinase 3 (PtMAPK3) regardless of P. parasitica infection. F. mosseae induced higher root salicylic acid (SA) concentrations, accompanied with up-regulation of SA synthesis genes (PtPAL1 and PtEPS1), regardless of being infected with P. parasitica or not. Jasmonic acid (JA) synthesis genes were down-regulated by mycorrhization in the absence of P. parasitica and up-regulated (except for PtAOC) by mycorrhization under P. parasitica infection. Moreover, F. mosseae stimulated higher expression of pathogenesis-related protein gene 1 (PtPR1), PtPR4, and PtPR5, especially under P. parasitica infection. F. mosseae inoculation increased levels of root lignin, calmodulin, and total soluble phenol and activities of root chitinase, phenylalanine ammonialyase, and β-1,3-glucanase, and decreased concentrations of root nitric oxide with or without P. parasitica infection. These results implied that F. mosseae elicited MAPKs cascades as well as SA- and calmodulin-mediated signal pathways to activate disease-defence genes, proteins, and compounds to early-warn P. parasitica infection for enhancing tolerance of root rot in trifoliate orange.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus is one of the widely planted economic fruit trees, of which China ranks first in the world in terms of planted area and production in citrus industry (He et al. 2020a). Citrus trees are vulnerable to various diseases in the field, including citrus root rot. Earlier studies had shown that citrus root rot was triggered mainly by Phytophthora parasitica in China (Zhu et al. 1993). In the early stage of infection, Phytophthora parasitica formed the haustoria, modified plant metabolic activities, and thus inhibited host defenses (O’Connell and Panstruga 2006). Pesticides are heavily used to mitigate the crop losses caused by P. parasitica which seriously damages the environment.
Symbiotic root endophytes can improve plant growth and enhance plant-disease defences (Gao et al. 2020; Yang et al. 2021). In particular, arbuscular mycorrhizal fungi (AMF), from the phylum Glomeromycota , build symbiotic associations with approximately 72% of terrestrial plants for increasing plant’s capacity to absorb nutrients beyond the nutrient depletion zone (Ferrol et al. 2019; Wu et al. 2019; Xie et al. 2020). Additionally, AMF improve the tolerance of plants in response to abiotic and biotic stress (Sarfir 1968; Xie et al. 2019; He et al. 2020b; Zhang et al. 2019, 2020; Zou et al. 2021). Inoculation with Funneliformis mosseae (formerly Glomus mosseae) significantly inhibited Pyrenochaeta infection and decreased the morbidity of red rot in Allium cepa (Sarfir 1968). Funneliformis mosseae and Rhizophagus irregularis collectively mitigated symptoms of Aphanomyces euteiches-induced root rot in pea (Slezack et al. 1999). Giovannetti et al. (1991) found that Glomus monosporum considerably reduced the formation of chlamydospores of Thielaviopsis basicola and the infection process in tobacco. As a result, it seems that mycorrhizalized plants have a high capacity to resist root rot, while the underlying mechanisms are not fully known.
Protein phosphorylation and de-phosphorylation processes are regulatory mechanisms involved in plant pathological processes (Pitzschke et al. 2009). Among them, protein phosphorylation is catalyzed by protein kinases including mitogen-activated protein kinases (MAPKs) (Meng and Zhang 2013). MAPK cascades have a dominant role in the amplification and transmission of signals (Aguilar et al. 2017) and thus help plants to resist pathogenic fungi, bacteria, nematodes, and insects (Wang et al. 2010).
Salicylic acid (SA) is known to be the primary signal in the regulation of plant immune responses (Chen et al. 2018). SA combines with SA-binding proteins (SABPs) to form SA–SABPs complexes, which can transfer the infection signal to trigger anaphylactic reactions (Alvarez 2000). Also, activation and interaction with transcription factors, such as non-expresser of pathogenesis-related gene 1 (NPR1), TGACG-binding factor (TGA), and WRKY (DNA binding transcription factors with highly conserved WRKYGDK domain), induced by the signal stimulus, increase the expression of PRs (Yu et al. 2001). On the other hand, jasmonic acid (JA) participates in the AMF-mediated resistance, while SA usually inhibits the biosynthesis of JA and JA-mediated defence responses (Robert-Seilaniantz et al. 2011). Nitric oxide (NO) stimulates SA accumulations, while SA induces NO production. It is not clear whether these signal responses are involved in AMF-induced tolerance against root rot.
The present work dually inoculated Funneliformis mosseae (an AM fungus) and P. parasitica (a root rot pathogen) on trifoliate orange (Poncirus trifoliata), and determined concentrations of root defence compounds, activities of root defence proteins, and relative quantities of root defence-related genes, to study the defence responses induced by AMF in trifoliate orange.
Materials and methods
Experimental design and microbial inoculations
The experiment consisted of two factors in a completely randomized blocked arrangement: the inoculation with or without F. mosseae and the infection with or without P. parasitica (Fig. 1). Each treatment was replicated five times, with three seedlings in each pot, resulting in a total of 20 pots (60 seedlings).
The AM fungal strain, F. mosseae (Nicol. & Gerd.) Schüßler and Walker, was propagated using white clover in pots, and the mycorrhizal fungal inoculum contained spores and the fungi-colonized root segments.
Three trifoliate orange seedlings having five leaves and without mycorrhization were transplanted into a 1.6-L pot. The pot contained 1.5 kg autoclaved mixture with soil and sand (5: 1, v/v) and 90 g of mycorrhizal inoculum. The treatment without AMF received an equivalent amount of autoclaved inocula plus 2 mL filtrate (25 µm filter) of mycorrhizal inocula.
Fourteen weeks after the mycorrhizal fungal inoculation, the pathogenic fungus P. parasitica was applied. Phytophthora parasitica was from the Citrus Research Institute, SWU/CAAS, Chongqing, China. The infected method of P. parasitica was followed with Cheng et al. (2020) in detail. A plug of sterile potato dextrose agar was placed onto the wound of root necks as the non-P. parasitica-infected controls. Both the P. parasitica-infected and non-P. parasitica-infected plants were covered with the moist sterile absorbent cotton. One week after the pathogen inoculation, the experiment ended and the treated plants were collected and divided into shoots and roots. Parts of roots were collected for the analysis of root AMF colonization rate, and the other roots were stored at – 80 °C after freezing in liquid nitrogen for the analysis of concentrations of SA, JA, NO, and calmodulin (CaM), activities of chitinase, β-1,3-glucanase, and phenylalanine ammonia-lyase (PAL), and expression of disease-related genes.
All the plants were placed in a greenhouse from April 8 to July 22, 2017, where the photon flux density ranged from 721 to 967 µmol/m2/s, with 25 °C/19 °C average day/night temperature and 68% relative air humidity.
Determinations of root mycorrhizal colonization
Root arbuscular mycorrhizas (10 root segments per plant and 5 plants per treatment) were stained with 0.05% trypan blue in lactoglycerol according to the protocol of Phillips and Hayman (1970). The microscopic observation was accorded to a fourfold objective lens multiplied by a 25-fold eyepiece. The diameter of one field was 300 scales. The extent of root mycorrhizal fungal colonization was calculated by He et al. (2020b).
Determinations of signal substances concentrations
The 0.2 g roots were ground in 1 mM phosphate buffers (pH 7.0) to extract NO, SA, and JA and homogenized in 0.6% NaCl solutions to extract CaM. The concentration of signal substances in roots was measured by double antibody sandwich-ELISA kits as per the user handbook (Shanghai Enzyme-linked Biotechnology Co., Ltd, Shanghai, China), along with the purified antibody of plant SA, JA, NO, and CaM as solid-phase antibody, horse radish peroxidase-labeled antibody, and the substrate 3,3′,5,5′-tetramethyl benzidine. The absorbance at 450 nm was determined by a Microplate Reader. All the determinations of the substances were repeated five times.
Determinations of disease-related compounds and proteins
Root total soluble phenol (TSP) and lignin concentrations were determined by the method of Kofalvi and Nassuth (1995) and Cahill and Mccomb (1992), respectively. Root β-1,3-glucanase and chitinase activities were determined by the protocol of Hu et al. (2017). Root PAL activity was carried out using the method described by Kofalvi and Nassuth (1995), where a unit of the enzyme activity was defined as the enzyme amount at 290 nm for a change of 0.01 unit of absorbance value per hour. All the above assays were replicated five times.
Relative expression of disease-related genes
We weighed 0.1 g of root samples, ground them in liquid nitrogen, and used an EASY spin Plus plant RNA kit to extract root total RNA. Then, 7 μL of RNA was reverse-transcribed into cDNA using a PrimeScript™ RT reagent kit with the gDNA eraser (Takara). qRT-PCR amplifications were conducted in a CFX96 Real-Time PCR Detection System (BIO-RAD) with the following composition: 3.5 μL sterile water, 0.5 μL cDNA, 5 μL SYBR GREEN PCR Master Mix (Applied Biosystem), 0.5 μL of 0.1 μM forward primer, and 0.5 μL of 0.1 μM reverse primer. These primers and gene ID for the selected genes, including pathogenesis-related (PR) genes (PtPR1, PtPR4, and PtPR5), phenylalanine ammonialyase 1 gene (PtPAL1), enhance enolpyruvylshikimate phosphate synthase 1 gene (PtEPS1), lipoxygenase gene (PtLOX), allene oxide synthase gene (PtAOS), allene oxide cyclase gene (PtAOC), and mitogen-activated protein kinase 3 gene (PtMPK3) were designed as per the Citrus sinensis database (Table 1). The relative gene expression was calculated using the 2−∆∆Ct method (Kenneth and Schmittgen 2001) in which β-actin acted as the control.
Statistical analysis
Data were subjected to the analysis of variance (SAS, v8.1), and significant differences between treatments were performed by the Duncan’s multiple range tests at the 0.05 level.
Results
Root mycorrhizal colonization
Root mycorrhizal colonization varied from 26.4 to 33.0%, while P. parasitica infection significantly inhibited root AMF colonization degree by 20%, compared with non-P. parasitica-infected mycorrhizal seedlings (Table 2).
Concentrations and activities of root defensive compounds
Root SA in mycorrhizal seedlings showed 33% higher concentrations under non-P. parasitica infection and 55% higher under P. parasitica infection, respectively, relative to non-mycorrhizal plants (Table 2). Compared with the non-AMF treatments, AMF inoculation distinctly reduced root JA concentrations of trifoliate orange by 43% under non-P. parasitica infection and 36% under P. parasitica infection. Compared with the non-AMF treatments, mycorrhizal inoculation significantly reduced root NO concentrations of trifoliate orange, whether infected with P. parasitica or not: 19% lower without P. parasitica infection and 11% lower with P. parasitica infection, respectively. In contrast, mycorrhizal plants showed significantly higher root CaM concentrations than control plants by 17% and 27% under non-P. parasitica infection and P. parasitica infection, respectively. Additionally, AMF inoculation induced significantly higher root TSP and lignin concentrations than non-AMF seedlings: 19% and 10% higher without P. parasitica infection and 28% and 31% higher with P. parasitica infection, respectively. Mycorrhizal colonization significantly increased root β-1,3-glucanase, chitinase, and PAL activities by 13%, 65%, and 37% without P. parasitica infection and by 13%, 61%, and 52% with P. parasitica infection, respectively.
Expression of root disease-related genes
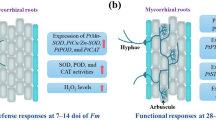
In the non-AMF-colonized plants, the PtPR4, PtLOX, and PtAOS expression was up-regulated by P. parasitica infection, whereas the PtPAL1 expression was down-regulated by P. parasitica infection (Fig. 2). In the mycorrhizal plants, P. parasitica infection up-regulated PtPR1, PtPR4, PtPR5, PtPAL1, and PtAOS gene expression levels, whereas down-regulated PtMAPK3 and PtAOC gene expression levels. Compared with non-AMF-colonized seedlings, AMF-colonized seedlings possessed 1182% and 240% higher expression levels of root PtMAPK3 in the absence and presence of P. parasitica infection, respectively. Compared with the non-AMF-colonized treatment, AMF treatment notably increased the expression of root PtPR1, PtPR4, and PtPR5 by 57%, 80% and 25% under the condition of non-P. parasitica infection, and 523%, 297% and 277% under the condition of P. parasitica infection. In contrast with the non-AMF-inoculated treatment, AMF-inoculated treatment notably increased the expression of root PtPAL1 and PtEPS1 by 107% and 210%, respectively, in trifoliate orange without P. parasitica infection. AMF inoculation also significantly promoted the expression of root PtPAL1 and PtEPS1 by 474% and 436% under P. parasitica infection, respectively, relative to non-mycorrhizal seedlings. Under non-P. parasitica infectious conditions, mycorrhizal inoculation reduced the expression of root PtLOX by 34%, in comparison with the non-mycorrhizal treatment. Apart from root PtAOC, which was down-regulated by 22%, the expression of the other two JA synthesis genes (PtLOX and PtAOS) was, respectively, increased by 56% and 23% under P. parasitica infection in mycorrhizal seedlings, compared with non-mycorrhizal seedlings.
Effects of Funneliformis mosseae inoculation and Phytophthora parasitica infection on the relative quantities of root defence-related genes in Poncirus trifoliata. The expression quantities of + F.m− P.p, − F.m + P.p, and + F.m + P.p treatments were compared with − F.m− P.p treatment. Data (means ± SD, n = 3) followed by different letters above the bars indicate significant differences (p < 0.05) between treatments. F.m inoculation with Funneliformis mosseae, and P.p infection by Phytophthora parasitica
Discussion
A week after the pathogen infection, there were relatively less visible decay areas in the root neck of AMF-colonized trifoliate orange plants than that of non-AMF-colonized plants, indicating the enhancement in tolerating root rot in mycorrhizal plants. The present work also showed that the pathogen infection with P. parasitica significantly reduced the root F. mosseae-colonized rate, indicating that P. parasitica had a suppressive role in mycorrhizal development. Similar result was reported by Ballhorn et al. (2014), who found that the infection of Colletotrichum gloeosporioides on leaves of soybean significantly reduced the colonization rate of AMF. Such inhibition might be the competition of carbon sources and infection sites between pathogens and beneficial endophytic fungi (Vos et al. 2014), thus leading to the decrease of mycorrhizal colonization rate.
Asai et al. (2002) found that the MAPKKK1-MAPKK4/5-MAPK3/6 cascade reaction chains were activated by flagellin which induced the expression of downstream WRKY22/WRKY29, and eventually activated the expression of relevant defence genes to enhance disease resistance. In the present study, mycorrhizal plants exhibited significantly higher expression of root PtMAPK3 than non-mycorrhizal plants regardless of P. parasitica infection, and AMF- and P. parasitica-infected plants presented the highest expression level. It seemed that AMF had stimulated effect on PtMAPK3 expression. Since SA generally restrains JA synthesis by up-regulating expression of WRKY transcription factors (WRKY62 and WRKY70), MAPK cascades keep in close contact with SA-mediated signal pathways (Dóczi et al. 2007). Hence, it was assumed from this study that MAPK cascades cross-talked with SA-mediated signal pathways by regulating expression of WRKY transcription factors. Furthermore, mycorrhizal fungal treatment increased root SA concentration but decreased root JA concentration under the condition of P. parasitica infection, indicating that mycorrhizal trifoliate orange plants preferred MAPK cascades and SA-mediated signal pathways to enhance the resistance to root rot.
The present work indicated that AMF inoculation dramatically increased root SA concentrations and the expression of root SA synthesis genes PtPAL1 and PtEPS1, regardless of the infection with or without P. parasitica. As reported by Zhang et al. (2013), AMF inoculation stimulated the phenolic synthesis through regulating SA signaling pathways. Park et al. (2009) reported that the SA-dependent signaling activated the expression of PRs in plants. In our study, AMF-inoculated treatment up-regulated the root PtPR1, PtPR4, and PtPR5 expression, especially in the plants with P. parasitica infection. Among PRs, PR1 has antifungal properties against several plant pathogenic fungi including Phytophthora spp. (Liu et al. 2019), and PR5 confers immunity from pathogen attacks (Monterio et al. 2003). Therefore, it was concluded that AMF induced expressions of PRs in host plants, which are of critical importance to resist root rot.
In addition to SA, JA also transfers the signal of pathogen attack to elicit plant defence responses (Sanders et al. 2000). Mehari et al. (2015) inoculated Botrytis cinerea on tomato plants and found that the plants were dependent on the JA response system to resist the pathogen. Besides, JA was involved in the resistance of Brassica napus to Plasmodiophora brassicae infection by Heteroconium chaetospira (Lahlali et al. 2014). While in the present work, compared with non-AM plants, AM plants without P. parasitica infection showed lower JA concentrations of roots, along with the down-regulation of PtLOX associated with JA synthesis. This is consistent with previous results conducted by Zhang et al. (2017), who found the inhibitive expression of PtLOX and the reduction of JA in trifoliate orange inoculated with three AMF species. In P. parasitica-infected plants, AMF treatment induced lower root JA concentrations but up-regulated the expression of root JA synthesis genes PtLOX and PtAOS (but not PtAOC). This suggests a distinct mechanism regarding the JA reduction after mycorrhization. SA and JA are antagonistic phytohormones that are controlled by transcription factors (Robert-Seilaniantz et al. 2011). The up-regulated expression of root PtMPK3 in P. parasitica-infected plants caused by mycorrhization indicated an activated response in SA–JA systems. Therefore, it was speculated that under the condition of P. parasitica infection, SA accumulation induced by AMF reversely inhibited JA synthesis genes in the downstream, which further influenced JA biosynthesis. Further studies still have to be analyzed focusing on the relation between JA and SA.
NO as a plant signal molecule activates defensive gene expression (Nagai et al. 2020). In the present work, AM plants presented lower root NO concentrations compared with non-AM plants in spite of the infection with and without P. parasitica. This might be because SA appears to activate antioxidant systems which inhibit NO production (Farivar and Brecher 1996). It is reported that AMF increased CaM (a signal in regulation of physiological processes) content to reduce the oxidative damage in drought-stressed citrus plants (Huang et al. 2014). The present study showed a significantly higher root CaM level in AMF-treated plants versus non-AMF-treated plants, regardless of P. parasitica infection. Xie et al. (2019) also observed the increase of CaM concentrations in roots of Xanthomonas amonopodis-infected trifoliate orange after inoculation with Paraglomus occultum. It suggests that mycorrhizal trifoliate orange is more likely dependent on CaM, but not NO, to transfer signals of the pathogen infection.
In this work, root chitinase and β-1,3-glucanase activities were induced by AMF inoculation irrespective of P. parasitica infection, indicating a better capacity to hydrolyze cell walls of P. parasitica in AM plants (Esquerré-Tugayé et al. 2000). Plant resistance to pathogenic fungi would be strengthened if the two enzymes work synergistically (Jongedijk et al. 1995; Hu et al. 2017). PAL takes part in the secondary metabolic process of phytoalexin, lignin, and phenolic compounds, and thus exerts the important role in resisting plant disease (Kim and Hwang 2014). In our study, AMF induced higher expression of root PtPAL1, accompanied with higher root PAL activities, no matter if infected with P. parasitica or not. Moreover, AMF induced significantly higher root TSP and lignin contents than non-mycorrhizal fungal treatment. Lignin increases the thickness of plant cell walls, constituting a mechanical barrier against pathogen invasion. Phenolic compounds restrain activities of pathogen polysaccharide enzymes, and accordingly reduce the destruction of host cell walls by pathogens further inhibiting pathogen infection (Du 2016). It was concluded that mycorrhizal trifoliate orange displayed higher PtPAL1 expression and subsequent PAL activities, which may accelerate the generation of TSP and lignin to suppress P. parasitica infection in citrus.
Conclusion
Funneliformis mosseae inoculation activated the MAPKs cascades to amplify and transfer the signals of P. parasitica infection and cross-talk with SA-mediated signal pathways, to enhance plant disease resistance via increased expression of defence genes, higher levels of disease-related proteins, and higher concentrations of other compounds implicated in disease resistance. As a result, in field, greater AM status in citrus plants will benefit the enhancement in resistance of root rot through field management. More studies under field conditions need to be undertaken to analyze AMF functioning on tolerating citrus root rot.
Author contribution statement
QSW and KK conceived the experiment. LT and YNZ conducted the experiment and analyzed the experimental data. LT wrote the first draft, and QSW revised the manuscript. All authors reviewed and approved the final draft.
References
Aguilar E, del Toro FJ, Canto T, Tenllado F (2017) Identification of MAPKs as signal transduction components required for the cell death response during compatible infection by the synergistic pair potato virus X-potato virus Y. Virology 509:178–184
Alvarez ME (2000) Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant Mol Biol 44:429–442
Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415:977–983
Ballhorn DJ, Younginger BS, Kautz S (2014) An aboveground pathogen inhibits belowground rhizobia and arbuscular mycorrhizal fungi in Phaseplus vulgaris. BMC Plant Biol 14:321
Cahill DM, Mccomb JA (1992) A comparison of changes in phenylalanine ammonia-lyase activity, lignin and phenolic synthesis in the roots of Eucalyptus calophylla (field resistant) and E. marginata (susceptible) when infected with Phytophthora cinnamomi. Physiol Mol Plant Pathol 40:315–332
Chen ZZ, Wang JG, Li Y, Zhong Y, Liao LG, Lu SG, Wang L, Wang XW, Chen SY (2018) Dry mycelium of Penicillum chrysogenum activates defense via gene regulation of salicylic acid and jasmonic acid signaling in Arabidopsis. Physiol Mol Plant Pathol 103:54–61
Cheng S, Tian L, Zou YN, Wu QS, Kuča K, Bora P (2020) Molecular responses of arbuscular mycorrhizal fungi in tolerating root rot of trifoliate orange. Not Bot Hortic Agrobot 48:558–571
Dóczi R, Brader G, Pettkószandtner A, Rajh I, Djamei A, Pitzschke A, Teige M, Hirt H (2007) The Arabidopsis mitogen-activated protein kinase kinase MKK3 is upstream of group C mitogen-activated protein kinases and participates in pathogen signaling. Plant Cell 19:3266–3279
Du XY (2016) Progress of phenylalanine ammoniase. J Mod Agric 7:24–26 (In Chinese)
Esquerré-Tugayé MT, Boudart G, Dumas B (2000) Cell wall degrading enzymes, inhibitory proteins, and oligosaccharides participate in the molecular dialogue between plants and pathogens. Plant Physiol Biochem 38:157–163
Farivar RS, Brecher P (1996) Salicylate is a transcriptional in hibitor of the inducible nitric oxide synthase in cultured cardiacfib rob lasts. Biol Chem 271:31585–31592
Ferrol N, Azcon-Aguilar C, Perez-Tienda J (2019) Arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: an overview on the mechanisms involved. Plant Sci 280:441–447
Gao WQ, Lu LH, Srivastava AK, Wu QS, Kuča K (2020) Effects of mycorrhizae on physiological responses and relevant gene expression of peach affected by replant disease. Agronomy 10:186
Giovannetti M, Tosi L, Torre GD (1991) Histological, physiological and biochemical interactions between vesicular-arbuscular mycorrhizae and Thielaviopsis basicola in tobacco plants. J Phytopathol 131:265–274
He JD, Chi GG, Zou YN, Shu B, Wu QS, Srivastava AK, Kuča K (2020a) Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Appl Soil Ecol 154:103592
He JD, Zou YN, Wu QS, Kuča K (2020b) Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci Hortic 262:108745
Hu N, Tu XR, Li KT, Ding H, Li H, Zhang HW, Tu GQ, Huang L (2017) Changes in protein content and chitinase and β-1,3-glucanase activities of rice with blast resistance induced by Ag-antibiotic 702. Plant Dis Pests 8:33–36
Huang YM, Srivastava AK, Zou YN, Ni QD, Han Y, Wu QS (2014) Mycorrhizal-induced calmodulin mediated changes in antioxidant enzymes and growth response of drought-stressed trifoliate orange. Front Microbiol 5:682–688
Jongedijk E, Tigelaar H, van Roekel JSC, Bres-Vloemans SA, Dekker I, van den Elzen PJM, Cornelissen BJC, Melchers LS (1995) Synergistic activity of chitinases and β-1,3-glucanases enhances fungal resistance in transgenic tomato plants. Euphytica 85:173–180
Kenneth JL, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and 2−ΔΔCt method. Methods 25:402–408
Kim DS, Hwang BK (2014) An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J Exp Bot 65:2295–2306
Kofalvi SA, Nassuth A (1995) Influence of wheat streak mosaic virus infection on phenylpropanoid metabolism and the accumulation of phenolics and lignin in wheat. Physiol Mol Plant Pathol 47:365–377
Lahlali R, McGregor L, Song T, Gossen BD, Narisawa K, Peng G (2014) Heteroconium chaetospira induces resistance to clubroot via upregulation of host genes involved in jasmonic acid, ethylene, and auxin biosynthesis. PLoS ONE 9:e94144
Liu Y, Liu QP, Tang YM, Ding W (2019) NtPR1a regulates resistance to Ralstonia solanacearum in Nicotiana tabacum via activating the defense-related genes. Biochem Biophys Res Commun 508:940–945
Mehari ZH, Elad Y, Rav-David D, Graber ER, Harel YM (2015) Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochae amendment involves jasmonic acid signaling. Plant Soil 395:31–44
Meng X, Zhang S (2013) MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol 51:245–266
Monteiro S, Barakat M, Piçarra-Pereira MA, Teixeira AR, Ferreira RB (2003) Osmotin and thaumatin from grape: a putative general defense mechanism against pathogenic fungi. Phytopathology 93:1505–1512
Nagai A, Torres PB, Duarte LML, Chaves ALR, Macedo AF, Floh ELS, de Oliveira LF, Zuccarelli R, dos Santos DYAC (2020) Signaling pathway played by salicylic acid, gentisic acid, nitric oxide, polyamines and non-enzymatic antioxidants in compatible and incompatible Solanum-tomato mottle mosaic virus interactions. Plant Sci 290:110274
O’Connell RJ, Panstruga R (2006) Tête à tête inside a plant cell: establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytol 171:699–718
Park JY, Jin J, Lee YW, Kang S, Lee YH (2009) Rice blast fungus (Magnaporthe oryzae) infects Arabidopsis via a mechanism distinct from that required for the infection of rice. Plant Physiol 149:474–486
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Pitzschke A, Schikora A, Hirt H (2009) MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol 12:421–426
Robert-Seilaniantz A, Grant M, Jones JD (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol 49:317–343
Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, Goldberg RB (2000) The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12:1041–1061
Sarfir GE (1968) The infiuence of vesicular arbuscular mycorrhiza on the resistance of onion to Phyrenochacta terreation. Thesis for M.S., University of Illinois, Urbaba
Slezack S, Dumas-Gaudot E, Rosendahl S, Kjoller R, Paynot MJ, Gianinazzi S (1999) Endoproteolytic activities in pea roots inoculated with the arbuscular mycorrhizal fungus Glomus mosseae and/or Aphanomyces euteiches in relation to bioprotection. New Phytol 142:517–529
Vos CM, Yang Y, De Cininck B, Cammue BPA (2014) Fungal (-like) biocontrol organisms in tomato disease control. Biol Control 74:65–81
Wang Y, Li J, Hou S, Wang X, Li Y, Ren D, Chen S, Tang X, Zhou JM (2010) A Pseudomonas syringae ADP-ribosyltransferase inhibits Arabidopsis mitogen-activated protein kinase kinases. Plant Cell 22:2033–2044
Wu QS, He JD, Srivastava AK, Zou YN, Kuča K (2019) Mycorrhizas enhance drought tolerance of citrus by altering root fatty acid compositions and their saturation levels. Tree Physiol 39:1149–1158
Xie MM, Zhang YC, Liu LP, Zou YN, Wu QS, Kuča K (2019) Mycorrhiza regulates signal substance levels and pathogen defense gene expression to resist citrus canker. Not Bot Hortic Agrobot 47:1161–1167
Xie MM, Zou YN, Wu QS, Zhang ZZ, Kuča K (2020) Single or dual inoculation of arbuscular mycorrhizal fungi and rhizobia regulates plant growth and nitrogen acquisition in white clover. Plant Soil Environ 66:287–294
Yang L, Zou YN, Tian ZH, Wu QS, Kuča K (2021) Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci Hortic 277:109815
Yu D, Chen C, Chen Z (2001) Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13:1527–1540
Zhang RQ, Zhu HH, Zhao HQ, Yao Q (2013) Arbuscular mycorrhizal fungi inoculation increases phenolic synthesis in clover roots via hydrogen peroxide salicylic acid and nitric oxide signaling pathways. J Plant Physiol 170:74–79
Zhang YC, Liu CY, Wu QS (2017) Mycorrhiza and common mycorrhizal network regulate the production of signal substances in trifoliate orange (Poncirus trifoliata). Not Bot Hortic Agrobot 45:43–49
Zhang YC, Zou YN, Liu LP, Wu QS (2019) Common mycorrhizal networks activate salicylic acid defense responses of trifoliate orange (Poncirus trifoliata). J Integr Plant Biol 61:1099–1111
Zhang F, Zou YN, Wu QS, Kuča K (2020) Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environ Exp Bot 171:103962
Zhu WS, Lan XY, Chen H, Su YC, Shen CR (1993) Study on citrus root rot. Acta Phytopathol Sin 2:7–8 (In Chinese with English abstract)
Zou YN, Zhang F, Srivastava AK, Wu QS, Kuča K (2021) Arbuscular mycorrhizal fungi regulate polyamine homeostasis in roots of trifoliate orange for improved adaptation to soil moisture deficit stress. Front Plant Sci 11:600792
Acknowledgements
This study was supported by the Hubei Provincial Department of Education (T201604).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Kuzniak-Gebarowska.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tian, L., Zou, YN., Wu, QS. et al. Mycorrhiza-induced plant defence responses in trifoliate orange infected by Phytophthora parasitica. Acta Physiol Plant 43, 45 (2021). https://doi.org/10.1007/s11738-021-03216-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-021-03216-2