Abstract
A number of recent reports suggest that the functional specialization of plant cells in storage organs can influence subcellular protein sorting, so that the fate of a recombinant protein tends to differ between seeds and leaves. In order to test the general applicability of this hypothesis, we investigated the fate of a model recombinant glycoprotein in the leaves and seeds of a leguminous plant, Medicago truncatula. Detailed analysis of immature seeds by immunofluorescence and electron microscopy showed that recombinant phytase carrying a signal peptide for entry into the endoplasmic reticulum was efficiently secreted from storage cotyledon cells. A second version of the protein carrying a C-terminal KDEL tag for retention in the endoplasmic reticulum was predominantly retained in the ER of seed cotyledon cells, but some of the protein was secreted to the apoplast and some was deposited in storage vacuoles. Importantly, the fate of the recombinant protein in the leaves was nearly identical to that in the seeds from the same plant. This shows that in M. truncatula, the unanticipated partial vacuolar delivery and secretion is not a special feature of seed cotyledon tissue, but are conserved in different specialized tissues. Further investigation revealed that the unexpected fate of the tagged variant of phytase likely resulted from partial loss of the KDEL tag in both leaves and seeds. Our results indicate that the previously observed aberrant deposition of recombinant proteins into storage organelles of seed tissue is not a general reflection of functional specialization, but also depends on the species of plant under investigation. This discovery will have an impact on the production of recombinant pharmaceutical proteins in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In plant cells, the endomembrane system differentiates in a tissue-specific manner, and is particularly complex in storage organs such as seeds, where it gives rise to a number of distinct storage organelles. In the endomembrane system of leaves, proteins lacking a specific targeting determinant follow the default pathway, which results in their secretion to the apoplast (reviewed by Vitale and Denecke 1999; Jurgens and Geldner 2002; Matheson et al. 2006). Proteins required elsewhere in the secretory pathway must therefore carry a targeting determinant to ensure they reach their correct destination (Pelham 1990; Neuhaus 1996; Vitale and Raikhel 1999). For example, recombinant proteins can be targeted to the endoplasmic reticulum (ER) by adding a C-terminal KDEL sequence. In some cases, this results in very efficient retrieval to the ER (Pagny et al. 2000; Sriraman et al. 2004) while in other cases some of the protein escapes the retrieval mechanism and passes through the Golgi (Ko et al. 2003; Triguero et al. 2005). Saturation of the KDEL receptor has also been demonstrated (Crofts et al. 1999).
In the case of seeds, most studies have focused on the formation of protein bodies and the targeting of seed storage proteins to protein storage vacuoles (PSVs). In a number of these studies, seed storage proteins have also been expressed in transgenic leaf cells to establish how the protein is deposited in protein storage vacuoles (Castelli and Vitale 2005). Some PSV sorting signals have been identified, but in addition to specific sequence motifs these may also comprise arrangements of hydrophilic/charged and hydrophobic amino acid residues on the protein surface (Neuhaus 1996). In the developing cotyledons of legumes and most other plants, the main storage proteins enter the ER, follow the secretory pathway to the Golgi apparatus and aggregate in PSVs. The synthesis and sorting of major and minor constituents of the PSV has been carefully studied in pea, showing that legumin, vicillin and SBP (sucrose binding protein) are sorted in the cis-cisternae of the Golgi complex and are transported via dense vesicles (Hillmer et al. 2001; Wenzel et al. 2005). In developing pumpkin cotyledons, storage proteins are transported directly from the ER to PSVs in dense precursor accumulating (PAC) vesicles (Hara-Nishimiura et al. 1998), and in wheat endosperm, glutenins are also transported to the storage vacuole bypassing the Golgi (Levanony et al. 1992). Recent reviews have summarized the various transport pathways to the PSV, thereby highlighting the typical transport mechanisms that are most prominent in seeds (Vitale and Hinz 2005; Robinson et al. 2005; Jolliffe et al. 2005).
Several recent reports have highlighted the intracellular localization of recombinant proteins expressed in seeds (Philip et al. 2001; Wright et al. 2001; Chikwamba et al. 2003; Yang et al. 2003; Arcalis et al. 2004; Nicholson et al. 2005; Drakakaki et al. 2006; Petruccelli et al. 2006; van Droogenbroeck et al. 2007). These studies have shown that recombinant proteins may be targeted in unexpected ways, which may therefore impact on the quality of such proteins expressed in storage tissues (Hood 2004). We recently compared the subcellular deposition of recombinant phytase in rice endosperm and leaves, concluding that the storage function of rice endosperm may determine whether or not the recombinant protein is secreted (Drakakaki et al. 2006). It is not clear whether such behavior is specific to the highly specialized cereal endosperm, or whether it occurs in the storage tissues of other plant species as well.
To address this question, we studied the deposition of recombinant phytase in the legume species Medicago truncatula. The mature seed of this plant species consists mainly of embryo tissue, and the cotyledons and embryo axis are the specialized storage tissues (Gallardo et al. 2003). We expressed phytase constitutively in transgenic M. truncatula plants, using an N-terminal signal peptide for entry into the secretory pathway. Versions of the protein were expressed with and without a C-terminal KDEL tag for ER retrieval. The untagged protein was secreted efficiently from cotyledon cells, whereas the KDEL variant became predominantly localized in the ER, although some leaked through to the apoplast and to the storage vacuole. We compare these results to those observed in the leaf tissues from the same plants, and discuss our findings in the context of previous studies involving recombinant proteins expressed in plants.
Materials and methods
Generation of transgenic material
Transformation of M. truncatula embryogenic line M9-10a (Neves et al. 1999) was carried out following the protocol described in Araujo et al. (2004) with slight modifications. The Agrobacterium strain used in this study was GV3101 and the Ti plasmid was pTRA (provided by Thomas Rademacher, Aachen) which includes a resistance to carbenicilin, therefore we used Timentin 500 mg/L to eliminate the Agrobacterium after the co-cultivation period (instead of carbenicilin). Transgenic plants were maintained in in vitro culture for several months until plants were transferred to soil to obtain seeds.
Preparation of total protein extract and partial purification of phytase
Soluble total protein extracts were obtained from leaves or seeds using maceration in liquid nitrogen. Extraction and partial purification was carried out by sequential precipitation with ammonium sulphate as described earlier (Arcalis et al. 2004). Proteins were separated by SDS-PAGE using 12% acrylamide gels stained with coomassie blue. For immunoblot analysis, anti-phytase anti-serum followed by anti-rabbit-phosphatase alkaline antibody (Sigma) was used.
Enzyme activity assay for phytase
Phytase activity in leaf samples was determined by detecting the release of free inorganic phosphate, as described by Lucca et al. (2001). Briefly, leaf extracts were diluted in 0.2 M imidazol–HCl, pH 6.5, and an equal volume of 10 mg/ml phytic acid was added. After defined time intervals at 37°C, aliquots of the reaction were removed, and the liberated phosphate was measured by the ammonium molybdate method. One FTU corresponds to 1 μmol of inorganic phosphate per min released from phytic acid.
Microscopy
Light and electron microscopy
Untransformed control and transgenic leaves, as well as control seeds and seeds expressing recombinant phytase were fixed in 4% (w/v) paraformaldehyde and 0.5% (w/v) glutaraldehyde in phosphate buffer (0.1 M, pH 7,4) overnight at 4°C. Samples were then dehydrated through an ethanol series and then infiltrated and polymerized in LR white resin. The combination of mild fixation and low temperature (−20°C) was necessary to increase the sensitivity of detection, but resulted in partial preservation of the tissue, and especially of membranes.
For light microscopy, 1 μm sections were stained in toluidine blue. For electron microscopy, sections showing silver interference colors were stained in 2% (w/v) aqueous uranyl acetate. The sections were observed using a Philips EM-400 transmission electron microscope (Philips, Eindhoven, The Netherlands).
Immunolabeling
Sections mounted either on glass slides for fluorescence microscopy or on gold grids for electron microscopy were preincubated in 5% (w/v) bovine serum albumin (BSA fraction V) in phosphate buffer (0.1 M, pH 7.4) and then incubated with polyclonal rabbit anti-phytase diluted 1:400 in phosphate buffer (0.1 M, pH 7.4). Sections were then treated with the secondary antibody diluted 1:30 in phosphate buffer (0,1 M pH 7.4), goat anti-rabbit Alexa Fluor 594 for fluorescence microscopy, and goat anti-rabbit labeled with 10 nm gold particles for electron microscopy. In order to identify the vacuoles in the cotyledon cells, sections were probed with polyclonal rabbit anti-α TIP and polyclonal rabbit anti-γ-TIP serum, diluted 1:400 in phosphate buffer 0.1 M pH 7.4.
Confocal microscopy
Developing seeds expressing recombinant phytase were fixed in 4% (w/v) paraformaldehyde overnight at 4°C. After several washes, 30 μm vibratome sections were placed on glass slides coated with 0.1% (w/v) poly-l-lysine (Sigma). Sections were dehydrated and rehydrated through an ethanol series and then treated with 2% (w/v) cellulase (Onozuka R-10 from Trichoderma viridae), Triton X-100 in phosphate buffer (0.1 M, pH 7.4) for 1 h and preincubated in 5% (w/v) BSA (fraction V) in phosphate buffer (0.1 M, pH 7.4). Tissue sections were then incubated with polyclonal rabbit anti-α-TIP serum (Jauh et al. 1999) diluted 1:400 in phosphate buffer (0.1 M, pH 7.4). The antigen–antibody binding reaction was revealed by applying goat anti-rabbit Alexa Fluor 594, diluted 1:30 in phosphate buffer (0.1 M, pH 7.4). Samples were then observed using a Leica TCS SP confocal microscope (Wetzlar, Germany).
Glycoanalysis
Glycosylation analysis of protein bands from SDS-PAGE was performed by treating the destained gel pieces with dithiotreitol, iodoacetamide, trypsin and peptide:N-glycosidase A and purifying the released glycans as described (Kolarich and Altmann 2000; Drakakaki et al. 2006). Extracted oligosaccharides were analyzed, with the kind permission of Prof. Dr. Gert Lubec (Medical University Vienna), on a Bruker Ultraflex MALDI-TOF/TOF mass spectrometer in positive, reflectron mode using 2,5-dihydroxybenzoic acid as matrix.
Results
Expression of recombinant phytase in Medicago plants
Medicago truncatula embryogenic line M9-10a (Neves et al. 1999) was transformed using Agrobacterium tumefaciens essentially as described by Araujo et al. (2004). Two constructs were introduced, both containing the phyA gene from Aspergillus niger under the control of the constitutive CaMV35S enhanced promoter, with an additional 5′ sequence encoding an N-terminal signal peptide for ER translocation. One construct had in addition a C-terminal KDEL tag for ER-retrieval. Eleven transgenic Medicago lines were selected, seven containing the untagged construct (SP-phy) and four containing the tagged construct (SP-phy-KDEL). Four representative lines were selected for further analysis, two expressing SP-phy (lines E and J) and two expressing SP-phy-KDEL (lines M and N). The phytase activities in these transgenic lines were 31.8 FTU/g (line E), 4.5 FTU/g (line J), 11.6 FTU/g (line M), and 122 FTU/g (line N), while no significant background activity was measured in the non-transgenic control plant. All transgenic plants appeared morphologically normal.
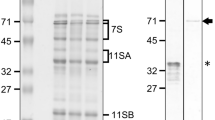
Total soluble protein was extracted from transgenic leaves and seeds, and western blots were carried out using a polyclonal anti-serum to detect the recombinant phytase. In the leaf extracts, phytase migrated as a double band (65–70 kDa) agreeing with previous results reported for the leaves of other plants (Ullah et al. 2002, 2003). There was no significant difference in molecular mass between SP-phy and SP-phy-KDEL (Fig. 1a). In the seed extracts, phytase migrated as a blurred band with a molecular mass of approximately 65 kDa (Fig. 1b).
SDS-PAGE and immunoblot of phytase from M. truncatula leaves and seeds. a Immunoblot detection of recombinant phytase in extracts from leaves of transgenic plants. Twenty micrograms of TSP (total soluble protein) were loaded in each lane. 1 Prestained protein marker; 2 line E expressing phytase SP-phy; 3 line J expressing phytase SP-phy; 4 line N expressing phytase SP-phy-KDEL; 5 line M expressing phytase SP-phy-KDEL. b Immunoblot detection of recombinant phytase in extracts from leaves (1) and seeds (2) of Line E. c Coomassie-stained SDS-PAGE of purified phytase. 1 Prestained protein marker; 2 purified phytase obtained from leaves of line N (SP-phy-KDEL); 3 Purified phytase obtained from leaves of line E (SP-phy). d Immunoblot corresponding to the gel shown in C using anti-phytase anti-serum. 1 Prestained protein marker; 2 purified SP-phy-KDEL from Line N; 3 purified SP-phy from line E. e Immunoblot corresponding to the gel shown in c using anti-KDEL anti-serum. 1 Prestained protein marker; 2 purified SP-phy-KDEL from line N; 3 purified SP-phy from line E. The phytase of higher molecular mass, which reacted with both anti-phytase anti-serum and anti-KDEL anti-serum, is marked by an asterisk
Medicago seed morphology
In order to study the deposition of phytase, it was necessary to characterize the cross sections of fixed and embedded Medicago seeds in detail using untransformed (supplementary Fig. S1) and transgenic seeds (Fig. 2). A cross section through the middle of the seed allows the radicle and cotyledons to be seen (Fig. 2a). Cotyledon cells were round and contained numerous electrondense vacuoles (2–4 μm) (Fig. 2b). The tonoplast surrounding these vacuoles was rich in the marker α-TIP (tonoplast intrinsic protein; Jauh et al. 1999) (Fig. 2c, d). In contrast, a serum against γ-TIP, which was used as a control, did not yield a signal (supplementary material S2b). These results were in line with the morphological appearance of the organelles, identifying them as PSVs. Each PSV was surrounded by a single layer of lipid droplets, with further droplets scattered within the cytoplasm and adjacent to the plasma membrane (Fig. 2b). M. truncatula cotyledon cells had thick cell walls and were loosely packed, revealing wide intercellular spaces (Fig. 2b). No morphological difference was observed between transgenic (Fig. 2b) and non-transformed cells (supplementary material S1b).
Morphology of cotyledon cells. a Seed cross section, line E stained with toluidine blue. Cotyledons (c), radicle (r). The outlined region corresponds to the area of the cotyledon studied in detail. b Cotyledon cell, line E (Electron microscopy after general non-specific contrasting). PSVs are highly electrondense and are surrounded by electrotransparent lipid droplets. Apoplast (apo), cell wall (cw), nucleus (n). c Localization of α-TIP in a cotyledon cell (Confocal optical section). Strong labeling is observed on the tonoplast of the PSVs. Nucleus (n). d Localization of α-TIP in a cotyledon cell (Electron microscopy). Note the gold probes decorating the tonoplast of the PSV (arrows). Bars: a, c 20 μm; b 1 μm, d 0.25 μm
SP-phy is secreted from Medicago cotyledons
In order to establish the exact location of SP-phytase in storage cotyledons, immunocytochemical detection of phytase was performed on seed sections from line E. The phytase signal was very clearly confined to the apoplast as expected for a secreted protein. No significant labeling was detected in any other compartment of the cotyledon cell (Fig. 3a). The distribution pattern was confirmed by electron microscopy. Thus, phytase accumulated in the apoplast, while the PSVs, cell walls and cytosol appeared mostly devoid of labeling (Fig. 3b).
Localization of SP-Phy (a, b) and SP-Phy-KDEL (c–e) in seed cotyledons. a Fluorescence microscopy. Phytase is secreted to the apoplast (arrow). Note the absence of signal within the cell. b Electron microscopy. Abundant gold probes decorate the apoplast (apo). Note the absence of labeling in the PSV, the cytoplasm, the lipid droplets (l), or in the cell wall (cw). c Fluorescence microscopy. Strong labeling can be seen within the cytoplasm (arrow heads). Significant signals are also visible in the PSVs (asterisk) and in the apoplast (arrows). d, e. Electron microscopy. Abundant gold particles are observed in the cytoplasm, where labeled ER-cisternae can be distinguished (c, arrow), and some signal is present in the apoplast (apo). Note the slight labeling within the PSV and the absence of labeling in the lipid droplets (l) or in the cell wall (cw). Bars: a, c 20 μm; b, d, e 0.5 μm
SP-phy-KDEL is mis-targeted in seeds and leaves
The localization of SP-phy-KDEL was studied in the developing cotyledons of line M (moderate expression) because the better performing line N failed to produce seeds. The phytase signal was detected in the cytoplasm between the PSVs, as expected for a protein accumulating in the ER. Surprisingly, labeling was also observed in the apoplast, although the signal was weaker than that seen in line E (Fig. 3c). Electron microscopy provided more details of the deposition pattern within the cotyledon cells. The abundant gold particles in the cytoplasm confirmed the presence of the recombinant protein within the ER. PSVs were labeled weakly, but no significant signal was detected in the lipid droplets or in any other intracellular compartment (Fig. 3d, e). Gold particles in the apoplast confirmed the unexpected secretion of phytase (Fig. 3d).
Given the unexpected localization of SP-phy-KDEL in seeds, we carried out the same localization study in leaves from lines M and N. The results were similar in both the lines, and those from line N are presented in Fig. 4. The phytase signal was clearly shown decorating ER-like structures in the cytoplasm, as would be expected for a KDEL-tagged protein. Surprisingly, the central vacuole also showed very strong labeling, indicating that the recombinant phytase was also delivered to this organelle (Fig. 4a). As in the seeds, SP-phy-KDEL was also seen to accumulate in the apoplast (Fig. 4b). Some unspecific background labeling was found in the chloroplasts.
Glycan profiles of SP-phy-KDEL
N-glycans are sequentially modified in different parts of the secretory pathway. The type of N-glycans found on a recombinant protein can therefore reveal its intracellular route. Small quantities of pure protein were obtained from phytase-enriched fractions of leaf extracts by sequential ammonium sulphate precipitation (Arcalis et al. 2004, Abranches et al. 2005). The protein was separated by SDS-PAGE and each band of the phytase doublet mentioned above was excised individually (Fig. 1c). The identity of each band was confirmed by peptide mass fingerprinting, and the extracted and dried tryptic peptides were then digested with PNGase A to release all the N-glycans.
Glycan masses were determined by MALDI-TOF mass spectrometry. The larger of the two SP-phy-KDEL forms (migrating at approximately 70 kDa) contained mainly oligomannosidic N-glycans with masses of 1,582 and 1,744 Da, respectively, corresponding to seven and eight mannose residues. Minor fractions included glycans bearing two N-acetylglucosamine residues at the non-reducing ends of the core (GnGnXF or GlcNAc2Man3(Xyl)(Fuc)GlcNAc2) or additionally with one or two Lewis a epitopes as often found attached to secreted plant glycoproteins (Fig. 5a).
N-glycan profiles on phytase bands: the two bands corresponding to phytase as isolated from line N were analyzed separately for their N-glycan profile by MALDI-TOF MS. Glycan structures were assigned on the basis of the molecular mass of the sodium adducts. a Oligomannosidic structures dominate in phytase of higher molecular mass, and in addition minor amounts of complex structures with terminal GlcNAc residues or Lewis a epitopes were identified. b The vacuolar type glycans MMX and MMXF were identified as the major N-glycan structures attached to phytase with the lower molecular mass
In contrast, the lower molecular mass phytase (migrating at approximately 65 kDa) contained mainly paucimannosidic-type N-glycans with either fucose and xylose (MMXF) or only xylose (MMX). These structures are representative of vacuolar proteins. Minor fractions included glycans typical of the cis-Golgi compartment (1,258 Da, Man5 or Man5GlcNAc2) (Fig. 5b).
The results from glycan analysis reflected precisely the immunolocalization data, suggesting that SP-phy-KDEL accumulates in the endoplasmic reticulum of leaf cells, but a proportion is diverted to the vacuole. The proportion carrying complex-type glycans with or without Lewis a epitopes probably corresponds to the phytase detected in the apoplast. These results prompted the question as to whether the KDEL sequence is still present on the mistargeted phytase. To address this question we subjected the phytase purified from line N to an immunoblot analysis using an anti-KDEL antibody. A comparison of Fig. 1D and E clearly demonstrates that the KDEL signal is only present on the higher molecular mass phytase glycoform, which contained mainly oligomannosidic glycan structures.
Discussion
To a certain extent, the final destination of a recombinant protein can be controlled by adding signals that the cell interprets as instructions for subcellular targeting. However, previous studies have shown that functional specialization of plant cells, particularly within storage tissues, results in deviations from the expected or “default” pathway (Philip et al. 2001; Wright et al. 2001; Chikwamba et al. 2003; Yang et al. 2003; Arcalis et al. 2004; Nicholson et al. 2005; Drakakaki et al. 2006; Petruccelli et al. 2006; van Droogenbroeck et al. 2007).
We have previously used different versions of the model fungal protein phytase to investigate recombinant protein accumulation and deposition in cereals. Phytase is a useful model because it is stable in all the plant cell compartments in which it has been expressed, is easily detected and has several N-glycosylation sites. In our previous studies, we have shown that a secreted version of phytase (SP-phy) behaves differently in rice endosperm compared to callus and leaf cells of the same plant. Specifically, SP-phy was secreted to the apoplast in leaf and callus cells as expected, but was retained in ER-derived prolamin bodies and in protein storage vacuoles in rice endosperm cells (Drakakaki et al. 2006). Similarly, in wheat endosperm cells, phytase also accumulated in storage organelles, and was not secreted to the apoplast (Arcalis et al. 2004). In contrast, efficient secretion of phytase had been demonstratd in tobacco leaves (Verwoerd et al. 1995).
Similarly unexpected results for other recombinant proteins expressed in storage tissues support the existence of tissue-specific protein sorting, especially in seeds. For example, Yang et al. (2003) expressed recombinant human lysozyme, and Nicholson et al. (2005) expressed a recombinant human immunoglobulin. In both cases, the proteins were expressed in rice seeds, and carried a signal peptide that should have ensured secretion. In the cases, the proteins accumulated inside the cell and were not secreted. Chikwamba et al. (2003) found that recombinant Escherichia coli heat-labile enterotoxin subunit B (LT-B) was deposited in the starch granules of maize seeds instead of being secreted by the default pathway. Furthermore, an antigenic glycoprotein from human cytomegalovirus was expressed in tobacco seeds and found in protein storage vacuoles (Tackaberry et al. 1999; Wright et al. 2001). These proteins also carried a signal peptide for secretion but no other known targeting determinant, and should have been secreted via the default pathway.
The intracellular deposition of all the proteins discussed above was attributed to the storage function of the seed tissues under investigation, especially cereal endosperm, which may be able to override the default pathway that directs proteins to the apoplast in leaf cells. Recently, Petruccelli et al. (2006) reported that a secretory version of a recombinant immunoglobulin was sorted to the matrix of PSVs in transgenic tobacco embryos, suggesting that the abnormal subcellular localization of secretory proteins in storage compartments may be a general feature of plants that can be extended to storage cotyledons and to the seeds of other plant species. We therefore set out to test this hypothesis by expressing recombinant secretory phytase in Medicago truncatula, a model legume whose cotyledons are specialized for protein storage. Our investigation revealed that storage cotyledons in Medicago seeds are perfectly capable of secreting recombinant phytase, showing that the specialization of protein sorting in storage tissues appears to be a species-dependent phenomenon.
The phytase signal was very clearly restricted to the intercellular space of the cotyledons, whereas no significant labeling was observed among the densely packed storage organelles, which we identified immunologically by labeling with antibodies against α-TIP, a well-established marker for the tonoplast of the PSV (Johnson et al. 1990; Jauh et al. 1999). Thus secretion of phytase appeared even more efficient than in mature leaves, where a minor portion of the recombinant protein was detected in the vacuole (Abranches et al. 2005).
For comparison, we included in our studies a KDEL-tagged version of phytase, which was predominantly retained in the ER. Even so, a portion of this SP-phy-KDEL was secreted and some was also delivered to storage vacuoles, even in leaf cells where efficient retention was anticipated. Interestingly, the very same subcellular localization was reported by Petruccelli et al. (2006) for a KDEL-tagged immunoglobulin in transgenic tobacco embryos. However, this unusual pattern was not observed in mature tobacco leaves, where the KDEL-tagged antibody was fully retained in the ER. Therefore the authors attributed the unusual deposition to specific characteristics of the protein sorting machinery in the endomembrane system of seeds. This conclusion cannot be reached in Medicago, where the fate of SP-phy-KDEL in leaves and seeds were very similar.
It has been documented previously that the KDEL-receptor may be saturated by the overload of proteins containing H/KDEL motifs (Crofts et al. 1999). Therefore we also confirmed the subcellular distribution of the recombinant protein in the leaves of a transgenic plant producing lower levels of SP-phy-KDEL. We could detect no difference in the distribution of the protein when comparing the high-expressing and low-expressing plants, which does not seem to support the saturation hypothesis in this case. Limited exposure of the KDEL sequence would be an alternative explanation, and the accessibility of the KDEL tag has previously been discussed as a potential explanation for differences in retrieval efficiency for different recombinant proteins (Sriraman et al. 2004). We also investigated the possible loss of the C-terminal KDEL sequence, and we could clearly show that the lower molecular mass phytase glycoform, containing vacuolar glycan structures with Golgi-specific modifications, was devoid of the KDEL sequence. It is therefore likely that the loss of the KDEL sequence accounts for the ability of some phytase to escape from the ER.
After escaping from the ER, a proportion of the SP-phy-KDEL population was obviously delivered to the vacuole. Diversion of KDEL-tagged proteins to the leaf vacuole has been noted occasionally, and a role for H/KDEL sequences in vacuolar sorting has been proposed for HDEL-tagged sporamin in BY2 suspension cells (Gomord et al. 1997). Similarly, Frigerio et al. (2001) reported that a mutant phaseolin protein carrying a KDEL signal was delivered to the vacuole of protoplasts, bypassing the Golgi complex. The pattern of phytase deposition in M. truncatula was confirmed by N-glycan analysis, the results of which were perfectly consistent with the immunolocalization data and allowed us to track the route of recombinant phytase through the cell. In contrast to the phaseolin study mentioned above, the SP-phy-KDEL from Medicago leaves contained Golgi-modified glycan structures, confirming the passage of phytase through the Golgi complex as indicated by immunohistochemical analysis. These glycans comprised mainly complex paucimannosidic structures indicative of vacuolar proteins, as well as diantennary structures with or without Lewis a epitopes, which are typical of extracellular proteins (Fitchette-Laine et al. 1997) and likely correspond to the phytase portion in the apoplast. Golgi-mediated vacuolar delivery of an endogenous HDEL-tagged protein has been shown for Bip, which is transported to the lytic vacuole via multivesicular bodies when HDEL-mediated retrieval from the cis-Golgi fails, and it has been speculated that this may occur due to a cryptic vacuolar sorting signal within the Bip sequence (Pimpl et al. 2006). A recombinant secretory IgA/G hybrid immunoglobulin was also detected in the vacuole after apparently passing through the Golgi (Frigerio et al. 2000) and this was also attributed to a cryptic vacuolar sorting signal in the alpha globulin chain (Hadlington et al. 2003).
A cryptic vacuolar targeting signal is rather unlikely to be present in our recombinant phytase given the fact that SP-phy lacking a KDEL sequence was secreted to the apoplast of storage cotyledon cells with no hint of vacuolar diversion. Although we have yet to determine the factors responsible for mistargeting SP-phy-KDEL, these factors are unlikely to reflect the specialization of storage tissues as suggested for other species, since the recombinant phytase behaves in largely the same manner in leaves. Indeed, the fact that phytase is secreted efficiently from storage cotyledon cells in M.truncatula is in complete contrast to the tendency towards intracellular retention seen in other species we, and others, have observed. It is therefore clear that functional specialization as a storage tissue does not preclude adherence to the default pathway for secreted proteins, but is more likely to depend on developmental and physiological factors specific for the species under investigation.
References
Abranches R, Marcel S, Arcalis E, Altmann F, Fevereiro P, Stoger E (2005) Plants as bioreactors: a comparative study suggests that Medicago truncatula is a promising production system. J Biotechnol 120:121–34
Araujo SS, Duque SRL, Dos Santos DM, Fevereiro PS (2004) An efficient transformation method to regenerate a high number of transgenic plants using a new embryogenic line of Medicago truncatula cv. Jemalong. Plant Cell Tiss Org Cult 78:123–131
Arcalis E, Marcel S, Altmann F, Kolarich D, Drakakaki G, Fischer R, Christou P, Stoger E (2004) Unexpected deposition patterns of recombinant proteins in post-endospermic reticulum compartments of wheat endosperm. Plant Physiol 136:3457–3466
Castelli S, Vitale A (2005) The phaseolin vacuolar sorting signal promotes transient, strong membrane association and aggregation of the bean storage protein in transgenic tobacco. J Exp Bot 56:1379–1387
Chikwamba RK, Scott MP, Mejia LB, Mason HS, Wang K (2003) Localization of a bacterial protein in starch granules of transgenic maize kernels. Proc Natl Acad Sci USA 100:11127–11132
Crofts AJ, Leborgne-Castel N, Hillmer S, Robinson DG, Phillipson B, Carlsson LE, Ashford DA, Denecke J (1999) Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell 11:2233–2248
Drakakaki G, Marcel S, Arcalis E, Altmann F, Gonzalez-Melendi P, Fischer R, Christou P, Stoger E (2006) The intracellular fate of a recombinant protein is tissue dependent. Plant Physiol 141:578–586
Fitchette-Lainé AC, Gomord V, Cabanes M, Michalski JC, Saint Macary M, Foucher B, Cavelier B, Hawes C, Lerouge P, Faye L (1997) N-glycans harbouring the Lewis a epitope are expressed at the surface of plant cells. Plant J 12:1411–1417
Frigerio L, Vine ND, Pedrazzini E, Hein MB, Wang F, Ma JK-C, Vitale A (2000) Assembly, secretion, and vacuolar delivery of a hybrid immunoglobulin in plants. Plant Physiol 123:1483–1493
Frigerio L, Pastres A, Prada A, Vitale A (2001) Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: selective use of an alternative route to vacuoles. Plant Cell 13:1109–1126
Gallardo K, Le Signor C, Vandekerckhove J, Thompson RD, Burstin J (2003) Proteomics of Medicago truncatula seed development establishes the time frame of diverse metabolic processes related to reserve accumulation. Plant Physiol 133:664–682
Gomord V, Denmat LA, Fitchette-Laine AC, Satiat-Jeunemaitre B, Hawes C, Faye L (1997) The C-terminal HDEL sequence is sufficient for retention of secretory proteins in the endoplasmic reticulum (ER) but promotes vacuolar targeting of proteins that escape the ER. Plant J 11:313–325
Hadlington JL, Santoro A, Nuttall J, Denecke J, Ma JK, Vitale A, Frigerio L (2003) The C-terminal extension of a hybrid immunoglobulin A/G heavy chain is responsible for its Golgi-mediated sorting to the vacuole. Mol Biol Cell 14:2592–2602
Hara-Nishimura I, Shimada T, Hatano K, Takeuchi Y, Nishimura M (1998) Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10:825–836
Hillmer S, Movafeghi A, Robinson DG, Hinz G (2001) Vacuolar storage proteins are sorted in the cis-cisternae of the pea cotyledon Golgi apparatus. J Cell Biol 152:41–50
Hood EE (2004) Where, oh where has my protein gone? Trends Biotechnol 22:53–55
Jauh GY, Phillips TE, Rogers JC (1999) Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 11:1867–1882
Johnson KD, Hofte H, Chrispeels MJ (1990) An intrinsic tonoplast protein of protein storage vacuoles in seeds is structurally related to a bacterial solute transporter (GIpF). Plant Cell 2:525–532
Jolliffe NA, Craddock CP, Frigerio L (2005) Pathways for protein transport to seed storage vacuoles. Biochem Soc Trans 33:1016–1018
Jurgens G, Geldner N (2002) Protein secretion in plants: from the trans-golgi network to the outer space. Traffic 3:605–613
Ko K, Tekoah Y, Rudd PM, Harvey DJ, Dwek RA, Spitsin S, Hanlon CA, Rupprecht C, Dietzschold B, Golovkin M, Koprowski H (2003) Function and glycosylation of plant-derived antiviral monoclonal antibody. Proc Natl Acad Sci USA 101:8013–8018
Kolarich D, Altmann F (2000) N-Glycan analysis by matrix-assisted laser desorption/ionization mass spectrometry of electro- phoretically separated nonmammalian proteins: application to peanut allergen ara h 1 and olive pollen allergen ole e 1. Anal Biochem 285:64–75
Levanony H, Rubin R, Altschuler Y, Galili G (1992) Evidence for a novel route of wheat storage proteins to vacuoles. J Cell Biol 119:1117–1128
Lucca P, Hurrell R, Potrykus I (2001) Genetic engineering approaches to improve the bioavailability and the level of iron in rice grains. Theor Appl Genet 102:392–397
Matheson LA, Hanton SL, Brandizzi F (2006) Traffic between the plant endoplasmic reticulum and golgi apparatus: to the golgi and beyond. Curr Opin Plant Biol 9:601–609
Neuhaus JM (1996) Protein targeting to the plant vacuole. Plant Physiol Biochem 34:217–221
Neves LO, Duque SRL, De Almeida JS, Fevereiro PS (1999) Repetitive somatic embryogenesis in Medicago truncatula ssp. Narborensis and M. truncatula Gaertn cv. Jemalong. Plant Cell Rep 18:398–405
Nicholson L, Gonzales-Menlendi P, van Dolleweerd C, Tuck H, Perrin Y, Ma JKC, Fischer R, Christou P, Stoger E (2005) A recombinant multimeric immunoglobulin expressed in rice shows assembly-dependent subcellular localization in endosperm cells. Plant Biotechnol J 3:115–127
Pagny S, Cabanes-Macheteau M, Gillikin JW, Leborgne-Castel N, Lerouge P, Boston RS, Faye L, Gomord V (2000) Protein recycling from the Golgi apparatus to the endoplasmic reticulum in plants and its minor contribution to calreticulin retention. Plant Cell 12:739–756
Pelham HR (1990) The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci 15:483–6
Petruccelli S, Otegui MS, Lareu F, Tran Dinh O, Fitchette AC, Circosta A, Rumbo M, Bardor M, Carcamo R, Gomord V, Beachy RN (2006) A KDEL-tagged monoclonal antibody is efficiently retained in the endoplasmic reticulum in leaves, but is both partially secreted and sorted to protein storage vacuoles in seeds. Plant Biotechnol J 4:511–527
Philip R, Darnowski DW, Maughan PJ, Vodkin LO (2001) Processing and localization of bovine β-casein expressed in transgenic soybean seeds under control of a soybean lectin expression cassette. Plant Sci 161:323–335
Pimpl P, Taylor JP, Snowden C, Hillmer S, Robinson DG, Denecke J (2006) Golgi-mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway. Plant Cell18:198–211
Robinson DG, Oliviusson P, Hinz G (2005) Protein sorting to the vacuoles of plants: a critical appraisal. Traffic 6:615–625
Sriraman R, Bardor M, Sack M, Vaquero C, Faye L, Fischer R, Finnern R, Lerouge P (2004) Recombinant anti-hCG antibodies retained in the endoplasmic reticulum of transformed plants lack core-xylose and core-α(1,3)-fucose residues. Plant Biotechnol J 2:279–287
Tackaberry ES, Dudani AK, Prior F, Tocchi M, Sardana R, Altosaar I, Ganz PR (1999) Development of biopharmaceuticals in plant expression systems: cloning, expression and immunological reactivity of human cytomegalovirus glycoprotein B (UL55) in seeds of transgenic tobacco. Vaccine 17:3020–3029
Triguero A, Cabrera G, Cremata JA, Yuen CT, Wheeler J, Ramirez NI (2005) Plant-derived mouse IgG monoclonal antibody fused to KDEL endoplasmic reticulum-retention signal is N-glycosylated homogeneously throughout the plant with mostly high-mannose-type N-glycans. Plant Biotechnol J 3:449–457
Ullah AH, Sethumadhavan K, Mullaney EJ, Ziegelhoffer T, Austin-Phillips S (2002) Cloned and expressed fungal PhyA gene in alfalfa produces a stable phytase. Biochem Biophys Res Commun 290:1343–1348
Ullah AH, Sethumadhavan K, Mullaney EJ, Ziegelhoffer T, Austin-Phillips S (2003) Fungal PhyA gene expressed in potato leaves produces active and stable phytase. Biochem Biophys Res Commun 306:603–609
Van Droogenbroeck B, Cao J, Stadlmann J, Altmann F, Colanesi S, Hillmer S, Robinson DG, Van Lerberge E, Terryn N, Van Montagu M, Liang M, Depicker A, De Jaeger G (2007) Aberrant localization and underglycosylation of highly accumulating single-chain Fv-Fc antibodies in transgenic Arabidopsis seeds. Proc Natl Acad Sci USA 104:1430–1435
Verwoerd T, Van Paridon P, Van Ooyen A, Hoekema A, Pen J (1995) Stable accumulation of Aspergillus niger phytase in transgenic tobacco leaves. Plant Physiol 109:1199–1205
Vitale A, Denecke J (1999) The endoplasmic reticulum—gateway of the secretory pathway. Plant Cell 11:615–628
Vitale A, Hinz G (2005) Sorting of proteins to storage vacuoles: how many mechanisms? Trends Plant Sci 10:316–323
Vitale A, Raikhel NV (1999) What do proteins need to reach different vacuoles? Trends Plant Sci 4:149–155
Wenzel D, Schauermann G, von Lupke A, Hinz G (2005) The cargo in vacuolar storage protein transport vesicles is stratified. Traffic 6:45–55
Wright KE, Prior F, Sardana R, Altosaar I, Dudani AK, Ganz PR, Tackaberry ES (2001) Sorting of glycoprotein B from the human cytomegalovirus to protein storage vesicles in seeds of transgenic tobacco. Transgenic Res 10:177–181
Yang D, Guo F, Liu B, Huang N, Watkins SC (2003) Expression and localization of human lysozyme in the endosperm of transgenic rice. Planta 216:597–603
Acknowledgments
The authors would like to thank Dr. John Rogers for providing polyclonal rabbit anti-α-TIP and anti-γ-TIP sera, Dr. Richard Twyman for critical assessment and help with manuscript preparation, and Dr. Thomas Rademacher for providing the pTRA vectors. The work was financially supported by DAAD and Fundacao para a Ciencia e Tecnologia, Portugal (POCI/BIA-BCM/55762/2004)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
425_2007_647_MOESM1_ESM.tif
Supplementary material S1: Morphology of untransformed cotyledon cells. a Seed cross section, stained with toulidine blue. Cotyledon cells packed with light blue stained vacuoles (arrows). b Cotyledon cell. (electron microscopy after general non-specific contrasting). PSVs are highly electrondense and are surrounded by electrotransparent lipid droplets (l). Cell wall (cw), nucleus (n). Bars a 5 μm, b 2 μm (TIF 4248 kb)
425_2007_647_MOESM2_ESM.tif
Supplementary material S2: Controls a Detection of phytase in WT leaves (Electron microscopy). No gold probes can be observed within the vacuole (v), chloroplast (chl). b Detection of gamma-TIP in cotyledon cells (Electron microscopy). No significant labeling is observed on the vacuole tonoplast (PSV). Cell wall (cw), lipid droplets (l). Bars 0.5 μm (TIF 1275 kb)
Rights and permissions
About this article
Cite this article
Abranches, R., Arcalis, E., Marcel, S. et al. Functional specialization of Medicago truncatula leaves and seeds does not affect the subcellular localization of a recombinant protein. Planta 227, 649–658 (2008). https://doi.org/10.1007/s00425-007-0647-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0647-3