Abstract
Wheat seed storage proteins (prolamins) are important for the grain quality because they provide a characteristic texture to wheat flour products. In wheat endosperm cells, prolamins are transported from the Endoplasmic reticulum to Protein storage vacuoles through two distinct pathways—a conventional pathway passing through the Golgi apparatus and an unconventional Golgi-bypassing pathway during which prolamins accumulate in the ER lumen, forming Protein bodies. Unfortunately, transport studies conducted previously achieved limited success because of the seed-specificity of the latter pathway and the multigene architecture of prolamins. To overcome this difficulty, we expressed either of the two families of wheat prolamins, namely α-gliadin or High-molecular-weight subunit of glutenin, in soybean seed, which naturally lacks prolamin-like proteins. SDS-PAGE analysis indicated the successful expression of recombinant wheat prolamins in transgenic soybean seeds. Their accumulation states were quite different—α-gliadin accumulated with partial fragmentation whereas the HMW-glutenin subunit formed disulfide-crosslinked polymers without fragmentation. Immunoelectron microscopy of seed sections revealed that α-gliadin was transported to PSVs whereas HMW-glutenin was deposited in novel ER-derived compartments distinct from PSVs. Observation of a developmental stage of seed cells showed the involvement of post-Golgi Prevacuolar compartments in the transport of α-gliadin. In a similar stage of cells, deposits of HMW-glutenin surrounded by membranes studded with ribosomes were observed confirming the accumulation of this prolamin as ER-derived PBs. Subcellular fractionation analysis supported the electron microscopy observations. Our results should help in better understanding of molecular events during the transport of prolamins in wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat storage proteins are major components of seed proteins in wheat and are known to be important factors in determining the quality of wheat grain. They have been extensively studied for several decades considering the fact that they form gluten during dough mixing and provide favorable textures to various wheat products (Shewry et al. 1995; Wieser 2007). These proteins mainly include gliadins and glutenins, which both belong to prolamins, a group of storage proteins associated with the grass family. They are hardly soluble in water or saline, similar to conventional prolamins, and have unique physicochemical properties distinct from each other. Gliadin is a monomeric protein characterized by its high viscosity and solubility in aqueous alcohols. Based on electrophoretic mobility and later on the basis of similarity of amino acid sequences, gliadin was classified into three major subtypes: α–gliadin, γ–gliadin, and ω–gliadin (Shewry et al. 1995; Wieser 2007). α–Gliadin and γ–gliadin, which account for more than half of the total gluten protein, consist of an N–terminal domain containing repetitive sequences enriched in proline and glutamine residues, and a globular C–terminal domain with several cysteine residues that form intramolecular disulfide bonds. In contrast, ω-gliadin is characterized by the absence of a cysteine-containing region. In contrast to the monomeric gliadin, glutenin is a polymeric protein that consists of a number of subunits crosslinked by intermolecular disulfide bonds. Polymers of glutenin subunits are viscoelastic and largely insoluble in aqueous alcohols unless their intermolecular bridges are broken under reducing conditions. Two different types of glutenin subunits are typically involved in the formation of such polymers: a 30–50 kDa Low-molecular-weight glutenin subunit (LMW-glutenin) and a 65–90 kDa High-molecular-weight glutenin subunit (HMW-glutenin) (Shewry et al. 1995; Wieser 2007). The HMW-glutenin subunit accounts for only a small part of the glutenin fraction compared with the LMW-glutenin subunit; their quantity and allelic composition, however, are closely related to the ability of glutenin to form large polymers and are thought to have a role in flour qualities, such as dough strength and breadmaking properties.
Seed storage proteins, including wheat prolamins, enter the endomembrane system. Generally, their nascent polypeptides are first targeted to the Endoplasmic reticulum (ER), where they are cotranslationally folded into their higher-order structures and begin to undergo post-translational modifications (Shewry et al. 1995). The folded proteins are then loaded into endomembrane transport pathways to specific cellular compartments for storage. In seed cells, storage albumins, storage globulins, and some prolamins are usually sorted into Protein storage vacuoles (PSVs). PSVs are specialized vacuolar compartments, commonly observed in seed cells, and differ from lytic vacuoles in vegetative tissues (Paris et al. 1996). In previous studies, it has been suggested that two different pathways mediate the transport of wheat prolamins to PSVs: a conventional Golgi-passing pathway of a branch of the anterograde secretory pathway and an unconventional Golgi-bypassing pathway. In the latter, prolamins aggregate in the ER lumen to form intermediate compartments called ER-derived Protein bodies (PBs), and are subsequently transported to PSVs by an unknown mechanism analogous to autophagy (Levanony et al. 1992). ER-derived PBs are usually described as organelles formed by massive accumulation of ER-resident proteins, and are also known as the final destination of prolamins of other cereals, such as maize and rice (Shewry et al. 1995).
Because of the involvement of multiple transport pathways and the multigene composition of natural wheat prolamins, it is difficult to elucidate how individual gliadins and glutenin subunits are sorted in the wheat endosperm. To overcome this difficulty, heterologous expression studies have been conducted using a variety of eukaryotic cell systems. Earlier studies expressing gliadins in yeast or Xenopus laevis oocytes showed that they were successfully targeted to their ER and entered the Golgi-mediated secretory pathway or accumulated in the ER lumen (Simon et al. 1990; Altschuler et al. 1993; Rosenberg et al. 1993; Altschuler and Galili 1994). Moreover, when various wheat prolamins were expressed in leaves or cells of transgenic tobacco, they formed structures similar to ER-derived PBs, and some of them were transported to vacuoles in a Golgi-dependent manner (Napier et al. 1997; Lombardi et al. 2009; Francin-Allami et al. 2011, 2013; Saumonneau et al. 2011). These studies indicate that some of the sorting machineries are conserved among various eukaryotic cells; however, plant seeds have unique transport pathways to PSVs (Herman et al. 2008; Arcalis et al. 2013), and Golgi-bypassing transport of prolamins also seems specific to seed cells (Bagga et al. 1995; Reyes et al. 2011). In view of these facts, extensive studies using seed systems are needed to understand the molecular events that occur during the transport of prolamins in wheat.
In the present study, we constructed transgenic soybean lines expressing a single wheat prolamin in a seed-specific manner to investigate its trafficking in seed cells. Soybean seeds are an advantageous host in that they lack endogenous prolamin genes and endomembrane protein transport in their cells is well characterized. We selected two different families of wheat prolamins, monomeric α-gliadin and highly oligomeric HMW-glutenin subunit, which themselves tend to be transported through the Golgi-passing pathway and the Golgi-bypassing pathway, respectively. Seeds of transgenic soybean lines successfully accumulated recombinant wheat prolamins. Subcellular localization analysis showed their targeting to different organelles: PSVs and ER-derived PBs. Based on these results, we conclude that singly expressed α-gliadin and HMW-glutenin are sorted differently in soybean seed cells, and have discussed the reasons for the difference.
Material and methods
Production of transgenic soybean
A cDNA clone from a wheat cultivar, Chinese Spring (GenBank accession: AK331110), which encodes the full-length protein sequence of an α-gliadin isoform (GenBank accession: ATL17013), was obtained from the cDNA library of KOMUGI, the wheat genetic resources database. A cDNA encoding the amino acid sequence of the HMW-glutenin Dy10 subunit (GenBank accession: AAU04841) was artificially synthesized with codon optimization for expression in soybean. These DNA fragments were inserted into the Xbal–Sacl site of a binary vector derived from pMDC123 (Yamada et al. 2014), placed downstream of the β–conglycinin α′ subunit promoter. Transformation of soybean was conducted according to the protocol previously described by Yamada et al. (2010). Briefly, the cotyledonary nodes of the soybean ‘Kariyutaka’ were wounded with a stainless-steel micro-brush and incubated with a suspension of Agrobacterium tumefaciens harboring the vector. Adventitious shoots were transferred to a selection medium containing Basta (Bayer Crop Science), and rooting explants were transplanted to the soil to obtain T1 seeds.
Plant material
Soybean plants were grown on gardening soil in pots placed in a greenhouse. The mature or developmental stages of the seeds were harvested and used for analysis.
Antiserum preparation
Rabbit antiserum against α-gliadin, HMW–glutenin 1Dy10 subunit, and two PDI family proteins, PDI-L1 (GenBank accession: BAG16714) and PDI-M (GenBank accession: NP_001236576), were prepared using recombinant proteins produced in Escherichia coli. An MBP-tag sequence was fused to the N–terminus of the recombinant HMW-glutenin subunit. Antisera against the β–conglycinin α′ subunit (Nishizawa et al. 2003), glycinin A1aB1b subunit (Yamada et al. 2014), and BiP (Mori et al. 2004) were also used in this study.
Protein extraction from soybean seeds
After removing seed coats and germs, dry soybean cotyledons were cut and vortexed with 50 µL/mg of extraction buffer (20 mM Tris–HCl pH 7.2, containing 10 g/L SDS and 2% v/v 2-mercaptoethanol). Samples were centrifuged at 10,000 × g for 5 min, and the supernatants were collected as total protein extracts. To investigate the effect of reducing agents on HMW-glutenin extraction, protein was extracted from dry transgenic seeds accumulating HMW-glutenin without 2-mercaptoethanol, and the extract was incubated at 37 °C for 1 h for protein degradation.
SDS-PAGE analysis
For conventional SDS-PAGE analysis, samples were mixed with SDS-PAGE sampling buffer (20 mM Tris–HCl pH 7.2, containing 10 g/L SDS, 2% v/v 2-mercaptoethanol, and 80 mg/L bromophenol blue), and the mixtures were boiled for 5 min. Samples for nonreducing SDS-PAGE were mixed with sampling buffer without 2-mercaptoethanol. Proteins were separated by SDS-PAGE using protein marker Protein MultiColor III (Biodynamics Laboratory Inc.). The patterns of total protein were analyzed by CBB staining. For western blot analysis, the proteins were transferred onto nitrocellulose membranes. The membranes were pre-incubated with PBSTM (20 mM sodium phosphate pH 7.6, containing 150 mM NaCl, 50 g/L nonfat dry milk [Megmilk Snow Brand], and 0.05% v/v Tween20) at room temperature for 30 min, and then treated with PBSTM containing rabbit antiserum against α-gliadin (1:5000 dilution), HMW-glutenin Dy10 subunit (1:50,000 dilution), or BiP (1:1000 dilution) at 25 °C for 60 min. They were rinsed with fresh PBSTM repeatedly and reacted with anti-rabbit IgG (Fc), AP conjugate secondary antibody (PROMEGA, 1:5000 dilution) in PBSTM at 25 °C for 60 min. After rinses with PBSTM and TBST (10 mM Tris–HCl pH 7.5 containing 150 mM NaCl and 0.05% v/v Tween20), the membranes were incubated with AP substrate-containing buffer (10 mM Tris–HCl pH 7.5 containing 0.33 mg/mL nitro blue tetrazolium chloride and 0.17 mg/mL 5–bromo–4–chloro–3–indolyl–phosphate) at 25 °C until adequate signals were detected.
Preparation of microscopy samples
For microscopy, soybean cotyledons were embedded in resin blocks after treatment with a conventional chemical fixation method. Small pieces, < 1 mm in length, were cut and incubated in chilled fixation buffer (10 mM sodium phosphate, pH 7.2, containing 40 g/L paraformaldehyde and 1% v/v glutaraldehyde) for 5 h. During the first 2 h of fixation, the tissues were degassed for better infiltration of fixatives. After fixation, the fixatives were removed by repeatedly exchanging the buffer with fresh 10 mM sodium phosphate buffer (pH 7.2). The fixed tissues were dehydrated in a series of ethanol gradient (from 30%–99.5% v/v) and sequentially infiltrated in LR white resin diluted with ethanol at a ratio of 1:2, 2:1, and 1:0. After a few days of infiltration, the samples were transferred into beam capsules filled with activator-containing resin, and the resin was polymerized by exposure to UV emission for 48 h at 4 °C.
Immunoelectron microscopy
Resin-embedded samples were sliced into 60 nm-thick ultra-thin sections using an ultramicrotome. The sections were collected on mesh nickel grids coated with formvar and evaporated carbon. They were pre-incubated with PBS (pH 7.2) containing 10 g/L bovine serum albumin (BSA) and were floated on a 30 µL droplet of BSA-PBST (PBS containing 10 g/L BSA and 0.1% v/v Tween20) containing primary rabbit antiserum at 25 °C for 1 h; the antiserum was specific to α-gliadin, HMW-glutenin, β-conglycinin α′ subunit, glycinin A1aB1b subunit, BiP, or PDIs, and was used at moderate dilutions. The serum-labeled sections were rinsed with BSA-PBST and incubated on a 30 µL droplet of BSA-PBST containing anti-rabbit IgG 15 nm gold particle conjugate (BBI solutions, 1:25 dilution) at 25 °C for 30 min. After sequential rinses with BSA-PBST, PBS, and DW, the immunolabeled sections were collected and subjected to electron staining with uranyl acetate and lead nitrate. Transmission electron microscopes H-7100 and H-7650 (Hitachi) were used for the examination. The developed films were scanned with an EPSON GT-X820 image scanner. The digital images obtained were modified using the GNU image manipulation program (GIMP) available at https://www.gimp.org.
Subcellular fractionation
Subcellular fractionation was carried out according to the method described by Wang et al. (2016), with some modifications. Essentially, 1 g of early to middle development stages of transgenic soybean cotyledons (weighing 200–300 mg) were ground in liquid nitrogen with a mortar and pestle, and suspended in 3 mL of suspension buffer (10 mM Tris–HCl, pH 7.2, containing 10 mM KCl, 1 mM DTT, 72 g/L sucrose, and either 5 mM MgCl2 or 5 mM EDTA) for 15 min. The suspension was filtered through four sheet-stacked gauze and centrifuged at 300 × g for 10 min. The resulting supernatant was separated on a discontinuous sucrose gradient (1.0–1.5–2.0 M, 3 ml each) with ultracentrifugation at 217,500 × g for 1 h using a CP 75β ultracentrifuge (Hitachi) and a P40ST swinging bucket rotor (Himac). Gradient buffers also contained 10 mM Tris HCl pH 7.2, 10 mM KCl, and either 5 mM MgCl2 or 5 mM EDTA. The resulting fractions collected from the bottom to top of the tubes (1 mL) were subjected to SDS-PAGE analysis.
Mass spectrometry analysis
For mass spectrometry analysis, a homogenate prepared from 9 g of transgenic cotyledon was subjected to sequential ultracentrifugation thrice in the presence of 5 mM EDTA. The first and second fractionations were performed as described above. For the third fractionation, samples were overlaid with five steps of discontinuous sucrose density gradient (40–45–50–55–60% g/g, 2 mL each) and were ultracentrifuged at 217,500 × g for 1 h. Before the second and third fractionation, samples containing HMW-glutenin were dialyzed with a gradient buffer containing 30% g/g sucrose. The protein sample was digested with trypsin and the resulting peptides were analyzed by LC–MS/MS. They were separated by liquid chromatography (LC) EASY-nLC II (Thermo Scientific) on a C18 column (0.075 × 150 mm, GL Sciences) using an acetonitrile gradient containing 0.1% formic acid as the mobile phase, and MS spectra were obtained with an LTQ Orbitrap Velos spectrometer (Thermo Scientific). The spectra were analyzed with Proteome Discoverer version.1.3 (Thermo Scientific). Mascot version.2.4 (Matrix Science) was used to search for the protein database of Glycine max and the genus Triticum.
Results
Construction of transgenic soybean lines
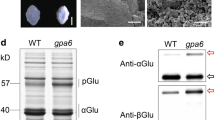
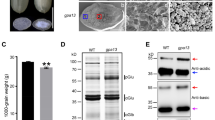
Agrobacterium-mediated transformation was performed to obtain transgenic soybean lines expressing wheat prolamins. For seed-specific expression, genes encoding α-gliadin or HMW-glutenin 1Dy10 subunits were placed under the control of the soybean β-conglycinin α′ subunit promoter (Fig. 1a). Transformed plants were selected based on their resistance to Basta. We named α-gliadin-expressing lines α-Gli and HMW-glutenin-expressing lines HMW-Glu, as described in the sections below. To confirm the accumulation of wheat prolamins in transgenic soybean, total protein extracts from dry seeds were analyzed by SDS–PAGE. CBB–stained gels did not show any clear differences in band patterns between the wild-type and transgenic lines (Fig. 1b); all three lines exhibited bands of subunits of β–conglycinin (7S globulin) and glycinin (11S globulin), which are major storage proteins of soybean seeds; however, no bands specific to the transgenic lines were found. However, immunoblotting using specific antisera indicated successful expression of the recombinant proteins (Fig. 1c). α-Gliadin was mainly detected as a band that migrated slightly less than that of the 32 kDa maker. This apparent molecular mass seems to correspond to the size of its mature form (33.4 kDa). Notably, an antiserum to α-gliadin also detected several minor bands, which mainly ranged from 27–32 kDa, forming a short ladder. The presence of such minor bands suggests that although α-gliadin was correctly synthesized in transgenic soybean, it was partially degraded into smaller fragments. The fragmentation was possibly mediated by endopeptidases in lytic compartments, such as vacuoles, as previously reported in transgenic tobacco expressing recombinant gliadins (Napier et al. 1997; Francin-Allami et al. 2011, 2013). One possibly-causal protease is asparagine-specific vacuolar processing enzyme (VPE), which in seeds usually has a role in maturation of vacuolar proteins, including legumin-like storage proteins (Yamada et al. 2020). In contrast to α–gliadin, HMW-glutenin was detected as a single band migrating near the 71 kDa marker band (Fig. 1c). Considering the molecular mass of its monomeric subunit (68.2 kDa), this band probably represents an intact monomer. The absence of HMW–glutenin in extracts without reducing agents suggests the formation of insoluble oligomers crosslinked by disulfide bonds (Fig. 1d). The possibility of non-specific cross-reactions was excluded because these antisera did not detect any immunoreactive band from the extracts of wild-type seeds.
SDS-PAGE analysis of protein extracts from transgenic soybean seeds. a Schematic representation of the vector construct used in this study. Genes for recombinant wheat prolamins were placed under the control of the seed-specific β-conglycinin α′ subunit promoter (p7SAP) and AtHSP terminator (TER). The Basta resistance gene cassette is indicated by Bastar. b The patterns of coomassie brilliant blue staining. Protein bands corresponding to soybean storage globulins are shown. c Western blot analysis using an antiserum specific for each wheat prolamin. The bands of mature α-gliadin and high-molecular-weight (HMW)-glutenin subunit are indicated by an asterisk and arrow, respectively. d Reducing or nonreducing SDS-PAGE of HMW-Glu seed extracts followed by western blot analysis. The presence or absence of 2-mercaptoethanol in extraction buffers and SDS-PAGE sampling buffers (2-MEext and 2-MEsmp, respectively) is indicated. The band of the HMW-glutenin subunit is indicated by an arrow. α: α-Gli line; H: HMW-Glu line; M: protein marker; W: wild-type control; 7S: β-conglycinin subunits; 11SA; acidic chains of glycinin subunits; 11SB: basic chains of glycinin subunits
Immunoelectron microscopy of mature seeds
Subcellular localization of α-gliadin and HMW-glutenin in transgenic soybean was investigated by immunoelectron microscopy of chemically fixed cotyledon cells. We first observed dry or entirely mature cotyledon cells, where storage proteins accumulate in their targeted compartments. Expectedly, antisera to α-gliadin or HMW–glutenin hardly immunolabeled wild-type cells (Supplementary Fig. S1). In contrast, the results of α-Gli and HMW-Glu seeds were clearly distinct. At these stages, α–Gli cotyledon cells showed normal cellular morphology, except that their PSVs had novel intravacuolar structures that had never been observed in wild-type cells. Immunolabeling of these structures with an antiserum to α-gliadin clearly indicated vacuolar targeting of this prolamin (Fig. 2a, b). These α-gliadin aggregates were < 1 to 7 µm in diameter, oval or non-spherical, and less electron-dense compared with the surrounding proteinaceous matrix of matured PSVs. Although their internal composition appeared largely uniform, they sometimes contained many electron-translucent inclusions (Supplementary Fig. S2). The morphological characteristics of α-gliadin aggregates described above made it easy to distinguish them from other intracellular compartments without any specific labeling. Thus, to better understand their nature and processes of formation, sections were also immunolabeled with an antiserum to the α′ subunit of β–conglycinin or the A1aB1b subunit of glycinin, which are components of soybean storage globulins. Storage globulins account for a major part of soybean seed protein and are massively stored in PSVs, forming a proteinaceous matrix. Both the antisera specifically immunolabeled the PSV matrix but hardly labeled the α-gliadin aggregates (Fig. 2c, d). These results suggest that recombinant α-gliadin accumulated in soybean PSVs, segregating from other vacuolar components, similarly to the natural prolamins of wheat and oat (Shewry et al. 1995). Such segregation might involve their solubility, surface hydrophobicity, and/or specific protein–protein interaction characteristics, as previously speculated for various types of seed storage proteins and recombinant proteins (Lending and Larkins 1989; Kim et al. 2002; Motoyama et al. 2010; Saberianfer et al. 2016; Arcalis et al. 2019).
Electron micrographs of mature α-Gli and HMW-Glu cotyledon cells. a–d Micrographs of mature stage of α-Gli cotyledon cells. a A cross-section of a cell accumulating α-gliadin. b Magnification of an α-gliadin-containing protein storage vacuole (PSV) on the upper right of a. c, d Intravacuolar segregation of α-gliadin from soybean globulins. e–h Micrographs of dry HMW-Glu cotyledon cells. e Accumulation of HMW-glutenin in cytosolic protein bodies (PBs). f A magnified clip of e. The corresponding region in e is outlined by a square. g, h Localization of endoplasmic reticulum (ER) chaperones to HMW-glutenin PBs. Sections were immunolabeled with an antiserum to α-gliadin (a, b), α′ subunit of β-conglycinin (c), A1aB1b subunit of glycinin (d), HMW-glutenin (e, f), BiP (g), or PDI-L1 (h). As well as the antisera to BiP and PDI-L1, that to PDI-M, another ER chaperone protein, also immunolabeled the PBs. Aggregates of α-gliadin and HMW-glutenin are indicated by asterisks. Gold particles immunolabeled to ER chaperones are indicated by arrowheads (g, h). CW: cell wall; N: nucleus; OB: oil body; SG: starch granule; V: PSV. Scale bars: (a) 10 µm; (b–h) 1 µm
In dry HMW-Glu cotyledon cells, newly formed structures specifically immunolabeled with an antiserum to HMW-glutenin were observed. These structures were 1–2 µm in diameter, nonspherical, and had a nearly uniform electron density similar to that of α-gliadin aggregates. However, unlike the gliadin aggregates, they were localized not in PSVs but in the cytosol (Fig. 2e, f). The HMW-glutenin subunit gene was designed to contain its native signal peptide, and the result of nonreducing SDS–PAGE suggested its oxidative folding in the ER lumen (Fig. 1d). Thus, these cytosolic aggregates seemed to be analogs of ER-derived prolamin PBs in cereal grains. However, electron microscopy at this stage showed no clear evidence of the presence of ER membranes surrounding the putative PBs, which is a prominent feature of conventional PBs. To clarify the origin of the structures, sections were immunolabeled with antisera to ER-resident chaperones, BiP or Protein disulfide isomerases (PDIs). As shown in Fig. 2g and h, both the antisera frequently immunolabeled the HMW-glutenin structures. Such labeling suggests they originate from the ER. Altogether, it was concluded that in transgenic soybean cotyledon cells, recombinant α-gliadin and HMW-glutenin are targeted to different endomembrane organelles; the former accumulates in PSVs while the latter accumulates in cytosolic PBs.
Immunoelectron microscopy of developing seeds
Observation of mature α-Gli cotyledon cells revealed the targeting of α-gliadin to soybean PSVs. However, there are multiple possible pathways from the ER to vacuoles, and it remains unclear how recombinant α-gliadin is transported to the PSVs. Thus, we performed extensive immunoelectron microscopy of an early-middle development stage of α-Gli cotyledon cells, in which expression of the recombinant wheat prolamin was expected to be upregulated. Immunolabeling of α-gliadin showed that this prolamin already started to accumulate in PSVs as in mature cells (Fig. 3a, b). The antiserum also labeled small aggregates (0.2–1 µm in diameter) localized in the prevacuolar compartments (PVCs; Fig. 3c). PVCs are intermediate organelles where endomembrane cargos accumulate before they are finally released into the vacuolar lumen by membrane fusion (Mori et al. 2004; Tse et al. 2004). PVCs of α–Gli seeds were apparently indistinguishable from those of wild-type seeds, except that some of them contained the prolamin aggregates. They also contained electron-dense aggregates of soybean storage globulins (Fig. 3d, e). Differences between α-gliadin and storage globulins aggregates in electron densities and immunolabeling specificity suggest their segregation in PVCs as well as in PSVs. Intriguingly, however, the two types of storage protein aggregates were sometimes associated (Fig. 3c–e). At this stage, the amount of these globulins in the PVCs appeared to greatly exceed that of α-gliadin. However, when the interior of PSVs was examined, α–gliadin aggregates sometimes had a good scale of size or amount comparable to storage globulin deposits (Fig. 3a). Despite observation of a number of sections, we unfortunately failed to clarify whether these large α-gliadin aggregates were also formed by transport through PVCs. This imbalance between PVCs and PSVs may be due to the promoter activity of the β-conglycinin α′ subunit, which terminates earlier than that of other storage globulin subunits. To compare patterns of immunolabeling among different proteins, subcellular localizations of ER-localized proteins were also investigated. When sections of developing α-Gli seeds were immunolabeled with an antiserum to BiP or PDIs, as expected, the antisera immunolabeled the ER lumen (Supplementary Fig. S3a, c). As shown in Supplementary Fig. S3b and d, a few gold particles were also observed in α-gliadin aggregates and globulin matrices inside PSVs, but such labeling was scarce.
Electron micrographs of developing α-Gli and HMW-Glu cotyledon cells.a–f Micrographs of early-middle stages of α-Gli cotyledon cells. a Accumulation of α-gliadin in a large PSV. b A high-magnification clip of an α-gliadin aggregate observed in a. c A prevacuolar compartment (PVC) containing a small α-gliadin aggregate. d, e Colocalization of α-gliadin and soybean globulins in the same PVCs. f Golgi apparatus immunolabeled with an antiserum to α-gliadin. g–l Micrographs of similar stages of HMW-Glu cotyledon cells. g HMW-glutenin PBs surrounded by ER membranes. h A high-magnification image of a HMW-glutenin PB attached by ribosome-like particles. i A magnified clip of h. j Localization of BiP to HMW-glutenin PBs. k, l Localization of soybean globulins to HMW-glutenin PBs. Sections were immunolabeled with an antiserum to α-gliadin (a–c, f), α′ subunit of β-conglycinin (d, k), A1aB1b subunit of glycinin (e, l), HMW-glutenin (g–i), or BiP (j). Aggregates of α-gliadin and HMW-glutenin are indicated by asterisks. Gold particles in d–f and j–i are indicated by arrowheads. Some of the visible ribosome-like particles on the surface of the PBs are indicated by arrows (h, i). The corresponding regions in a and h shown as magnified clips of (b and i) are outlined by a square. CW: cell wall; ER: endoplasmic reticulum; G: Golgi apparatus; OB: oil body; SG: starch granule; V: PSV. Scale bars: (a) 5 µm; (b–l) 500 nm
In Arabidopsis and tobacco cells, PVCs are also regarded as Multivesicular bodies (MVBs), post-Golgi compartments formed through endosome maturation (Tse et al. 2004; Scheuring et al. 2011). PVCs of soybean seeds are also post-Golgi compartments, and they play a major role in the transport of storage proteins to PSVs (Mori et al. 2004). Consistently, some cisternae of the Golgi apparatus and Golgi-associated vesicles were also immunolabeled with the antiserum against α-gliadin (Fig. 3f; Supplementary Fig. S4a, b). The labeling on the Golgi-related structures was weak and infrequent, possibly due to the low concentrations of recombinant α-gliadin. However, it still seemed specific, because they were apparently absent in wild-type cells (Supplementary Fig. S4c, d). The involvement of the Golgi apparatus in vacuolar transport of wheat prolamins has been suggested in a number of previous studies, while its contribution in wheat endosperm cells appears limited to the earliest stages of grain development (Parker 1982; Kim et al. 1988; Stenram et al. 1991; Levanony et al. 1992; Loussert et al. 2008; Tosi et al. 2009). Previous studies also revealed that the seeds of some plants, including wheat and soybean, have unconventional trafficking pathways in which storage proteins aggregate in the lumen of rough ER and are subsequently transported to PSVs, bypassing the Golgi apparatus (Levanony et al. 1992; Kinney et al. 2001; Mori et al. 2004). In α–Gli seeds, however, no α–gliadin aggregate was observed in the ER. In summary, the results suggest that the Golgi-passing pathway has a central role in vacuolar transport of α-gliadin in transgenic soybean seeds, as well as in transgenic tobacco (Francin-Allami et al. 2013).
To investigate how HMW–glutenin PBs are formed in transgenic soybean, an early-middle developing stage of HMW-Glu cotyledon cells was also observed. At this stage, HMW–glutenin was observed as aggregates outside PSVs as well as at the mature stage (Fig. 3g; Supplementary Fig. S5a). These aggregates were already compatible with the PBs of dry seeds in maximum size (< 2 µm in diameter), and notably, appeared to be enclosed by membranous structures partly or entirely studded by ribosomes (Fig. 3h, i). The presence of such surrounding membranes, as well as specific immunolabeling with an antiserum to BiP (Fig. 3j), suggests the accumulation of HMW-glutenin in the ER lumen forming ER-derived PBs. As shown in Fig. 3g–l, some of the PB membranes were tightly bound to the internal HMW–glutenin aggregates, similar to those of typical prolamin PBs. However, others expanded abnormally (> 3 µm in maximum diameter) and were detached from the prolamin deposits, forming a large electron-translucent luminal space (Supplementary Fig. S5b, c). Such unusual PBs might be artifacts resulting from poor fixation of samples, or analogs of small spherical PBs in vesicular ERs in rice (Bechtel and Juliano 1980). Further studies are required to elucidate their true origin. Antisera to vacuolar-targeted soybean storage globulins also immunolabeled the PBs (Fig. 3k, l), even though the frequencies of gold particles seemed much lower than those in PSVs or PVCs. Such labeling is possibly the result of nonspecific trapping of ER-resident proteins into PBs, as previously shown in transgenic tobacco cells (Saberianfar et al. 2015).
Subcellular fractionation analysis
To confirm the accumulation of recombinant HMW-glutenin in ER-derived PBs by biochemical experiments, subcellular fractionation analysis was performed. Homogenates of developing HMW-Glu cotyledons were ultracentrifuged on a three-step discontinuous sucrose density gradient, and the resulting fractions were analyzed by SDS-PAGE. The results showed that a major part of storage globulins, which is a marker of PSVs and PVCs, was concentrated in the light fractions up to the interface between gradient buffers containing 1.0 or 1.5 M sucrose (Fig. 4a, fraction 1 to 4 in the upper image). In contrast, HMW-glutenin was mainly detected in fractions containing 2.0 M sucrose (Fig. 4a, fraction 8–10 in the lower image). Sedimentation of recombinant HMW-glutenin into the heavy fractions clearly indicated its accumulation independent of PSVs. The effect of metal ion chelation was also examined to demonstrate the binding of ribosomes to PB membranes. Figure 4a shows the pattern of fractionation in the presence of Mg2+, which stabilizes the binding of ribosomes to ER membranes. Under the condition, BiP, an ER marker protein, showed two discontinuous peaks: it was detected not only from the relatively light fractions, but also from the heavy fractions containing HMW-glutenin (Fig. 4a, fractions 1–4 and 7–9 in the middle image, respectively). In contrast, in the presence of EDTA, a chelating agent that reduces the binding of ribosomes to the ER by removing the ions from the solutions, BiP was excluded from the heavy fractions to form a continuous pattern (Fig. 4b, fraction 1–7 in the middle image). The addition of EDTA also affected the pattern of HMW-glutenin. It was distributed over a broader range of fractions, although it was still enriched in the heaviest fractions and was largely separated from storage globulins (Fig. 4b, the upper and lower images, fraction 4–10 for HMW-glutenin, and 1–4 for storage globulins). These results, as well as immunoelectron microscopy observations, suggest the formation of HMW-glutenin PBs and binding of ribosomes to their membranes.
Subcellular fractionation of HMW-Glu cotyledon homogenates. a, b The fractionation pattern in the presence of MgCl2 (a) or EDTA (b). Cellular homogenates were fractionated by ultracentrifugation on three steps of a discontinuous sucrose gradient (1.0–1.5–2.0 mol⋅L−1). Proteins in the resulting fractions were separated by SDS-PAGE and were analyzed by coomassie brilliant blue staining or western blotting using antisera against BiP and HMW-glutenin (HMW-Glu). H: homogenates before fractionation
Furthermore, we carried out three rounds of continuous fractionation of HMW-Glu homogenates to purify HMW-glutenin PBs. Some of the resulting fractions were positive for immunoblotting with an antiserum against HMW-glutenin. LC/MS/MS analysis of these fractions indicated that they contained recombinant prolamin (Supplementary Fig. S6). However, a considerable level of signal from vacuolar proteins was detected as contaminants, suggesting the incompleteness of organelle isolation.
Discussion
The transport of wheat storage prolamins is highly complicated and difficult to understand. Heterologous expression is a powerful approach that simplifies the matter and allows ontarget studies based on established knowledge or experimental systems. Several plant hosts, including nonseed tobacco tissue and cereal endosperm, have been used to investigate the trafficking of gliadins and glutenin subunits (Shani et al. 1994; Napier et al. 1997; Lombardi et al. 2009; Francin-Allami et al. 2011, 2013; Saumonneau et al. 2011; Zhang et al. 2013; Oszvald et al. 2014). However, to the best of our knowledge, this is the first study to investigate how wheat prolamins are transported in the cells of dicotyledonous seeds, which naturally do not accumulate prolamin proteins. Notably, soybeans express storage proteins in living cotyledonous parenchyma cells, while wheat does so in starchy endosperm cells, which finally undergo programmed cell death. Nevertheless, the cells are similarly capable of accumulating substantial storage protein mass, and largely share machineries to sort endomembrane proteins. Therefore, the usage of the soybean expression system could facilitate the understanding of prolamin transport in wheat.
In wheat endosperm, gliadins and glutenins are targeted to PSVs via two distinct pathways: a Golgi-passing pathway and a Golgi-bypassing pathway forming ER-derived PBs (Levanony et al. 1992). On the contrary, in transgenic soybean seeds, only α–gliadin accumulated in PSVs, probably depending on the former pathway, and the HMW-glutenin subunit was deposited in the ER as cytosolic PBs without subsequent transport to PSVs. Similar trafficking of these prolamins has been reported in nonseed tissues of transgenic tobacco (Saumonneau et al. 2011; Francin-Allami et al. 2013), and is thought to be caused by their different molecular properties. During endomembrane transport of newly synthesized prolamins, PB formation is one of the most important events because it not only inhibits their entrance into the Golgi-passing pathway but is the first step of the Golgi-bypassing pathway in seeds. Its dependency on the efficient assembly of luminal proteins has been suggested in a number of studies, and owing to this ability, α-gliadin and HMW-glutenin are quite different. Whereas gliadin is a monomeric protein, HMW-glutenin is highly oligomeric and its subunits can form more than two intermolecular disulfide bonds, which are required for chain branching and formation of large oligomers (Shewry et al. 1995; Wieser 2007). Such superiority in oligomerization ability discriminates them not only from monomeric gliadins but also from LMW-glutenin subunits, which usually form only two intermolecular bonds for linage extension of oligomers (Wieser 2007). These intermolecular bonds facilitate ER retention or PB formation of prolamins, probably by stabilizing their assembly (Altschuler and Galili 1994; Pompa and Vitale 2006; Mainieri et al. 2014). In fact, when various wheat prolamins, including α-gliadin, γ-gliadin, LMW-glutenin subunit, and HMW-glutenin subunit, were singly expressed in transgenic tobacco, their long-term stability in the ER was dependent on the ability to form the bonds (Saumonneau et al. 2011; Francin-Allami et al. 2013). In addition, among these wheat prolamins, only the HMW-glutenin subunit showed complete retention in the ER. In the present study, the HMW-glutenin subunit in transgenic soybean formed insoluble oligomers crosslinked through disulfide bonds. Thus, it seems reasonable to speculate that its accumulation as PBs is also due to the formation of such oligomers.
Intermolecular disulfide bonds are covalent bonds, and thus, they surely have a role in prolamin sorting. However, in transgenic tobacco, monomeric gliadins can transiently accumulate in the ER, forming PB-like structures until they are finally transported to vacuoles, possibly through the Golgi-passing pathway (Francin-Allami et al. 2011, 2013), and PB formation of HMW-glutenin subunits is independent of their formation of insoluble oligomers (Shani et al. 1994). Such disulfide-independent PB formation of wheat prolamins is thought to be mainly driven by noncovalent interactions between their repetitive domains, and the involvement of nonrepetitive domains has also been suggested (Francin-Allami et al. 2011, 2013). The repetitive domains of gliadins and LMW-glutenin subunits are located at their N-terminus and account for half of their polypeptide, whereas HMW-glutenin subunits have much longer domains, sandwiched between the short N- and C-terminal domains (Shewry et al. 1995; Wieser 2007). The strength of their noncovalent interactions per molecule has been suggested to be dependent on the number of repeats in this domain or on its length itself, and that changes in such properties can influence their accumulation behavior in the ER (Altschuler et al. 1993; Feeney et al. 2003; Saumonneau et al. 2011). Thus, even though accurate estimation is difficult because of low sequence similarity, it is likely that gliadins are also inferior to HMW-glutenin subunits in the ability to noncovalently aggregate. The modest interaction between gliadin molecules is consistent with an in vitro observation that γ-gliadin can reversibly assemble into condensed droplets through liquid–liquid phase separation (Sahli et al. 2019). Such reversible aggregation may be sufficient for gliadins to form PB-like structures in transgenic tobacco, but not to entirely escape being transported to vacuoles.
In contrast to gliadins in transgenic tobacco, there is no evidence of the accumulation of α-gliadin in the ER of transgenic soybean. Such inconsistencies between different expression systems have also been reported for maize γ-zein, which is usually described as a PB-forming protein (Geli et al. 1994; Foresti et al. 2008; Llop-Tous et al. 2010). Llop-Tous (2010) speculated that this may be attributable to whether the expression level of prolamin, or its concentration in the ER lumen, is sufficient for its efficient assembly. A later study revealed that PB formation is a concentration-dependent phenomenon, although highly aggregating proteins can form PBs even at a low expression level (Saberianfar et al. 2015). Moreover, phase separation of the γ–gliadin solution also requires a certain prolamin concentration (Sahli et al. 2019). Thus, it is possible that the low expression level of α–gliadin in transgenic soybean, which was indicated by SDS-PAGE analysis of seed protein extracts, eliminated its PB formation and resulted in its efficient transport through the Golgi-passing pathway. Notably, soybean seeds also form ER-derived PBs when they overexpress proteins other than cereal prolamins. Kinney et al. (2001) found that the knockdown of α/α′ subunits of β–conglycinin leads to the accumulation of endogenous proglycinin in ER-derived PBs. Mori et al. (2004) investigated multiple soybean lines with different compositions of storage globulin and revealed that the enrichment of group I subunit of glycinin, which is less soluble than group II subunit in the vicinity of neutral pH, tends to yield more PBs. Schmidt and Herman (2008) established transgenic soybean lines accumulating ER-targeted GFP in their seeds, as much as more than 7% of total seed protein, and the recombinant protein formed PBs as well as EGFP transiently overexpressed in N. benthamiana leaves (Saberianfar et al. 2015). Such reports suggest that soybean can accumulate a variety of proteins in ER-derived PBs, as long as they have a highly aggregating nature and/or are sufficiently concentrated in the ER. Moreover, there may be a biological reason why PBs are not normally found in soybean seeds, despite their efficient formation under the unusual conditions mentioned above.
Protein trafficking to PSVs has been well characterized in soybean seeds using storage globulins as model cargos. Transport of their major subunits is mediated by Vacuolar sorting receptors (VSRs), a family of plant-specific transmembrane proteins (Nishizawa et al. 2003; Maruyama et al. 2006). These receptors recognize cargo proteins by binding to vacuolar sorting signals (VSSs), which are usually described as a small peptide sequence or a conformational patch of the cargo (Xiang et al. 2013). In the present study, α–gliadin was colocalized with globulins in PVCs, where cargos of VSR were deposited before reaching the vacuoles. Although crosstalk between the PVCs and other compartments makes it difficult to conclude, this colocalization is possibly the result of a similar sorting of α-gliadin and globulins. According to databases, such as Expression Atlas (EMBL-EBI), homologs of VSR are also expressed in wheat endosperm. Although gliadins and glutenin subunits have no known types of VSSs, various contexts of peptide sequences are recognized as functional signals (Maruyama et al. 2006; Luo et al. 2014). In view of these facts, wheat prolamins may be novel types of VSR cargos. This possibility could be clarified by in vitro or in vivo experimental approaches previously established.
Another, but not exclusive possibility, is that α-gliadin binds to other cargo proteins possessing VSSs, and is passively carried by the binding partners. Notably, Wenzel et al. (2005) revealed that pea 11S legumin, whose VSSs also remain uncharacterized, distributes equally in vacuolar-targeted Dense vesicles (DVs), while homologs of Sucrose-binding protein (SBP), another family of vacuolar storage protein, have potentially functional VSSs and tend to be localized in the peripheral region of the DVs. Vacuolar sorting of endomembrane proteins potentially does not require the recognition of every single cargo molecule by receptors, and aggregation enables indirect sorting of a mass of storage proteins via protein–protein interaction with bona fide cargos. Therefore, even if α-gliadin does not have any functional VSSs, its aggregating nature might be sufficient for its correct sorting. In transgenic soybean, storage globulins may be a major binding partner of α-gliadin, considering most of their subunits have known VSSs and are highly expressed in cotyledon cells. The observation that recombinant α-gliadin aggregates in PVCs sometimes associated with storage globulin deposits also seems consistent with this speculation. However, it should be considered that VSR-mediated cargo sorting is already completed in PVCs (Robinson and Neuhaus 2016), and that the molecular states of the storage proteins in the PVCs would not exactly reflect those during their sorting processes, because of the differences in luminal conditions, in terms of pH and/or ion concentrations, for example. Conversely, efficient aggregation of HMW-glutenin subunit in the soybean ER could have interfered the initiation of its receptor-mediated vacuolar sorting.
Whereas the process of PB formation has been extensively studied, the mechanism regulating their downstream transport, which shows dynamic plasticity in seed cells, is entirely elusive. In soybean seeds, HMW-glutenin PBs in the present study remained cytosolic during seed maturation, as well as in the GFP-accumulating PBs reported by Schmidt and Herman (2008). In contrast, some or most of the proglycinin PBs were finally integrated into PSVs (Kinney et al. 2001; Mori et al. 2004), similar to in the cases of PAC vesicles of pumpkin cotyledon or castor bean endosperm (Hara-Nishimura et al. 1998). Such divergent targeting dynamics of the soybean PBs might be dependent on the nature of the accumulating proteins, or the cellular environment, including gene subsets participating in their sorting. Moreover, in the case of PBs of cereal prolamins, previous studies have reported some of their unique transport characteristics. During wheat grain development, the Golgi-bypassing pathway involving ER-derived PBs formation accounts for a major part of prolamin transport to PSVs, while the Golgi-passing pathway apparently plays a limited role (Levanony et al. 1992). Although little is known about the mechanism underlying this pathway, its spatiotemporal regulation in cereal grains has been suggested. In wheat endosperm cells, this pathway is barely operative at the earliest stages of development, but later makes a predominant contribution (Kim et al. 1988; Levanony et al. 1992), whereas in maize grains, it is specific to aleurone cells (Reyes et al. 2011). Electron microscopy studies revealed that this pathway is initiated by autophagic engulfment of prolamin PBs by unknown vesicles which finally incorporate with PSVs (Levanony et al. 1992; Reyes et al. 2011). Furthermore, Reyes et al. (2011) also showed that the vesicles develop into atypical compartments described as “autophagic PVCs,” which contain not only prolamin PBs but also other cargos from the cytoplasm and post-Golgi compartments. Notably, these compartments are likely to be independent of conventional autophagy because they are not immunolabeled with antibodies against ATG8, a marker protein localized to autophagosome membranes. In previous studies, it has been suggested that this pathway is conserved even in dicotyledonous plants. When maize zeins were heterologously expressed in transgenic tobacco and soybean, they formed ER-derived PBs, and some of them were transported to vacuoles in a seed-specific manner (Bagga et al. 1995; Kim and Krishnan 2004, 2019). The vacuolar transport of zein PBs in transgenic soybean seems inconsistent with the absence of HMW-glutenin PBs observed in PSVs. However, because this transport seemed to be a rare case in transgenic dicotyledonous seeds and the number of HMW-glutenin PBs in a cross section of a cell was not much large, whether the HMW-glutenin PBs are never transported to PSVs remains unclear.
In the present study, transgenic soybean expresses only a single gliadin or glutenin subunit gene; therefore, their molecular properties are likely to be a predominant factor determining their transportation mode. Nevertheless, the effect of heterologous expression should be also considered. Conversely, wheat prolamin often consists of several types of gliadins and glutenin subunits. Wheat prolamin transport would depend on the nature of the whole prolamin aggregate rather than that of each component, whose sorting is affected by interactions with others. In addition, the composition of newly-synthesized prolamin in wheat endosperm cells differs depending on their development stage, location in the tissue, or environmental factors, and possibly, so does its transport (Tosi 2012). Rubin et al. (1992) has previously demonstrated that prolamin enriched in PB-forming HMW-glutenin subunits tends to be transported through the Golgi-bypassing pathway. However, the detailed relationship between wheat prolamin composition and its transport mode, as well as the underlying mechanisms, remains unclear. Exploring how co-expression of wheat prolamins in dicotyledonous seeds alters their transport compared to in the case of single expression, or whether approximation of prolamin composition in the transgenic seeds to that in wheat cells reconstitutes its natural transport could offer interesting insights. Exploring such questions would reveal the compositional regulation of wheat prolamin transport, supplementing the somewhat opaque results in the case of wheat.
Data availability
The main data in the study are included in the manuscript.
References
Altschuler Y, Galili G (1994) Role of conserved cysteines of a wheat gliadin in its transport and assembly into protein bodies in Xenopus oocytes. J Biol Chem 269(9):6677–6682
Altschuler Y, Rosenberg N, Harel R, Galili G (1993) The N- and C-terminal regions regulate the transport of wheat γ-gliadin through the endoplasmic reticulum in Xenopus oocytes. Plant Cell 5:443–450
Arcalis E, Stadlmann J, Rademacher T, Marcel S, Sack M, Altmann F, Stoger E (2013) Plant species and organ influence the structure and subcellular localization of recombinant glycoproteins. Plant Mol Biol 83:105–117
Arcalis E, Ibl V, Hilscher J, Rademacher T, Avesani L, Morandini F, Bortesi L, Pezzotti M, Vitale A, Pum D, De Meyer T, Depicker A, Stoger E (2019) Russell-like bodies in plant seeds share common features with prolamin bodies and occur upon recombinant protein production. Front Plant Sci 10:777
Bagga S, Adams H, Kemp JD, Sengupta-Gopalan C (1995) Accumulation of 15-kilodalton zein in nove1 protein bodies in transgenic tobacco. Plant Physiol 107:13–23
Bechtel DB, Juliano BO (1980) Formation of protein bodies in the starchy endosperm of rice (Oryza sativa L.): a re-investigation. Ann Bot 45:503–509
Feeney KA, Wellner N, Gilbert SM, Halford NG, Tatham AS, Shewry PR, Belton PS (2003) Molecular structures and interactions of repetitive peptides based on wheat glutenin subunits depend on chain length. Biopolym 72(2):123–131
Foresti O, De Marchis F, de Virgilio M, Klein EM, Arcioni S, Bellucci M, Vitale A (2008) Protein domains involved in assembly in the endoplasmic reticulum promote vacuolar delivery when fused to secretory GFP, indicating a protein quality control pathway for degradation in the plant vacuole. Mol Plant 1(6):1067–1076
Francin-Allami M, Saumonneau A, Lavenant L, Bouder A, Sparkes I, Hawes C, Popineau Y (2011) Dynamic trafficking of wheat γ-gliadin and of its structural domains in tobacco cells, studied with fluorescent protein fusions. J Exp Bot 62(13):4507–4520
Francin-Allami M, Bouder A, Popineau Y (2013) Comparative study of wheat low-molecular-weight glutenin and α-gliadin trafficking in tobacco cells. Plant Cell Rep 32(1):89–101
Geli IM, Torrent M, Ludevid D (1994) Two structural domains mediate two sequential γ-zein targeting: protein endoplasmic reticulum retention and protein body formation. Plant Cell 6:1911–1922
Hara-Nishimura I, Shimada T, Hatano K, Takeuchi Y, Nishimura M (1998) Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10:825–836
Herman EM (2008) Endoplasmic reticulum bodies: solving the insoluble. Curr Opin Plant Biol 11(6):672–679
Kim WS, Krishnan HB (2004) Expression of an 11 kDa methionine-rich delta-zein in transgenic soybean results in the formation of two types of novel protein bodies in transitional cells situated between the vascular tissue and storage parenchyma cells. Plant Biotechnol J 2(3):199–210
Kim WS, Krishnan HB (2019) Impact of co-expression of maize 11 and 18 kDa δ-zeins and 27 kDa γ-zein in transgenic soybeans on protein body structure and sulfur amino acid content. Plant Sci 280:340–347
Kim WT, Franceschi VR, Krishnan HB, Okita TW (1988) Formation of wheat protein bodies: involvement of the golgi apparatus in gliadin transport. Planta 176(2):173–182
Kim CS, Woo YM, Clore AM, Burnett RJ, Carnerio NP, Larkins BA (2002) Zein protein interactions, rather than the asymmetric distribution of zein mRNAs on endoplasmic reticulum membranes, influence protein body formation in maize endosperm. Plant Cell 14:655–672
Kinney AJ, Jung R, Herman EM (2001) Cosuppression of the α subunits of β-conglycinin in transgenic soybean seeds induces the formation of endoplasmic reticulum–derived protein bodies. Plant Cell 13:1165–1178
Lending CR, Larkins BA (1989) Changes in the zein composition of protein bodies during maize endosperm development. Plant Cell 1:1011–1023
Levanony H, Rubin R, Altschuler Y, Galili G (1992) Evidence for a novel route of wheat storage proteins to vacuoles. J Cell Biol 119(5):1117–1128
Llop-Tous I, Madurga S, Giralt E, Marzabal P, Torrent M, Ludevid MD (2010) Relevant elements of a maize γ-zein domain involved in protein body biogenesis. J Biol Chem 285(46):35633–35644
Lombardi A, Barbante A, Cristina PD, Rosiello D, Castellazzi CL, Sbano L, Masci S, Ceriotti A (2009) A Relaxed specificity in interchain disulfide bond formation characterizes the assembly of a low-molecular-weight glutenin subunit in the endoplasmic reticulum. Plant Physiol 149(1):412–423
Loussert C, Popineau Y, Mangavel C (2008) Protein bodies ontogeny and localization of prolamin components in the developing endosperm of wheat caryopses. J Cereal Sci 47(3):445–456
Luo F, Fong YH, Zeng Y, Shen J, Jiang L, Wong KB (2014) How vacuolar sorting receptor proteins interact with their cargo proteins: crystal structures of apo and cargo-bound forms of the protease-associated domain from an Arabidopsis vacuolar sorting receptor. Plant Cell 26(9):3693–3708
Mainieri D, Morandini F, Maîtrejean M, Saccani A, Pedrazzini E, Vitale A (2014) Protein body formation in the endoplasmic reticulum as an evolution of storage protein sorting to vacuoles: insights from maize γ-zein. Front Plant Sci 5:331
Maruyama N, Mun LC, Tatsuhara M, Sawada M, Ishimoto M, Utsumi S (2006) Multiple vacuolar sorting determinants exist in soybean 11S globulin. Plant Cell 18(5):1253–1273
Mori T, Maruyama N, Nishizawa K, Higasa T, Yagasaki K, Ishimoto M, Utsumi S (2004) The composition of newly synthesized proteins in the endoplasmic reticulum determines the transport pathways of soybean seed storage proteins: transport pathways of soybean seed storage proteins. Plant J 40(2):238–249
Motoyama T, Okumoto Y, Tanisaka T, Utsumi S, Maruyama N (2010) Co-expression of α′ and β subunits of β-conglycinin in rice seeds and its effect on the accumulation behavior of the expressed proteins. Transgenic Res 19:819–827
Napier JA, Richard G, Turner MFP, Shewry PR (1997) Trafficking of wheat gluten proteins in transgenic tobacco plants: γ-gliadin does not contain an endoplasmic reticulum-retention signal. Planta 203:448–494
Nishizawa K, Maruyama N, Satoh R, Fuchikami Y, Higasa T, Utsumi S (2003) A C-terminal sequence of soybean β-conglycinin α′ subunit acts as a vacuolar sorting determinant in seed cells. Plant J 34(5):647–659
Oszvald M, Tamas L, Shewry PR, Tosi P (2014) The trafficking pathway of a wheat storage protein in transgenic rice endosperm. Ann Bot 113(5):807–815
Paris N, Stanley CM, Jones RL, Rogers JC (1996) Plant cells contain two functionally distinct vacuolar compartments. Cell 85(4):563–572
Parker ML (1982) Protein accumulation in developing endosperm of a high-protein line of Triticum DicoCcoides. Plant, Cell Environ 5:37–43
Pompa A, Vitale A (2006) Retention of a bean phaseolin/maize γ-zein fusion in the endoplasmic reticulum depends on disulfide bond formation. Plant Cell 18(10):2608–2621
Reyes FC, Chung T, Holding D, Jung R, Vierstra R, Otegui MS (2011) Delivery of prolamins to the protein storage vacuole in maize aleurone cells. Plant Cell 23(2):769–784
Robinson DG, Neuhaus J-M (2016) Receptor-mediated sorting of soluble vacuolar proteins: myths, facts, and a new model. J Exp Bot 67(15):4435–4449
Rosenberg N, Shimoni Y, Altschuler Y, Levanony H, Volokita M, Galili G (1993) Wheat (Triticum aestivum L.) γ-gliadin accumulates in dense protein bodies within the endoplasmic reticulum of yeast. Plant Physiol 102:61–69
Rubin R, Levanony H, Galili G (1992) Evidence for the presence of two different types of protein bodies in wheat endosperm. Plant Physiol 99:718–724
Saberianfar R, Joensuu JJ, Conley AJ, Menassa R (2015) Protein body formation in leaves of Nicotiana benthamiana: a concentration-dependent mechanism influenced by the presence of fusion tags. Plant Biotechnol J 13(7):927–937
Saberianfar R, Sattarzadeh A, Joensuu JJ, Kohalmi SE, Menassa R (2016) Protein bodies in leaves exchange contents through the endoplasmic reticulum. Front Plant Sci 7:693
Sahli L, Renard D, Solé-Jamault V, Giuliani A, Boire A (2019) Role of protein conformation and weak interactions on γ-gliadin liquid-liquid phase separation. Sci Rep 9(1):13391
Saumonneau A, Rottier K, Conrad U, Popineau Y, Guéguen J, Francin-Allami M (2011) Expression of a new chimeric protein with a highly repeated sequence in tobacco cells. Plant Cell Rep 30(7):1289–1302
Scheuring D, Viotti C, Krüger F, Künzl F, Sturm S, Bubeck J, Hillmer S, Frigerio L, Robinson DG, Pimpl P, Schumacher K (2011) Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell 23(9):3463–3481
Schmidt MA, Herman EM (2008) Proteome rebalancing in soybean seeds can be exploited to enhance foreign protein accumulation. Plant Biotechnol J 6(8):832–842
Shani N, Rosenberg N, Kasarda DD, Galilil G (1994) Mechanisms of assembly of wheat high molecular weight glutenins inferred from expression of wild-type and mutant subunit in transgenic tobacco. J Biol Chem 269(12):8924–8930
Shewry PR, Napier JA, Tatham AS (1995) Seed storage proteins: structures and biosynthesis. Plant Cell 7:945–956
Simon R, Altschuler Y, Rubin R, Galili G (1990) Two closely related wheat storage proteins follow a markedly different subcellular route in Xenopus laevis oocytes. Plant Cell 2:941–950
Stenram U, Heneen WK, Skerritt JH (1991) Immunocytochemical localization of wheat storage proteins in endosperm cells 30 days after anthesis. J Exp Bot 42:1347–1355
Tosi P (2012) Trafficking and deposition of prolamins in wheat. J Cereal Sci 56:81–90
Tosi P, Parker M, Gritsch CS, Carzaniga R, Martin B, Shewry PR (2009) Trafficking of storage proteins in developing grain of wheat. J Exp Bot 60(3):979–991
Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L (2004) Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16:672–693
Wang G, Wang G, Wang J, Du Y, Yao D, Shuai B, Han L, Tang Y, Song R (2016) Comprehensive proteomic analysis of developing protein bodies in maize (Zea mays) endosperm provides novel insights into its biogenesis. J Exp Bot 67(22):6323–6335
Wenzel D, Schauermann G, von Lüpke A, Hinz G (2005) The cargo in vacuolar storage protein transport vesicles is stratified. Traffic 6:45–55
Wieser H (2007) Chemistry of gluten proteins. Food Microbiol 24(2):115–119
Xiang L, Etxeberria E, den Ende WV (2013) Vacuolar protein sorting mechanisms in plants. FEBS J 280(4):979–993
Yamada T, Watanabe S, Arai M, Harada K, Kitamura K (2010) Cotyledonary node pre-wounding with a micro-brush increased frequency of Agrobacterium-mediated transformation in soybean. Plant Biotechnol 27(2):217–220
Yamada T, Mori Y, Yasue K, Maruyama N, Kitamura K, Abe J (2014) Knockdown of the 7S globulin subunits shifts distribution of nitrogen sources to the residual protein fraction in transgenic soybean seeds. Plant Cell Rep 33(12):1963–1976
Yamada K, Basak AK, Goto-Yamada S, Tarnawska-Glatt K, Hara-Nishimura I (2020) Vacuolar processing enzymes in the plant life cycle. New Phytol 226:21–31
Zhang W, Sangtong V, Peterson J, Scott MP, Messing J (2013) Divergent properties of prolamins in wheat and maize. Planta 237(6):1465–1473
Acknowledgements
We are thankful to the National Bio-Resource Project KOMUGI for providing the cDNA clone used in this study. Plant culture was supported by the Development and Assessment of Sustainable Humanosphere (DASH) System at the Research Institute for Sustainable Humanosphere, Kyoto University, Japan. The electron microscopy study was partly supported by Keiko Okamoto-Furuta and Haruyasu Kohda (Division of Electron Microscopic Study, Center for Anatomical Studies, Graduate School of Medicine, Kyoto University). We thank Eiko Okuda (Kyoto University) for technical assistance and M. Suzuki, S. Noguchi, and Y. Kitsui (Hokkaido University) for technical assistance with plant transformation and tissue culture. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by a JSPS KAKENHI Grant (JP24658286) to NM.
Author information
Authors and Affiliations
Contributions
TY and NM designed the experiments; TY prepared the transgenic soybean; YM performed the analyses on transgenic soybean; YM, TY, and NM contributed to the writing of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsuoka, Y., Yamada, T. & Maruyama, N. Wheat α-gliadin and high-molecular-weight glutenin subunit accumulate in different storage compartments of transgenic soybean seed. Transgenic Res 31, 43–58 (2022). https://doi.org/10.1007/s11248-021-00279-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-021-00279-2