Abstract
A cDNA encoding a type 2 metallothionein (MT) was isolated from Azolla filiculoides, termed AzMT2, accession no. AF482470. The AzMT2 transcript was expressed in sterile A. filiculoides that were free of the cyanobiont Anabaena azollae after erythromycin treatment, proving that AzMT2 is encoded by the fern genome. AzMT2 RNA expression was enhanced by the addition of Cd+2, Cu+2, Zn+2 and Ni+2 to the growth medium. The transcript level of AzMT2 correlated with the metal content in the plants. Temporal analysis of AzMT2 expression demonstrated that Cd2+ and Ni2+ induction of AzMT2 RNA expression occurred within 48 h. AzMT2-enhanced expression responded more intensely to the toxic Cd and Ni ions in A. filiculoides suggesting that AzMT2 may participate in detoxification mechanism. The more moderate response of AzMT2 to Zn and Cu ions, which are essential micronutrients, suggest a role for AzMT2 in metal homeostasis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil and water pollution by toxic heavy metals is a major environmental concern worldwide. Heavy metals can readily enter bio-geochemical cycles, and so major efforts should be made to prevent or minimize their distribution and damage. Phytoremediation is a novel technology for environmental clean-up whereby metal-accumulating plants remove, detoxify or stabilize pollutants from contaminated sites (Salt et al. 1995). All plants, terrestrial and aquatic, have the ability to acquire and accumulate metal ions such as Fe2+, Mn2+, Zn2+ and Cu2+, which are essential for plant growth and development. Some plants can also accumulate non-essential toxic metal ions. Consequently, the concept has emerged that plants can be used to remove toxic metals from soil and water thereby contributing to the remediation of polluted sites (Salt et al. 1999). Some aquatic plants have been investigated for their potential to improve wastewater quality because of their ability to grow in water polluted by heavy metals (Reimer and Duthie 1993). Plants have evolved a suite of mechanisms that control the uptake and accumulation of both essential and non-essential heavy metals. These mechanisms include the chelation and sequestration of heavy metals by various ligands. Two well-characterized peptide ligands for heavy metals in plant cells are phytochelatins and metallothioneins (Cobbett and Goldsbrough 2002).
Metallothioneins (MTs) are low molecular weight (4–10 kDa), cysteine-rich, metal-binding proteins that bind metals via the thiol groups of cysteine residues (Hamer 1986; Robinson et al. 1993; Zenk 1996). Since MTs were first purified from horse kidney as Cd-binding proteins in 1956, MT genes have been identified throughout the animal and plant kingdoms and in some prokaryotes (Cobbett and Goldsbrough 2002). MTs typically contain two metal binding, cysteine-rich domains that provide a dumb-bell conformation for metal chelation. MT proteins have been classified based on the arrangement of Cys residues (Binz and Kägi 1999); Class I includes primarily mammalian MTs containing 20 highly conserved Cys residues and Class II includes MTs from plants and fungi, as well as invertebrate animals. The plant Class II MT proteins can be further classified into four groups based on their amino acid sequence, in particular the position of cysteine residues (Robinson et al. 1993; Cobbett and Goldsbrough 2002). Based on the analysis of MT RNA expression in a number of plant species, type 1 MT genes are expressed more abundantly in roots than leaves, whereas type 2 MT genes are expressed primarily in the leaves (Zhou and Goldsbrough 1994, 1995; Hsieh et al. 1995, 1996). Type 3 includes many MTs identified as being expressed during fruit ripening, and type 4 MTs, exemplified by the wheat Ec protein are expressed only in seeds (reviewed by Cobbett and Goldsbrough 2002).
The relationship between MT expression and metal concentration in different organisms suggests that MTs could be an effective reporter of environmental conditions (Morris et al. 1999). The isolation and characterization of MTs from model bioindicator organisms could also contribute to our understanding of the biological response to biomonitoring pollutants in the environment (Morris et al. 1999).
The aquatic fern Azolla filiculoides, a traditional biofertilizer that forms a symbiosis with the N2-fixing cyanobacterium Anabaena azollae, is distributed worldwide and is used as a green manure by rice farmers. A. filiculoides is an exceptionally efficient producer of biomass because of its high photosynthetic bioproductivity and simple vegetative propagation, and it is easily converted to a source of available nitrogen in flooded rice fields (Lumpkin and Pluncknett 1980; Sela et al. 1988).
Earlier studies in our lab have examined the uptake, accumulation and distribution of toxic heavy metals by A. filiculoides grown in the presence of 1–20 mg/l of Cd, Cu, Zn, Cr and Ni ions, and described the immobilization of these metals in the cell wall and other insoluble components of A. filiculoides biomass (Sela et al. 1988, 1989, 1990; Tel-Or et al. 1997).
To increase our understanding of the mechanisms that contribute to metal detoxification and accumulation in A. filiculoides, we have isolated and characterized a gene encoding a metallothionein protein. RNA expression of this MT gene is enhanced when A. filiculoides is exposed to a number of toxic metals. The expression of MT genes in a common aquatic plant may also provide a molecular tool to monitor heavy-metal pollution in aquatic ecosystems.
Materials and methods
Plant growth and nutrition
A. filiculoides Lamarck (collected from botanical garden Tel-Aviv University, Israel) was grown in the phytotron at 27/22°C day/night, in 500 ml containers containing IRRI (International Rice Research Institute) mineral growth medium (Watanable et al. 1977). The IRRI medium contained 174 mg/l K2SO4, 147 mg/l CaCl2, 169 mg/l MgSO4, 144 mg/l H3PO4, 3 mg/l Fe chelate, 138 mg/l NaH2PO4, 0.16 mg/l CuSO4, 3.6 mg/l MnCl2, 0.4 mg/l ZnSO4, 0.8 mg/l NaMoO4, 5.6 mg/l H3BO3 and 0.1 mg/l CoCl2.
Isolation of AzMT2
Total RNA was isolated from A. filiculoides as described by Oren-Benaroya et al. (2004). Poly(A)+ RNA was isolated from 7.5 μg total RNA using Oligotex mRNA Mini Kit (Qiagen). cDNA was prepared using Omniscript RT kit (Qiagen). All steps were performed according to the manufacturer’s protocols. cDNAs encoding MT genes were isolated by PCR using A. filiculoides cDNA as template. PCR was first performed using two degenerate oligonucleotides based on the published DNA sequences encoding MTs in other plant species: 5′-ATGTCTTGCTGYGGAGGAARCTGT-3′ (sense) and 5′-ACAAKYGCARGGGTYACASKTGCA-3′ (antisense) (Giordani et al. 2000) (Y=C/T, R=A/G, K=G/T, S=G/C). After sequencing, specific primers were designed: 5′-ATGTCTTGCTGTGGAGGAAA-3′ (sense) and 5′-TTAGCATCGGCAAGGATT-3′ (antisense). To determine the origin of the MT cDNA, primers for nifB (accession no. J05111) were used: 5′-ATCCCTGCTACAGCGAAGAA-3′ (sense) and 5′-CCAGCAATTCCCAGAACTGT-3′ (antisense).
Thermocycling was performed using an initial denaturation at 94°C for 3 min followed by 36 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 40 s with a final 10 min extension period at 72°C, using Taq polymerase (JMR Holding, Sevenoaks Kent, UK). After agarose gel electrophoresis, the amplified fragment was isolated using High Pure PCR Product Purification Kit (Roche) and cloned into pGEM-T Vector System I (Promega). Plasmids were isolated by QIAprep Spin Miniprep Kit (Qiagen) and sequenced.
Multiple sequence alignments were performed using ClustalW 1.6 and edited with Gendoc (http://www.psc.edu/biomed/gendoc).
Antibiotic treatment
A. filiculoides plants were grown in the phytotron at 27/22°C day/night in IRRI medium with or without 60 μg/ml erythromycin (Sigma) for 25 days.
Scanning electron microscope (SEM)
Samples were fixed in 5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2 for 2 h at 4°C. The samples were rinsed twice in 0.1 M phosphate buffer and dehydrated in graded series of ethyl alcohol, dried to the critical point and coated with colloidal gold. The observations were made using a Jeol JSM-35 C microscope (Forni et al. 1991). Five plants were scanned and for each plant five leaf cavities were scanned for A. azollae.
Semi-quantitative RT-PCR
For RT-PCR we used the specific primers for AzMT2 and the following primers for Ubq10: 5′-CGATTACTCTTGAGGTGGAG-3′ (sense) and 5′-AGACCAAGTGAAGTGTGGAC-3′ (antisense). In addition to the specific primers, the reaction mixtures contained 2 μl of the cDNA and 0.6 unit of Taq polymerase (JMR Holding, Sevenoaks Kent, UK), in a total volume of 10 μl. Annealing temperature was 64°C for AzMT2 and 60°C for Ubq10. Reactions were subjected to 28 cycles for AzMT2 and 20 cycles for Ubq10. After separation of the products on a 1.5% agarose gel, the DNA was transferred to a Hybond N+ membrane. To visualize the products, probes were labeled by PCR DIG Synthesis Kit (Roche), according to the manufacturer’s protocol. Membranes were prehybridized with DIG Easy Hyb solution for 30 min at 42°C, followed by hybridization in the same solution containing denatured DIG-labeled probe at 42°C overnight. The membranes were washed to a final stringency of 0.1×SSC and 0.1% SDS at 68°C. The membranes were subjected to immunological detection with anti-digoxigenin-AP conjugate and CDP-Star, followed by exposure to X-ray film (Fuji, Tokyo, Japan). Hybridization signals were analyzed using the ImageScanner (BioScience, Amersham). The signals from cDNA series were quantified by densitometry of autoradiograms and normalized to Ubq using ImageJ software.
Metal treatments
For the dose response of A. filiculoides to the four heavy metal treatments, Cd(NO3)2, CuSO4, ZnSO4 or NiSO4 were added to final concentrations of 10 μM, 100 μM and 1 mM. Plants were grown in the presence of each of the heavy metals, separately, for 4 days. Control plants were grown without supplementary heavy metals. In A. filiculoides, for the time course response to the four heavy metals, the same metal salts were used at final concentration of 100 μM in the growth medium. Plant samples were collected at 0, 12, 24 and 48 h.
Metal analysis
Metal contents of the A. filiculoides plants were determined by ICP analysis. Plant samples were collected, washed thoroughly in distilled water, dewatered and then oven dried (80°C, 24 h). The dry samples were hand-milled, mixed to homogeneity and 250 mg aliquots were digested in 4 ml concentrated HNO3 at 120°C for 10 min. After cooling, the digests were diluted to 25 ml with distilled water, filtered through Whatman paper No. 1 and analyzed for metal content by Inductive Coupled Plasma (ICP) Spectroscopy (Spectro Flame, Kleve, Germany).
Statistics
Each treatment was conducted in triplicate, and each experiment was conducted at least three times. Data are presented as mean ± SD, unless otherwise indicated. The t-tests were preformed using the algorithm embedded into Microsoft Excel (Microsoft Corporation, Seattle, WA, USA). The term “significant” is used in the text only when the change in question was confirmed to be significant (P<0.05) using t-test.
Results
Isolation of AzMT2
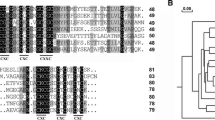
A large number of cDNA sequences encoding MTs have been isolated from higher plants. Conserved amino acid sequences in plant MTs were used to design PCR primers to amplify cDNAs for MTs from A. filiculoides. Primers were designed based on conserved residues in type 1 and type 2 plant MTs. However, a PCR product was obtained only with the primers designed for type 2 MT sequences. The amplified 234 bp cDNA sequence (accession no. AF482470) contained an open reading frame of 78 amino acids (Fig. 1). The predicted protein contains two cysteine-rich domains in the terminal regions of the protein, characteristic of MTs, and has been named AzMT2.
Sequence alignment of the predicted protein sequence of A. filiculoides MT with sequence from representative plants (from which GeneBank code and species name is reported). Sequences were aligned by ClustalW program. Identical and highly similar amino acids are labeled with black boxes, and less similar ones are labeled with gray boxes
The amino acid sequence of A. filiculoides MT2 was analyzed by the BLAST program from the National Center for Biotechnology Information (NCBI) (Altschul et al. 1990) and shows high homology with many other plant MT proteins (Fig. 1). Comparisons using ClustalW 1.6 between the A. filiculoides MT sequence and those of MTs from Brassica oleracea (accession no. AF200712), Pimpinella brachycarpa (accession no. AF093585), Oenanthe javanica (accession no. AF017787), Nicotiana glutinosa (accession no. U46543) and Pringlea antiscorbutica (accession no. AY333930) showed protein sequence identities between 34% and 40%.
Plant MTs have been divided into different types based on the arrangement of cysteine residues in the amino- and carboxy-terminal domains (Robinson et al. 1993; Rauser 1999). The predicted amino acid sequence of AzMT2 contains a conserved amino-terminal domain typical of type 2 plant MTs (xxCCxxxCxCxxxCxCxxxCxxC, where C indicates cysteine and x indicates any other amino acid), although AzMT2 lacks the last two Cys residues. Similarly, the carboxy-terminal domain of AzMT2 contains a conserved cysteine-rich domain (CxCxxxCxCxxCxC) typical of type 2 MTs. Therefore, AzMT2 represents a MT protein with significant amino acid sequence similar to type 2 MTs in plants.
The origin of the AzMT2 gene; a product of the fern or the cyanobiont?
The RNA used as the template for cDNA synthesis and subsequent PCR was prepared from plants carrying the A. azollae symbiont. This raises the formal possibility that the amplified MT cDNA sequence was derived from the symbiont rather than the plant. To test this possibility, plants were grown for 25 days in IRRI medium containing the antibiotic erythromycin in order to eliminate the A. azollae. Under these conditions the A. filiculoides plants showed symptoms of senescence compared to control plants grown in medium without antibiotics (Fig. 2a). The leaf cavities were examined by SEM to determine whether the antibiotic treatment had cured the plants of the A. azollae cyanobacterium (Fig. 2b). The same RT-PCR product for AzMT2 was obtained from the control plants and from plants treated with erythromycin (Fig. 2c). Both RT-PCR products were sequenced and shown to be identical to that for AzMT2. To confirm there was no trace of A. azollae in the antibiotic-treated plants, RT-PCR was performed with primers for the A. azollae gene nifB. No RT-PCR product of nifB (228 bp) was detected from the erythromycin-treated A. azollae-free plant (Fig. 2d, lane 1), while the untreated A. filiculoides carrying A. azollae produced the expected PCR product from nifB (Fig. 2d, lane 2) demonstrating the presence of the cyanobiont. These results indicate that the AzMT2 gene is indeed derived from the host fern and that MT RNA expression in the experiments that are described here cannot be attributed to the A. azollae symbiont.
a–d The distribution of AzMT2 and nifB genes in A. filiculoides and A. azollae. a After 25 days of growth in 60 μg/ml erythromycin (antibiotic) medium (1), the plants showed symptoms of senescence due to effect of the drug on the cyanobacterium compared to plants untreated with erythromycin (2). b SEM photographs of the empty leaf cavity of A. filiculoides treated with erythromycin (1) and untreated control (2). c RT-PCR results using AzMT2 primers for erythromycin-treated (lane 1) and untreated (lane 2). d RT-PCR results using nifB primers for erythromycin-treated (lane 1) and untreated controls (lane 2). L, GeneRuler 100 bp DNA ladder plus (MBI Fermentas)
Heavy metals induce accumulation of AzMT2 transcripts in A. filiculoides plants
In some plants expression of MT genes can be enhanced by various metals. To determine if heavy metals induce RNA expression of AzMT2, A. filiculoides plants were grown for 2 weeks in IRRI medium and then transferred to medium containing Cd(NO3)2, CuSO4, ZnSO4 or NiSO4 at final concentrations of 10 μM, 100 μM and 1 mM. The plants grown in the presence of 1 mM Cd(NO3)2 did not survive (nitrate is easily assimilated by A. filiculoides with no influence on heavy metal response; data not shown). Plants were harvested after 4 days and analyzed for metal content and RNA expression of AzMT2. No toxic effects of either Ni2+, Zn2+ or Cu2+ at the concentrations used were observed on A. filiculoides plants. The content of Cd2+, Zn2+, Ni2+ and Cu2+ in the A. filiculoides plants increased along with the concentration of these metals in the IRRI growth medium (Fig. 3), and were about 20–30-fold higher in the plant dry weight than the concentration of the metals present in the medium. Semi-quantitative RT-PCR was used to examine the RNA expression of AzMT2 in plants exposed to metals (Fig. 4). These experiments indicated that the expression of AzMT2 increased dramatically in response to each of these metals. Elevated levels of AzMT2 RNA were observed in response to relatively low concentrations (10 μM) of cadmium, zinc and copper, whereas 100 μM nickel was required to evoke a similar response. Treatment with Cd(NO3)2 concentrations higher than 100 μM was toxic to the plants after 4 days.
Metal concentration in the plants after 4 days of exposure. Plants were exposed to Cd(NO3)2 (black), NiSO4 (stripes), CuSO4 (white) or ZnSO4 (dots) in IRRI medium for 4 days. The results describe average of three replicates of metal content in A. filiculoides plants that was determined by ICP spectroscopy and is expressed in mg metal/kg DW (dry weight) of plants, the error bars are standard deviations. Means ±SD, n=3. Treatment means with different letters differ significantly by the t-test (P<0.05)
a–d The effect of different heavy metals concentrations on transcript level of AzMT2. A. filiculoides plants were exposed to different concentrations of Cd (a), Ni (b), Zn (c) and Cu (d) in IRRI medium for 4 days. RNA was isolated from treated and control (IRRI medium) plants, cDNA was prepared and subjected to semi-quantative RT-PCR analysis. Ubq10 was used as a constitutive gene. Transcript analysis was conducted as described in Materials and methods. Graphs represent the relative expression levels of AzMT2 for each experiment as quantified by densitometry and normalized to the respective Ubq10 levels. Note: no RNA was isolated from 1 mM Cd treatment (a), plants were unable to survive in the presence of 1 mM Cd
To examine the time course of metal uptake and induction of AzMT2 gene expression, plants were exposed to 100 μM Cd(NO3)2, NiSO4, CuSO4 or ZnSO4 for 0, 12, 24 and 48 h. With the exception of zinc, uptake of metals was rapid, reaching at least 75% of the maximum content after 12 h (Fig. 5). Furthermore, there was no significant increase in metal content between 24 h and 48 h. RNA was isolated and used to prepare cDNA for semi-quantitative RT-PCR experiments to examine the expression of AzMT2 RNA (Fig. 6). The results of these experiments indicated that AzMT2 RNA did not change markedly after 12 h exposure to any of these metals. Only after 48 h treatment with cadmium or nickel, there was a prominent increase in AzMT2 RNA expression.
Metal concentration in A. filiculoides plants after different exposure period. Plants were exposed to 100 μM of Cd (NO3)2 (black), NiSO4 (stripes), CuSO4 (white) or ZnSO4 (dots) in IRRI medium for 0, 12, 24 and 48 h. The results describe average of three replicates of metal content in A. filiculoides plants that was determined by ICP spectroscopy and is expressed in mg metal/kg DW (dry weight) of plants, the error bars are standard deviations. Means ±SD, n=3. Treatment means with different letters differ significantly by the t-test (P<0.05)
a–d The response time of AzMT2 expression to heavy metals in A. filiculoides. The plants were exposed to 100 μM of Cd (a), Ni (b), Zn (c) and Cu (d) in IRRI medium for 0, 12, 24 and 48 h. RNA was isolated from treated and control (0 h) plants, cDNA was prepared and subjected to semi-quantative RT-PCR analysis. Ubq10 was used as a constitutive gene. Transcript analysis was conducted as described in Materials and methods. Graphs represent the relative expression levels of AzMT2 for each experiment as quantified by densitometry and normalized to the respective Ubq10 levels
Discussion
Following our earlier report on the ability of A. filiculoides to accumulate metal ions (Sela et al. 1988), the experiments reported here provide clear evidence for the presence of a MT gene in the aquatic fern A. filiculoides, and describe the RNA expression of this gene in response to various metals. The developing interest in the potential use of aquatic plants for phytoremediation of environments contaminated with toxic heavy metals calls for better understanding of the mechanisms involved in metal ion binding, sequestration and detoxification in these plants.
Expression of MTs can be induced by a variety of stimuli, including elevated concentrations of essential and non-essential heavy metals (Kägi 1993). In view of their metal binding capacity, it has been suggested that MTs may play a role in the homoeostasis of essential metal ions and detoxification of heavy metals such as Cd2+ (Hamer 1986). The regulation of AzMT2 in A. filiculoides by Zn2+ and Cu2+, both essential nutrients, and by Cd2+ and Ni2+, two non-essential toxic metal ions, suggests a role for AzMT2 in both metal nutrition and metal detoxification in A. filiculoides plants.
A. filiculoides forms a symbiosis between the host fern and the cyanobiont A. azollae. Because cyanobacteria have been reported to contain and express MT genes (Shi et al. 1992), it is important to determine if AzMT2 is encoded by the genome of the host or the cyanobiont. Treatment of A. filiculoides with erythromycin results in loss of its cyanobiont, as observed by SEM shown in Fig. 2. RT-PCR analysis with the nifB gene confirmed that erythromycin effectively eliminated the endosymbiont (Fig. 2). RT-PCR analysis also showed that AzMT2 is expressed in cyanobiont-free A. filiculoides, demonstrating that the AzMT2 gene is encoded in the genome of the host fern. In previous studies we have shown that the host fern is the major accumulator of toxic heavy metals such as Cd2+ while the endosymbiotic A. azollae in the leaf cavity does not play a major role in metal uptake and storage (Sela et al. 1988).
Plant MTs have been divided into different types based on the arrangement of cysteines in domains 1 and 2 (Rauser 1999). The A. filiculoides MT cDNA isolated in our experiments encodes a protein with two cysteine-rich regions. These regions show significant identity with the N- and C-terminal domains of different type 2 MTs from plants.
Our attempts to identify a cDNA from a type 1 MT gene in A. filiculoides were not successful. Type 1 MT genes are expressed more abundantly in roots than leaves, whereas type 2 MT genes are expressed primarily in the leaves (Zhou and Goldsbrough 1994, 1995; Hsieh et al. 1995, 1996). In A. filiculoides, roots play a very limited role in mineral uptake, which is mainly conducted by ventral leaves (Tel-Or et al. 1997). If a type 1 MT gene was present but not expressed in the tissue used for RNA isolation, it would not have been detected by RT-PCR. Additional experiments, including PCR analysis of genomic DNA and RNA from plants grown under different conditions, will be necessary to determine if the A. filiculoides genome contains multiple types of MT genes as found in higher plants.
The metal-enhanced expression of AzMT2 may reflect both the direct metal effect as well as the oxidative stress caused by the toxic metals. Additional experiments using pro-oxidants such as hydrogen peroxide or paraquat, or environmental conditions that trigger oxidative stress, such as excessive light or temperature extremes, may identify the primary cause for metal-enhanced expression of AzMT2. Furthermore, expression of AzMT2 in MT-deficient yeast may resolve whether this protein imparts tolerance by metal binding or by other mechanisms.
The RNA expression of MT genes in other species has been shown to be affected by metal ions (De Miranda et al. 1990; Kawashima et al. 1991; Foley and Singh 1994; Zhou and Goldsbrough 1994, 1995; Morris et al. 1999; Giordani et al. 2000). Exposure of A. filiculoides to elevated concentrations of Cd2+, Ni2+, Cu2+ and Zn2+ resulted in induction of the AzMT2 gene (Fig. 4). Of the metals tested, Cd2+ was the most effective inducer of AzMT2 and the most toxic, with plants unable to survive in the presence of 1 mM Cd2+. MTs together with phytochelatins may play an important role in detoxification of Cd2+ by A. filiculoides plants, but to the best of our knowledge, no studies have analyzed the role of phytochelatins in metal resistance of A. filiculoides to cadmium and other metals.
Contrasting results have been reported for the expression of MT genes in response to metals in different plant species. For example, Cu2+ had either no effect or repressed MT gene expression in V. faba, Mimulus guttatus and soybean (De Miranda et al. 1990; Kawashima et al. 1991; Foley and Singh 1994). In contrast, MT gene expression was enhanced by Cu2+ in Arabidopsis (Zhou and Goldsbrough 1995), Fucus (Morris et al. 1999) and P. oceanica (Giordani et al. 2000).
Temporal analysis of AzMT2 expression in response to 100 μM of four metals is described in Fig. 6. Only Cd2+ and Ni2+, the more toxic ions, enhanced expression of AzMT2 within 48 h. Copper and zinc, as essential metals, are taken up and incorporated in metabolic pathways and may not reach the critical intracellular concentration required to enhance AzMT2 expression within 48 h. However, longer exposure to copper and zinc, shown in Figs. 3 and 4, may lead to accumulation of the critical intracellular concentration required to enhanced expression of AzMT2 by copper and zinc. Thus, we suggest that AzMT2 is involved in homeostasis of copper and zinc as essential metals, once theire intracellular concentration reaches critical concentration. In contrast, cadmium and nickel are taken up and accumulated at high intracellular concentrations that lead to overexpression of AzMT2 within 48 h. These results suggest that elevated expression of MT may contribute to Cd2+ detoxification at 100 μM and Ni2+ at levels of 1 mM in the growth medium.
Purified Arabidopsis MT, Vicia faba MT and Pisum sativum MT expressed in E.coli have shown metal-binding ability (Tommey et al. 1991; Foley et al. 1997; Murphy et al. 1997). Even though we did not test the metal-binding ability of AzMT2, but based on the identity of AzMT2 to MTs from other plants and the proven ability of plant MTs to bind metals, AzMT2 very likely binds metals as well. The response of AzMT2 expression to the four metals suggests that AzMT2 may play a role either as a “trafficking” metal chaperone, targeting the metal-bound protein to an intracellular compartment, or it may be involved in the long-term storage of metal ions in A. filiculoides. Although MTs are expressed ubiquitously and conserved in plants, determining their function remains a future challenge (Cobbett and Goldsbrough 2002).
In summary, AzMT2 from A. filiculoides, encodes a MT protein and RNA expression of this gene is regulated by the four metal ions tested, representing both essential nutrients (Zn2+ and Cu2+) and toxic heavy metals (Cd2+ and Ni2+). AzMT2 is proposed to be involved in both the homeostasis of essential metals as well as detoxification of toxic metals in A. filiculoides.
Abbreviations
- MT:
-
Metallothionein
- IRRI:
-
International rice research institute
- SEM:
-
Scanning electron microscopy
- ICP:
-
Inductive coupled plasma
- RT-PCR:
-
Reverse transcription polymerase chain reaction
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Binz PA, Kägi JHR (1999) Metallothionein: Molecular evolution and classification. In: Klaassen C (ed) Metallothionein IV. Birkhäuser, pp7–13
Cobbett CS, Goldsbrough PB (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–183
De Miranda JR, Thomas MA, Thurman DA, Tomsett AB (1990) Metallothionein genes from the flowering plants Mimulus guttatus. FEBS Lett 260:277–280
Foley RC, Singh KB (1994) Isolation of a Vicia faba metallothionein-like gene: expression in foliar trichomes. Plant Mol Biol 26:435–444
Foley RC, Liang ZM, Singh KB (1997) Analysis of type 1 metallothionein cDNAs in Vicia faba. Plant Mol Biol 33:583–591
Forni C, Tel-Or E, Bar E, Grilli Caiola M (1991) Effects of antibiotic treatments on Azolla-Anabaena and Arthrobacter. Plant Soil 137:151–155
Giordani T, Natali L, Maserti BE, Taddei S, Cavallini A (2000) Characterization and expression of DNA sequences encoding putative Type-II metallothioneins in the seagrass Posidonia oceanica. Plant Physiol 123:1571–1581
Hamer DH (1986) Metallothionein. Annu Rev Biochem 55:913–951
Hsieh HM, Liu WK, Chang A, Huang PC (1996) RNA expression patterns of a type 2 metallothionein-like gene from rice. Plant Mol Biol 32:525–529
Hsieh HM, Liu WK, Huang PC (1995) A novel stress-inducible metallothionein-like gene from rice. Plant Mol Biol 28:381–389
Kägi JHR (1993) Evolution, structure and chemical activity of class I metallothioneins: an overview. In: Suzuki KT, Imura N, Kimura M (eds) Metallothionein III. Birkhäuser, pp 29–55
Kawashima I, Inokuchi Y, Chino M, Kimura M, Shimizu N (1991) Isolation of gene for a metallothionein protein from soybean. Plant Cell Physiol 32:913–916
Lumpkin TA, Pluncknett DL (1980) Azolla: botany, physiology and use as a green manure. Economic Bot 34:111–153
Morris CA, Nicolaus B, Sampson V, Harwood JL, Kille P (1999) Identification and characterization of a recombinant metallothionein protein from a marine alga, Fucus vesiculosus. Biochem J 338:553–560
Murphy A, Zhou J, Goldsbrough PB, Taiz L (1997) Purification and immunological identification of metallothioneina 1 and 2 from Arabidopsis thaliana. Plant Physiol 113:1293–1301
Oren-Benaroya R, Zamski E, Tel-Or E (2004) L-Myo-inositol 1-phosphate synthase in the aquatic fern Azolla filiculoides. Plant Physiol Biochem 42:97–102
Rauser WE (1999) Structure and function of metal chelators produced by plants: the case for organic acids, amino acids, phytin and metallothionein. Cell Biochem Biophys 31:19–48
Reimer P, Duthie HC (1993) Concentrations of zinc and chromium in aquatic macrophytes from the Sudbury and Muskoka regions of Ontario, Canada. Environ Pollut 79:261–265
Robinson NJ, Tommey AM, Kuske C, Jackson PJ (1993) Plant metallothioneins. Biochem J 295:1–10
Salt DE, Benhamou N, Leszczyniecka M, Raskin I, Chen I (1999) A possible role for rhizobacteria in water treatment by plant roots. Inter J Phytorem 1:67–79
Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley BD, Chen I, Raskin I (1995) Phytoremediation:a novel strategy for removal of toxic metals from the environment using plants. Biotechnology 13:468–474
Sela M, Fritz E, Huttermann A, Tel-Or E (1990) Studies on cadmium localization in the water fern Azolla. Physiol Plant 79:547–553
Sela M, Garty J, Tel-Or E (1989) The accumulation and the effect of heavy-metals on the water fern Azolla filiculoides. New Phytol 112:7–12
Sela M, Tel-Or E, Fritz E, Huttermann A (1988) Localization and toxic effects of cadmium, copper, and uranium in Azolla. Plant Physiol 88:30–36
Shi J, Lindsay WP, Huckle JW, Morby AP, Robinson NJ (1992) Cyanobacterial metallothionein gene expressed in Escherichia coli: metal-binding properties of the expressed protein. FEBS Lett 303:159–163
Tel-Or E, Sela M, Ravid S (1997) Biofiltration of heavy metals by the aquatic fern Azolla. In: Rosen D, Tel-Or E, Hadar Y, Chen Y (eds) Modern agriculture and the environment. Kluwer Academic Publishers, pp 431–442
Tommey AM, Shi J, Lindsay WP, Urwin PE, Robinson NJ (1991) Expression of pea gene PsMT A in E.coli: metal-binding properties of expressed protein. FEBS Lett 292:48–52
Watanable I, Espinas CR, Berja NS, Alimaguo BV (1977) The utilization of the Azolla–Anabena complex as nitrogen fertilizer. Int Rice Res 11:1–5
Zenk MH (1996) Heavy metal detoxification in higher plants. Gene 179:21–30
Zhou JM, Goldsbrough PB (1994) Functional homologs of animal and fungal metallothionein genes from Arabidopsis. Plant Cell 6:875–884
Zhou JM, Goldsbrough PB (1995) Sructure, organization and expression of metallothionein gene family in Arabidopsis. Mol Gen Genet 248:318–328
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schor-Fumbarov, T., Goldsbrough, P.B., Adam, Z. et al. Characterization and expression of a metallothionein gene in the aquatic fern Azolla filiculoides under heavy metal stress. Planta 223, 69–76 (2005). https://doi.org/10.1007/s00425-005-0070-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0070-6