Abstract
Iris lactea var. chinensis (I. lactea var. chinensis) is a widely adapted perennial species with a high level of copper tolerance. To evaluate the role of metallothioneins (MTs) in copper tolerance in I. lactea var. chinensis, a full-length cDNA homologue of MT2, designated IlMT2b (GenBank accession No. AB907788), was cloned using the RACE-PCR method. The expression level of IlMT2b in the leaves and roots of I. lactea var. chinensis was induced in response to copper (Cu) treatment. Ectopic expression of IlMT2b in Arabidopsis thaliana increased the Cu concentration and reduced H2O2 production in the transgenic plants. After treatment with 50 and 100 μM Cu, the root length of two transgenic seedlings was respectively about 1.5- and 3-fold longer than that of the wild-type. Together, these results suggested that IlMT2b may represent a useful target gene for the phytoremediation of Cu-polluted soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The metallothionein (MT) family is one of the most important classes to protect plants from heavy metal toxicity (Hall 2002). It has features of low molecular weight, rich cysteine (Cys) content and strong metal-binding ability (Samardzic et al. 2010). Based on distribution of cysteine residues in the amino- and carboxy-terminal regions, MTs can be divided into four categories in plants (Cobbett and Goldsbrough 2002). Type 1 contains two Cys-rich domains with metal binding motif Cys–X–Cys (X represents another amino acid), while type 2 is composed of Cys–Cys, Cys–X–X–Cys and Cys–X–Cys in the N-terminal region and Cys–X–Cys in the C-terminal region. Phytochelatins are described as type 3 which have the structure of [γ-Glu–Cys]n–X polymers, whereas type 4 has three cysteine-rich domains.
Copper (Cu) is an essential micronutrient for various plant physiological processes via the form of Cu-dependent enzymes (Cobbett and Goldsbrough 2002), but excess copper accumulation always results in plant poisoning (Harris and Gitlin 1996). Plant MTs have important roles in seed germination (Zhou et al. 2012), salinity stress (Kumar et al. 2012), drought stress (Samardzic et al. 2010), and low temperature stress (Xue et al. 2009). The most important role of plants MTs is in tolerance to metals, such as Cu, cadmium (Cd) and zinc (Zn) (Lv et al. 2012; Ren et al. 2012; Xia et al. 2012a, b). However, few MTs genes have been isolated from Cu-tolerant plant species. Iris lactea var. chinensis is a widely adapted perennial species, which is also a known Cu hyperaccumulator (Zhang et al. 2007). However, little is known about the molecular mechanism of Cu tolerance and accumulation in this species. The purpose of our study was to experimentally test if IlMT2b can represent a useful target gene for the phytoremediation of Cu-polluted soil.
Materials and Methods
Plants of Iris lactea var. chinensis (Fisch.) Koidz. were collected from the Iris Resource Collection Garden of the Institute of Botany, Jiangsu Province and Chinese Academy of Science. Plants were grown in pots containing 1/2 Hoagland nutrient solution (Han et al. 2007). Seedlings with 10 cm height were selected to optimize uniformity of the experiment. For quantitative real-time PCR (qRT-PCR), 100 μM CuSO4 stress treatment was given to plants. Roots and leaves were sampled at 0, 1, 3, 6, 12 and 24 h after treatment. All samples were collected immediately into liquid nitrogen and then stored at −80 °C until used to extract RNA.
For gene cloning, I. lactea var. chinensis seedlings were grown in 1/2 Hoagland nutrient solution and then 100 μM CuSO4 treatment for 6 h. Total RNA was extracted from I. lactea var. chinensis leaves using the TRIzol reagent (TaKaRa, Japan) according to the manufacturer’s instructions. The first cDNA strand was synthesized using Oligo (dT)18 and SuperScript III reverse transcriptase (Invitrogen, USA), following the manufacturer’s instructions, and used as a template for a 25-µL PCR. Three specific primers (GSP1, GSP2, GSP3) were designed for the 5′ rapid application of cDNA ends (RACE) reaction based on the sequence of an Iris EST (EX953691) (Tang et al. 2009). The 5′ RACE method was acquired following the procedures described by Liu et al. (2012). Finally, a pair primer (IlMT2b-S, IlMT2b-X) was designed to amplify the complete IlMT2b open reading frame (ORF), followed by confirmation of the amplicon by DNA sequencing. The sequences for all of the above primers are given in Table 1. The IlMT2b cDNA ORF was identified using the ORF finder program. Multiple peptide alignments and the phylogeny of the sequences were derived using the DNAman software package (version 5.2.2.0; Lynnon Biosoft, St Louis, QC, CA).
The RNA purity was assessed spectrophotometrically at 260/280 and 260/230 nm. The integrity of the purified RNA was checked by denatured agarose gel electrophoresis. qRT-PCR assays were conducted and assessed following the procedures described by Gu et al. (2011). The gene-specific pair IlMT2b-RT-S/-X (sequences given in Table 1) amplified a 151-bp IlMT2b fragment, while IlUBC-S/-X (sequences given in Table 1) amplified a 224-bp fragment of the reference gene (Iris ubc, GenBank accession EX953716) (Gu et al. 2014).
IlMT2b coding sequence was amplified using a forward primer (IlMT2bc-S) incorporating a Sma I restriction site, and a reverse primer (IlMT2bc-X) incorporating a Xba I restriction site. Sma I-Xba I digested amplicons were inserted into pCAMBIA1301-220 to generate a 35S: IlMT2b construct, which was introduced into Arabidopsis thaliana Col-0 (ecotype Columbia) via the floral dip method (Liu et al. 2012). Transformed lines were selected by germination on a standard medium containing 20 mg/L hygromycin, and confirmed by subsequent RT-PCR and qRT-PCR (sequences given in Table 1). T2 generation A. thaliana was used for identifying IlMT2b function.
Seeds of wild-type and two IlMT2b transgenic lines were germinated on Murashige and Skoog (MS) agar medium containing 0, 50 μM CuSO4, or 100 μM CuSO4 in 9-mm-diameter plates. The plate was maintained vertically in the culture room for 10 days to compare the root length. At least 10 seedlings of each line were measured. For Cu accumulation and H2O2 content, four-leaf stage wild type and transgenic A. thaliana seedlings grown on MS agar medium were potted into a 1:1:1 perlite: vermiculite: soilrite mixture and grown for 4 weeks, then plants were transferred to a growth regulator-free Hoagland and Arnon’s (1950) culture medium. After 2 days, 50 μM CuSO4, or 100 μM CuSO4 was added to the medium and plants were sampled after 7 days growth. The Cu concentration was determined by atomic absorption spectrometry as followed by Lv et al. (2012). H2O2 content was determined according to Veljovic-Jovanovic et al. (2002).
Results were expressed as means ± standard errors. SPSS v13.0 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel 2003 (Microsoft, Redmond, WA, USA) were used for statistical analysis. To determine differences among treatments for each variable at each sampling time point, a one-way analysis of variance (ANOVA) was employed.
Results and Discussion
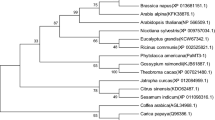
The full-length cDNA IlMT2b sequence (AB907788) was isolated by RT-PCR and RACE based on an EST created by Tang et al. (2009). It consisted of 422 nucleotides, of which 237 bp represented an ORF encoding 78 residues. The predicted gene product is a protein of molecular mass 7.64 kDa and a pI of 4.31, containing a conserved CC, CXC and CXXC domain in the N-terminal, and three CXC units in the C-terminal position (Fig. 1a). The alignment of the IlMT2b sequence with those of homologous MT2b proteins showed levels of similarity ranging between 49.38 % and 65.82 %, and suggested a close phylogenetic relationship with NpMT2 (Fig. 1b). To gain the expression pattern of IlMT2b, qRT-PCR was carried out to analyze samples from leaf and root of Cu stressed plants. From 0 to 3 h treatment, IlMT2b transcript abundance increased gradually in leaf and there was no significant difference in root (Fig. 2). The transcription accumulation of IlMT2b in leaf and root was dramatically increased in response to Cu treatment after 6 h, and then decreased. The four types of plant MTs differ in sequences (Cobbett and Goldsbrough 2002). Compared with homologues from other species, the positions of MT2 Cys residues are conserved (Fig. 1). So, we concluded that the IlMT2b gene encodes a type-2 MT protein. Differential expression of MT2 genes varies among plant species (Ahn et al. 2012; Ren and Zhao 2009). In the present study, IlMT2b was induced by Cu stress. The same Cu inducible expression was found in the AtMT2a in leaves and AtMT2b in roots (Guo et al. 2003), while BcMT2 and TcMT2 were unaffected by Cu treatment (Lv et al. 2012; Roosens et al. 2005). These findings suggest that different plant MTs have distinct functions in Cu tolerance and homeostasis (Lv et al. 2012).
The deduced peptide sequence of IlMT2b (marked in box) and related MT2. a Peptide alignment. Conserved sequences are shown underlined. b The phylogeny of IlMT2b and related MT2s. Bootstrap values of each branch of the derived tree are given. The genes encoding the amino acid sequences and their GenBank accession numbers are: CeMT2b (Colocasia esculenta; Q19LA2), AbMT2b (Atropa belladonna; CAC40757), AtMT2b (Arabidopsis thaliana; AED90465), HiMT2b (Hirschfeldia incana; AGQ45633), NnMT2b (Nelumbo nucifera; ABN46988), PtMT2b (Populus trichocarpa; AAT02525), IpMT2 (Ilex paraguariensis; AFP93964), PmMT2 (Plantago major; CAH59436), LbMT2 (Limonium bicolor; ABL10086), SmMT2b (Salvia miltiorrhiza; ABR92330), PsMT2 (Pisum sativum; BAD18383), SdMT2 (Sesbania drummondii; ABQ44281), ZmMT2 (Zea mays; ACG42263), IlMT2b (Iris. lactea var. chinensis; AB907788), NpMT2 (Narcissus pseudonarcissus; AAL16908), OsMT2b (Oryza sativa; Q5JM82). IlMT2b is in box
To further study the function of IlMT2b, 27 lines of hygromycin-resistant A.thaliana plants were selected as putative transgenic plantlets, 21 of which were positive in a glucuronidase (GUS) assay (data not shown). Based on the PCR and RT-PCR assays, two independent transgenic A. thaliana lines (35S:IlMT2b-1, 35S:IlMT2b-2) which showed higher levels of transgene expression were selected for further experiments (Fig. 3a, b). For the Cu tolerance analysis of IlMT2b-overexpressing A. thaliana, two transgenic lines of T2 progeny and wild-type plants were grown in normal and different Cu concentrations conditions. After 10 days of growth on the MS agar medium under normal conditions, no obvious difference in the root length between wild-type and transgenic seedlings was observed (Fig. 4a, b). After treatment with 50 and 100 μM Cu, the root length of two transgenic seedlings was respectively about 1.5- and 3-fold longer than that of the wild-type (Fig. 4c–f). Although wild-type and transgenic seedlings grew normally, their root lengths were inhibited under the two Cu concentrations compared to control. There was no significant root length difference between the two transgenic lines. As root length has been considered as a reliable standard for measuring heavy metal tolerance (Gasic and Korban 2007), our work demonstrated that heterologous IlMT2b expression has provided evidence for Cu tolerance in A. thaliana. Interestingly, conflicting results have been reported on BjMT2. Ectopic expression of BjMT2 reduced root growth in the absence of Cu exposure, whereas in the presence of Cu stress, root growth in wild-type and BjMT2 transgenic lines was identical (Zhigang et al. 2006). However, the root length of BcMT2 transgenic lines was longer under Cu stress (Lv et al. 2012). It is possible that the MT2-mediated root elongation reduction is related to interference of excess MT2 with the cellular redox balance or signalling processes (Mir et al. 2004; Thomas et al. 2005). Thus, more studies are under way to elucidate the MT2-mediated root growth mechanism.
PCR and qRT-PCR validation of transgenic. a RT-PCR demonstrating the heterologous expression of IlMT2b in A. thaliana. The AtUBQ sequence was used as an internal control; b qRT-PCR based on mRNA. Transcript abundance was normalized against the expression of the constitutively expressed AtUBQ. The PCR for each sample was replicated three times, and the data efficiency of each reaction was 2−∆∆Ct. WT wild type A. thaliana; 35S: IlMT2b-1 and 35S:IlMT2b-2, two presumptive IlMT2b-carrying transgenic lines
Tolerance to Cu in wild-type and two IlMTb transgenic lines. Seeds were germinated on MS agar medium containing 0 μM (a, b), 50 μM (c, d), or 100 μM (e, f) CuSO4, and petri dishes were placed in a vertical orientation upon onset of growth. After 10 days of growth, root lengths of two IlMT2b lines and WT were measured. An asterisk indicates a significant difference (by the t test at p < 0.05) from WT of the same treatment. Values correspond to mean ± SE of 10 plants
The concentration of Cu within the plants reflects their ability to cope with Cu stress. Copper concentrations in wild-type and transgenic seedlings were higher in plants exposed to 100 µM Cu than in those exposed to 50 µM Cu (Fig. 5). However, Cu concentrations in the two transgenic seedlings were higher than in the wild-type exposed to the two copper concentrations (Fig. 5). It is likely that high levels of IlMT2b expression may be required for Cu homeostasis (Roosens et al. 2004). Some observations support the hypothesis. Thomas et al. (2003) observed that overexpression of the yeast metallothionein (CUP 1) in tobacco plants results in two to three times the copper content as that of the control plants. Furthermore, a predominant Cu-homeostasis function has also been found in TcMT3 (Roosens et al. 2004). In A. thaliana, constitutive expression of AtMT2b increased Cu accumulation in the yeast Dcup1 mutant (Guo et al. 2003, 2008). Also, a pea class-2 MT increased Cu tolerance and accumulation, when overexpressed in Escherichia coli and A. thaliana (Evans et al. 1992).
Cu accumulation in leaves of wild-type and two IlMTb transgenic lines exposed to 50 or 100 μM Cu stess for 7 days. WT: wild type A. thaliana; 35S:IlMT2b-1 and 35S:IlMT2b-2: IlMT2b transgenic lines. An asterisk indicates a significant difference (by the t test at p < 0.05) from WT of the same treatment. Values correspond to mean ± SE of of 10 plants
Copper stress can lead to the generation of reactive oxygen species (ROS), such as H2O2 (Kumar et al. 2012). In our study, H2O2 accumulation in wild-type and transgenic seedlings was significantly increased, as seen by measuring H2O2 content during Cu stress (Fig. 6). However under 50 μM Cu stress, the leaves of two IlMT2b transgenic lines showed remarkably less accumulation of H2O2 content compared to wild-type (Fig. 6). This contrast in H2O2 levels between wild-type and transgenic lines was noticed even under 100 μM Cu stress (Fig. 6). In animals, MTs act as antioxidants against ROS (Palmiter 1998). However, plant MTs may also provide protection against ROS during abiotic stress. In our study, injured leaves were shown to contain lower concentrations of H2O2 in two IlMT2b transgenic lines under Cu stress (Fig. 6). It is possible that the ROS scavenging in plants might have been affected by overexpression of IlMT2b, and this may have resulted in the observed Cu tolerance in transgenic lines. Similar results have been reported previously. For example, AtMT2a mediates ROS balance during oxidative stress (Zhu et al. 2009). BcMT1 and BcMT2 decreased production of Cu induced ROS (Lv et al. 2012), while OsMT1e-P conferred multiple abiotic stress tolerance in tobacco via ROS scavenging (Kumar et al. 2012). Based on these studies, it was proposed that higher ROS scavenging ability resulted in increased tolerance to abiotic stress, and that ROS may act as secondary messengers in redox signal transduction (Xue et al. 2009).
In the present study, we described the isolation of a MT2b cDNA from I. lactea var. chinensis and showed that its transcription accumulation is induced by an exogenous supply of Cu. Its heterologous expression in A. thaliana demonstrated that the IlMT2b transgenic lines enhanced the tolerance and accumulation of Cu. Further, we also showed that IlMT2b overexpressing plants accumulated lower amounts of hydrogen peroxide (H2O2) under Cu stress. Based on these results, IlMT2b may serve as an important target gene for phytoremediation of Cu-contaminated soils.
References
Ahn YO, Kim SH, Lee J, Kim HR, Lee HS, Kwak SS (2012) Three Brassica rapa metallothionein genes are differentially regulated under various stress conditions. Mol Biol Rep 39:2059–2067
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Evans KM, Gatehouse JA, Lindsay WP, Shi J, Tommey AM, Robinson NJ (1992) Expression of the pea metallothionein-like gene PsMT A in Escherichia coli and Arabidopsis thaliana and analysis of trace metal ion accumulation: implications for PsMT A function. Plant Mol Biol 20:1019–1028
Gasic K, Korban SS (2007) Expression of Arabidopsis phytochelatin synthase in Indian mustard (Brassica juncea) plants enhances tolerance for Cd and Zn. Planta 225:1277–1285
Gu C, Liu L, Xu C, Zhao, Y, Zhu, X, Huang, S (2014) Reference gene selection for quantitative real-time RT-PCR normalization in Iris. lactea var. chinensis roots under cadmium, lead, and salt stress conditions. Sci World J, Article 532713, 7
Gu C, Chen S, Liu Z, Shan H, Luo H, Guan Z, Chen F (2011) Reference gene selection for quantitative real-time PCR in Chrysanthemum subjected to biotic and abiotic stress. Mol Biotechnol 49:192–197
Guo W, Bundithya W, Goldsbrough PB (2003) Characterization of the Arabidopsis metallothionein gene family: tissue-specific expression and induction during senescence and in response to copper. New Phytol 159:369–381
Guo W, Meetam M, Goldsbrough PB (2008) Examining the specific contributions of individual Arabidopsis metallothioneins to copper distribution and metal tolerance. Plant Physiol 146:1697–1706
Hall J (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Han Y, Yuan H, Huang S, Guo Z, Xia B, Gu J (2007) Cadmium tolerance and accumulation by two species of Iris. Ecotoxicology 16:557–563
Harris Z, Gitlin J (1996) Genetic and molecular basis for copper toxicity. Am J Clin Nutr 63:836S–841S
Kumar G et al (2012) Clustered metallothionein genes are co-regulated in rice and ectopic expression of OsMT1e-P confers multiple abiotic stress tolerance in tobacco via ROS scavenging. BMC Plant Biol 12:107
Liu Z et al (2012) Heterologous expression of a Nelumbo nucifera phytochelatin synthase gene enhances cadmium tolerance in Arabidopsis thaliana. Appl Biochem Biotech 166:722–734
Lv Y, Deng X, Quan L, Xia Y, Shen Z (2012) Metallothioneins BcMT1 and BcMT2 from Brassica campestris enhance tolerance to cadmium and copper and decrease production of reactive oxygen species in Arabidopsis thaliana. Plant Soil 367:507–519
Mir G, Domènech J, Huguet G, Guo W-J, Goldsbrough P, Atrian S, Molinas M (2004) A plant type 2 metallothionein (MT) from cork tissue responds to oxidative stress. J Exp Bot 55:2483–2493
Palmiter RD (1998) The elusive function of metallothioneins. P Natl Acad Sci USA 95:8428–8430
Ren Y, Zhao J (2009) Functional analysis of the rice metallothionein gene OsMT2b promoter in transgenic Arabidopsis plants and rice germinated embryos. Plant Sci 176:528–538
Ren Y, Liu Y, Chen H, Li G, Zhang X, Zhao J (2012) Type 4 metallothionein genes are involved in regulating Zn ion accumulation in late embryo and in controlling early seedling growth in Arabidopsis. Plant Cell Environ 35:770–789
Roosens NH, Bernard C, Leplae R, Verbruggen N (2004) Evidence for copper homeostasis function of metallothionein (MT3) in the hyperaccumulator Thlaspi caerulescens. FEBS Lett 577:9–16
Roosens NH, Leplae R, Bernard C, Verbruggen N (2005) Variations in plant metallothioneins: the heavy metal hyperaccumulator Thlaspi caerulescens as a study case. Planta 222:716–729
Samardzic JT, Nikolic DB, Timotijevic GS, Jovanovic ZS, Milisavljevic M, Maksimovic VR (2010) Tissue expression analysis of FeMT3, a drought and oxidative stress related metallothionein gene from buckwheat (Fagopyrum esculentum). J Plant Physiol 167:1407–1411
Tang S et al (2009) EST and EST-SSR marker resources for Iris. BMC Plant Biol 9:72
Thomas JC et al (2003) Yeast metallothionein in transgenic tobacco promotes copper uptake from contaminated soils. Biotechnol Progr 19:273–280
Thomas JC, Perron M, LaRosa PC, Smigocki AC (2005) Cytokinin and the regulation of a tobacco metallothionein-like gene during copper stress. Physiol Plantarum 123:262–271
Veljovic-Jovanovic S, Noctor G, Foyer CH (2002) Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol Biochem 40:501–507
Xia Y, Lv Y, Yuan Y, Wang G, Chen Y, Zhang H, Shen Z (2012a) Cloning and characterization of a type 1 metallothionein gene from the copper-tolerant plant Elsholtzia haichowensis. Acta Physiol Plant 34:1819–1826
Xia Y et al (2012b) Overexpression of Elsholtzia haichowensis metallothionein1 (EhMT1) in tobacco plants enhances copper tolerance and accumulation in root cytoplasm and decreases hydrogen peroxide production. J Hazard Mater 233–234:65–71
Xue T, Li X, Zhu W, Wu C, Yang G, Zheng C (2009) Cotton metallothionein GhMT3a, a reactive oxygen species scavenger, increased tolerance against abiotic stress in transgenic tobacco and yeast. J Exp Bot 60:339–349
Zhang K, Tong H, Huang S, Yuan H (2007) Effect of Cu stress on Cu accumulation and other nutrient element absorption of Iris pseudacaorus and I. lactea var. chinensis. J Plant Res Environ 1:18–22
Zhigang A, Cuijie L, Yuangang Z, Yejie D, Wachter A, Gromes R, Rausch T (2006) Expression of BjMT2, a metallothionein 2 from Brassica juncea, increases copper and cadmium tolerance in Escherichia coli and Arabidopsis thaliana, but inhibits root elongation in Arabidopsis thaliana seedlings. J Exp Bot 57:3575–3582
Zhou Y et al (2012) Overexpression of Nelumbo nucifera metallothioneins 2a and 3 enhances seed germination vigor in Arabidopsis. Planta 235:523–537
Zhu W, Zhao D, Miao Q, Xue T, Li X, Zheng C (2009) Arabidopsis thaliana metallothionein, AtMT2a, mediates ROS balance during oxidative stress. J Plant Physiol 52:585–592
Acknowledgments
The study was supported by the JiangSu Provincial Key LAB Foundation For Plant EX SITU Conservation (Grant No. 201201), National Natural Science Foundation of China (Grant No. 31301807) and the Science Foundation of JiangSu (Grant No. BK20130734).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, CS., Liu, LQ., Deng, YM. et al. The Heterologous Expression of the Iris lactea var. chinensis Type 2 Metallothionein IlMT2b Gene Enhances Copper Tolerance in Arabidopsis thaliana . Bull Environ Contam Toxicol 94, 247–253 (2015). https://doi.org/10.1007/s00128-014-1444-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1444-x