Abstract
La3+ ions are known to antagonize Ca2+ and are used as a Ca2+ channel blocker but little is known on the direct effects of La3+. Micromolar La3+ concentrations promoted root growth while higher concentrations were inhibitory. The uptake of La3+ in maize root protoplasts revealed a membrane binding component (0.14 and 0.44 pmol min−1 protoplast−1 for 100 and 1,000 μM La3+) followed by a slower concentration and time-dependent uptake. Uptake was reduced by Ca2+, but had no substantial effect on other ions. La3+ shifted microtubule organization from random to parallel but caused aggregation of microfilaments. Our data suggest that La3+ is taken up into plant cells and affects growth via stabilization of the cytoskeleton.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
La3+ (LAN) is a member of the rare-earth elements, which include 15 trivalent elements with similar properties. LAN was reported to function as a membrane-impermeable calcium antagonist (Thomson et al. 1973; Pantoja et al. 1992), and induces various responses including Ca2+-channel inhibition and Ca2+-related gene expression (Thomson et al. 1973; Pantoja et al. 1992; Jackson and Hall 1993; Bush 1995; Polisensky and Braam 1996; Rock and Quatrano 1996).
LAN has growth promoting and quality improving effects on wheat and rice, and is used as fertilizer (Guo 1985; Tang and Xiao 1996). LAN increased root growth (Diatloff et al. 1995a, 1995b) and spike production in wheat (Meehan et al. 1993), and promoted root and shoot growth in Phaseolus radiatus and Brassica pekinensis (Velasco et al. 1979). LAN promoted reproductive growth of Nicotiana tabacum and A. thaliana (He and Loh 2000; Sun et al. 2003) but inhibited the formation of shoot buds (Mundhara and Rashid 2002). In addition, LAN inhibited graviperception (Belyavskaya 1996) and gravitropic response in corn (Collings et al. 1992) and snapdragon spikes (Friedman et al. 1998). However, the mechanism and physiological processes of LAN activity remain obscure. To understand the physiological role of LAN in plants, cellular effects must be studied. One of the most important questions is whether LAN enters the cell or not. LAN uptake was inhibited at the casparian strips (Whitson and Murray 1974; Pellegrini at al. 1991) and there has been some debate as to whether LAN can across the cell membrane at all (Lehmann et al. 2000; Gao et al. 2003). Although there is binding and electrostatic attraction of LAN to plasma membranes (Abe and Takeda 1988; Yermiyahu et al. 1997), it is not clear whether LAN directly or indirectly affects a calcium-dependent signal transduction pathway. Ultrastructural studies may not be sensitive enough to accurately depict the actual distribution of LAN in situ (Lehmann et al. 2000), therefore a kinetic uptake study was performed.
Although its uptake remains controversial, LAN has been linked to intracellular processes such as inhibition of intracellular ion channels (Tester 1990), inductions of several genes (Polisensky and Braam 1996; Rock and Quatrano 1996), binding of proteins and mimicking Ca2+ action inside the cell (Belyavskaya 1996). These effects may depend on LAN uptake, but we do not know how LAN crosses membranes, or the time course of uptake. Confounding the problem of ion uptake in plant cells is the cell wall (CW), which is composed of diverse cross-linked components including microfibrils and other cross-linked carbohydrates, pectin, proteins, and ions (Cosgrove 1997). Therefore the CW acts as an ion exchanger with the surrounding solution. The CW might be a barrier that reduces the effective LAN concentration and thus inhibits transport across the plasma membrane. Protoplasts, as a CW-free system, offer the unique advantage of a tested physiological and versatile system for studying various aspects of plant signaling mechanisms (for review, see Sheen 2001), and we quantified LAN uptake using protoplasts.
Cell growth depends on the rate of cell division and the extent of subsequent elongation. Both functions are tightly linked to microtubules (MTs). MTs affect division by organizing the pre-prophase band and formation of the cell plate, and contribute to cell elongation by orienting cellulose microfibrils (Giddings and Staehelin 1991; Wymer and Lloyd 1996). Similar to MTs, actin is involved in numerous fundamental cellular processes, including regulation of cell-shape, cell motility, and transport. The cytoskeletal organization is controlled by cellular events and depends on calcium/calmodulin-related processes (Cyr and Palevitz 1995). The calcium dependency suggests that LAN might affect the cytoskeleton. However, to our knowledge there is no report investigating the direct effect of LAN on the organization of cytoskeleton to date. Because protoplasts are thought to have no predetermination of cell shape and cytoskeleton (Hasezawa and Syono 1988), we hypothesize that LAN can be taken up into the cell and that it mediates the organization of MTs and microfilaments (MFs). To our knowledge, this is the first report on integral LAN effects including uptake, growth, and cytoskeletal organization. These data provide a basis for interpreting the physiological and biochemical effects of LAN on plant cells.
Materials and methods
Plant material
Seeds of maize (Zea mays L. cv. Merit & Pioneer 3085) were soaked in deionized water overnight, planted between wet paper towels in plastic trays, and grown vertically in the dark at 24°C for about 2 days. Seedlings with 2- to 3-cm-long, straight primary roots were used for the experiments.
Measurement of root growth
Maize seedlings were transferred to a 60 ml clear plastic container filled with 5 mM MES/Tris buffer, pH 6.2. Seedlings were mounted on the wall with the terminal 10–15 mm of the root immersed in the buffer. The buffer was continuously aerated at about 25 ml/min. After allowing the roots to adapt over a period of 1 h, growth was recorded using a computer-based video-digitizer system (Hasenstein 1991), using a video camera (Panasonic WV-BP310) with a 50 mm lens. The position of the root tips was recorded at 2-min intervals at a resolution of 26 μm/pixel. Growth was measured over 5 h in MES/TRIS buffer (5 mM, pH 6.2) with 0, 1, 10, 100, 1,000 μM and 10 mM LaCl3. Each combination was measured four times with two repeats each.
Isolation of protoplasts
Protoplasts were prepared from harvested roots and digested overnight in 20 ml osmoticum [0.6 M mannitol, 5 mM MES/Tris (pH 6.0), 1 mM CaCl2, 0.2 mM MgCl2] containing 2% cellulase, 0.2% pectolyase Y-23 (Kikkoman, Tokyo, Japan) and 0.05% bovine serum albumin. Protoplasts were filtered through 85-µm mesh, and centrifuged (200 g, 10 min). The sedimented protoplasts were resuspended in osmoticum and layered on top of osmoticum containing 0.6 M sucrose instead of mannitol. After centrifugation (90 g, 10 min) protoplasts at the interphase were collected and washed three times in osmoticum with or without 1 mM CaCl2. Protoplasts were pelleted (130 g, 5 min), resuspended in osmoticum, and counted in a hemocytometer.
Vital staining of protoplasts
Fluorescein diacetate (5 mg) was dissolved in 1 ml acetone and mixed with 20 ml osmoticum to 0.05% (w/v) solution. A 0.25 ml aliquot of the protoplast suspension was added to 1 ml staining solution, gently mixed and examined on an epifluorescence photomicroscope (Nikon Eclipse E600 FN, excitation 420–490 nm).
La3+ uptake and ion load
Protoplast suspensions were divided into aliquots and 1 M LaCl3 stock solution was added to establish final concentrations of 1 µM, 100µM and 1 mM LaCl3. After 5 min, and 0.5, 1.5, 3 and 5 h, aliquots of protoplast suspension were removed and washed three times in osmoticum by centrifugation (130 g; 5 min). The protoplasts were digested with 1 ml 70% HNO3 for 30 min, diluted with distilled H2O to 10 ml, and analyzed using inductively coupled plasma spectrometry (ICP-OES, Perkin Elmer Optima 3000). Ion quantities were calculated as micrograms per 106 protoplasts.
Immunofluorescence staining
Freshly isolated protoplasts were washed with 0.6 M mannitol and fixed for 2 h in 3% formaldehyde, 0.4 M mannitol, 5% dimethylsulfoxide in PHEMD buffer (60 mM PIPES, 25 mM HEPES, 2 mM MgCl2 and 10 mM EGTA, pH 7.0). Protoplasts were washed in PHEMD buffer (pH 7.0) three times and transferred to slides coated with Mayer’s Albumen histological adhesive and covered by a thin agarose-gelatin film (Brown and Lemmon 1995). Cells were treated with 1% Triton-X for 20 min and incubated in rat anti-yeast tubulin (Clone Yol 1/34, Harlan Sera-Lab, Loughborough, UK) for at least 2 h. Monoclonal anti-pea actin antibody was a gift from R. Cyr, Pennsylvania State University (Andersland et al.1994). Sections were washed with PHEMD buffer three times. This was followed by incubation with secondary antibody conjugated to fluorescein isothiocyanate (goat against rat-IgG for MTs and rabbit anti-mouse IgG for F-actin; Accurate Chemical & Scientific, Westbury, N.Y.). After mounting (ProLong Anti-fade Kit, Molecular Probes, Eugene, Ore.) the slides were observed using a confocal laser scanning electron microscope (MRC-1024 ES; Bio-Rad, Richmond, Calif.), with excitation at 488 nm and emission at 520 nm. Images were processed with Adobe Photoshop 7.0.
Statistical analysis
Growth data and LAN uptake was analyzed using repeated-measure analysis of variance [SAS PROC GLM Version 8, 1999; SAS Institute, Cary, N.C.] with paired comparisons using Tukey-adjusted least-square means. Data were transformed when necessary to meet assumptions of normality and homogeneity of variance.
Results
Effect of La3+ on primary root growth
High LAN concentrations (>100 μM) inhibited root growth and low concentrations (<1 μM) promoted growth (Fig. 1). Elongation was proportional to the external LAN concentration (F4,35=19.43, P<0.001) and time (F6,30=5.28, P<0.001). The growth rate decreased immediately after exposure to 10 mM LAN (P=0.005); 1 mM LAN inhibited growth after 100 min (P=0.0196) of application and 100 μM LAN required 180 min before it showed an inhibitory effect (P=0.002). Application of 1 μM LAN resulted in growth promotion that was significant after 220 min (P=0.031). Over a time period of 4 h, the rates of root elongation increased 10% with 1 µM but decreased 28%, 42%, 55% at 100 µM, 1 mM and 10 mM LAN, respectively. After 24 h, roots exposed to 1 mM LAN were swollen and scaly, which was most pronounced in the elongation zone (Fig. 2).
La3+ uptake in protoplasts
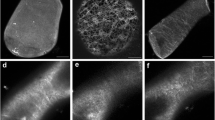
The protoplasts (yield 2×106 protoplasts per gram tissue) were spherical with well-organized internal structure (Fig. 3a). After LAN treatment, the size of the vacuole increased and changed its shape (Fig. 3c) but did not affect the ratio of viable protoplasts. However, the fluorescence intensity (Fig. 3d) was reduced compared to the control (Fig. 3b).
LAN associated with corn root protoplasts increased over time (F4,5=1,023.93, P<0.001, Fig. 4) proportionally to the external concentration (F3,8=105.47, P<0.001, data not shown). The temporal change of LAN measured in the protoplast fraction consisted of two phases. At 1 mM LAN, LAN increased rapidly to a level of about 100 μg per 106 protoplasts (Fig. 4). After 3 h, uptake resumed and increased steadily until the end of measurements. This distribution is in line with the concept that LAN is initially absorbed by the membrane. The time course of uptake after 1 mM and 100 μM LAN was similar (Fig. 4). In addition to a concentration dependency, LAN uptake was also dependent on Ca2+ in the wash medium; 1 mM Ca2+ in the wash medium reduced LAN signal to about one-fourth of the value without Ca2+ but the uptake dynamic remained the same (Fig. 5). Adding Ca2+ ionophore A23187 (P>0.05, data not shown) before LAN did not alter LAN uptake, regardless of the presence of Ca2+ in the wash medium.
a Uptake of La3+ in corn root protoplasts after application of 0, 1, 100, or 1,000 μM La3+; N=3±SE, *P<0.05, **P<0.01. b Subtraction of the linear portion of uptake (stippled line) from the total fraction yields the adsorbed quantity (- · -), which was numerically estimated as a function of the type y=It × exp(rt), see Table 1
Influence of La3+ on other ions
After 5 h, the addition of LAN slightly increased the level of Ca2+ in the presence of 1 and 100 μM LAN, but had no effect at 1 mM (Fig. 6 c). LAN decreased K+ levels during the first 30 min but had no long-term effect (Fig. 6b). The Mg2+ level was not affected (Fig. 6a).
Effect of La3+ on the organization of MTs
Protoplast MTs showed no preferential arrangement in the majority of newly isolated protoplasts (Figs. 7a, 8a). Three hours after isolation, wavy MTs gradually developed (Fig. 8a–c). Cells with transversely organized MTs elongated in response to 1 μM LAN (Fig. 7b). The MTs appeared brighter and formed numerous bundles. Exposure of more than 5 h to LAN concentrations >1 mM depolymerized MTs (Fig. 7c).
a Freshly isolated corn root protoplasts typically showed poorly aligned microtubules (MTs). b In the presence of 1 μM La3+ unusually elongated protoplasts with radial MTs were observed. c After exposure to 1 mM LaCl3 for more than 5 h, death of many cells was common and accompanied by fragmented MTs. Images are projections of optical stacks produced with a confocal microscope. Bar 10 μm
Organization of MTs in corn root protoplasts without LAN (a–c) and in response to 1 mM LAN (d–f). Untreated cell after 1 h (a), 3 h (b), and 5 h (c) in osmoticum showed poorly organized MTs. Application of LAN for 1 h (d), 3 h (e) and 5 h (f), resulted in increasingly better organized MTs with parallel organization. Images are composites of combined optical sections obtained with a confocal microscope. Bar 10 μm
Compared to controls (Fig. 8a–c), 1 mM LAN resulted in more aligned MTs, which developed into a parallel array after 3 h. At 5 h, realignment to parallel MTs was complete (Fig. 8d–f). However, in some cases, MTs depolymerized. The parallel MT organization was similar at 100 μM and 1 mM LaCl3 (data not shown).
Effect of La3+ on the organization of F-actin
Newly isolated protoplasts contained disorganized and evenly distributed F-actin filaments around the nucleus (Fig. 9a). In some protoplasts, LAN application changed the alignment of F-actin, with the filaments aggregated around the nucleus (Fig. 9b). In dying cells, most F-actin filaments depolymerized and only some fragments remained near the nucleus (Fig. 9c). F-actin was evenly distributed and did not change in control protoplasts but vacuolization increased (Fig. 10a, b). LAN (1 μM) disrupted actin filaments. Over time, filaments formed bundles (Fig. 10c) and aggregation of the actin network occurred (Fig. 10d) with actin strands radiating from the nucleus. Higher concentrations (0.1 and 1 mM LAN) modified the actin network similarly. After 5 h, thick bundles formed surrounding the enlarged vacuoles (Fig. 10e, f).
Microfilaments in freshly isolated corn root protoplasts were disorganized (a), but aggregated after exposure to 1 mM La3+ for 3 h (b). Fragmentation of microfilaments (MFs) was indicative of dying cells and was promoted by exposure to 1 mM La3+ (c). Images are projections of optical sections obtained with a confocal microscope. Bar 10 μm
Organization of F-actin in corn root protoplasts after exposure to 0 (a, b), 1 (c, d), 100 (e) and 1,000 μM (f) La3+. F-actin in control cell incubated in osmoticum for 1 (a) and 5 h (b) showed random orientation and thin F-actin filaments. After adding 1 µM La3+ for 1 h (c) and 5 h (d), filaments aggregated; 100 (e) and 1,000 μM La3+ (f) for 5 h caused F-actin bundling. Images are projections of optical sections obtained with a confocal microscope. Bar 10 μm
Discussion
La3+ effects on root elongation growth
The majority of studies have regarded LAN as a probe for calcium-related events in plants (Thomson et al. 1973; Pantoja et al. 1992, Jackson and Hall 1993; Bush 1995; Polisensky and Braam 1996; Rock and Quatrano 1996). However, the ability of low concentrations of LAN (<1 µM) to promote elongation (Fig. 1) is consistent with earlier reports of the growth-promoting action of LAN (Velasco et al. 1979; Diatloff et al. 1995a, 1995b; He and Loh 2000). These effects of LAN at >100 μM (Figs. 1, 2) are similar to those of aluminum on maize root growth (Blancaflor et al. 1998). However, compared to the rapid inhibition by Al3+ (Kochian 1995), LAN exhibited slower inhibition that was dependent on exposure time (Fig. 1). The complex results of LAN on root growth suggest mechanisms that cannot be attributed to non-membrane-permeable calcium probes. Therefore, the mechanism behind LAN’s mode of action requires assessment of whether LAN can cross the cell membrane.
La3+ uptake
Because uptake data are confounded by La binding to the external membrane, it is important to separate the two processes. The observed time course of LAN accumulation (Fig. 4) is consistent with two processes, an exponential phase of adsorption to the membrane and actual uptake. Adsorption is the difference between (linear) uptake and the observed measurements. A numerical analysis of the data (Table 1) indicates that adsorption is concentration-dependent and that lower LAN concentrations result in faster adsorption. The adsorption (Table 1, Fig. 4) of lower LAN concentrations results in faster adsorption equilibrium, which indicates that uptake of low LAN concentrations are difficult to assess. The concentration dependency of the adsorption process and the slower adsorption at higher concentrations (Table 1) is in line with membrane saturation. Because adsorption dominates the early phase of LAN interaction with protoplasts and cells, the uptake curve shows a flat interval. This observation may have contributed to the notion that LAN does not cross the cell membrane (Thomson et al. 1973; Leonard et al. 1975; Pantoja et al. 1992; Lehmann et al. 2000). However, the notion that LAN can cross the membrane is in line with the observed changes in the cytoskeleton (Figs. 7,8 9 10). Although the data support La uptake in protoplasts, the situation in intact cells or roots may differ substantially. The CW is known to have numerous charged binding sites and adsorption is likely to reduce the effective LAN concentration and reduce transport into cells. This scenario is also supported by the observation that LAN affected only the concentrations of its most similar ion, Ca2+, but had no effect on Mg2+ or K+ (Fig. 6). Although studies in vesicles showed that calcium ionophores facilitate LAN exchange (Wang et al. 1998), our data suggest that A23187 did not affect LAN uptake, possibly because the applied concentration of ionophore (1 µM) was not optimal for ionophore-catalyzed transport (Wang et al. 1998).
La3+ and the cytoskeleton
Growth inhibition and swelling of roots (Fig. 2) and protoplasts (Fig. 3) after LAN treatment suggest that LAN affects the cytoskeleton. Because isolated protoplasts retain their cell identity and differentiated state (Sheen 2001), and the behavior of cortical MTs in regenerating protoplast is similar to that of MTs in developing tissue (Wymer et al. 1996), the cytoskeletal changes are likely to be representative of those occurring in intact roots. However, protoplasts are discontinuous and therefore do not provide the spatial cues of cytoskeletal arrays associated with intact tissue. The enzymatic removal of the CW produces sphere-shaped protoplasts with disorganized cortical arrays of MTs and F-actin (Figs. 7a, 9a), as shown previously (Hasezava and Syono 1988; Wymer et al. 1996; Collings et al. 1998). The increased stability after 1 µM LAN application can be inferred from enhanced brightness, bundling, and the high proportion of elongated cells (Fig. 7b), which indicate MT stabilization (Weerdenburg and Seagull 1987; Melan 1990). Because cortical MTs normally acquire their organization before affecting elongation (Hasezava and Syono 1988), we hypothesize that LAN-induced growth promotion relies on (enhanced) MT organization. In contrast, inhibitory concentrations of LAN (>100 µM) resulted in disorganization or delayed organization of MTs, which is likely to affect microfibril deposition (Ledbetter and Porter 1964; Cyr 1994). MT reorientation during LAN incubation (Fig. 8) is similar to the effect of taxol (Kuss-Wymer and Cyr 1992) or aluminum (Blancaflor et al. 1998), which also inhibit elongation. Compared with the reaction of MTs in intact roots, where reorientation occurs 24 h after LAN application (data not shown), the LAN-induced reorganization in protoplasts is rapid and indicates that LAN affects growth via MT organization.
Although F-actin did not change its orientation after LAN treatment, remarkable changes were observed. F-Actin appeared either as single filaments or thin bundles in controls and LAN-treated cells (Figs. 9a, 10a–d). The thickness of F-actin bundles increased proportionally to the concentration of LAN, indicating LAN-impaired function of fine actin filaments (Ketelaar and Emons 2001). Fine filaments are associated with regions of active growth or cell repair, whereas bundled actin may impede vesicle trafficking (Foissner et al. 1996; Miller et al. 1999). Aggregation of F-actin is therefore consistent with growth inhibition at >100 µM LAN (Fig. 1). In addition to changes in MTs, the deformation of vacuoles (Fig. 10e, f) may affect cell shape. The accumulation of numerous, smaller vacuoles is likely to contribute to cell distortions and scaly surface morphology (Fig. 3). Actin reorganization may also affect the transport of ions and metabolites across the plasma membrane (Schweibert et al. 1994; Tilly et al. 1996).
Possible mechanism of La3+
Although our experiments illustrate significant effects of LAN on root growth, the fundamental mechanism remains unclear. It is possible that LAN is active by itself or functions via calcium (Thomson et al. 1973; Pantoja et al. 1992). Calcium-related effects can result from a competitive interaction with Ca2+ (Fig. 5). However, independent LAN effects can be deduced from the temporal disparity between uptake and subsequent effects. LAN exhibited a fast effect on growth but the effects on the cytoskeleton were delayed. If LAN acts only as a Ca2+ competitor, all effects observed should show a similar lag. Because growth and cytoskeletal responses showed different lag times, complex and multiple LAN effects are likely, such as ion signaling and homeostasis (Fig. 6) and interaction with ion channels (Osawa and Matsumoto 2002) or binding to aromatic rings, carboxyl group and phospholipids (Abe and Takeda 1988). The similarities between LAN and Al effects suggest effects on root growth through interactions with the CW, plasma membrane, or symplasm (Kochian 1995). The data presented here illustrate that LAN is taken up into cells and that it has effects beyond the role of a Ca2+ antagonist. Future studies will characterize LAN-induced gene activation.
Abbreviations
- CW :
-
Cell wall
- ICP-OES :
-
Inductively coupled plasma spectrometry
- LAN :
-
Lanthanum
- MF :
-
Microfilament
- MT :
-
Microtubule
References
Abe S, Takeda J (1988) Effects of LaCl3 on surface charges, dielectrophoresis, and electrofusion of barley protoplasts. Plant Cell Physiol 87:389–394
Andersland JM, Fisher DD, Wymer CL, Cyr RJ, Parthasarathy MV (1994) Characterization of a monoclonal antibody prepared against plant actin. Cell Motil Cytoskeleton 29:339–344
Belyavskaya NA (1996) Calcium and graviperception in plants: inhibitor analysis. Int Rev Cytol 168:123–185
Blancaflor EB, Jones DL, Gilroy S (1998) Alterations in the cytoskeleton accompany aluminum induced growth inhibition and morphological changes in primary roots of maize. Plant Physiol 118:159–172
Brown RC, Lemmon BE (1995) Methods in plant immunolight microscopy. Methods Cell Biol 49:85–107
Bush SD (1995) Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol 46:95–122
Collings DA, White RG, Overall RL (1992) Ionic current changes associated with the gravity-induced bending response in roots of Zea mays L. Plant Physiol 100:1417–1426
Collings DA, Allen NS, Shibaoka (1998) Plasma membrane-associated actin in bright Yellow 2 tobacco cells. Plant Physiol 118:917–928
Cosgrove DJ (1997) Assembly and enlargement of the primary cell wall in plants. Annu Rev Cell Dev Biol 13:171–201
Cyr RJ (1994) Microtubules in plant morphogenesis: role of the cortical array. Annu Rev Cell Biol 10:153–180
Cyr RJ, Palevitz BA (1995) Organization of cortical microtubules in plant cells. Curr Opin Cell Biol 7:65–71
Diatloff E, Smith FW, Asher CJ (1995a) Rare earth elements and plant growth. II. Responses of corn and mungbean to low concentrations of lanthanum in dilute, continuously flowing nutrient solutions. J Plant Nutr 18:1977–1986
Diatloff E, Smith FW, Asher CJ (1995b) Rare earth elements and plant growth. III. Responses of corn and mungbean to low concentrations of cerium in dilute, continuously flowing nutrient solutions. J Plant Nutr 18:1987–2003
Foissner I, Lichtscheidl IK, Wasteneys GO (1996) Actin-based vesicle dynamics and exocytosis during wound wall formation in Characean internodal cells. Cell Motil Cytoskeleton 35:35–48
Friedman H, Meir S, Rosenberger I, Halevy AH, Kaufman PB, Philosoph-Hadas S (1998) Inhibition of the gravitropic response of snapdragon spikes by the calcium-channel blocker lanthanum chloride. Plant Physiol 118:483–492
Gao YS, Zeng FL, Yi A, Ping S, Jing LH (2003) Research of the entry of rare earth elements Eu3+ and La3+ into plant cell. Biol Trace Element Res 91:253–265
Giddings TH, Staehelin LA (1991) Microtubule-mediate control of microfibril deposition: a re-examination of the hypothesis. In: Lyoyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, San Diego, pp 85–99
Guo B (1985) Present and future situation of rare earth in China’s agronomy. In: Xu G, Xia J (eds) Proceedings of international conference on rare earth development and applications. Science Press, Beijing, pp 1522–1602
Hasenstein KH (1991) Measurement of circumnutation in maize roots. Micrograv Sci Technol 4:262–266
Hasezawa S, Syono K (1988) Rearrangement of cortical microtubules in elongating cells derived from tobacco protoplasts—a time course observation by immunofluorescence microscopy. J Plant Physiol 133:46–51
He YW, Loh CS (2000) Cerium and lanthanum promote floral initiation and reproductive growth of Arabidopsis thaliana. Plant Sci 159:117–124
Jackson C, Hall JL (1993) A fine structural analysis of auxin-induced elongation of cucumber hypocotyls, and the effects of calcium antagonists and ionophores. Ann Bot 72:193–204
Ketelaar T, Emons AC (2001) The cytoskeleton in plant cell growth: lessons from root hairs. New Phytol 152:409–418
Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46:237–260
Kuss-Wymer CL, Cyr RJ (1992) Tobacco protoplasts differentiate into elongate cells without net microtubule depolymerization. Protoplasma 110:425–430
Ledbetter MC, Porter KR (1964) Morphology of microtubules of plant cells. Science 144:872–874
Lehmann H, Stelzer R, Holzamer S, Kunz U, Gierth M (2000) Analytical electron microscopical investigations on the apoplastic pathways of lanthanum transport in barley roots. Planta 211:816–822
Leonard RT, Nagahashi G, Thomson WW (1975) Effect of lanthanum on ion absorption in corn roots. Plant Physiol 55:542–546
Meehan B, Peverill K, Skroce A (1993) The impact of bioavailable rare earth elements in Australia agricultural soils. In: Australia soil and plant analysis. Australia: First National Workshop on Soil and Plant Analysis. pp 36–41
Melan MA (1990) Taxol maintains organized microtubule patterns in protoplasts which lead to resynthesis of organized cell wall microfibrils. Protoplasma 153:169–177
Miller DD, DeRuitjer NCA, Bisseling T, Emons AM (1999) The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharides as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J 17:141–154
Mundhara R, Rashid A (2002) Stimulation of shoot-bud regeneration on hypocotyl of Linum seedlings, on a transient withdrawal of calcium: effect of calcium, cytokinin and thidiazuron. Plant Sci 162:211–214
Osawa H, Matsumoto H (2002) Aluminum triggers malate-independent potassium release via ion channels from the root apex in wheat. Planta 215:405–412
Pantoja O, Gelli A, Blumwald E (1992) Voltage-dependent calcium channels in plant vacuoles. Science 255:1567–1570
Pellegrini L, Epiardlahaye M, Penot M (1991) Use of lanthanum as a marker of apoplastic transportation in cystoseira-Nodicalis (Fucales, Cystoseiraceae) Can J Bot 69:18–25
Polisensky DH, Braam J (1996) Cold-shock regulation of the Arabidopsis TCH genes and the effects of modulating intracellular calcium levels. Plant Physiol 111:1271–1279
Rock CD, Quatrano RS (1996) Lanthanide ions are agonists of transient gene expression in rice protoplasts and act in synergy with ABA to increase Em gene expression. Plant Cell Rep 15:371–376
Schwiebert EM, Mills JW, Stanton BA (1994) Actin-based cytoskeleton regulates a chloride channel and cell volume in a renal cortical collecting duct cell line. J Biol Chem 269:7081–7089
Sheen J (2001) Signal transduction in maize and Arabidopsis mesophyll protoplast. Plant Physiol 127:1466–1475
Sun Y, Liu DL, Yu ZQ, Zhang Q, Bai J, Sun D (2003) An apoplastic mechanism for short-term effects of rare earth elements at lower concentrations. Plant Cell Environ 26:887–896
Tang R, Xiao G (1996) Rare earths. In: Wang K, Tang R, Xu H, Luo X (eds) Trace elements in life sciences. China Measuring Publishing Press, Beijing, pp 450–509
Tester M (1990) Plant ion channels: whole-cell and single-channel studies. New Phytol 114:305–340
Thomson WW, Platt KA, Campbell N (1973) The use of lanthanum to delineate the apoplastic continuum in plants. Cytobios 8:57–62
Tilly BC, Edixhoven MJ, Tertoolen LGJ, Morii N, Saitoh Y, Narumiya S, de Jonge HR (1996) Activation of the osmo-sensitive chloride conductance involves P21rho and is accompanied by a transient reorganization of the F-actin cytoskeleton. Mol Biol Cell 7:1419–1427
Velasco JR, Domingo LE, Lansangan AS, Sierra ZN (1979) Cultural studies on coconut Cadang: reaction of plants to rare earths, thalium and certain soil samples. Philipp J Coconut Stud 4:1–13
Wang E, Taylor RW, Pfeiffer DR (1998) Mechanism and specificity of lanthanide series cation transport by ionophores A23187, 4-BrA23187, and ionomycin. Biophys J 75:244–254
Weerdenburg C, Seagull RW (1987) The effect of taxol and colchicines on microtubule and microfibril arrays in elongating plant cells in culture. Can J Bot 66:1707–1716
Whitson G, Murray F (1974) The Casparian strip as a barrier to the movement of lanthanum in corn roots. Science 183:670–671
Wymer C, Lloyd C (1996) Dynamic microtubules: implication for cell wall patterns. Trends Plant Sci 1:222–228
Wymer CL, Wymer SA, Cosgrove DJ, Cyr RJ (1996) Plant cell responds to external forces and the response requires intact microtubules. Plant Physiol 110:425–430
Yermiyahu U, Rytwo G, Brauer DK, Kinraide TB (1997) Binding and electrostatic attraction of lanthanum (La3+) and aluminum (Al3+) to wheat root plasma membranes. J Membr Biol 159:239–252
Acknowledgements
We thank Dr. Susan Mopper for assistance with the statistical analyses and Dr. Tom Pesacreta for assistance with the microscopy. This work was supported by NASA (grant no. NAG10-0190) and the Graduate Student Organization of UL Lafayette.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, M., Hasenstein, K.H. La3+ uptake and its effect on the cytoskeleton in root protoplasts of Zea mays L.. Planta 220, 658–666 (2005). https://doi.org/10.1007/s00425-004-1379-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1379-2