Abstract
Plant root architecture modulates during developmental stages and adjusts with the environmental condition. The cytosolic calcium which is a ubiquitous secondary messenger in all eukaryotes strongly affects the root system in Arabidopsis thaliana (L.) Heynh. We proposed that calcium chloride gradients affect PIN2 expression, which in term modulates root architecture and lateral root emergence. In the present study, the root development of PIN2 overexpressing lines of A. thaliana were investigated on different CaCl2 concentrations. This study found that the abundance of PIN2 protein has a direct effect on the root curvature with respect to CaCl2 gradient. In the presence of low concentration of CaCl2, PIN2 protein is stabilized and its subsequent accumulation lead to straight root architecture. However, as the CaCl2 concentration were increased, PIN2 protein destabilized and subsequently degraded which in turn showed a wavy root phenotype. On the other hand, different concentrations of CaCl2 did not show any effect on PIN2 gene at transcript level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Plants being sessile, often experience environmental stresses (biotic and abiotic) that lead to decrease in plant growth and crop productivity [1, 2]. A major feature of plant development is that it is continuously modified by interactions with the environment, thereby enabling plants to cope with the constraints of their sessile lifestyle. A quintessential example is the developmental plasticity of the three-dimensional deployment of the root (the root architecture) in response to the soil environment [3]. This plasticity is critical for a plant’s ability to forage the soil for nutrients and water and contributes to a plant’s competitive fitness. Root architecture is determined by the pattern of root branching (lateral root formation) and by the rate and direction of growth of individual roots [4]. The formation of lateral roots (LRs) is a reiterative process. LRs form along the length of the primary root and these first order LRs give rise to second order LRs which themselves can form the third order LRs etc. [5, 6]. Several studies have shown that how differences in the composition of the growth medium can alter root architecture [4]. A good example is the LR proliferation and curvature of LRs and primary roots into resource-rich growth media zones such as those enriched in NO3 or phosphate [7, 8].
On the other hand, less is understood regarding how plant root architecture is modulated in response to different stress conditions, even though this response may be vital for the plant’s ability to compete for soil resources. A survey of plant responses to potassium nitrate, phosphate, auxin and oxilipins is characterized by the increase of LRs, inhibition of primary root and formation of wavy roots [9, 10]. The mild chronic stress stimulated stress-induced morphogenic response, which comprises of inhibition of root elongation, localized stimulation of cell division and alteration of cell differentiation. It is suggested that modulation of root architecture is thus typified by growth redistribution rather than by cessation of growth [3]. For example the reduction in root elongation by the osmotic or salinity stress is reported in cotton, maize and bean [2, 11–13]. In most studies using Arabidopsis, osmotic or salt stress severely repressed the formation of LRs [14–17]. Osmotic stress appears to inhibit LR formation after LR initiation and affects the formation of autonomous LRs from LRPs [14].
In the light of these reports regarding the effect of salt stress on Arabidopsis root architecture, the effect of CaCl2 salt on Arabidopsis root was investigated in the present study. To understand the effect of in vitro calcium stress, we demonstrated morphogenic responses induced in roots of A. thaliana transgenic lines overexpessing PIN2 after application of different concentration of CaCl2 salt. Indeed, we observed visible effect of CaCl2 on primary root architecture and LR emergence in A. thaliana.
MATERIALS AND METHODS
Plant material and growth conditions.Arabidopsis thaliana (L.) Heynh. (Col-0) homozygous PIN2 overexpressing lines were provided by the “Cell signalling lab,” Division of Plant Sciences, Gyeongsang National University, Korea. They used floral dip method and generated transgenic Arabidopsis lines, and MS medium containing cephalosporin and kanamycin were used for select the transgenic plants of T1 and T2 generations. Furthermore, homozygous T3 progenies were confirmed by RT-PCR and were used for these experiments.
Arabidopsis lines overexpressing PIN2 were tested for root architecture and lateral root emergence. The seeds were sterilized and plated on half the norm of MS [18] with 1.5% sucrose at 4°C in darkness for 48 h of stratification, then transferred to a growth chamber under condition of 20 to 22°C with a 16/8 h light/dark cycle. Five days old seedling with 1.0 to 1.5 cm in length were used for experiments.
CaCl2treatment. To check the root response on calcium stress, PIN2 overexpressing plants were grown on media supplemented with different concentration of CaCl2. The five-day-old seedlings were transferred on the calcium deficient media supplemented with various concentrations (0, 0.01, 0.1, 1, 3 and 10 mM) of calcium chloride. The seedling roots were positioned vertically and the initial sited of the root tip were marked. The plates were reoriented by 90° for gravitropic response and the gravitropic response was digitized using (Canon CKI-PowerShot-GII) to observe the root architecture, primary root length and lateral root emergence. All the experiments were conducted with three biological replicates.
PIN2expression at protein level. To study the expression of PIN2 at potein level, Western Blotting carried out to check the PIN2 abundance after subjecting to different concentrations of CaCl2. The proteins of the PIN2 overexpressing lines were extracted from ten-day-old plant. Antibodies were diluted as follows: 1 : 1000 for anti-PIN2; 1 : 4000 for anti-rabbit. The picture was developed in dark room using x-ray developing procedure. Each experiment was repeated three times to check the reproducibility.
PIN2expression at RNA level. To study the change in the PIN2 expression; RT-PCR was carried-out in ArabidopsisPIN2 overexpressing line after subjecting to different concentrations of CaCl2. The RNA was extracted by RNeasy Plant Mini Kit (Qiagen) from ten-day-old plant (prior to the fact that maximum affect of calcium ion be explored). Two micrograms of DNAse-treated total RNA was used as a template for first-strand cDNA synthesis with Superscript III Reverse Transcriptase (Invitrogen) and oligo (dT) primer. The ACTIN2 gene was used as a reference with a primer 5'-CCTTCGTCTTGATCTTGCGG-3' and 5'-AGCGATGGCTGGAACAGAAC-3' and to detect PIN2 transcript: 5'-AAGTCACGTACATGCATGTG-3' and 5'-AGATGCCAACGATAATGAGTG-3' primer were used. Twenty-microliter reactions were set-up for each reaction and amplified through 30 PCR cycles and subsequently, 15 µL of the PCR product was run on 1% agrose gel for gel-documentation.
RESULTS
CaCl2 Stress Decreased LRs and Caused Wavy Root Phenotype
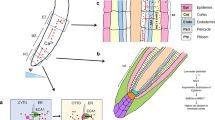
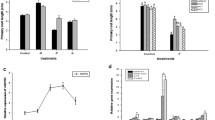
Calcium is an essential plant nutrient, involved in various structural roles in the cell wall and membranes differentiation. Calcium is also an intracellular messenger, which coordinates numerous developmental signals and environmental challenges. Here we observed that calcium ion concentration produced wave phenotype as the concentration increases from 0.01 up to 10 mM CaCl2 (Fig. 1a). It was found that the wave root formation prominently observed at 3 and 10 mM of CaCl2 in the calcium deficient media.
Mild CaCl2 Stress Stimulates Root Growth and Lateral Root Proliferation
To examine how CaCl2 stress affects Arabidopsis root growth and LRs proliferation, the response of Arabidopsis seedlings were tested on increasing CaCl2 concentration. Fig. 1b shows that increasing CaCl2 level up to 0.1 mM stimulate the production of LRs, where seedlings exhibited virtually twofold number of LRs compared with the control plants (with 0 mM CaCl2). However, the numbers of LRs were decreased by transferring the seedlings to 1 mM CaCl2 containing medium, and 10 mM CaCl2 stress caused almost complete inhibition of LRs development. These results propose an acclimation response of LR development to mild levels of CaCl2 stress. Similarly, root length was also increased at lower concentration of CaCl2, i.e., from 0 and 0.01 mM, however its length decreased again after the application of 0.1–10 mM CaCl2 (Fig. 1c).
Phenotype Reverting Assay
With the increase of calcium ion concentration, the PIN2 overexpressing plants showed a wavy root phenotype. We have found that wavy root phenotype revert to straight phenotype back when transplant from high CaCl2 level to low (Fig. 2). Thus, we confirmed that the wavy root phenotype appearance depends on calcium ion concentration.
Calcium-dependent wavy root phenotype got straight at 0 mM CaCl2. R-I–transplant three-day-old plants to a medium containing different concentration of CaCl2; R-II–clustered the plant (from the media having different concentration of CaCl2: R-I) on 0 mM Ca2+ containing media; consequently the curvature of the root change to normal, i.e., wavy phenotype (W) reverts into straight (S: R-II).
It is previously investigated by many researchers that calcium is fully involved in various structural roles in the cell wall and cell membranes differentiation which ultimately bring changes in the root emergence and the number of LLR emergence. With the increase of CaCl2, the LR emergence/number increased up to 1 mM and decreased with the increase of CaCl2 concentration from 3 mM (Fig. 2).
Ca2+ Stress-Induced Gravitropic Responses Are Related to PIN2 Protein Levels
It’s known that PIN2 protein play an important role in the redistribution of auxin and subsequently gravitropic responses [19]. It was assumed that the expression of PIN2 protein is modified by calcium concentration. We tested the level of PIN2 protein in the seedling overexpressing PIN2. Under low CaCl2 concentration (0 to 0.1 mM) Western Blot showed high intensity/abundance of PIN2 expression and the plant showed normal gravitropic response with a straight root phenotype. Surprisingly, above 0.1 mM of CaCl2 PIN2 protein abundance was reduced and ultimately defused and plant showed wavy root phenotype. Unlike PIN2 expression at protein level, the PIN2 transcript abundance was not affected with the increase or decrease of CaCl2 concentration (Fig. 3). These results suggested that calcium stress represses the abundance and distribution of PIN2 protein influencing the response to gravity.
DISCUSSION
Variation in the supply and distribution of inorganic nutrients affect root development either directly (changes in the external concentration of the nutrient) or indirectly (changes in the internal nutrient status of the plant) resulting trophomorphogenic responses induces in plants [20]. Calcium is one of the major inorganic nutrient elements. In higher plants, it is available in the form of Ca2+ with 5000 mg/kg (0.5%) concentration in dry tissue. It is previously investigated by many workers that within the cell Ca2+ is fully involved in the formation of middle lamella, essential for cell membrane formation, acts as second messenger in metabolic regulation, help to stabilize the structure of chromosomes and an activator of many enzymes [21]. Similarly, the deficiency of Ca2+ within cells causes disintegration of growing meristematic region of the root, stem and leaves and chlorosis along the margins of younger leaves and malformation of younger leaves [22].
Under various concentrations of CaCl2 (0, 0.01, 0.1, 1, 3 and 10 mM), the number of LRs were significantly increased at 0.01 and 0.1 mM CaCl2 than the control seedlings as shown in Fig. 1a. Interestingly, the LRs were reduced and the plant showed slightly negative response to 1 mM CaCl2, but at this stage, the LRs were still significantly more than the control plant. We found that mild CaCl2 concentration (up to 0.1 mM) increase LRs proliferation in PIN2, but increase concentration of CaCl2 decrease LRs proliferation which was further conformed by 3 and 10 mM CaCl2 as shown in Fig. 1b. It was found that the numbers of LRs significantly decreased at 3 and 10 mM of CaCl2, and the primary root was developed almost without lateral root proliferation. These results revealed that mild calcium stress stimulated stress-induced morphogenic response in Arabidopsis roots [3], however enhanced CaCl2 concentration cause toxicity to plant and inhibited root growth [23, 24]. It has been elaborated that LR primordium (LRP) initiation phase dependent on a root tip-localized IAA source, and LRP emergence phase dependent on leaf-derived IAA up to 10 days after germination in A. thaliana [25, 26]. Our results suggested that uptake of CaCl2 up to 0.1 mM stimulate the developmental stages of lateral root formation resulting more highly branched root system in A. thalianaPIN2 overexpressing lines but extreme high concentration of CaCl2 inhibit the developmental stages of LRt, assuming that synthesis of tip-localized IAA and leaf-derived IAA is remarkably sensitive to stress of CaCl2. Hence, mild CalCl2 stress stimulates LR proliferation in A. thaliana.
For the first time it was hypothesized that wavy root phenotype of Arabidopsis is a gravity-induced touch-response to the medium surface and the root tip changed direction in an obstacle-avoidance manoeuvre [27]. Then Simmons et al. [28] proposed that, a combination of gravitropism and circumnutation (natural oscillating growth) act as the major stimuli to induced root waving in A. thaliana. Subsequently, Buer et al. [29] demonstrated that the gaseous environment within the Petri dish can override gravitational effects and the medium ion concentrations and gelling polymers modify the wave response. We found that CaCl2 stress-induced wavy root phenotypes are related to PIN2 protein levels. Western blot analysis showed normal PIN2-expression in control plant with straight primary root architecture, but when the abundance of PIN2-expression was reduced and ultimately diffused with high CaCl2 concentration [25], the plants showed wavy primary root consequently. The wavy root formation was prominently observed at 3 and 10 mM of CaCl2 in the calcium deficient media. These results suggested that high concentration of CaCl2 limits the abundance of PIN2 protein which have direct effect on the wavy root phenotype development.
To conclude, low concentration of CaCl2 brings morphogenic responses inducing root length and LRt emergence in A. thaliana. However, higher concentration of CaCl2 inhibit root growth, LR emergence and causes wavy root phenotype. LR emerge after several developmental stages occur in plants and depend on growth regulator therefore we suggested that CaCl2 interfere in main physiological events which have direct effect on LRs proliferation. We have also concluded that abundance of PIN2 protein directly affect wavy root phenotype. High concentration of CaCl2 represses the abundance of PIN2 protein in response to gravity resulting the plant showed wavy root phenotype.
REFERENCES
Ullah, A., Sun, H., Yang, X., and Zhang, X., Drought coping strategies in cotton: increased crop per drop, Plant Biotechnol. J., 2017, vol. 15, p. 271.
Ullah, A., Sun, H., Yang, X., and Zhang, X., A novel cotton WRKY gene, GhWRKY6-like, improves salt tolerance by activating the ABA signaling pathway and scavenging of reactive oxygen species, Physiol. Plant., 2018, vol. 162, p. 439.
Zolla, G., Yair, M., Heimer, and Barak, S., Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots, J. Exp. Bot., 2010, vol. 61, p. 211.
Malamy, J.E., Intrinsic and environmental response pathways that regulate root system architecture, Plant Cell Environ., 2005, vol. 28, p. 67.
Ullah, A., Manghwar, H., Shaban, M., Khan, A.H., Akbar, A., Ali, U., Ali, E., and Fahad, S., Phytohormones enhanced drought tolerance in plants: a coping strategy, Environ. Sci. Pollut. Res., 2019, vol. 25, p. 33103.
Chen, Q., Liu, Y., Maere, S., Lee, E., van Isterdael, G., Xie, Z., Xuan, W., Lucas, J., Vassilileva, V., Kitakura, S., Marhavý, P., Wabnik, K., Geldner, N., Benková, E., Le, J., et al., A coherent transcriptional feed-forward motif model for mediating auxin-sensitive PIN3 expression during lateral root development, Nat. Commun., 2015, vol. 6: 8821.
Linkohr, B.I., Williamson, L.C., Fitter, A.H., and Leyser, H.M.O., Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis,Plant J., 2002, vol. 29, p. 751.
Malamy, J.E. and Ryan, K.S., Environmental regulation of lateral root initiation in Arabidopsis,Plant Physiol., 2001, vol. 127, p. 899.
Potters, G., Pasternak, T.P., Guisez, Y., Palme, K.J., and Jansen, M.A., Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci., 2007, vol. 12, p. 98.
Potters, G., Pasternak, T.P., Guisez, Y., and Jansen, M.A.K., Different stresses, similar morphogenic responses: integrating a plethora of pathways, Plant Cell Environ., 2009, vol. 32, p. 158.
Croser, C., Renault, S., Franklin, J., and Zwiazek, J., The effect of salinity on the emergence and seedling growth of Picea mariana, Picea glauca, and Pinus banksian-a,Environ. Pollut., 2001, vol. 115, p. 9.
Akhtar, S.S., Andersen, M.N., Naveed, M., Zahir, Z.A., and Liu, F., Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize, Funct. Plant Biol., 2015, vol. 42, p. 770.
Talaat, N.B., Ghoniem, A.E., Abdelhamid, M.T., and Shawky, B.T., Effective microorganisms improve growth performance, alter nutrients acquisition and induce compatible solutes accumulation in common bean (Phaseolus vulgaris L.) plants subjected to salinity stress, Plant Growth Regul., 2015, vol. 75, p. 281.
Deak, K.I. and Malamy, J., Osmotic regulation of root system architecture, Plant J., 2005, vol. 43, p. 17.
Ding, Z.J., Yan, J.Y., Li, C.X., Li, G.X., Wu, Y.R., and Zheng, S.J., Transcription factor WRKY 46 modulates the development of Arabidopsis lateral roots in osmotic/salt stress conditions via regulation of ABA signaling and auxin homeostasis, Plant J., 2015, vol. 84, p. 56.
Xiong, L., Wang, R.G., Mao, G., and Koczan, J.M., Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid, Plant Physiol., 2006, vol. 142, p. 1065.
Kim, S., Kang, J.Y., Cho, D.I., Park, J.H., and Kim, S.Y., ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance, Plant J., 2004, vol. 40, p. 75.
Murashige, T. and Skoog, F., A revised medium for rapid growth and bio assays with tobacco tissue cultures, Physiol. Plant., 1962, vol. 15, p. 473.
Rahman, A., Takahashi, M., Shibasaki, K., Wu, S., Inaba, T., Tsurumi, S., and Baskin, T.I., Gravitropism of Arabidopsis thaliana roots requires the polarization of PIN2 toward the root tip in meristematic cortical cells, Plant Cell, 2010, vol. 22, p. 1762.
Prinzenberg, A.E., Barbier, H., Stich, B., Salt, D.E., and Reymond, M., Relationships between growth, growth response to nutrient supply, and ion content using a recombinant inbred line population in Arabidopsis, Plant Physiol., 2010, vol. 154, p. 1361.
Hepler, P.K., Calcium: a central regulator of plant growth and development, Plant Cell, 2005, vol. 17, p. 2142.
Karthika, K.S., Rashmi, I., and Parvathi, M.S., Biological functions, uptake and transport of essential nutrients in relation to plant growth, in Plant Nutrients and Abiotic Stress Tolerance, Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., and Hawrylak-Nowak, B., Eds., Singapore: Springer, 2018, p. 1.
Ullah, A., Mushtaq, H., Fahad, S., Shah, A., and Chaudhary, H.J., Plant growth promoting potential of bacterial endophytes in novel association with Olea ferrugin-ea and Withania coagulans,Microbiology, 2017, vol. 86, p. 119.
Julkowska, M.M. and Testerink, C., Tuning plant signaling and growth to survive salt, Trends Plant Sci., 2015, vol. 20, p. 586.
Ditengou, F.A., Teale, W.D., Kochersperger, P., Flittner, K.A., Kneuper, I., van der Graaff, E., Nziengui, H., Pinosa, F., Li, X., Nitschke, R., Laux, T., and Palme, K., Mechanical induction of lateral root initiation in Arabid-opsis thaliana,Proc. Natl. Acad. Sci. USA, 2008, vol. 105, p. 18818.
Ullah, A., Akbar, A., Luo, Q., Khan, A.H., Manghwar, H., Shaban, M., and Yang, X., Microbiome diversity in cotton rhizosphere under normal and drought conditions, Microb. Ecol., 2019, vol. 77, p. 429.
Okada, K. and Shimura, Y., Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus, Science, 1990, vol. 250, p. 274.
Simmons, C., Söll, D., and Migliaccio, F., Circumnutation and gravitropism cause root waving in Arabidopsis thaliana,J. Exp. Bot., 1995, vol. 46, p. 143.
Buer, C.S., Masle, J., and Wasteneys, G.O., Growth conditions modulate root-wave phenotypes in Arabidopsis, Plant Cell Physiol., 2000, vol. 41, p. 1164.
Author information
Authors and Affiliations
Contributions
M. Nisar designed the project, conducted experiments and wrote the paper. A. Ullah and H. Park modified and reviewed the paper. Z. Ali, A. Ali, R. Aman and S.F. Wadood helped M. Nisar in the experiments.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants as objects of research.
Rights and permissions
About this article
Cite this article
Nisar, M., Ali, Z., Ali, A. et al. CaСl2 Salt Signaling in Primary Root Architecture and Lateral Root Emergence in Arabidopsis thaliana. Russ J Plant Physiol 67, 515–520 (2020). https://doi.org/10.1134/S1021443720030176
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443720030176