Abstract
Some species of the holoparasitic flowering plant genus Cuscuta, like C. reflexa, have retained a plastid genome that encodes photosynthesis-related gene products as well as the plastid-encoded RNA polymerase (PEP). In contrast, other species like C. gronovii and C. subinclusa have lost the rpo genes coding for the PEP subunits while photosynthetic genes have been retained. In order to ensure expression of the photosynthesis-related genes in the absence of PEP, a number of adaptations within the plastid genome were required that enable gene transcription mediated exclusively by the nuclear-encoded plastid RNA polymerase (NEP). In this study we analyzed promoter sequence conservation and transcription start sites of a typical PEP gene of non-parasitic plants, rbcL, which codes for the large subunit of ribulose bisphosphate carboxylase/oxygenase. We show that despite high sequence conservation of the coding region of rbcL among different Cuscuta species and tobacco, the 5′ non-coding regions of C. gronovii and C. subinclusa have suffered extensive deletions encompassing the PEP promoter that is present in C. reflexa and tobacco. Primer-extension analyses enabled the identification of transcripts initiated at NEP promoter motifs in C. gronovii and C. subinclusa that are not detectable in the 5′ non-coding region of C. reflexa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcription of plastid genes is mediated by a plastid-encoded RNA polymerase (PEP) in addition to one or two nuclear-encoded phage-type RNA polymerases (Hedtke et al. 1997; Chang et al. 1999; Hess and Börner 1999; Bligny et al. 2000). Each polymerase recognizes a distinct set of promoters. While PEP promoter sequences resemble the promoters of eubacterial genes (Igloi and Kössel 1992; Weihe and Börner 1999; Liere and Maliga 2001), NEP transcription starts at a YRTA-motif that shows great homology to bacteriophage and mitochondrial promoters (Hajdukiewicz et al. 1997; Kapoor et al. 1997; Liere and Maliga 1999; Weihe and Börner 1999). Whereas NEP is thought to be responsible mainly for the transcription of housekeeping genes, including the transcription of the subunits of the PEP enzyme, PEP is mainly responsible for the transcription of photosynthesis-related genes (Allison et al. 1996; Hajdukiewicz et al. 1997; Krause et al. 2000).
Previous studies have shown that in C. reflexa, despite the deletion of some plastid genes (Haberhausen et al. 1992; Haberhausen and Zetsche 1994), the plastid-encoded RNA polymerase (PEP) is still retained (Krause et al. 2003). Since C. reflexa accumulates chlorophyll and performs photosynthesis at low levels (Hibberd et al. 1998; van der Kooij et al. 2000) the existence of PEP is consistent with the notion that PEP is needed for the transcription of photosynthesis-related genes (Allison et al. 1996; deSantis-Maciossek et al. 1999). In contrast, in two other species (C. gronovii and C. subinclusa) the rpo genes are absent from the plastid genome although the capacity to perform photosynthesis at low levels has been retained (van der Kooij et al. 2000). A transfer of functional rpo genes to the nuclear genome and thus the import of the PEP subunits have been ruled out by Southern Blot analysis and PCR (Krause et al. 2003) so that NEP has to be responsible for the expression of photosynthesis-related genes at levels sufficient to allow for photosynthesis. This is in clear contrast to observations that were made with PEP-deficient plastids of non-parasitic plants obtained either by mutagenesis of the plastid genome or by induction of plastid ribosome deficiency. All these PEP-deficient plants are characterized by achlorophyllous tissue that lacks photosynthetic capacity (Falk et al. 1993; Hess et al. 1993; Allison et al. 1996; deSantis-Maciossek et al. 1999; Krause et al. 2000).
A switch from PEP- to NEP-driven transcription in some Cuscuta species has so far only been observed for the gene encoding the 16S rRNA (Krause et al. 2003) that in non-parasitic plants already contains promoters for both PEP and NEP. Photosynthesis-related genes, like the plastid rbcL gene, in contrast, normally possess only a promoter that is recognized by PEP (Shinozaki and Sugiura 1982; Mullet et al. 1985; Orozco et al. 1990; Allison et al. 1996). Since transcripts of this gene were detected in a number of Cuscuta species, both with and without PEP (van der Kooij et al. 2000), this gene is a good candidate for the analysis of the consequences of PEP-deficiency on the promoter structures of photosynthesis genes.
Materials and methods
Plant material
Cuscuta reflexa, C. gronovii and C. subinclusa were kept in a greenhouse at the University of Kiel on Pelargonium zonale as the host plant, as described before (van der Kooij et al. 2000; Krause et al. 2003). Nicotiana tabacum L. cv. Xanthi, which was used as a non-parasitic control plant, was grown in a greenhouse.
PCR amplification and sequence analysis
The primers used for PCR amplification of fragments of the rbcL gene were designed based on the published sequence of the tobacco plastid DNA (Wakasugi et al. 1998; positions in parentheses):
-
atpB.P5-uni (56495–56012) 5′-TGTAAAACGACGGCCAGTCTGTGTCAATCACTTCC-3′;
-
atpB.P4-uni (56747–56765) 5′-GTAAAACGACGGCCAGTAGAACCAGAAGTAGTAGG-3′;
-
rbcL-for-uni (57610–57629) 5′-GTAAAACGACGGCCAGTAGACTAAAGCAAGTGTTG-3′;
-
rbcL.P1-rev (57654–57636) 5′-AGGAAACAGCTATGACCGTACTCTTTAACACCAGC-3′;
-
rbcL.P5-rev (57735–57754) 5′-AGGAAACAGCTATGACCGCTTCTTCAGGTGGAACT-3′;
-
rbcL.P3-uni (58328–58342) 5′-GTAAAACGACGGCCAGTACATGCGAAGAAATG-3′;
-
rbcL.P2-rev (58342–58328) 5′-CAGGAAACAGCTATGACCATTTCTTCGCATGTACC-3′;
-
rbcL.P4-rev (59056–59039) 5′-CAGGAAACAGCTATGACTTTCTCCTTATCCTTC-3′.
Different combinations of these primers were used to amplify overlapping fragments.
The PCR products were sequenced directly after purification from agarose gels as described by Krause et al. (2003). After alignment of the overlapping fragments, the resulting rbcL sequences from C. gronovii and C. subinclusa were deposited in the EMBL database under accession numbers AJ320207 (C. gronovii) and AJ320210 (C. subinclusa).
Primer extensions
Primer extensions were carried out essentially as described by Krause et al. (2003) with 10–50 μg of total RNA on a Li-Cor DNA sequencer (Model 4000 L; MWG Biotech) using the following IRD800-labeled primer (complementary nucleotides 57624–57595 in tobacco): 5′-ACTTGCTTTAGTCTCTGTTTGTGGTGACAT-3′.
Results
Amplification and sequence analysis of the rbcL gene
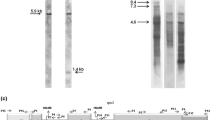
The 5′-region of the rbcL gene containing the promoter sequence as well as the first 700 nucleotides of the coding region were amplified in separate reactions with primer pairs derived from the tobacco plastid DNA sequence. While the PCR with the primers specific for the coding region revealed no size differences of the amplified fragments in C. reflexa, C. gronovii, C. subinclusa or tobacco, amplification of the intergenic region between atpB and rbcL yielded differently sized PCR products (Fig. 1a,b)
Conservation of the rbcL gene from different Cuscuta species. a,b PCR amplification of fragments from the intergenic region between atpB and rbcL (a) and the first 700 bp of the rbcL coding region (b). Ethidium bromide-stained gels containing the PCR fragments of C. gronovii (C.g.), C. reflexa (C.r.) and C. subinclusa (C.s.) are shown below a schematic depiction of the amplified region. The sizes of bands from the molecular weight standard (left lane) are given on the left. C. odorata (C. o.) lacking the rbcL gene (van der Kooij et al. 2000) and tobacco (N.t.) were included as a negative and positive controls, respectively. The genes (grey boxes) are not drawn to scale. Arrows indicate the direction of transcription of the rbcL gene. c Sequence comparison of the 5′-region of the rbcL gene and part the coding region. The numbering of the nucleotides refers to the tobacco sequence and starts with nucleotide number 1 at the start codon. Nucleotides identical to the rbcL sequence of N. tabacum are given in white on a black background. A hyphen (-) indicates that a nucleotide is missing with respect to the tobacco sequence. The black bars mark the position of the σ70-type promoter (‘−10’ and ‘−35’-boxes) of the tobacco rbcL gene. GenBank accession numbers are NC_001879 (N. tabacum), X61698 (C. reflexa), AJ320207 (C. gronovii) and AJ320210 (C. subinclusa)
Sequence analysis demonstrated that the coding regions of the rbcL gene in the three Cuscuta species were highly homologous to each other and to the rbcL gene of tobacco (Fig. 1c). Comparison of the rbcL 5′-non-coding sequences of the three Cuscuta species and tobacco revealed that C. gronovii and C. subinclusa have suffered several large deletions in this area. The characteristic promoter sequence motif for the plastid-encoded RNA polymerase (PEP) is strongly altered in C. gronovii and completely absent from the C. subinclusa sequence. In contrast, C. reflexa has suffered only a few changes in the region containing the PEP promoter sequence (Fig. 1c).
Expression of the rbcL gene
The detection of the mature rbcL mRNA and the accumulation of the corresponding protein (van der Kooij et al. 2000) in the three Cuscuta species implies that the gene is actively transcribed and functional in the plastids. In non-parasitic plants, all transcripts of this gene are initiated at a PEP promoter. As PEP is absent in C. gronovii and C. subinclusa (Krause et al. 2003), it was important to determine the transcription start sites and thus gain further information on the promoters that are involved in directing rbcL gene transcription. For this purpose we mapped the 5′-ends of rbcL transcripts by primer-extension reactions. In the case of C. reflexa the 5′-end of the rbcL transcript mapped to position −200 relative to the start of the coding region of the rbcL mRNA. The sequence preceding this 5′-end resembles the sequence of the published transcription start site of tobacco at −180 (Shinozaki and Sugiura 1982; Mullet et al. 1985; Allison et al. 1996). The signal corresponding to position −59 of tobacco rbcL represents the processed transcript (Mullet et al. 1985). In the other two Cuscuta species, 5′-ends were mapped to completely different positions. In C. subinclusa and C. gronovii, signals were obtained at positions −116 and −108, respectively, relative to the translation start site of the mRNA (Fig. 2). Both 5′-ends are preceded by sequences that contain a YRTA-box as well as a GAA-motif. These sequence motifs are known to be associated with NEP-based transcription (Weihe and Börner 1999) and were found at a similar distance from the start site of transcription in many other genes (Fig. 3).
Mapping of 5′-ends of rbcL transcripts. The 5′-ends of the rbcL transcripts were detected by primer-extension analysis and are indicated by black arrowheads on the right of each panel. For comparison, sequences were generated using the same primers that were used for the primer-extension reactions. For each species, the sequence ladders are shown on the left next to the lane with the primer extensions, respectively. The loading order (AGCT) is indicated above. Promoter consensus motifs (PEP −10/−35-boxes and NEP YRT- and GAA-boxes) that were found in the regions immediately upstream of the primer extension signals are shown on the left of each panel
Alignment of NEP promoter sequences. Sequences upstream of the 5′-ends of the rbcL transcripts of C. subinclusa and C. gronovii were compared with published sequences of the NEP promoters of different genes from various species. The conserved consensus motifs (YRTA-box and GAA-box, Weihe and Börner 1999) are indicated by bold upper case letters
Discussion
In non-parasitic higher plants the plastid-encoded RNA polymerase (PEP) is thought to be mainly responsible for the transcription of genes associated with photosynthesis (Allison et al. 1996; deSantis-Maciossek et al. 1999), while the nuclear-encoded RNA polymerase(s) (NEP) are mainly responsible for transcription of housekeeping genes (Maliga 1998; Hess and Börner 1999). In most holoparasitic plants the genes encoding PEP are missing, and transcription of plastid genes depends exclusively on NEP (Morden et al. 1991; Wolfe et al. 1992; Thalouarn et al. 1994; Lusson et al. 1998). C. gronovii and C. subinclusa, though being green and capable of performing photosynthesis at low levels (van der Kooij et al. 2000), lack PEP likewise as white parasitic plants like Cuscuta odorata (van der Kooij et al. 2000) and Epifagus virginiana (Morden et al. 1991; Wolfe et al. 1992). Among the Cuscuta species analyzed so far (van der Kooij et al. 2000), only C. reflexa is known to possess a PEP. In order to elucidate how plastid genes associated with photosynthesis are transcribed in the absence of PEP, the transcription initiation sites and promoter sequences of rbcL transcripts from C. gronovii and C. subinclusa were investigated.
The rbcL gene belongs to the group of genes that is exclusively transcribed by PEP in non-parasitic plants (Mullet et al. 1985; Allison et al. 1996; Hajdukiewicz et al. 1997). With the exception of C. odorata, all Cuscuta species analyzed so far possess and express the rbcL gene (van der Kooij et al. 2000), though some of them lack PEP (Krause et al. 2003). As shown in this study the loss of PEP is accompanied by alterations in the promoters of the rbcL genes. The rbcL transcripts in C. gronovii and C. subinclusa are initiated from NEP promoter sequences that are neither present in C. reflexa nor in the non-coding region of rbcL from non-parasitic plants. The transcripts detected by primer-extension reactions are likely to be true primary transcripts since in contrast to the processed rbcl transcripts of tobacco they could not be ligated to an RNA linker (data not shown). The method of RNA-linker ligation is an alternative to capping experiments (Vera and Sugiura 1992), which can be used to distinguish between original 5′-ends and those that result from processing (Miyagi et al. 1998). The newly evolved NEP promoter sequences in the Cuscuta species lacking PEP consist of a GAA-motif followed by a YRTA-motif (Hübschmann and Börner 1998; Liere and Maliga 1999; Weihe and Börner 1999). Similar alterations in the promoter region have been observed also in the rrn promoter sequence of different Cuscuta species. Though non-parasitic plants possess both NEP and PEP promoter elements in the rrn promoter, a new NEP promoter at a different position from that found in non-parasitic plants is used for transcription of the operon in Cuscuta species lacking PEP (Krause et al. 2003).
In the achlorophyllous Lathraea clandestina, rbcL transcripts were found to have an unusually long 5′-non-coding region (Lusson et al. 1998). In spite of high sequence conservation, the rbcL PEP promoter appears not to be used. Instead, based on sequence comparisons, an NEP promoter was proposed that could take over transcription of the rbcL gene (Lusson et al. 1998). However, no attempts to positively identify the transcription start site of rbcL in this species have been reported so far.
In contrast to achlorophyllous Lathraea clandestina, both C. gronovii and C. subinclusa are capable of synthesizing chlorophylls and performing photosynthesis, albeit at low levels. Obviously NEP-mediated expression of the rbcL gene, and most likely further genes associated with photosynthesis, is sufficient to allow for photosynthetic carbon fixation at a low level. In contrast to C. gronovii and C. subinclusa, tobacco rpo mutants are not capable of performing photosynthesis. One explanation for this could be that the mutants show severe disturbances in processing of primary transcripts (Krause et al. 2000; Legen et al. 2002) as a secondary effect of the loss of PEP, which could negatively influence translation efficiency. It is hypothesized that parasitic plants may cope with the loss of PEP not only by evolution of new promoter elements, but, possibly, by an adaptation of posttranscriptional mechanisms enabling the stabilization and correct processing of NEP-derived transcripts required for building up a photosynthetic apparatus.
Abbreviations
- atpB :
-
Gene coding for the β-subunit of the plastid ATPase
- NEP :
-
Nuclear-encoded plastid RNA polymerase
- PEP :
-
Plastid-encoded plastid RNA polymerase
- rbcL :
-
Gene coding for the large subunit of Rubisco
- Rubisco :
-
Ribulose bisphosphate carboxylase/oxygenase
References
Allison LA, Simon LD, Maliga P (1996) Deletion of rpoB reveals a second distinct transcription system in plastids of higher plants. EMBO J 15:2802–2809
Bligny M, Courtois F, Thaminy S, Chang C-C, Lagrange T, Baruh-Wolff J, Stern D, Lerbs-Mache S (2000) Regulation of plastid rDNA transcription by interaction of CDF2 with two different RNA polymerases. EMBO J 19:1851–1860
Chang CC, Sheen J, Bligny M, Niwa Y, Lerbs-Mache S, Stern DB (1999) Functional analysis of two maize cDNAs encoding T7-like RNA polymerases. Plant Cell 11:911–926
deSantis-Maciossek G, Kofer W, Bock A, Schoch S, Maier RM, Wanner G, Rüdiger W, Koop HU, Herrmann RG (1999) Targeted disruption of the plastid RNA polymerase genes rpoA, B and C1: molecular biology, biochemistry and ultrastructure. Plant J 18:477–489
Falk J, Schmidt A, Krupinska K (1993) Characterization of plastid DNA transcription in ribosome deficient plastids of heat-bleached barley leaves. J Plant Physiol 141:176–181
Haberhausen G, Zetsche K (1994) Functional loss of all ndh genes in an otherwise relatively unaltered plastid genome of the holoparasitic flowering plant Cuscuta reflexa. Plant Mol Biol 24:217–222
Haberhausen G, Valentin K, Zetsche K (1992) Organisation and sequence of photosynthetic genes from the plastid genome of the holoparasitic flowering plant Cuscuta reflexa. Mol Gen Genet 232:154–161
Hajdukiewicz PTJ, Allison LA, Maliga P (1997) The two RNA polymerases encoded by the nuclear and the plastid compartments transcribe distinct groups of genes in tobacco plastids. EMBO J 16:4041–4048
Hedtke B, Börner T, Weihe A (1997) Mitochondrial and chloroplast phage-type RNA polymerases in Arabidopsis. Science 277:809–811
Hess W, Börner T (1999) Organellar RNA polymerases of higher plants. Int Rev Cytol 190:1–59
Hess W, Prombona A, Fiedler B, Subramanian A, Börner T (1993) Chloroplast rps15 and the rpoB/C1/C2 gene cluster are strongly transcribed in ribosome-deficient plastids: evidence for a functioning non-chloroplast-encoded RNA polymerase. EMBO J 12:563–571
Hibberd JM, Bungard RA, Press MC, Jeschke WD, Scholes JD, Quick WP (1998) Localization of photosynthetic metabolism in the parasitic angiosperm Cuscuta reflexa. Planta 205:506–513
Hübschmann T, Börner T (1998) Characterization of transcript initiation sites in ribosome-deficient barley plastids. Plant Mol Biol 36:493–496
Igloi T, Kössel H (1992) The transcriptional apparatus of chloroplasts. Crit Rev Plant Sci 10:525–558
Kapoor S, Suzuki J, Sugiura M (1997) Identification and functional significance of a new class of non-consensus-type plastid promoters. Plant J 11:327–337
Krause K, Maier RM, Kofer W, Krupinska K, Herrmann RG (2000) Disruption of plastid-encoded RNA polymerase genes in tobacco: expression of only a distinct set of genes is not based on selective transcription of the plastid chromosome. Mol Gen Genet 263:1022–1030
Krause K, Berg S, Krupinska K (2003) Plastid transcription in the holoparasitic plant genus Cuscuta: parallel loss of the rrn16 PEP-promoter and of the rpoA and rpoB genes coding for the plastid-encoded RNA polymerase. Planta 216:815–823
Legen J, Kemp S, Krause K, Profanter B, Herrmann R, Maier R (2002) Comparative analysis of plastid transcription profiles of entire plastid chromosomes from tobacco attributed to wild-type and PEP-deficient transcription machineries. Plant J 31:171–188
Liere K, Maliga P (1999) In vitro characterization of the tobacco rpoB promoter reveals a core sequence motif conserved between phage-type plastid and plant mitochondrial promoter. EMBO J 18:249–257
Liere K, Maliga P (2001) Plastid RNA polymerases in higher plants. Regulation of photo-synthesis. In: Aro E-M, Anderson B (eds) Kluwer, Rotterdam, pp 29–39
Lusson N, Delavault PM, Thalouarn PA (1998) The rbcL gene from the nonphotosynthetic parasite Lathraea clandestina is not transcribed by a plastid-encoded RNA polymerase. Curr Genet 34:210–215
Maliga P (1998) Two plastid RNA polymerases of higher plants: an evolving story. Trends Plant Sci 3:4–6
Miyagi T, Kapoor S, Sugita M, Sugiura M (1998) Transcript analysis of the tobacco plastid operon rps2/atpI/H/F/A reveals the existence of a non-consensus type II (NCII) promoter upstream of the atpI coding sequence. Mol Gen Genet 257:299–307
Morden CW, Wolfe KH, dePamphilis CW, Palmer JD (1991) Plastid translation and transcription genes in a non-photosynthetic plant: intact, missing and pseudo genes. EMBO J 10:3281–3288
Mullet JE, Orozco EM, Chua N-H (1985) Multiple transcripts for higher plant rbcL and atpB genes and localization of the transcription initiation site of the rbcL gene. Plant Mol Biol 4:35–54
Orozco EM Jr, Chen LJ, Eiler RJ (1990) The divergently transcribed rbcL and atpB genes of tobacco plastid DNA are separated by nineteen base pairs. Curr Genet 17:65–71
Shinozaki K, Sugiura M (1982) The nucleotide sequence of the tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase-oxygenase. Gene 20:91–102
Silhavy D, Maliga P (1998) Mapping of promoters for the nucleus-encoded plastid RNA polymerase (NEP) in the iojap maize mutant. Curr Genet 33:340–344
Thalouarn P, Theodet C, Russo N, Delavault P (1994) The reduced plastid genome of a non-photosynthetic angiosperm Orobanche hederae has retained the rbcL gene. Plant Physiol Biochem 32:233–242
van der Kooij TAW, Krause K, Dörr I, Krupinska K (2000) Molecular, functional and ultrastructural characterisation of plastids from six species of the parasitic flowering plant genus Cuscuta. Planta 210:701–707
Vera A, Sugiura M (1992) Combination of in vitro capping and ribonuclease protection improves the detection of transcription start sites in chloroplasts. Plant Mol Biol 19:309–311
Wakasugi T, Sugita M, Tsudzuki T, Sugiura M (1998) Updated map of tobacco chloroplast DNA. Plant Mol Biol Rep 16:231–241
Weihe A, Börner T (1999) Transcription and the architecture of promoters in chloroplasts. Trends Plant Sci 4:169–170
Wolfe KH, Morden CW, Palmer JD (1992) Function and evolution of a minimal plastid genome from a non-photosynthetic parasitic plant. Proc Natl Acad Sci USA 89:10648–10652
Xie G, Allison LA (2002) Sequences upstream of the YRTA core region are essential for transcription of the tobacco atpB NEP promoter in chloroplasts in vivo. Curr Genet 41:176–182
Acknowledgement
S. Berg received a PhD grant from the Friedrich-Naumann foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berg, S., Krause, K. & Krupinska, K. The rbcL genes of two Cuscuta species, C. gronovii and C. subinclusa, are transcribed by the nuclear-encoded plastid RNA polymerase (NEP). Planta 219, 541–546 (2004). https://doi.org/10.1007/s00425-004-1260-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1260-3