Abstract
Expression of a cDNA encoding the castor bean (Ricinus communis L.) oleate Δ12-hydroxylase in the developing seeds of Arabidopsis thaliana (L.) Heynh. results in the synthesis of four novel hydroxy fatty acids. These have been previously identified as ricinoleic acid (12-hydroxy-octadec-cis-9-enoic acid: 18:1-OH), densipolic acid (12-hydroxy-octadec-cis-9,15-enoic acid: 18:2-OH), lesquerolic acid (14-hydroxy-eicos-cis-11-enoic acid: 20:1-OH) and auricolic acid (14-hydroxy-eicos-cis-11,17-enoic acid: 20:2-OH). Using mutant lines of Arabidopsis that lack the activity of the FAE1 condensing enzyme or FAD3 ER Δ-15-desaturase, we have shown that these enzymes are required for the synthesis of C20 hydroxy fatty acids and polyunsaturated hydroxy fatty acids, respectively. Analysis of the seed fatty acid composition of transformed plants demonstrated a dramatic increase in oleic acid (18:1) levels and a decrease in linoleic acid (18:2) content correlating to the levels of hydroxy fatty acid present in the seed. Plants in which FAD2 (ER Δ12-desaturase) activity was absent showed a decrease in 18:1 content and a slight increase in 18:2 levels corresponding to hydroxy fatty acid content. Expression of the castor hydroxylase protein in yeast indicates that this enzyme has a low level of fatty acid Δ12-desaturase activity. Lipase catalysed 1,3-specific lipolysis of triacylglycerol from transformed plants demonstrated that ricinoleic acid is not excluded from the sn-2 position of triacylglycerol, but is the only hydroxy fatty acid present at this position.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The predominant fatty acid in the storage triacylglycerol (TAG) of castor seeds (Ricinus communis) is the unusual hydroxy fatty acid ricinoleic acid (12-hydroxy-octadec-cis-9-enoic acid: 18:1-OH). The presence of this fatty acid at high levels (nearly 90% of total fatty acids) gives castor oil the unique properties that make it a valuable industrial raw material.
The synthesis of ricinoleic acid in the castor seed has been studied in vitro using microsomal membrane preparations from developing endosperm. The hydroxy fatty acid is formed by the hydroxylation of oleic acid (18:1) esterified to the sn-2 position of the membrane lipid phosphatidylcholine (PC; Bafor et al. 1991). The reaction requires oxygen and utilizes the cytochrome b 5 electron transport system (Moreau and Stumpf 1981; Smith et al. 1992). A cDNA encoding the oleate 12-hydroxylase from castor has been cloned and the deduced amino acid sequence of the protein (CFAH12) shows high homology to the endoplasmic reticulum (ER) oleate 12-desaturases (FAD2) of higher plants (van de Loo et al. 1995). Expression of the hydroxylase in transgenic Arabidopsis thaliana resulted in the accumulation of four novel hydroxy fatty acids in the seed oil (Broun and Somerville 1997). These were identified as ricinoleic acid (18:1-OH), densipolic acid (12-hydroxy-octadec-cis-9,15-enoic acid: 18:2-OH), lesquerolic acid (14-hydroxy-eicos-cis-11-enoic acid: 20:1-OH) and auricolic acid (14-hydroxy-eicos-cis-11,17-enoic acid: 20:2-OH). The biosynthesis of the C20 and polyunsaturated hydroxy fatty acids was proposed to be a result of the activity of the FAE1 condensing enzyme and ER FAD3 desaturase (Fig. 1), respectively (Broun and Somerville 1997).
An oleate 12-hydroxylase gene has also been isolated from the desert mustard plant, Lesquerella fendleri. Expression of the gene in transgenic Arabidopsis (Broun et al. 1998a) and yeast (Broun et al. 1998b) has demonstrated that the Lesquerella hydroxylase protein (LFAH12) is a bifunctional enzyme. It has both hydroxylase and desaturase activities acting on 18:1 to produce 18:1-OH and also 18:2. As L. fendleri seed oil contains nearly 60% lesquerolic acid (20:1-OH) and very little ricinoleic acid (Hayes et al. 1995) it was hypothesized that newly formed ricinoleic acid resulting from LFAH12 activity was rapidly elongated to lesquerolic acid. Further evidence in support of this pathway comes from the recent isolation of a seed-specific condensing enzyme from L. fendleri (Moon et al. 2001) with high specificity towards 18:1-OH.
Heterologous expression of either CFAH12 or LFAH12 in transgenic Arabidopsis results in the accumulation of relatively low levels (>17%) of hydroxy fatty acids in the seed oil (van de Loo et al. 1995; Broun and Somerville 1997; Broun et al. 1998a). The ability to produce high levels of these fatty acids in a transgenic oilseed would provide an alternative to castor, which is at present the only commercial source of hydroxy fatty acid-containing oil. To gain a better understanding of the factors that limit hydroxy fatty acid accumulation in transgenic Arabidopsis, we have conducted a detailed analysis of the seed lipids of plants expressing either CFAH12 of LFAH12 in their seeds.
Materials and methods
Gene constructs
The castor hydroxylase cDNA (CFAH12, accession no. U22378) and the Lesquerella fendleri hydroxylase gene (LFAH12, accession no. AF016103) were obtained from Prof. C.R. Somerville (Carnegie Institution of Washington, Stanford, Calif., USA). Due to lack of information concerning the length of the LFAH12 promoter, a 2,188-bp region of genomic DNA directly upstream of the LFAH12 coding region was amplified by PCR using the forward primer 5′-GCGAATTCTGAGCTCATCAGTTACT-3′ and the reverse primer 5′-TGCGGATCCTAACAGTCATTAATCTAT-3′, with the genomic clone as a template. PCR conditions used were: 1 min of initial denaturation at 94 °C followed by 33 cycles of 94 °C for 40 s, 55 °C for 40 s and 72 °C for 3 min using Pfu polymerase (Stratagene). The resulting PCR product, referred to as the Lesquerella hydroxylase promoter, was cut with EcoRI and BamHI and cloned into the vector pGEM-11Zf(−) (Promega) to create plasmid pMS30.
To generate the Lesquerella hydroxylase promoter–castor hydroxylase cDNA–nos terminator (LR) and Lesquerella hydroxylase promoter–Lesquerella hydroxylase–nos terminator (LL) cassettes, the promoter from pMS30, appropriate hydroxylase coding region and nos terminator (from the vector pJD330; Shaul and Galili 1992) were assembled in the vector pGEM 3Zf (−) (Promega). The LR and LL expression cassettes were subsequently released by restriction digestion and subcloned into the binary vector pRD400 (Datla et al. 1992) to create the vectors pMS37 and pMS42, respectively. These vectors were used to transform Agrobacterium tumefaciens, strain GV3101 (pMP90; Koncz and Schell 1986) by heat shock. Transformed cells were selected by plating on kanamycin (100 μg/ml).
For expression in yeast, the coding regions of the castor and Lesquerella hydroxylases were cloned separately into the vector pESC-TRP (Stratagene) downstream of the GAL10 promoter to create the vectors pMS259 and pMS229, respectively. The constructs also contained the coding region of tobacco cytochrome b 5 (Smith et al. 1994) cloned in sense orientation downstream of the GAL1 promoter.
Arabidopsis mutant lines and plant transformation
The mutant lines of Arabidopsis thaliana (L.) Heynh. used were all of the Columbia ecotype. Plants referred to as "wild type" are Columbia-2 ecotype. The fad2/fae1 mutant line was made by crossing the fad2 mutant AL63 (designated as fad2-4; Miquel and Browse 1992) with the fae1 mutant AC56 (Kunst et al. 1992). A fad3 line (N8034, fad3-2) was obtained from the Nottingham Arabidopsis Stock Center, University of Nottingham, UK. The fad3/fae1 double mutant line was made by crossing the undesignated fad3 mutant AJ70 with the fae1 mutant AC56. Mutant lines AL63, AC56 and AJ70 were generated by Dr. L. Kunst using ethyl methyl sulfonate (EMS). Line AC56 carries a Q160K point mutation in the FAE1 gene (Dusty Post-Beittenmiller, Department of Biochemistry, University of Missouri–Columbia, personal communication). The FAD2 gene from AL63 was sequenced in our laboratory and has a P280L point mutation. Arabidopsis plants were transformed using the floral dip method (Clough and Bent 1998). Screening for transformants was done on 50 μg/ml kanamycin as described previously (Katavic et al. 1994). Plants were grown under continuous light at 20 °C in Terra-lite Redi-earth (W.R. Grace. Canada, Ajax, Ontario, Canada).
Seed fatty acid analysis
For the determination of the fatty acid composition of mature seeds, around 100 seeds from each plant were transmethylated in 1 ml of 1 M HCl in methanol (Supelco) with 300 μl hexane at 80 °C for 2 h. After cooling to room temperature, 1 ml 0.9% NaCl was added to each tube and fatty acid methyl esters (FAMES) were extracted in the hexane phase. FAMES were separated by gas–liquid chromatography (GC) as described previously (Kunst et al. 1992).
Transformation and lipid analysis of yeast
Yeast cells (Saccharomyces cerevisiae strain YPH499; Stratagene) were transformed by a lithium acetate procedure (Ausubel et al. 1995) and grown on selective medium lacking tryptophan and containing 2% glucose. Hydroxylase expression was induced by streaking cells onto selective medium plates containing 2% galactose followed by incubation at 30 °C for 5 days. For fatty acid analysis, cells were scraped from the plates and resuspended in 2 ml of 1 M HCl in methanol (Supelco). FAMES were prepared and analysed by GC as described for seeds.
TLC analysis and lipase digestion of seed neutral lipids
Total lipids were extracted from 150 mg of seeds by grinding in 1 ml of a 2:1 chloroform/methanol mixture. Ground seeds and solvent were transferred to a glass tube and the mixture made up to 4 ml by the addition of more of the chloroform/methanol mixture. Following the addition of 1 ml of 0.9% NaCl, the chloroform phase was transferred to a clean tube and evaporated to dryness under a stream of N2. Lipids were resuspended in a small volume of chloroform and stored at −20 °C. Neutral lipids were separated by TLC (silica gel G60 plates; EM Separations Technology) with hexane/diethyl ether/acetic acid (140:60:2, by vol.) as the developing solvent. Lipids were visualized by staining with iodine. Triacylglycerols with one or two hydroxy fatty acids were eluted from the gel with a small volume of chloroform/methanol (2:1, v/v) and positional analysis was conducted using Rhizopus arrhizus lipase (Sigma) as described by Bafor et al. (1990). Lipase hydrolysis products were separated by TLC on silica gel G60 plates with hexane/diethyl ether/acetic acid (70:140:3, by vol.) and visualized by lightly staining with iodine. For fatty acid analysis, lipid fractions were scraped from the TLC plate into glass tubes and FAMES were prepared and analysed by GC as described above.
Statistical analysis
Statistical analysis was conducted using the GraphPad InStat program (Graphpad Software, San Diego, USA).
Results
FAD2 and FAE1 are involved in hydroxy fatty acid biosynthesis
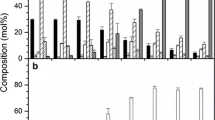
Heterologous expression of the castor hydroxylase cDNA in wild-type Arabidopsis results in the accumulation of 18:1-OH and three other hydroxy fatty acids (Fig. 2b), that have been identified as 18:2-OH, 20:1-OH and 20:2-OH (Broun and Somerville 1997). To determine whether the FAD3 desaturase and FAE1 condensing enzyme were involved in the synthesis of these fatty acids, as previously suggested (Broun and Somerville 1997), we expressed the castor hydroxylase cDNA, under the control of a seed-specific promoter, in a variety of mutant lines of Arabidopsis. Expression in a fad2/fae1 double mutant line, which lacks the activity of both the ER Δ12 desaturase (FAD2) and the FAE1 condensing enzyme, resulted in the accumulation of the C18 hydroxy fatty acids 18:1-OH and 18:2-OH, with no detectable C20 hydroxy fatty acids present (Fig. 2c). Seeds of a transformed fad3 mutant line, which lacks ER Δ15 desaturase (FAD3) activity, only produced the monounsaturated hydroxy fatty acids 18:1-OH and 20:1-OH (Fig. 2d). In a fad3/fae1 double mutant line expressing the castor hydroxylase, 18:1-OH was the only hydroxy fatty acid that could be detected (Fig. 2e). The fatty acid compositions of the seeds shown in Fig. 2 are given in Table 1.
Gas-chromatographic analysis of FAMES prepared from mature seeds of wild-type Arabidopsis and transformed lines expressing the castor hydroxylase cDNA. a Untransformed wild type; b wild type + castor hydroxylase; c fad2/fae1 double mutant + castor hydroxylase; d fad3 mutant + castor hydroxylase; e fad3/fae1 double mutant + castor hydroxylase
High levels of 18:1 do not result in a major increase in hydroxy fatty acid accumulation
To determine whether the level of hydroxy fatty acid accumulation in the seeds of transformed plants was related to substrate (18:1) content, we expressed the castor hydroxylase in two mutant lines of Arabidopsis that differed in the 18:1 content of their seeds. The fad2/fae1 line was chosen as a high-oleate line as these plants produce around 80–85% 18:1 in their seed oil. For comparison, we transformed a fad3 mutant line that contains around 15% 18:1 in its seed oil. Both lines were transformed with the castor hydroxylase cDNA as the LR cassette. Plants were grown under the same conditions of temperature and light. Hydroxy fatty acid content, as a percentage of total seed fatty acid, was determined for 32 randomly chosen plants from each line (Fig. 3). Both lines showed a similar range of hydroxy fatty acid content. The population of transformed fad2/fae1 plants had a slightly higher average hydroxy fatty acid content (5.6% vs. 4.3%) and higher maximum (9.7% vs. 8.2%) than the fad3 plants. Statistical analysis of the data (t-test) indicated that the difference between the two groups was unlikely to be caused by random sampling (P=0.038). We have transformed a large number of Arabidopsis plants of the lines indicated in Fig. 2 with either the castor or Lesquerella hydroxylase. The hydroxy fatty acid content of seeds of these lines is almost always less than 20% of total seed fatty acids.
Hydroxy fatty acid contents of seeds from individual Arabidopsis transformants expressing the castor hydroxylase cDNA. Seeds were collected from 32 randomly chosen primary transformants from each population and hydroxy fatty acid content (expressed as a percentage of total seed fatty acid) was determined by GC. a fad2/fae1 double mutant + castor hydroxylase; b fad3 mutant + castor hydroxylase
Relationship between hydroxy fatty acid content and the accumulation of other seed fatty acids
The fatty acid composition data from seeds of transformed plants was used to examine the relationships between total hydroxy fatty acid content and the level of each non-hydroxy fatty acid. Seeds were harvested from individual plants of the fad2/fae1 double mutant line transformed with the castor hydroxylase. FAMES were prepared from total seed lipids and analysed by GC. The percentage of each major fatty acid was then plotted against the percentage of total hydroxy fatty acids for all plants in the group (Fig. 4a). The double mutant plants showed a significant reduction in the 18:1 content of the seed that strongly correlated to the percentage of hydroxy fatty acids present. Data analysis indicated that the correlation was linear within the range of hydroxy fatty acid content seen in these samples. A slight positive correlation was observed between the levels of hydroxy fatty acids and those of the saturated fatty acids 16:0 and 18:0. Levels of these fatty acids were slightly higher in plants with higher hydroxy fatty acid content. Comparison of 18:2 levels showed a significant increase in the amount of this fatty acid corresponding to the level of hydroxy fatty acids in the seed oil. The increase was significant and linear as judged by a high R 2 values (R 2=0.95) when the data were subjected to linear regression analysis. No significant correlation was observed between 18:3 levels and hydroxy fatty acid content. Levels of 18:2-OH increased in proportion to the increase in total hydroxy fatty acids (Fig. 5a).
Relationship between hydroxy fatty acid content and the levels of non-hydroxy fatty acids in the seeds of Arabidopsis lines expressing the castor hydroxylase cDNA. Total seed fatty acid composition was determined for individual plants in the two transformed populations shown in Fig. 3. The percentage composition of each major fatty acid was plotted against the percentage of total hydroxy fatty acids in the sample. R 2 values determined for the linear regression lines shown were 0.94 and 0.95 for 18:1 and 18:2, respectively, in the transformed fad2/fae1 double mutant line and 0.68 and 0.82 for 18:1 and 18:2 in the fad3 mutant lines
Relationship between 18:2-OH and total hydroxy fatty acid content in seeds of plants from a fad2/fae1 double mutant population expressing the castor hydroxylase. b Relationship between 20:1-OH and total hydroxy fatty acid content in seeds from plants from a fad3 mutant population expressing the castor hydroxylase. c Relationship between ODP and total hydroxy fatty acid content in seeds from a population of fad3 plants expressing the castor hydroxylase. ODP was calculated for each plant and plotted against total hydroxy fatty acid content
In contrast to the data from the fad2/fae1 plants, expression of the castor hydroxylase in the fad3 mutant line, which has an active FAD2 desaturase resulted in a dramatic increase in 18:1 levels and a decrease in 18:2 content correlating closely to the level of hydroxy fatty acids in the seed (Fig. 4b). This trend was also seen in populations of wild-type plants expressing the castor hydroxylase (data not shown). No obvious changes in the levels of the other major fatty acids, 16:0, 18:0, 18:3 and 20:1, were observed in these plants. A correlation between the levels of 20:1-OH relative to total hydroxy fatty acid content was seen (Fig. 5b), with plants containing higher hydroxy fatty acid levels having correspondingly higher 20:1-OH content. The amount of 20:1 in these lines did not increase despite the increase in the levels of 18:1.
To assess the overall effect of hydroxy fatty acid production on the utilization of oleate in the seeds of plants expressing the castor hydroxylase we calculated the Oleate Derivative Proportion (ODP) values for plants from the fad3 population described above. ODP is a modification of that defined by Singh et al. (2001) and is defined here as the total of all major fatty acids derived from oleate (18:2% + 18:3% + 20:1% + 18:1-OH% + 20:1-OH%) divided by the sum of oleate and its derivatives (18:1% + 18:2% + 18:3% + 20:1% + 18:1-OH% + 20:1-OH%). Untransformed fad3 plants have an ODP value of around 0.74. In plants expressing the hydroxylase there was a significant inverse relationship between ODP and hydroxy fatty acid content (Fig. 5c). For example, the ODP value was reduced to 0.59 in the plant with the highest percentage of hydroxy fatty acids (8.16%).
The castor hydroxylase has a low level of desaturase activity
As shown in Fig. 4a, seed-specific expression of the castor hydroxylase in a FAD2-deficient line of Arabidopsis (the fad2/fae1 double mutant line) results in an increase in 18:2 content corresponding to the level of hydroxy fatty acid present. A similar but greater increase in 18:2 content was observed in seeds expressing the Lesquerella hydroxylase (data not shown). As this enzyme has been shown to be bifunctional, exhibiting both hydroxylase and desaturase activity, it is likely that the observed increase in 18:2 is due to the desaturase activity of the Lesquerella enzyme. To determine whether the castor hydroxylase also has desaturase activity, we expressed the hydroxylase cDNA in yeast. For comparison, we transformed yeast with the Lesquerella hydroxylase. Transformed yeast expressing the castor hydroxylase (Fig. 6b) accumulated low levels of 18:1-OH, but also produced 18:2, which was absent in cells transformed with the empty expression vector (Fig. 6a). Traces of 16:1-OH and 16:2 were also seen. As gas chromatographs of FAMES prepared from control cells showed a small peak with a similar retention time to 16:2 it is not possible to accurately determine the 16:1 desaturase activity of the enzyme without in vitro assays. Cells expressing the Lesquerella hydroxylase accumulated significant amounts of 16:2, 18:2 and 18:1-OH and a small amount of 16:1-OH (Fig. 6c). As shown in Table 2, only low levels of hydroxy fatty acids were detected in yeast expressing either of the two hydroxylases.
In Arabidopsis, ricinoleic acid is the only hydroxy fatty acid present in the sn-2 position of TAG
The seed lipids from three lines of Arabidopsis, all expressing the castor hydroxylase, were separated by TLC. Visualization of lipids by staining with iodine showed that the oil from all three lines contained TAG species that co-chromatographed with TAG from castor bean containing one (1-OH-TAG) and two (2-OH-TAG) hydroxy fatty acid residues (Fig. 7). In the oil sample from the transformed fad2/fae1 double mutant plants, TAG species containing one or two hydroxy fatty acids accounted for nearly 38% of the total TAG molecules (Table 3). Total hydroxy fatty acid content in this oil was around 11%. The fatty acid composition of the various lipid species from the transformed fad2/fae1 double mutant and fad3 mutant lines was determined by GC analysis (Table 3). The results from both lines indicated that the 1-OH-TAG and 2-OH-TAG species contained slightly lower proportions of hydroxy fatty acid than the 33% and 66% that would be expected. In addition to TAG, hydroxy fatty acids were also found in the polar lipid fraction. In the lipids from the transformed fad3 line, 20:1-OH was not present in the polar lipids but was abundant in the 2-OH-TAG fraction. Although this lipid class only represented 1.9% of the total seed lipids it contained nearly one-third of the 20:1-OH present in the seed oil.
TLC separation of seed neutral lipids. Lanes: 1, castor bean seed oil; 2, fad2/fae1 double mutant expressing the castor hydroxylase: 3, untransformed fad2/fae1 double mutant; 4, fad3 mutant expressing the castor hydroxylase; 5, fad3/fae1 double mutant expressing the castor hydroxylase; 6, seed oil from wild-type Arabidopsis
To determine whether hydroxy fatty acids were present in the sn-2 position of TAG, we conducted 1,3-specific lipolysis of the 1-OH-TAG and 2-OH-TAG fractions from the oil of the transformed fad2/fae1 double mutant plants. The products of the lipase digestion were separated by TLC (Fig. 8a). Lipase digestion of both TAG species was incomplete but both contained two separate species of monoacylglycerol (MAG), one of which co-chromatographed with hydroxy MAG (OH-MAG) prepared by lipase treatment of castor TAG. Diacylglycerol (DAG) species with 2-hydroxy fatty acids (2-OH-DAG) were seen in the digestion products of the 2-OH-TAG. GC analysis of the MAG fractions indicated that the fatty acid present in the OH-MAG was 18:1-OH (Fig. 8b) whereas the major fatty acid in the non-hydroxy MAG was 18:1. No significant amounts of 18:2-OH were detected in the OH-MAG fraction. A similar lipase digestion was conducted on 1-OH-TAG and 2-OH-TAG from fad3 plants that were expressing the castor hydroxylase. Although the seed oil from these plants contained both 18:1-OH and 20:1-OH, the OH-MAG fraction contained only 18:1-OH (data not shown). Due to the incomplete digestion of lipids, and the small amount of material available, it was not possible to accurately determine the proportion of TAG molecules containing 18:1-OH in the sn-2 position in either study.
TLC separation of TAG species after partial digestion with Rhizopus arrhizus lipase. Lanes: 1, fatty acids from castor oil; 2, castor oil + lipase; 3, 1-OH-TAG from transformed fad2/fae1 double mutant plants + lipase; 4, 2-OH-TAG from transformed fad2/fae1 double mutant plants + lipase; 5, oil from wild-type Arabidopsis + lipase. FAs Unesterified fatty acids, ? unidentified lipid. b Gas chromatographic analysis of FAMES prepared from MAG and OH-MAG lipid species. To quantify fatty acids, a 17:1 FAME standard was added to each sample
Discussion
Expression of either the castor or Lesquerella hydroxylase in the seeds of wild-type Arabidopsis results in the synthesis of four different hydroxy fatty acids. Both enzymes are known to utilize oleate as substrate and the presence of polyunsaturated and C20 hydroxy fatty acids has thus been suggested to be the result of the further metabolism of ricinoleate catalysed by the ER FAD3 desaturase and FAE1 condensing enzymes (Broun and Somerville 1997; Broun et al. 1998a). We have confirmed the involvement of these enzymes by expressing the castor hydroxylase in Arabidopsis mutants that lack the activity of one or both of these proteins (Fig. 1). The ability of FAD3 to desaturate 18:1-OH is supported by the evidence of a number of groups (Engeseth and Stymne 1996; Reed et al. 1997, 2000) who demonstrated that the hydroxy group of ricinoleic acid is able to substitute for a double bond in substrate recognition by the FAD3 desaturase. The order of synthesis of the C20 hydroxy fatty acids is still, however, uncertain. The absence of these fatty acids in plants without a functional FAE1 condensing enzyme demonstrates the involvement of the enzyme but does not clearly indicate whether 20:1-OH results from the elongation of 18:1-OH or the hydroxylation of 20:1. It seems likely that the first step is the hydroxylation of 18:1. For example, in vitro studies using developing seeds of Lesquerella fendleri suggest that the Lesquerella hydroxylase does not efficiently hydroxylate 20:1 (Reed et al. 1997) and yet wild-type Arabidopsis plants expressing this hydroxylase synthesize 20:1-OH and 20:2-OH. Furthermore, the hydroxylation of 20:1 would require its esterification to PC, as this complex lipid, and not acyl-CoA, is the substrate for hydroxylation (Bafor et al. 1991). Very long chain fatty acids (VLCFAs) are generally excluded from membrane lipids of developing Arabidopsis seeds and also the sn-2 position of TAG (Norton and Harris 1983).
The identification of the enzymes required to synthesize polyunsaturated and C20 hydroxy fatty acids will enable the production of oils with a variety of hydroxy fatty acid profiles. Our results also demonstrate that it is possible to produce oil in Arabidopsis that contains ricinoleic acid as the only hydroxy fatty acid. The ability to produce pure ricinoleate in an oilseed crop such as Brassica napus may provide a viable alternative to castor oil for industrial applications.
Expression of the castor hydroxylase in a fad2/fae1 double mutant background resulted in a decrease in the levels of oleic acid corresponding to the hydroxy fatty acid content of the seed (Fig. 4a). Expression of the hydroxylase in a fad3 mutant line of Arabidopsis that contains an active FAD2 desaturase, however, resulted in a significant increase in 18:1 levels and a decrease in 18:2 content. The changes appeared to be linear over the range of hydroxy fatty acid content observed. This correlation was also seen in wild-type seeds expressing the hydroxylase and was observed both with the castor and Lesquerella hydroxylases. Calculation of ODP values for plants in the transformed fad3 line indicates that there is a significant decrease in the utilization of oleate in these lines. The increase in 18:1 content of seeds expressing the castor hydroxylase is in agreement with the results of Broun and Somerville (1997). Their work, however, reported an increase in 18:1 content in seeds of a transformed fad2 mutant whereas in our study 18:1 levels decrease. The reason for this discrepancy appears to be due to the fact that the fad2 mutant used in the earlier study (fad2-3 allele, Miquel and Browse 1992) still has a low level of FAD2 activity in the developing seeds.
Suppression of FAD2 activity is a phenomenon that has also been reported to result from the heterologous expression of fatty acid conjugase, acetylenase and epoxygenase enzymes in Arabidopsis and other oilseeds (Cahoon et al. 1999, 2002; Singh et al. 2001; Thomaeus et al. 2001). All of these enzymes catalyse the synthesis of unusual fatty acids using either oleate or linoleate as substrate, with modification of the acyl group occurring at the Δ12 position. With the exception of the Δ12-epoxygenase from Euphorbia lagascae (Cahoon et al. 2002), these enzymes are all members of the FAD2 family of enzymes (Shanklin and Cahoon 1998). This observation has led to a number of suggestions as to the mechanism of the suppression. These include homology-dependant gene silencing (HDGS), a mechanism that now appears unlikely and that has been discussed in detail by Singh et al. (2001), competition for substrate, and the possibility of negative interactions at the protein level. Availability of substrate does not appear to be a limiting factor in the accumulation of hydroxy fatty acids in Arabidopsis. We transformed lines with high 18:1 content (fad2/fae1 double mutant, 85% 18:1) and relatively low levels of 18:1 (fad3 mutant, 15% 18:1) and observed little difference in levels of hydroxy fatty acid accumulation. Although there may be other factors that limit hydroxy fatty acid accumulation, such as incorporation into TAG, it seems likely that there would be an observable difference when large populations of transformed plants were analysed. The inhibition of FAD2 activity in plants expressing the Δ12-epoxygenase from Euphorbia lagascae, which is clearly a cytochrome P450 enzyme (Bafor et al. 1993; Cahoon et al. 2002), suggests that negative interaction between the endogenous FAD2 protein and the introduced enzyme, based on a high degree of structural similarity, may not be the mechanism responsible for this phenomenon. A feature that both the FAD2-like fatty acid-modifying enzymes and the Euphorbia epoxygenase appear to share, however, is that they utilize fatty acids esterified to the membrane lipid PC as substrate (Stymne et al. 1987; Bafor et al. 1991, 1993). Studies conducted on developing seeds of Arabidopsis expressing a variety of FAD2-like enzymes has demonstrated that the unusual fatty acid product of these enzymes is not efficiently removed from PC after synthesis (Thomaeus et al. 2001). The build up of these fatty acids in PC has therefore been suggested to be a factor contributing to the inhibition of FAD2 activity (Thomaeus et al. 2001). An observation that may support this is that the levels of 20:1 in seeds are unaffected by the presence of the hydroxylase. The elongation of 18:1 to 20:1 is thought to occur while the fatty acid is esterified to CoA and not PC (Fehling and Mukherjee 1991). A better understanding of this phenomenon will be essential if plants containing high levels of unusual fatty acids are to be produced.
An increase in 18:2 content has not previously been reported in plants expressing the castor hydroxylase. Expression of the hydroxylase in yeast indicates that the enzyme has a low level of desaturase activity, which likely contributes to the observation in plants. Unlike the Lesquerella hydroxylase, which is clearly bifunctional (Broun et al. 1998a), the castor enzyme appears to be a highly efficient hydroxylase. The desaturase activity of this protein is further evidence of the similarity between the mechanism of desaturation and hydroxylation in this class of proteins (Broadwater et al. 2002).
Analysis of the neutral lipids of Arabidopsis plant expressing the castor hydroxylase show that in this species 18:1-OH is not excluded from the middle (sn-2) position of the TAG molecule. Partial lipolysis of the hydroxy TAG fractions produced both OH-MAG and 2-OH-DAG. Ricinoleic acid was, however, the only hydroxy fatty acid found in this position. The presence of this fatty acid may indicate that 18:1-OH-CoA is a substrate of the sn-2 acyltransferase for TAG assembly. Alternatively, a relatively low rate of removal of 18:1-OH from PC may allow the fatty acid to enter the TAG pool by interconversion of PC to DAG and subsequent acylation of the sn-3 position. The absence of 18:2-OH from the sn-2 position of TAG and its low abundance in polar lipids may indicate that the mechanism of the incorporation of this fatty acid into TAG is different from that of 18:1-OH. The absence of 20:1-OH from the sn-2 position of TAG and from polar lipids reflects the distribution of VLCFAs in wild-type Arabidopsis. The reason for the relative enrichment of this hydroxy fatty acid in 2-OH-TAG is unknown. The mechanism of hydroxy fatty acid incorporation into TAG is clearly an area that is still poorly understood. Understanding how these fatty acids are metabolized in transgenic plants will be a key factor in increasing the levels of hydroxy fatty acids that can be produced in transgenic seed oil.
Abbreviations
- DAG:
-
diacylglycerol
- ER:
-
endoplasmic reticulum
- FAE1:
-
Fatty Acid Elongation 1
- FAMES:
-
fatty acid methyl esters
- MAG:
-
monoacylglycerol
- ODP:
-
Oleate Derivative Proportion
- PC:
-
phosphatidylcholine
- TAG:
-
triacylglycerol
- VLCFA:
-
very long chain fatty acid (C20 and longer)
- X:Y:
-
a fatty acid containing X carbons with Y double bonds
References
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, Albright LM, Coen DM, Varki A (eds) (1995) Current protocols in molecular biology. Wiley, New York
Bafor M, Jonsson L, Stobart AK, Stymne S (1990) Regulation of triacylglycerol biosynthesis in embryos and microsomal preparations from the developing seeds of Cuphea lanceolata. Biochem J 272:31–38
Bafor M, Smith MA, Jonsson L, Stobart K, Stymne S (1991) Ricinoleic acid biosynthesis and triacylglycerol assembly in microsomal preparations from developing castor-bean (Ricinus communis) endosperm. Biochem J 280:507–514
Bafor M, Smith MA, Jonsson L, Stobart K, Stymne S (1993) Biosynthesis of vernoleate (cis-12-epoxyoctadeca-cis-9-enoate) in microsomal preparations from developing endosperm of Euphorbia lagascae. Arch Biochem Biophys 303:141–151
Broadwater JA, Whittle E, Shanklin J (2002) Desaturation and hydroxylation: Residues 148 and 324 of Arabidopsis FAD2, in addition to substrate chain length, exert a major influence in partitioning of catalytic specificity. J Biol Chem 277:15613–15620
Broun P, Somerville C (1997) Accumulation of ricinolic, lesquerolic and densipolic acids in seeds of transgenic Arabidopsis plants that express a fatty acyl hydroxylase cDNA from castor bean. Plant Physiol 113:933–942
Broun P, Boddupalli S, Somerville C (1998a) A bifunctional oleate 12-hydroxylase: desaturase from Lesquerella fendleri. Plant J 13:201–210
Broun P, Shanklin J, Whittle E, Somerville C (1998b) Catalytic plasticity of fatty acid modification enzymes underlying chemical diversity of plant lipids. Science 282:1315–1317
Cahoon EB, Carlson TJ, Ripp KG, Schweiger BJ, Cook GA, Hall SE, Kinney AJ (1999) Biosynthetic origin of conjugated double bonds: production of fatty acid components of high-value drying oils in transgenic soybean embryos. Proc Natl Acad Sci USA 96:12935–12940
Cahoon EB, Ripp KG, Hall SE, McGonigle BM (2002) Transgenic production of epoxy fatty acids by expression of a cytochrome P450 enzyme from Euphorbia lagascae seed. Plant Physiol 128:615–624
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Datla RSS, Hammerlindl JK, Panchuk B, Pelcher LE, Keller W (1992) Modified binary plant transformation vectors with the wild-type gene encoding NPTII. Gene 211:383–384
Engeseth N, Stymne S (1996) Desaturation of oxygenated fatty acids in Lesquerella and other oil seeds. Planta 198:238–245
Fehling E, Mukherjee KD (1991) Acyl-CoA elongase from a higher plant (Lunaria annua): metabolic intermediates of very long chain acyl-CoA products and substrate specificity. Biochim Biophys Acta 1082:239–246
Hayes DG, Kleiman R, Phillips BS (1995) The triglyceride composition, structure and presence of estolides in the oils of Lesquerella and related species. J Am Oil Chem Soc 72:559–569
Katavic V, Haughn GW, Reed D, Martin M, Kunst L (1994) In planta transformation of Arabidopsis thaliana. Mol Gen Genet 245:363–370
Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue specific expression of chimeric genes by a novel type of Agrobacterium binary vector. Mol Gen Genet 204:383–396
Kunst L, Taylor DC, Underhill EW (1992) Fatty acid elongation in developing seeds of Arabidopsis thaliana. Plant Physiol Biochem 30:425–434
Miquel M, Browse J (1992) Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. Biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J Biol Chem 267:1502–1509
Moon H, Smith MA, Kunst L (2001) A condensing enzyme from the seeds of Lesquerella fendleri that specifically elongates hydroxy fatty acids. Plant Physiol 127:1635–1643
Moreau RA, Stumpf PK (1981) Recent studies of the enzymatic synthesis of ricinoleic acid by developing castor bean. Plant Physiol 67:672–676
Norton G, Harris, JF (1983) Triacylglycerols in oilseed rape during seed development. Phytochemistry 22:2703–2707
Reed DW, Taylor DC, Covello PS (1997) Metabolism of hydroxy fatty acids in developing seeds in the genera Lesquerella (Brassicaceae) and Linum (Linaceae). Plant Physiol 114:63–68
Reed DW, Schafer UA, Covello PS (2000) Characterization of the Brassica napus extraplastidial linoleate desaturase by expression in Saccharomyces cerevisiae. Plant Physiol 122:715–720
Shanklin J, Cahoon EB (1998) Desaturation and related modifications of fatty acids. Annu Rev Plant Physiol Plant Mol Biol 49:611–641
Shaul O, Galili G (1992) Increased lysine synthesis in tobacco plants that express high levels of bacterial dihydrodipicolinate synthase in their chloroplasts. Plant J 2:203–209
Singh S, Thomaeus S, Lee M, Stymne S, Green A (2001) Transgenic expression of a Δ12-epoxygenase gene in Arabidopsis seeds inhibits accumulation of linoleic acid. Planta 212:872–879
Smith MA, Jonsson L, Stymne S, Stobart K (1992) Evidence for cytochrome b5 as an electron donor in ricinoleic acid biosynthesis in microsomal preparations from developing castor bean (Ricinus communis L.). Biochem J 287:141–144
Smith MA, Stobart AK, Shewry PR, Napier JA (1994) Tobacco cytochrome b5: cDNA isolation, expression analysis and in vitro protein targeting. Plant Mol Biol 25:527–537
Stymne S, Griffiths G, Stobart K (1987) Desaturation of fatty acids on complex lipid substrates. In: Stumpf PK et al (eds) The metabolism, structure and function of plant lipids. Plenum, New York, pp 405–412
Thomaeus S, Carlsson AS, Stymne S (2001) Distribution of fatty acids in polar and neutral lipids during seed development in Arabidopsis thaliana genetically engineered to produce acetylenic, epoxy and hydroxy fatty acids. Plant Sci 161:997–1003
van de Loo FJ, Broun P, Turner S, Somerville C (1995). An oleate 12-hydroxylase from castor (Ricinus communis) is a fatty acyl desaturase homologue. Proc Natl Acad Sci USA 92:6743–6747
Acknowledgements
The authors acknowledge the financial support of Linnaeus Plant Sciences Inc. and The Science Council of British Columbia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, M.A., Moon, H., Chowrira, G. et al. Heterologous expression of a fatty acid hydroxylase gene in developing seeds of Arabidopsis thaliana . Planta 217, 507–516 (2003). https://doi.org/10.1007/s00425-003-1015-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1015-6