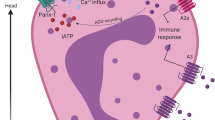
Abstract
Neutrophil granulocytes are exposed to widely varying microenvironmental conditions when pursuing their physiological or pathophysiological functions such as fighting invading bacteria or infiltrating cancer tissue. Examples for harsh environmental challenges include among others mechanical shear stress during the recruitment from the vasculature or the hypoxic and acidotic conditions within the tumor microenvironment. Chemokine gradients, reactive oxygen species, pressure, matrix elasticity, and temperature can be added to the list of potential challenges. Transient receptor potential (TRP) channels serve as cellular sensors since they respond to many of the abovementioned environmental stimuli. The present review investigates the role of TRP channels in neutrophil granulocytes and their role in regulating and adapting neutrophil function to microenvironmental cues. Following a brief description of neutrophil functions, we provide an overview of the electrophysiological characterization of neutrophilic ion channels. We then summarize the function of individual TRP channels in neutrophil granulocytes with a focus on TRPC6 and TRPM2 channels. We close the review by discussing the impact of the tumor microenvironment of pancreatic ductal adenocarcinoma (PDAC) on neutrophil granulocytes. Since neutrophil infiltration into PDAC tissue contributes to disease progression, we propose neutrophilic TRP channel blockade as a potential therapeutic option.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neutrophil granulocytes are major constituents of the cellular immune system and serve as the first-line defense to invading pathogens. On the other hand, they also contribute to pathological conditions such as chronic inflammatory diseases and cancer. They are exposed to widely varying microenvironmental conditions. The environmental signals acting on neutrophil granulocytes include, but are not limited to, (i) shear forces during the recruitment from the vasculature, (ii) chemoattractants guiding them to sites of infection or inflammation, (iii) mechanical properties (e.g., elasticity, pressure) of the surrounding tissue encountered during chemotaxis, (iv) an acidic and hypoxic environment that may also have an increased concentration of reactive oxygen species (ROS), and (v), finally, the temperature may diverge from the normal body temperature of 37 °C. Hence, neutrophil granulocytes must have developed mechanisms to adapt and respond to these environmental cues in order to function properly despite the potentially harsh environmental challenges. This requires the presence of sensors in the plasma membrane of neutrophil granulocytes. Accordingly, they express many different receptors in their plasma membrane ranging from G protein-coupled chemoattractant receptors to innate immune receptors. Their role in adapting the function of neutrophil granulocytes to external stimuli is well described, and we refer to recent reviews on this topic (e.g., [27, 38]).
Many of the receptor-dependent signaling cascades such as those triggered by G protein-coupled chemoattractant receptors utilize intracellular Ca2+ ions as a second messenger. Consequently, ion channels mediating the influx of Ca2+ ions into neutrophil granulocytes are involved in transducing the external signals. The molecular nature of the channels regulating the influx of Ca2+ into neutrophil granulocytes and their physiological significance are not yet well described. They include TRP and ORAI channels. TRP channels are a large family of cation channels. Most of them are Ca2+ permeable. Their function can be described in very general terms as that of “cellular sensors” [99]. Some TRP channels are also known to be activated by the environmental cues listed above. TRPC1 and TRPM7 channels are involved in mechanosensation [33, 35, 137], TRPM2 channels serve as ROS sensors [135] and are pH sensitive [138], TRPC6 channels are activated in a hypoxic environment [97, 98], and TRPV1 and TRPM8 are temperature sensitive.
It is our aim to review the current knowledge on TRP channels in neutrophil granulocytes. We refer to a recent review for more information on the function of ORAI channels in neutrophil granulocytes [12]. We will discuss the concept of neutrophilic TRP channels being sensors, transducers, and possibly modifiers of the microenvironment. This discussion will be preceded by a brief overview of some of the main physiological functions of neutrophil granulocytes. We will then provide a critical overview on the available electrophysiological evidence for TRP (and other ion) channels in neutrophil granulocytes and examine the roles of TRP channels in neutrophil functions. The review will be closed with a more visionary discussion of the role of TRP channels in tumor-associated neutrophil granulocytes in pancreatic ductal adenocarcinoma (PDAC).
Physiological functions of neutrophil granulocytes
Neutrophil granulocytes are derived from the hematopoietic system in the bone marrow in a steady process of self-renewal and differentiation. However, during inflammation, the capacity of steady-state granulopoiesis is often exceeded, which induces a distinct program of accelerated production known as “emergency” granulopoiesis [77]. After granulopoiesis, mature granulocytes are released from the bone marrow to the blood stream from where they are recruited to sites of infection or inflammation.
Pathogens or inflammatory foci lead tissue macrophages and mast cells to produce cytokines like TNF-α or interleukins that induce the activation of endothelial cells and the expression of adhesion molecules on their cell surface. This triggers a well-defined cascade of events to recruit (neutrophil) granulocytes from the blood stream. A number or recent reviews outline this process in great detail (e.g., [64, 78]). The recruitment cascade includes the capturing, rolling, adhesion, intravascular migration, and diapedesis through the endothelial layer of postcapillary venules. Selectins and intercellular adhesion molecule 1 (ICAM-1) in endothelial cells as well as P-selectin glycoprotein ligand 1 (PSGL1) and β2-integrins (LFA1 = αLβ2 integrin; MAC1 = αMβ2 integrin) in granulocytes are crucial adhesion receptors for these processes. Neutrophil granulocytes are activated by chemoattractants that are presented on the endothelial surface. This triggers conformational changes of integrins leading to a strengthening of the adhesion of neutrophil granulocytes to endothelial cells that is crucial for the ensuing extravasation [47]. After leaving the blood vessels, the cells have to follow chemoattractant gradients to reach the site of inflammation.
The ability to migrate is one of the key characteristics of neutrophil granulocytes. Migration comes into play during intravascular crawling which enables neutrophil granulocytes to reach a proper site to cross the endothelial barrier. An impairment of intravascular crawling, e.g., by the deficiency of αMβ2 integrin (MAC1), leads to markedly delayed transmigration because neutrophil granulocytes have to emigrate through nonoptimal sites [112]. After leaving the blood vessels, the cells migrate through the tissue to reach the site of inflammation and fight pathogens. Recent evidence indicates that the so-called reverse migration contributes to the resolution of an inflammation, i.e., the migration of neutrophil granulocytes away from an inflammatory focus [13, 114].
Cell migration is described as a repeated cycle of protrusion of the cell front, attachment of this lamellipodium to the matrix or another cell, and retraction of the rear end (uropod) in association with release of adhesion structures. It is accompanied by temporally and spatially coordinated fluxes of ions and water across the plasma membrane [48, 88, 129]. Directional migration and chemotaxis require a cellular asymmetry. One of the signals underlying cell polarization of migrating cells including immune cells is a front-to-rear gradient with an increasing intracellular Ca2+ concentration towards the rear end [6, 75]. The increased Ca2+ concentration at the rear end is related to myosin II contraction [11], calpain-mediated cleavage of focal adhesions [10], and local ion channel activity [128]. In addition to the global front-to-rear gradient, there are local Ca2+ and signaling microdomains at the cell front promoting directed migration [148, 149]. To obtain a more quantitative insight into the mechanisms underlying chemotaxis, a number of mathematical models such as the polarized local excitation and global inhibition (LEGI-BEN) model have been developed [54].
Phagocytosis and ROS production
Neutrophil granulocytes, similarly to macrophages and dendritic cells, are specialized phagocytes. They internalize potentially dangerous particles or cellular debris in a receptor-mediated manner. Being the most abundant phagocytes in the bloodstream, they are indispensable in preventing pathogen dissemination. Particles are opsonized with antibodies or complement molecules which bind to Fc and complement receptors expressed on phagocytes. Phagocytes like neutrophil granulocytes engulf and entrap these particles into phagosomes. During “phagosomal maturation,” they fuse with other vesicular compartments providing enzymes and an acidic (in macrophages) or initially alkaline (in neutrophil granulocytes) environment for particle digestion [73, 154].
Phagocytosis and activation of PAMP (pathogen-associated molecular pattern molecules) receptors and also phorbol esters induce the production of reactive oxygen species (ROS) [100]. Neutrophil granulocytes produce ROS predominately inside the phagosomes, while eosinophil granulocytes release ROS extracellularly to fight comparably large parasites [69]. Oxygen consumption of activated neutrophil granulocytes rises during the “oxidative burst” which is driven by NADPH oxidase 2 (NOX2). NOX2 consists of membrane-bound and cytosolic components. Gp91phox and p22phox (flavocytochrome b558) reside mainly (85%) in membranes of specific granules. The remaining 15% are expressed in secretory vesicles and the plasma membrane [157]. The membrane-bound NOX2 complex mediates electron release from NADPH and their translocation into the phagosome or outside the cell. Due to its electrogenicity, NOX activity is regulated by the membrane potential and requires the flux of charge-balancing counterions [154]. Electrons derived from NADPH oxidation reduce molecular oxygen to superoxide anion (O2−) which in turn is converted to H2O2 by superoxide dismutase (SOD). Myeloperoxidase (MPO) then mediates the reaction of H2O2 and Cl− to form the strong bactericidal molecule hypochlorous acid (HOCl). The oxidative burst is accompanied by an intracellular acidification which is compensated by the Na+/H+ exchanger (NHE1) and voltage-dependent proton channels (HV1; see below) [89].
Electrophysiology of neutrophil granulocytes, do TRP channels contribute?
Neutrophil granulocytes are notoriously complicated cells to patch-clamp. Their lifespan after isolation is very limited without special treatment. They activate easily and sometimes even spontaneously. The complications connected to the experimental work have left the literature about patch-clamp measurements of neutrophil granulocytes manageable: ~70 publications have been found (as of February 2018) in PubMed using “neutrophil patch-clamp” as search input. However, the search parameter “neutrophil conductance” has yielded already more than 300 hits. This section focuses exclusively on patch-clamp measurements of neutrophil granulocytes with some additions from the related eosinophil granulocytes. We will begin by briefly reviewing the main conductances in patch-clamped neutrophil granulocytes.
Potassium conductance
Like in heart muscle, skeletal muscle, and other cells, there is an inwardly rectifying K+ conductance measurable in newt neutrophil granulocytes [60]. The inwardly rectifying K+ conductance was detected in newt eosinophil and basophil granulocytes, too. Two more reports identified it as being mediated by Kir2.1 in human eosinophil and mouse neutrophil granulocytes [80, 145]. It has a decreased conductance at low internal pH, no conductance at 0 K+, and shows a voltage-dependent block by cesium and barium. Interestingly, Krause and Welsh [67] could not find an inwardly rectifying K+ conductance in their initial patch-clamp study of human neutrophil granulocytes which would contradict the previously mentioned publications. Instead, Krause and Welsh found a Ca2+-activated K+ conductance that was activated by the Ca2+ ionophore ionomycin in 2 mM CaCl2 bath solution, and blocked by barium, but not by charybdotoxin, apamin, quinine, or 4-aminopyridine. A Ca2+-activated K+ conductance was also detected in eosinophil granulocytes [121, 122]. Two types of channels were reported with single-channel conductances of ~ 10 and 22 pS, respectively. Krause and Welsh [67] measured a depolarization-activated inwardly rectifying potassium conductance, which has not been reported by other scientists. It would be intriguing to have a second look at this conductance. The once proposed expression of large conductance Ca2+-activated K+ channels (KCa1.1), however, could not be confirmed neither in human neutrophil [31] nor in eosinophil granulocytes [36] so the respective publication had to be withdrawn. In non-excitable cells, Ca2+-activated K+ channels are often seen as a means to maintain the electrical driving force for influx of Ca2+ across the plasma membrane: the depolarizing effect of Ca2+ influx is circumvented by the simultaneous activation of Ca2+-sensitive K+ channels which keep the cell membrane potential at hyperpolarized values [39]. Furthermore, it has been reported that eosinophil granulocytes contain a limited number (~25 channels per cell) of ATP-dependent K+ channels (KATP) [2, 131].
Chloride conductance
Phagocytes appear to have a very high intracellular Cl− concentration (~ 100 mmol/l) [56] so that opening of Cl− channels will lead to Cl− efflux and volume changes. This is relevant for neutrophil granulocytes that undergo marked shape changes during migration. Shape changes are usually accompanied by changes of the cell volume [51]. However, a more recent study using a fluorescent Cl− indicator reported a considerably lower intracellular Cl− concentration in neutrophil granulocytes (~ 30 mmol/l; [52]). Krause and Welsh [67] are the first to report Ca2+-activated Cl− currents in human neutrophil granulocytes. A few years later, a mechanosensitive but Ca2+-insensitive Cl− conductance, with a single-channel conductance of ~ 1.5 pS estimated from noise analysis, was described [141]. This conductance has much in common with the typical volume-sensitive Cl− current expressed in many different cell types. A small Cl− current was detected in neutrophil granulocytes activated by TNF-α or fMLF [111]. Its pharmacological characterization revealed that it is only blocked by ethacrynic acid. It is distinct from the volume-sensitive Cl− conductance. Neutrophil granulocytes from LRCC8A knock-out mice have almost no detectable swelling-activated chloride current anymore [4]. This corroborates the idea that LRCC8A may be a part of the swelling-activated Cl− conductance. Finally, eosinophil granulocytes may contain a Cl− conductance that was ascribed to ClC-3 [130]. To the best of our knowledge, we found no electrophysiological evidence for CFTR expression. Neutrophil granulocytes also express glycin receptors (GlyRα2), ligand-gated Cl− channels. They are translocated to the plasma membrane upon lysophosphatidylcholine (LPC) stimulation and modulate TRPM2 channels by setting the intracellular Cl− concentration [52].
Proton conductance
The presence of a proton conductance is well established in neutrophil granulocytes, and it has been measured by various scientific groups multiple times [21]. Using noise analysis, a single-channel conductance of ~ 10 fS was initially estimated for neutrophilic proton channels [17]. The channel is pH and voltage-dependent and also expressed in other immune cells such as eosinophil and basophil granulocytes or lymphocytes [8, 40, 93, 124, 126]. Two scientific groups report proton currents to be augmented in the presence of an elevated cytosolic Ca2+ concentration [40, 126]. The prime function of proton currents is the pH and charge compensation during the respiratory burst in phagocytes [92]. In addition, it maintains Ca2+ influx during migration of neutrophil granulocytes by stabilizing the cell membrane potential [30]. The measurement of proton currents was also used to quantify the activity of the Na+/H+ antiporter in neutrophil granulocytes via patch-clamp [18].
Electron conductance
Electron currents were initially measured in eosinophil [127] and shortly thereafter in neutrophil granulocytes [19]. The electron conductance is small and carried by the translocation of electrons across the plasma membrane via the NADPH oxidase NOX2 which translocates ~300 electrons per second [15, 66]. At − 40 mV, the average electron current amounts to − 2.3 pA in human neutrophil granulocytes. In Table 1, we estimated a conductance for the oxidase of approximately 0.5 fS. However, this has to be taken with a grain of salt because of the non-linear voltage dependence of the oxidase [20].
Non-selective cation conductance
Patch-clamp electrophysiology of neutrophil granulocytes started in 1986 with a publication describing two non-selective and Ca2+-activated cation channels with conductances of 18–24 and 4–6 pS, respectively [152]. Their molecular nature, however, remained unknown. Heiner et al. detected TRPM2-mediated currents in neutrophils and HL-60 cells [43]. TRPM2 channels were exclusively activated when ADP-ribose (ADPR) or NAD were added to the pipette solution. Single-channel measurements showed a conductance of 55–63 pS. The single-channel events were exclusively detected in inward direction [43]. A follow-up paper by the same group determined the concentration of ADP-ribose to be 5 μM in unstimulated and 3.9 μM in stimulated neutrophil granulocytes. Addition of Ca2+ amplified the effect of ADP-ribose on the channel. Cyclic ADP-ribose (cADPR) did not trigger any TRPM2 currents [45]. An earlier description focused on TRPM2 single-channel events and detected events in inward and outward direction with conductances of 58 and 76 pS, respectively [123], exclusively triggered by ADP-ribose and NAD. The same publication detected TRPM2 currents in the eosinophil cell line EOL1 that were triggered by 0.5 mM ADP-ribose. A detailed investigation of TRPM2 agonists in neutrophil granulocytes and overexpression systems revealed that activation by cADPR can be potentiated by NAADP and that 100 μM of H2O2 lowers the concentration of ADPR needed to activate TRPM2 in neutrophil granulocytes [70]. ADPR-induced TRPM2 channel activity is further modulated by simultaneously changing the intracellular and extracellular Cl− concentration ([Cl−]i/[Cl−]e). Half-maximal activation occurs at a [Cl−]i/[Cl−]e of ~ 18 mmol/l [52].
In addition, there are experiments showing a Mg2+-inhibited current in neutrophil granulocytes which is a typical property of TRPM7 channels [43]. TRPM7 currents are upregulated in TRPM2−/− macrophages [3]. It is not known whether this is the case in neutrophil granulocytes as well. The mRNA screen of neutrophil granulocytes reported the expression of several TRP channels such as TRPV1, TRPV2, TRPC6, TRPM2, TRPM4, and TRPV4 [16, 43] but interestingly not TRPM7.
The functional expression of P2X1 channels was revealed in neutrophil granulocytes by using the activator αβ-methylene ATP [72]. However, this paper contained no detailed analysis of the αβ-methylene ATP-induced current. Thus, more measurements are clearly needed. To the best of our knowledge, P2X7 channels have not yet been demonstrated with patch-clamp experiments [79] which is in contrast to functional assays revealing their impact on IL-1β secretion of neutrophil granulocytes [59].
Store-operated channels
There are only few publications providing direct electrophysiological evidence for store-operated Ca2+ channels in neutrophil granulocytes [22, 125]. Heiner et al. demonstrated store-operated cation current in HL-60 cells, however, failed to detect them in human neutrophil granulocytes [44].
Capacity measurements in neutrophil granulocytes
Neutrophil granulocytes as well as many other cells from the immune system are able to degranulate. This process is comparable with membrane vesicle fusion in neurons which leads to characteristic changes of membrane capacitance that can be detected with patch-clamp technique [102,103,104]. Reported capacity values of unstimulated human neutrophil granulocytes range between 2 and 5 pF (e.g., [17, 43, 67, 141]). Vesicle fusion involves the formation of a conductive pore. During patch-clamp experiments, this can be mistaken for an ion channel with a unitary conductance of 35–380 pS. Thus, it cannot be dismissed that sightings of high conductance single-channel events rather reflect neutrophil degranulation than ion channel activity. There are publications demonstrating this special case of conductance during patch-clamp measurements [5, 76].
Taken together, patch-clamp studies of human neutrophil granulocytes revealed the presence of several conductances (Table 1). K+, Cl−, and proton conductances have been reported by several groups and with high consistency. Many of these experiments were done before TRP channels were discovered in human tissue [161]. Prior to the discovery of human TRP channels, there was only a single report of nonspecific cation channels in neutrophil granulocytes [152]. Unfortunately, these recordings were not reproduced later. Until now, TRPM2 is by far the best electrophysiologically characterized TRP channel in neutrophil granulocytes. It is intriguing, however, that there is not a single publication mentioning TRPM2-mediated single-channel events during the respiratory burst. The high unitary conductance of TRPM2 (50–70 pS) would generate single-channel currents of 3.6 pA at a holding potential of − 60 mV. In neutrophil granulocytes, single-channel events of this size would be readily recorded even in the whole-cell configuration [43, 70]. Therefore, it is possible that these channels are silent during the respiratory burst. This could be due to their pH sensitivity. TRPM2 channels are closed by a drop of the intracellular or extracellular pH [28, 138]. During the respiratory burst, a massive amount of protons is produced by oxidizing NADPH causing an intracellular acidification [89]. Moreover, the membrane potential of neutrophil granulocytes depolarizes to + 10 mV during the respiratory burst when stimulated with fMLP or even + 60 mV in the presence of PMA [119]. Accordingly, the electrical driving force for TRPM2-mediated cation fluxes (e.g., Ca2+ and Na+) are largely diminished during an oxidative burst.
From the reviewed data, it becomes obvious that there is a great lack of knowledge concerning the electrophysiological fingerprinting of molecularly identified ion channels in neutrophil granulocytes in general and of TRP channels in particular. This strongly contrasts with an increasing number of publications showing the involvement of different TRP channels in regulating the function of neutrophil granulocytes. Thus, there is a strong need for a systematic electrophysiological investigation of the ion channels functionally expressed in neutrophil granulocytes. Such measurements should be performed in a highly standardized way and meticulously account for the state of activation of neutrophil granulocytes and the environmental conditions. A detailed description of the patch-clamp technique using human neutrophil granulocytes has been published recently [90].
Function of neutrophil granulocytes depends on TRP channels
TRPC channels
TRPC6 is one of the best studied TRP channels in neutrophil granulocytes, despite the fact that electrophysiological proof for TRPC6 channel activity in these cells is still lacking. At least in human neutrophil granulocytes, oleoyl-acetyl-glycerol (OAG)-activated and TRPC6-mediated cation currents could not be detected [43]. So far, only Ca2+ imaging and Mn2+ quenching experiments were performed to assess the ion conductive properties of TRPC6 channel activity in neutrophil granulocytes and to complement behavioral assays. These studies provide firm evidence for an important role of TRPC6 channels in neutrophil recruitment. TRPC6 affects MIP-2 (CXCL2)-induced migration of neutrophil granulocytes and actin arrangement [16]. Furthermore, chemotaxis, recruitment to the peritoneal cavity, and chemoattractant-induced Ca2+ mobilization of murine neutrophil granulocytes strongly depend on TRPC6 channels [74]. Surprisingly, there appears to be a striking degree of selectivity of TRPC6 channels for some of the chemoattractants. Neutrophil granulocytes from TRPC6−/− mice fail almost completely to chemotax towards CXCR2 ligands, while their response to fMLP is absolutely normal [74]. TRPC6 channels are required for CXCL1-stimulated receptor-operated Ca2+ influx which leads to Akt phosphorylation and actin polymerization during the initial phase of stimulation. A similar apparent specificity has also been found for ORAI1 channels and C5a receptors [136]. Chemotaxis of neutrophil granulocytes towards fMLP is TRPC1 dependent [75]. Importantly, the absence of TRPC1 and TRPC6 channels affects almost exclusively the steering mechanism during chemotaxis since the speed of migration is practically identical with that of cells from the wild-type littermates [74, 75].
The exact mechanisms that cause the selective perturbation of chemotaxis as opposed to a general defect in cell migration upon deletion of TRPC1 and TRPC6 channels have not yet been elucidated. However, this observation is consistent with the notion that both channels reinforce the biased formation of pseudopods into the direction of the chemotactic gradient [95] or the biased activity of an excitable network underlying the polarized local excitation and global inhibition (LEGI-BEN) model [54]. Along these lines, TRPC6 channels could be involved setting the sensitivity for gradient detection by locally amplifying intracellular signaling cascades at the cell front. Such a positive feedback loop requiring Ca2+ influx was shown in migrating macrophages. In these cells, inhibition of extracellular Ca2+ influx leads to a decay of chemoattractant signals [32].
TRPC6 channels have also been proposed to underlie the Ca2+ influx into neutrophil granulocytes that is induced by PAF and augmented by E-selectin [83]. This is relevant for the early steps of the recruitment cascade when neutrophil granulocytes are captured by selectins on endothelial cells. Unpublished observations from our laboratory showed that TRPC6-mediated Ca2+ influx strengthens the β2 integrin- and ICAM1-dependent adhesion of neutrophil granulocytes to endothelial cells. A crucial role of TRPC6 channels in endothelial cells for the recruitment of neutrophil granulocytes has been revealed by the use of the targeted knock-out of TRPC6 channels in endothelial cells [156]. In endothelial cells, TRPC6 channels interact with PTEN [61]. This finding may have important implications for neutrophil granulocytes in which PTEN is crucial for the integration of signals from different chemoattractants. If such interaction also occurred in neutrophil granulocytes, TRPC6 channels could indirectly play a role in the prioritization of different chemoattractant signals [46].
It has been known for many years that chemokinetic stimulation of neutrophil granulocytes triggers intracellular Ca2+ transients [57]. Interestingly, Ca2+ signaling is affected in opposite ways by the deletion of TRPC6 or TRPC1 channels. Expectedly, CXCL1-stimulated TRPC6−/− neutrophils exhibit attenuated Ca2+ transients [74]. In contrast, fMLP induces an elevated Ca2+ influx into TRPC1−/− neutrophil granulocytes [75]. A likely explanation for this apparent paradox is given by the observation that TRPC1 decreases the Ca2+ selectivity of heteromeric TRPC channels. Accordingly, the deletion of TRPC1 increases their Ca2+ permeability [142].
TRPM2 channels
The role of TRPM2 channels was studied by using three different knock-out mouse models. The first TRPM2−/− mice were generated by deleting the transmembrane segments 5 and 6 including the pore-forming TRPM2 domain (129S4/SvJae* C57BL/6J genetic background; [158]). In 2011, GlaxoSmithKline generated another TRPM2−/− mouse model by deletion of the third and fourth transmembrane domains of the channel protein (129P2/OlaHsd* C57BL/6J genetic background; [62]). A third TRPM2−/− mouse was generated in 2013, with the same domains of the channel deleted as in the first KO mouse and an additionally introduced stop codon. Mice were also of C57BL/6 background [85]. The deletion of CD38 can at least partially mimic the effects of a TRPM2 knockout (reviewed in [63]. CD38 is an ectoenzyme that produces a number of the physiological TRPM2 agonists. TRPM2 channels affect recruitment and function of neutrophil granulocytes in two different ways, either indirectly or directly.
Neutrophil granulocytes are indirectly affected by the deletion of TRPM2 channels in other immune cells (e.g., macrophages) because the cytokine production in these cells is regulated by TRPM2 channels. Depending on the disease model, TRPM2 deletion leads to increased cytokine production [23] or to decreased cytokine production [41, 81, 84, 158]. Accordingly, the clinical outcome may be deteriorated or improved upon TRPM2 channel deletion. In a dextran sulfate sodium-induced colitis inflammation model, the outcome of TRPM2−/− mice is better than that of wild-type littermates. There is a lower CXCL2 and IFN-γ production so that neutrophil infiltration into the inflamed colon is reduced and produces less damage [158]. Similar protective effects of TRPM2 knock-out are seen in studies on neuroinflammation (carrageenan-induced inflammation and sciatic nerve injury) and in a model of postoperative ileus. TRPM2−/− macrophages and microglia produce less CXCL2 resulting in decreased neutrophil infiltration [41, 81]. Likewise, the IL-2, IFN-γ, and IL-17 production is lower in TRPM2−/− T cells when probed in an experimental autoimmune encephalomyelitis (EAE) model [84]. Contrary to these results, endotoxin-induced lung inflammation in TRPM2−/− mice is associated with increased recruitment of neutrophil granulocytes, cytokine production, and diminished survival [23]. In the case of a Listeria monocytogenes infection, the reduced production of inflammatory cytokines in TRPM2−/− mice is detrimental because cells of the innate immune system are not stimulated adequately [62].
Furthermore, recruitment of neutrophil granulocytes to a site of inflammation is indirectly affected by the deletion of TRPM2 channels in endothelial cells. When endothelial TRPM2 channels are activated by oxidants generated by neutrophil granulocytes, this will lead to an opening of the endothelial cell junctions so that neutrophil granulocytes can transmigrate [87].
Several direct effects of TRPM2 channels on the function of neutrophil granulocytes have been reported. TRPM2 channels mediate a sensitization of neutrophil granulocytes towards the simultaneous stimulation with LTB4 and H2O2. The resulting increase of the [Ca2+]i was proposed to enhance their adhesion to endothelial cells, e.g., via activation of Pyk2. In the setting of a myocardial ischemia/reperfusion injury model, recruitment of neutrophil granulocytes is promoted by TRPM2 channels so that wild-type mice have a worse outcome than TRPM2−/− mice [49]. Other studies, however, question the contribution of neutrophil granulocytes to cardiac ischemia/reperfusion injury [50, 86]. These studies rather showed that TRPM2 channels expressed in cardiac myocytes are more important. They protect the heart from ischemia/reperfusion injury by preserving mitochondrial function in cardiomyocytes.
The role of TRPM2 channels in regulating migration and chemotaxis of neutrophil granulocytes is discussed quite controversially. Based on a pharmacological approach, it was proposed that TRPM2 channels are required for chemotaxis of neutrophil granulocytes towards fMLP [108]. This was confirmed with neutrophils from TRPM2−/− mice. Using a Boyden chamber assay, Yamamoto et al. found that chemotaxis of TRPM2−/− neutrophil granulocytes towards fMLP is impaired while chemotaxis towards CXCL2 is not affected. Moreover, less TRPM2−/− neutrophil granulocytes migrate into the abdominal cavity upon intraperitoneal fMLP injection [158].
In contrast, TRPM2−/− neutrophil granulocytes from the GlaxoSmithKline mouse strain [62] have a slightly improved migratory and chemotactic behavior when assessed on a fibronectin- or fibrinogen-coated glass surface [155]. Migration speed and chemotaxis indices in response to different end target and intermediary chemoattractants are increased by 15–30%, and more neutrophil granulocytes are recruited into the lungs of TRPM2−/− mice upon intratracheal injection of fMLF. Mechanistically, the migration phenotype was explained by the following sequence of events: ROS produced by the neutrophil granulocytes themselves leads to the oxidation of a cysteine residue of the TRPM2 channel. Upon oxidation, the TRPM2 channel protein binds to the formyl peptide receptor 1 (FPR1) and thereby triggers the internalization of both proteins so that FPR1 signaling at the level of the cell membrane is terminated. It was concluded that this mechanism does not rely on conductive properties of the channel and it was interpreted as a negative feedback control mechanism or “stop signal” for neutrophil granulocytes migrating towards a site of bacterial infection [155]. However, given the relatively small effect on migration, it is likely that other (indirect) effects such as increased vascular permeability amplify the contribution of TRPM2 channels to neutrophil recruitment.
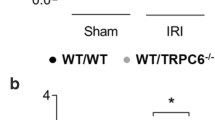
It has also been reported that migration of neutrophil granulocytes chemokinetically stimulated with H2O2 and LTB4 is not affected by the deletion of TRPM2 channels [49]. This coincides with our observations. We evaluated a potential contribution of TRPM2 channels to the migratory and chemotactic behavior of murine neutrophil granulocytes [158] that have been embedded in a three-dimensional collagen matrix [74]. As depicted in Fig. 1, the absence of TRPM2 channels has no impact on chemotaxis in gradients of fMLP, C5a, KC, and LTB4. The speed of migration is not different in the two genotypes either.
TRPM2 channels are not involved in neutrophil chemotaxis within a three-dimensional collagen matrix in gradients of chemoattractants. The experimental procedures for isolating murine neutrophils, preparing collagen gels, setting up, and analyzing chemotaxis experiments were described previously [74, 75]. Chemotaxis was monitored by means of time-lapse videomicroscopy in 10-s intervals for 30 min. a Trajectories of WT and TRPM2−/− neutrophils [158] in KC/CXCL1 and fMLP gradients that have been normalized to common starting points. The mean trajectories are superimposed as bold gray lines. n = 80 cells from N = 4 independent experiments for each genotype. b The net movement of neutrophils into the direction of the chemotactic gradient as a function of time. The direction of the chemotactic gradient is represented by the Y coordinate. The position along the Y coordinate is given as mean ± SEM. c Summary of the chemotaxis experiments. The chemotaxis indices are calculated as the ratios of the net distances traveled into the direction of the chemotactic gradient divided by the total distances covered during the course of the experiments. LTB4: N = 3, n = 60 cells; C5a: N = 3, n = 60 cells. Values are given as mean ± SEM. There are no significant differences between the two genotypes
It is evident that the published results on the role of TRPM2 channels in neutrophil granulocytes are quite discrepant. It has been speculated that this could be due to the use of different mouse models. However, in our view, the type of disease models and experimental procedures are more likely causes for the apparently contradictory results.
Several studies in other cell types showed that TRPM2 channels are expressed intracellularly. In dendritic cells, they are found in late endosomal and lysosomal compartments and mediate intracellular Ca2+ release. This appears not to be the case in neutrophil granulocytes. TRPM2−/− dendritic cells chemotax less efficiently in transwell assays towards CXCL12 and CCL19 gradients, and have altered Ca2+ fluxes [144]. In macrophages, intracellularly expressed TRPM2 channels promote phagosomal maturation and acidification and thereby contribute to increased bacterial clearance in different models of bacterial infection [24, 117, 160]. So far, a role of TRPM2 channels for phagosome maturation in neutrophil granulocytes has not been shown [37]. Given the differences between macrophage and neutrophil phagosomes [154], findings from the former cannot be automatically extrapolated to the latter. Accordingly, there are only few reports indicating a role of TRPM2 channels related to ROS production and bacterial killing in neutrophil granulocytes. Bacterial killing induced by LPC depends on a glycine receptor α2 (GlyRα2)-mediated increase of the intracellular Cl− concentration in neutrophil granulocytes which in turn causes an activation of TRPM2 channels. Bacterial killing is reduced upon TRPM2 channel silencing caused by lower Ca2+ influx resulting in decreased p38 phosphorylation [52]. Elastase release is also mediated by p38/MAPK signaling [109]. p38 phosphorylation is reduced in TRPM2−/− neutrophil granulocytes when they are stimulated with fMLP. Accordingly, TRPM2−/− neutrophil granulocytes release less elastase which in turn leads to reduced bacterial clearance [118].
TRPM7 channels
Using Boyden chamber assays, chemotaxis of neutrophil-like HL60 cells towards the pro-inflammatory cyclophilin A and their invasion into Matrigel® was shown to involve TRPM7 channels. However, the underlying TRPM7-regulated mechanisms were not elucidated in detail [153]. In this context, it is interesting to note that at least in bone marrow-derived macrophages, the lack of TRPM2 channels leads to an upregulation of TRPM7 channels [3]. To the best of our knowledge, it has not yet been determined whether this is the case in neutrophil granulocytes, too.
TRPV4 channels
Neutrophil granulocytes also express TRPV4 channels [16]. Their role in neutrophil function was investigated in an acid-induced lung injury model that mimics the aspiration of acidic gastric juice [159]. TRPV4 channels mediate sustained Ca2+ influx and ROS production when neutrophil granulocytes are activated with PAF. Comparing the degree of lung injury in chimeric mice with TRPV4 knock-out in stromal and/or myeloid cells pointed to a more prominent role for stromal TRPV4 channels. However, infiltration of lung tissue with TRPV4−/− neutrophil granulocytes is lower when compared to that of wild-type cells. This is consistent with in vitro results showing reduced adhesion to and chemotaxis of TRPV4−/− neutrophil granulocytes to PAF across a layer of endothelial cells. The common link of TRPV4-regulated behavioral traits of neutrophil granulocytes was seen in the Ca2+-dependent activation of Rac.
Neutrophil granulocytes in pancreatic ductal adenocarcinoma—a potential role for TRP channels?
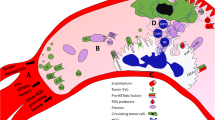
The preceding sections have shown that TRP channels regulate a number of different neutrophil functions. We will now discuss whether the microenvironment, to which neutrophil granulocytes are exposed to, can impact on their behavior in a TRP channel-dependent manner. We will do this exemplarily for pancreatic ductal adenocarcinoma (PDAC) which is the fourth leading cause of cancer-associated deaths in Europe with a 5-year survival rate of only 5% [9]. PDAC is characterized by a microenvironment with special physico-chemical properties. It contains abundant amounts of extracellular matrix (ECM) proteins within the tumor (desmoplasia), primarily secreted by activated pancreatic stellate cells. The hypovascular and desmoplastic tumor microenvironment accounts for 90% and tumor cells only for 10% of the tumor mass [94]. In addition to ECM, it contains different non-malignant cell types like pancreatic stellate cells, cancer-associated fibroblasts (CAFs), endothelial cells, and immune cells including neutrophil granulocytes [94, 96]. The high metabolic demand of tumor and immune cells together with limited supply of metabolic substrates due to the compression of the few blood vessels by high intratumoral pressure [116, 143] causes severe hypoxia in PDAC [29, 65]. There is intense mutual activation of cancer and stromal cells through paracrine signaling via growth factors, hypoxia, ROS, and altered pH.
A neutrophil infiltrate is an important constituent of the microenvironment of many tumors including PDAC [82, 115, 133]. A high density of intratumoral neutrophil granulocytes is associated with poor patient survival [105, 133]. Neutrophil granulocytes are recruited CXCR2 dependently to the PDAC stroma by cytokines such as CXCL5 that are secreted by the tumor cells [105]. In PDAC, they elicit immunosuppressive activity leading to immune evasion of the tumor cells [105, 113] and drive metastasis [139]. Other mechanisms by which neutrophil granulocytes can contribute to an unfavorable patient prognosis include promoting metastasis by trapping circulating tumor cells in neutrophil extracellular nets [14] or by reprogramming pancreatic stellate cells [82].
The observation that CXCR2 signaling is crucial for recruiting neutrophil granulocytes to the PDAC stroma is important for the context of this review. We have already mentioned that chemotaxis of neutrophil granulocytes towards CXCR2 ligands relies on TRPC6 channels [74]. In addition, endothelial [156] and neutrophilic (unpublished observations from our laboratory) TRPC6 channels also regulate transendothelial migration through and CXCL1-induced adhesion of neutrophil granulocytes to a layer of endothelial cells, respectively. TRPC6 channels are also expressed in pancreatic stellate cells. They are stimulated under hypoxic conditions, thereby promoting pancreatic stellate cell activation as evidenced by increased migration and cytokine secretion [98]. Whether neutrophilic TRPC6 channels are also activated in the hypoxic PDAC stroma is currently unknown. Thus, inhibiting TRPC6 channels which are potent modulators of cytokine-dependent activation of neutrophil granulocytes and pancreatic stellate cells would target several mechanisms related to functions of these cells that promote disease progression. This overlaps with the impact of CXCR2 inhibition which is also expressed in neutrophil granulocytes and stromal cells [55]. This could also be relevant for other tumors such as breast cancer [132] or non-small cell lung cancer [146] that exhibit neutrophil infiltration as well and whose progression and metastasis are also driven by CXCR2 signaling.
There are two further properties of the PDAC microenvironment that are expected to lead to the activation of neutrophilic TRP channels within the tumor stroma. PDAC is characterized by increased ROS levels which are suggested to elicit anti-apoptotic and pro-survival effects and increase the chemo-resistance of the cancer cells [26, 151]. Since TRPM2 channels function as ROS sensors [34], the activation of neutrophilic TRPM2 channels [155] in the tumor stroma is to be expected. The PDAC stroma is also characterized by a stiff ECM [68] and a massively elevated tissue pressure [116]. We have shown that the activation of pancreatic stellate cells by an elevated ambient pressure occurs TRPC1 dependently [35] and that TRPC1 channels contribute to mechanosignaling during cell migration [33]. TRPC1 channels are also expressed in neutrophil granulocytes where they have a role in chemotaxis [75]. In pancreatic stellate cells, the expression of two other mechanosensitive TRP channels is regulated by the ambient pressure: TRPM7 and TRPV4 [35]. These channels are also expressed in neutrophil granulocytes. Thus, one could speculate that neutrophilic TRPC1, TRPM7, and TRPV4 channels are mechanically activated within the PDAC stroma. To the best of our knowledge, however, mechanically induced Ca2+ signaling let alone the involved Ca2+ permeable ion channels have not yet been investigated in much detail in neutrophil granulocytes. However, a study showing that P-selectin-induced Ca2+ signaling is force dependent in HL-60 cells may be taken as proof-of-principle for mechanically induced Ca2+ signaling in neutrophils [53]. It is also known that cytokine (IL8, CXCL16) or hormone (atrial natriuretic factor, ANF) stimulation of neutrophils impacts on their own mechanical properties [91, 140]. Whether this can be causally linked to the activation of mechanosensitive TRP channels remains to be determined. The inverse relation has been found in cardiomyocytes. TRPC1 knockdown prevents the hypertrophic response of cardiomyocytes and inhibits the mRNA upregulation of ANF [106].
So far, our discussion suggests that TRP channels can have a substantial share in regulating the function of neutrophil granulocytes within the PDAC microenvironment. However, the relative importance of TRP channel activity for regulating neutrophil function is complicated by the special pH landscape of the diseased pancreas [110]. Postprandially, the cells of the pancreatic ducts secrete copious amounts of HCO3− with concentrations reaching up to 150 mmol/l [101]. The equimolar amount of protons is extruded basolaterally so that the pancreatic interstitium is acidified intermittently. In PDAC, the physiological, intermittent acidification of the pancreatic interstitium is superimposed by the typical acidosis of the tumor stroma. We proposed that the acid milieu acts as a double-edged sword during PDAC development: it prevents premalignant lesions to undergo rapid tumorigenesis, whereas in later tumor stages, the acid pH promotes PDAC progression [110]. The question is how the pH landscape in PDAC will affect neutrophil granulocytes and how this is regulated by TRP channels. Several of the neutrophilic TRP channels (TRPM2, TRPM7, TRPV4) are pH sensitive [58, 134, 138] so that, e.g., TRPM2 and TRPV4 may not be active in the acid tumor microenvironment. An acid extracellular environment has seemingly contradictory effects on the behavior of neutrophil granulocytes. On the one hand, suspended neutrophil granulocytes are activated and the response to fMLP is augmented by an acidification (pH 6.5) of the extracellular solution [147]. On the other hand, migration and chemotaxis of fMLP-stimulated neutrophils on a coated glass surface is inhibited by an extracellular and more so by an intracellular acidification [7, 42, 71]. The mechanisms by which extra- and intracellular protons are detected are not yet fully clarified [107]. In addition to proton-sensing G protein-coupled receptors [150], the pH sensitivity of some important neutrophilic TRP channels could be way by which the environmental acid-base status is sensed and translated into neutrophil behavior.
Summary and outlook
Figure 2 provides a synopsis of the current knowledge and open questions about TRP channel function in neutrophil granulocytes. Our review shows that the role of TRP channels in function of neutrophil granulocytes is still far from being fully understood. The use of knock-out mouse models has greatly expanded our knowledge and led to the identification of putative therapeutic strategies involving the targeting of TRP channels. Thus, the available evidence indicates that blocking TRPC6 channels could be an attractive complementation of CXCR2 targeting in PDAC and other cancer entities. Such findings add to the growing appreciation of the therapeutic potential of ion channels in cancer (see [25] for a series of reviews on this topic). We would like to emphasize that ion channels such as TRP channels in cells of the tumor stroma including neutrophil granulocytes need to be considered, too [97]. Being membrane proteins, they are in a strategic position to sense and transmit signals between cancer cells and components of the tumor microenvironment [1]. Importantly, these considerations also apply to an “inflammatory microenvironment” which shares many similarities with the tumor microenvironment. Acidity, hypoxia, cytokines, and ROS are also central elements of the inflammatory microenvironment.
Function of TRP channels in neutrophil granulocytes and remaining open questions. The role of several TRP channels in neutrophil recruitment and chemotaxis is well established. However, it remains to be determined whether the relative importance of these channels is modified by environmental stressors to which neutrophil granulocytes are exposed
On the other hand, we have noted that the electrophysiological characterization of neutrophilic TRP channels and other ion channels still remains rather rudimentary. Moreover, it has become apparent that the impact of the microenvironment is usually not well recapitulated in many in vitro studies. This is particularly important for assessing TRP channel function because they respond to a great variety of physico-chemical properties of the microenvironment. Finally, the function of TRP channels expressed in intracellular membranes has to be studied in more detail. Most of the publications on ion transport across phagosomal membranes have been performed in macrophages. It is for example not yet known whether intracellular TRPM2 and TRPC6 channels elicit similar functions in macrophages and neutrophil granulocytes [24, 120]. Thus, there are still many exciting discoveries to be made in the field of neutrophilic ion channels.
References
Arcangeli A (2011) Ion channels and transporters in cancer. 3. Ion channels in the tumor cell-microenvironment cross talk. Am J Physiol Cell Physiol 301:C762–C771
Bankers-Fulbright JL, Kephart GM, Loegering DA, Bradford AL, Okada S, Kita H, Gleich GJ (1998) Sulfonylureas inhibit cytokine-induced eosinophil survival and activation. J Immunol 160:5546–5553
Beceiro S, Radin JN, Chatuvedi R, Piazuelo MB, Horvarth DJ, Cortado H, Gu Y, Dixon B, Gu C, Lange I, Koomoa DL, Wilson KT, Algood HM, Partida-Sanchez S (2017) TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol 10:493–507
Behe P, Foote JR, Levine AP, Platt CD, Chou J, Benavides F, Geha RS, Segal AW (2017) The LRRC8A mediated “swell activated” chloride conductance is dispensable for vacuolar homeostasis in neutrophils. Front Pharmacol 8:262
Breckenridge LJ, Almers W (1987) Currents through the fusion pore that forms during exocytosis of a secretory vesicle. Nature 328:814–817
Brundage RA, Fogarty KE, Tuft RA, Fay FS (1991) Calcium gradients underlying polarization and chemotaxis of eosinophils. Science 254:703–706
Cao S, Liu P, Zhu H, Gong H, Yao J, Sun Y, Geng G, Wang T, Feng S, Han M, Zhou J, Xu Y (2015) Extracellular acidification acts as a key modulator of neutrophil apoptosis and functions. PLoS One 10:e0137221
Capasso M, Bhamrah MK, Henley T, Boyd RS, Langlais C, Cain K, Dinsdale D, Pulford K, Khan M, Musset B, Cherny VV, Morgan D, Gascoyne RD, Vigorito E, DeCoursey TE, MacLennan IC, Dyer MJ (2010) HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat Immunol 11:265–272
Carrato A, Falcone A, Ducreux M, Valle JW, Parnaby A, Djazouli K, Alnwick-Allu K, Hutchings A, Palaska C, Parthenaki I (2015) A systematic review of the burden of pancreatic cancer in Europe: real-world impact on survival, quality of life and costs. J Gastrointest Cancer 46:201–211
Chang SJ, Chen YC, Yang CH, Huang SC, Huang HK, Li CC, Harn HI, Chiu WT (2017) Revealing the three dimensional architecture of focal adhesion components to explain Ca2+-mediated turnover of focal adhesions. Biochim Biophys Acta 1861:624–635
Chen YT, Chen YF, Chiu WT, Wang YK, Chang HC, Shen MR (2013) The ER Ca2+ sensor STIM1 regulates actomyosin contractility of migratory cells. J Cell Sci 126:1260–1267
Clemens RA, Lowell CA (2015) Store-operated calcium signaling in neutrophils. J Leukoc Biol 98:497–502
Colom B, Bodkin JV, Beyrau M, Woodfin A, Ody C, Rourke C, Chavakis T, Brohi K, Imhof BA, Nourshargh S (2015) Leukotriene B4-neutrophil elastase axis drives neutrophil reverse transendothelial cell migration in vivo. Immunity 42:1075–1086
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L (2013) Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 123:3446–3458
Cross AR, Erickson RW, Ellis BA, Curnutte JT (1999) Spontaneous activation of NADPH oxidase in a cell-free system: unexpected multiple effects of magnesium ion concentrations. Biochem J 338:229–233
Damann N, Owsianik G, Li S, Poll C, Nilius B (2009) The calcium-conducting ion channel transient receptor potential canonical 6 is involved in macrophage inflammatory protein-2-induced migration of mouse neutrophils. Acta Physiol (Oxford) 195:3–11
DeCoursey TE, Cherny VV (1993) Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys J 65:1590–1598
DeCoursey TE, Cherny VV (1994) Na+-H+ antiport detected through hydrogen ion currents in rat alveolar epithelial cells and human neutrophils. J Gen Physiol 103:755–785
DeCoursey TE, Cherny VV, Zhou W, Thomas LL (2000) Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc Natl Acad Sci U S A 97:6885–6889
DeCoursey TE, Morgan D, Cherny VV (2003) The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 422:531–534
DeCoursey TE (2016) The intimate and controversial relationship between voltage-gated proton channels and the phagocyte NADPH oxidase. Immunol Rev 273:194–218
Demaurex N, Rawlings SR, Krause KH, Jaconi ME, Lew PD, Schlegel W (1994) Combination of microfluorimetric monitoring of cytosolic calcium and pH with patch clamp electrophysiological recordings in neutrophil granulocytes. Methods Enzymol 238:308–320
Di A, Gao XP, Qian F, Kawamura T, Han J, Hecquet C, Ye RD, Vogel SM, Malik AB (2011) The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol 13:29–34
Di A, Kiya T, Gong H, Gao X, Malik AB (2017) Role of the phagosomal redox-sensitive TRP channel TRPM2 in regulating bactericidal activity of macrophages. J Cell Sci 130:735–744
Djamgoz MB, Coombes RC, Schwab A (2014) Ion transport and cancer: from initiation to metastasis. Philos Trans R Soc Lond Ser B Biol Sci 369:20130092
Donadelli M, Dando I, Zaniboni T, Costanzo C, Dalla Pozza E, Scupoli MT, Scarpa A, Zappavigna S, Marra M, Abbruzzese A, Bifulco M, Caraglia M, Palmieri M (2011) Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis 2:e152
Dorward DA, Lucas CD, Chapman GB, Haslett C, Dhaliwal K, Rossi AG (2015) The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am J Pathol 185:1172–1184
Du J, Xie J, Yue L (2009) Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J Gen Physiol 134:471–488
Duffy JP, Eibl G, Reber HA, Hines OJ (2003) Influence of hypoxia and neoangiogenesis on the growth of pancreatic cancer. Mol Cancer 2:12
El Chemaly A, Okochi Y, Sasaki M, Arnaudeau S, Okamura Y, Demaurex N (2010) VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. J Exp Med 207:129–139
Essin K, Salanova B, Kettritz R, Sausbier M, Luft FC, Kraus D, Bohn E, Autenrieth IB, Peschel A, Ruth P, Gollasch M (2007) Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity. Am J Physiol Cell Physiol 293:C45–C54
Evans JH, Falke JJ (2007) Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc Natl Acad Sci U S A 104:16176–16181
Fabian A, Bertrand J, Lindemann O, Pap T, Schwab A (2012) Transient receptor potential canonical channel 1 impacts on mechanosignaling during cell migration. Pflügers Arch 464:623–630
Faouzi M, Penner R (2014) TRPM2. Handb Exp Pharmacol 222:403–426
Fels B, Nielsen N, Schwab A (2016) Role of TRPC1 channels in pressure-mediated activation of murine pancreatic stellate cells. Eur Biophys J 45:657–670
Femling JK, Cherny VV, Morgan D, Rada B, Davis AP, Czirjak G, Enyedi P, England SK, Moreland JG, Ligeti E, Nauseef WM, DeCoursey TE (2006) The antibacterial activity of human neutrophils and eosinophils requires proton channels but not BK channels. J Gen Physiol 127:659–672
Foote JR, Behe P, Frampton M, Levine AP, Segal AW (2017) An exploration of charge compensating ion channels across the phagocytic vacuole of neutrophils. Front Pharmacol 8:94
Futosi K, Fodor S, Mocsai A (2013) Reprint of neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 17:1185–1197
Gao YD, Hanley PJ, Rinne S, Zuzarte M, Daut J (2010) Calcium-activated K+ channel (KCa3.1) activity during Ca2+ store depletion and store-operated Ca2+ entry in human macrophages. Cell Calcium 48:19–27
Gordienko DV, Tare M, Parveen S, Fenech CJ, Robinson C, Bolton TB (1996) Voltage-activated proton current in eosinophils from human blood. J Physiol 496(Pt 2):299–316
Haraguchi K, Kawamoto A, Isami K, Maeda S, Kusano A, Asakura K, Shirakawa H, Mori Y, Nakagawa T, Kaneko S (2012) TRPM2 contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J Neurosci 32:3931–3941
Hayashi H, Aharonovitz O, Alexander RT, Touret N, Furuya W, Orlowski J, Grinstein S (2008) Na+/H+ exchange and pH regulation in the control of neutrophil chemokinesis and chemotaxis. Am J Physiol Cell Physiol 294:C526–C534
Heiner I, Eisfeld J, Halaszovich CR, Wehage E, Jungling E, Zitt C, Lückhoff A (2003) Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J 371:1045–1053
Heiner I, Eisfeld J, Lückhoff A (2003) Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium 33:533–540
Heiner I, Eisfeld J, Warnstedt M, Radukina N, Jungling E, Luckhoff A (2006) Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem J 398:225–232
Heit B, Robbins SM, Downey CM, Guan Z, Colarusso P, Miller BJ, Jirik FR, Kubes P (2008) PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat Immunol 9:743–752
Herter J, Zarbock A (2013) Integrin regulation during leukocyte recruitment. J Immunol 190:4451–4457
Hind LE, Vincent WJ, Huttenlocher A (2016) Leading from the back: the role of the uropod in neutrophil polarization and migration. Dev Cell 38:161–169
Hiroi T, Wajima T, Negoro T, Ishii M, Nakano Y, Kiuchi Y, Mori Y, Shimizu S (2013) Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovasc Res 97:271–281
Hoffman NE, Miller BA, Wang J, Elrod JW, Rajan S, Gao E, Song J, Zhang XQ, Hirschler-Laszkiewicz I, Shanmughapriya S, Koch WJ, Feldman AM, Madesh M, Cheung JY (2015) Ca2+ entry via TRPM2 is essential for cardiac myocyte bioenergetics maintenance. Am J Physiol Heart Circ Physiol 308:H637–H650
Hoffmann EK, Lambert IH, Pedersen SF (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89:193–277
Hong CW, Kim TK, Ham HY, Nam JS, Kim YH, Zheng H, Pang B, Min TK, Jung JS, Lee SN, Cho HJ, Kim EJ, Hong IH, Kang TC, Lee J, Oh SB, Jung SJ, Kim SJ, Song DK (2010) Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine.GlyR alpha 2/TRPM2/p38 MAPK signaling. J Immunol 184:4401–4413
Huang B, Ling Y, Lin J, Fang Y, Wu J (2016) Mechanical regulation of calcium signaling of HL-60 on P-selectin under flow. Biomed Eng Online 15:153
Iglesias PA, Devreotes PN (2012) Biased excitable networks: how cells direct motion in response to gradients. Curr Opin Cell Biol 24:245–253
Ijichi H, Chytil A, Gorska AE, Aakre ME, Bierie B, Tada M, Mohri D, Miyabayashi K, Asaoka Y, Maeda S, Ikenoue T, Tateishi K, Wright CV, Koike K, Omata M, Moses HL (2011) Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest 121:4106–4117
Ince C, Thio B, van Duijn B, van Dissel JT, Ypey DL, Leijh PC (1987) Intracellular K+, Na+ and Cl− concentrations and membrane potential in human monocytes. Biochim Biophys Acta 905:195–204
Jaconi ME, Rivest RW, Schlegel W, Wollheim CB, Pittet D, Lew PD (1988) Spontaneous and chemoattractant-induced oscillations of cytosolic free calcium in single adherent human neutrophils. J Biol Chem 263:10557–10560
Jiang J, Li M, Yue L (2005) Potentiation of TRPM7 inward currents by protons. J Gen Physiol 126:137–150
Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E (2016) Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1beta secretion in response to ATP. Nat Commun 7:10555
Kawa K (1989) Electrophysiological properties of three types of granulocytes in circulating blood of the newt. J Physiol 415:211–231
Kini V, Chavez A, Mehta D (2010) A new role for PTEN in regulating transient receptor potential canonical channel 6-mediated Ca2+ entry, endothelial permeability, and angiogenesis. J Biol Chem 285:33082–33091
Knowles H, Heizer JW, Li Y, Chapman K, Ogden CA, Andreasen K, Shapland E, Kucera G, Mogan J, Humann J, Lenz LL, Morrison AD, Perraud AL (2011) Transient receptor potential melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc Natl Acad Sci U S A 108:11578–11583
Knowles H, Li Y, Perraud AL (2013) The TRPM2 ion channel, an oxidative stress and metabolic sensor regulating innate immunity and inflammation. Immunol Res 55:241–248
Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175
Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M (2000) Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys 48:919–922
Koshkin V, Lotan O, Pick E (1997) Electron transfer in the superoxide-generating NADPH oxidase complex reconstituted in vitro. Biochim Biophys Acta 1319:139–146
Krause KH, Welsh MJ (1990) Voltage-dependent and Ca2+-activated ion channels in human neutrophils. J Clin Invest 85:491–498
Lachowski D, Cortes E, Pink D, Chronopoulos A, Karim SA, PM J, Del Rio Hernandez AE (2017) Substrate rigidity controls activation and durotaxis in pancreatic stellate cells. Sci Rep 7:2506
Lacy P, Abdel-Latif D, Steward M, Musat-Marcu S, Man SF, Moqbel R (2003) Divergence of mechanisms regulating respiratory burst in blood and sputum eosinophils and neutrophils from atopic subjects. J Immunol 170:2670–2679
Lange I, Penner R, Fleig A, Beck A (2008) Synergistic regulation of endogenous TRPM2 channels by adenine dinucleotides in primary human neutrophils. Cell Calcium 44:604–615
Lardner A (2001) The effects of extracellular pH on immune function. J Leukoc Biol 69:522–530
Lecut C, Frederix K, Johnson DM, Deroanne C, Thiry M, Faccinetto C, Maree R, Evans RJ, Volders PG, Bours V, Oury C (2009) P2X1 ion channels promote neutrophil chemotaxis through Rho kinase activation. J Immunol 183:2801–2809
Lee WL, Harrison RE, Grinstein S (2003) Phagocytosis by neutrophils. Microbes Infect 5:1299–1306
Lindemann O, Umlauf D, Frank S, Schimmelpfennig S, Bertrand J, Pap T, Hanley PJ, Fabian A, Dietrich A, Schwab A (2013) TRPC6 regulates CXCR2-mediated chemotaxis of murine neutrophils. J Immunol 190:5496–5505
Lindemann O, Strodthoff C, Horstmann M, Nielsen N, Jung F, Schimmelpfennig S, Heitzmann M, Schwab A (2015) TRPC1 regulates fMLP-stimulated migration and chemotaxis of neutrophil granulocytes. Biochim Biophys Acta 1853:2122–2130
Lollike K, Borregaard N, Lindau M (1995) The exocytotic fusion pore of small granules has a conductance similar to an ion channel. J Cell Biol 129:99–104
Manz MG, Boettcher S (2014) Emergency granulopoiesis. Nat Rev Immunol 14:302–314
Marki A, Esko JD, Pries AR, Ley K (2015) Role of the endothelial surface layer in neutrophil recruitment. J Leukoc Biol 98:503–515
Martel-Gallegos G, Rosales-Saavedra MT, Reyes JP, Casas-Pruneda G, Toro-Castillo C, Perez-Cornejo P, Arreola J (2010) Human neutrophils do not express purinergic P2X7 receptors. Purinergic Signal 6:297–306
Masia R, Krause DS, Yellen G (2015) The inward rectifier potassium channel Kir2.1 is expressed in mouse neutrophils from bone marrow and liver. Am J Physiol Cell Physiol 308:C264–C276
Matsumoto K, Kawanaka H, Hori M, Kusamori K, Utsumi D, Tsukahara T, Amagase K, Horie S, Yamamoto A, Ozaki H, Mori Y, Kato S (2018) Role of transient receptor potential melastatin 2 in surgical inflammation and dysmotility in a mouse model of post-operative ileus. Am J Physiol Gastrointest Liver Physiol
Mayer P, Dinkic C, Jesenofsky R, Klauss M, Schirmacher P, Dapunt U, Hackert T, Uhle F, Hansch GM, Gaida MM (2018) Changes in the microarchitecture of the pancreatic cancer stroma are linked to neutrophil-dependent reprogramming of stellate cells and reflected by diffusion-weighted magnetic resonance imaging. Theranostics 8:13–30
McMeekin SR, Dransfield I, Rossi AG, Haslett C, Walker TR (2006) E-selectin permits communication between PAF receptors and TRPC channels in human neutrophils. Blood 107:4938–4945
Melzer N, Hicking G, Gobel K, Wiendl H (2012) TRPM2 cation channels modulate T cell effector functions and contribute to autoimmune CNS inflammation. PLoS One 7:e47617
Miller BA, Wang J, Hirschler-Laszkiewicz I, Gao E, Song J, Zhang XQ, Koch WJ, Madesh M, Mallilankaraman K, Gu T, Chen SJ, Keefer K, Conrad K, Feldman AM, Cheung JY (2013) The second member of transient receptor potential-melastatin channel family protects hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 304:H1010–H1022
Miller BA, Hoffman NE, Merali S, Zhang XQ, Wang J, Rajan S, Shanmughapriya S, Gao E, Barrero CA, Mallilankaraman K, Song J, Gu T, Hirschler-Laszkiewicz I, Koch WJ, Feldman AM, Madesh M, Cheung JY (2014) TRPM2 channels protect against cardiac ischemia-reperfusion injury: role of mitochondria. J Biol Chem 289:7615–7629
Mittal M, Nepal S, Tsukasaki Y, Hecquet CM, Soni D, Rehman J, Tiruppathi C, Malik AB (2017) Neutrophil activation of endothelial cell-expressed TRPM2 mediates transendothelial neutrophil migration and vascular injury. Circ Res 121:1081–1091
Mogilner A, Oster G (2003) Polymer motors: pushing out the front and pulling up the back. Curr Biol 13:R721–R733
Morgan D, Capasso M, Musset B, Cherny VV, Rios E, Dyer MJ, DeCoursey TE (2009) Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc Natl Acad Sci U S A 106:18022–18027
Morgan D, Decoursey TE (2014) Analysis of electrophysiological properties and responses of neutrophils. Methods Mol Biol 1124:121–158
Morikis VA, Radecke C, Jiang Y, Heinrich V, Curry FR, Simon SI (2015) Atrial natriuretic peptide down-regulates neutrophil recruitment on inflamed endothelium by reducing cell deformability and resistance to detachment force. Biorheology 52:447–463
Murphy R, DeCoursey TE (2006) Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta 1757:996–1011
Musset B, Morgan D, Cherny VV, MacGlashan DW Jr, Thomas LL, Rios E, DeCoursey TE (2008) A pH-stabilizing role of voltage-gated proton channels in IgE-mediated activation of human basophils. Proc Natl Acad Sci U S A 105:11020–11025
Neesse A, Krug S, Gress TM, Tuveson DA, Michl P (2013) Emerging concepts in pancreatic cancer medicine: targeting the tumor stroma. Onco Targets Ther 7:33–43
Neilson MP, Veltman DM, van Haastert PJ, Webb SD, Mackenzie JA, Insall RH (2011) Chemotaxis: a feedback-based computational model robustly predicts multiple aspects of real cell behaviour. PLoS Biol 9:e1000618
Nielsen MF, Mortensen MB, Detlefsen S (2016) Key players in pancreatic cancer-stroma interaction: cancer-associated fibroblasts, endothelial and inflammatory cells. World J Gastroenterol 22:2678–2700
Nielsen N, Lindemann O, Schwab A (2014) TRP channels and STIM/ORAI proteins: sensors and effectors of cancer and stroma cell migration. Br J Pharmacol 171:5524–5540
Nielsen N, Kondratska K, Ruck T, Hild B, Kovalenko I, Schimmelpfennig S, Welzig J, Sargin S, Lindemann O, Christian S, Meuth SG, Prevarskaya N, Schwab A (2017) TRPC6 channels modulate the response of pancreatic stellate cells to hypoxia. Pflugers Arch 469:1567–1577
Nilius B, Szallasi A (2014) Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev 66:676–814
Norton LJ, Zhang Q, Saqib KM, Schrewe H, Macura K, Anderson KE, Lindsley CW, Brown HA, Rudge SA, Wakelam MJ (2011) PLD1 rather than PLD2 regulates phorbol-ester-, adhesion-dependent and Fcγ-receptor-stimulated ROS production in neutrophils. J Cell Sci 124:1973–1983
Novak I, Haanes KA, Wang J (2013) Acid-base transport in pancreas—new challenges. Front Physiol 4:380
Nusse O, Lindau M (1988) The dynamics of exocytosis in human neutrophils. J Cell Biol 107:2117–2123
Nusse O, Lindau M (1990) GTP gamma S-induced calcium transients and exocytosis in human neutrophils. Biosci Rep 10:93–103
Nusse O, Lindau M (1993) The calcium signal in human neutrophils and its relation to exocytosis investigated by patch-clamp capacitance and Fura-2 measurements. Cell Calcium 14:255–269
Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, Jacobs RC, Ye J, Patel AA, Gillanders WE, Fields RC, DeNardo DG, Hawkins WG, Goedegebuure P, Linehan DC (2017) Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. https://doi.org/10.1136/gutjnl-2017-313738
Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H (2007) Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol 42:498–507
Okajima F (2013) Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell Signal 25:2263–2271
Partida-Sanchez S, Gasser A, Fliegert R, Siebrands CC, Dammermann W, Shi G, Mousseau BJ, Sumoza-Toledo A, Bhagat H, Walseth TF, Guse AH, Lund FE (2007) Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by adp-ribose, the major product generated by the CD38 enzyme reaction. J Immunol 179:7827–7839
Partrick DA, Moore EE, Offner PJ, Meldrum DR, Tamura DY, Johnson JL, Silliman CC (2000) Maximal human neutrophil priming for superoxide production and elastase release requires p38 mitogen-activated protein kinase activation. Arch Surg 135:219–225
Pedersen SF, Novak I, Alves F, Schwab A, Pardo LA (2017) Alternating pH landscapes shape epithelial cancer initiation and progression: focus on pancreatic cancer. BioEssays 39
Perez-Cornejo P, Arreola J, Law FY, Schultz JB, Knauf PA (2004) Volume-sensitive chloride channels do not mediate activation-induced chloride efflux in human neutrophils. J Immunol 172:6988–6993
Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P (2006) Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med 203:2569–2575
Pickup MW, Owens P, Gorska AE, Chytil A, Ye F, Shi C, Weaver VM, Kalluri R, Moses HL, Novitskiy SV (2017) Development of aggressive pancreatic ductal adenocarcinomas depends on granulocyte colony stimulating factor secretion in carcinoma cells. Cancer Immunol Res 5:718–729
Powell D, Tauzin S, Hind LE, Deng Q, Beebe DJ, Huttenlocher A (2017) Chemokine signaling and the regulation of bidirectional leukocyte migration in interstitial tissues. Cell Rep 19:1572–1585
Powell DR, Huttenlocher A (2016) Neutrophils in the tumor microenvironment. Trends Immunol 37:41–52
Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21:418–429
Qian X, Numata T, Zhang K, Li C, Hou J, Mori Y, Fang X (2014) Transient receptor potential melastatin 2 protects mice against polymicrobial sepsis by enhancing bacterial clearance. Anesthesiology 121:336–351
Qian X, Zhao H, Chen X, Li J (2018) Disruption of transient receptor potential melastatin 2 decreases elastase release and bacterial clearance in neutrophils. Innate Immun 24:122–130
Rada BK, Geiszt M, Kaldi K, Timar C, Ligeti E (2004) Dual role of phagocytic NADPH oxidase in bacterial killing. Blood 104:2947–2953
Riazanski V, Gabdoulkhakova AG, Boynton LS, Eguchi RR, Deriy LV, Hogarth DK, Loaec N, Oumata N, Galons H, Brown ME, Shevchenko P, Gallan AJ, Yoo SG, Naren AP, Villereal ML, Beacham DW, Bindokas VP, Birnbaumer L, Meijer L, Nelson DJ (2015) TRPC6 channel translocation into phagosomal membrane augments phagosomal function. Proc Natl Acad Sci U S A 112:E6486–E6495
Saito M, Sato R, Hisatome I, Narahashi T (1996) RANTES and platelet-activating factor open Ca2+-activated K+ channels in eosinophils. FASEB J 10:792–798
Saito M, Sato R, Munoz NM, Herrnreiter A, Oyaizu M, Kasugai H, Narahashi T, Leff AR (1997) Association of granular exocytosis with Ca2+-activated K+ channels in human eosinophils. Am J Phys 273:L16–L21
Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K (2001) Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293:1327–1330
Schilling T, Gratopp A, DeCoursey TE, Eder C (2002) Voltage-activated proton currents in human lymphocytes. J Physiol 545:93–105
Schrenzel J, Demaurex N, Lew DP, Krause KH (1995) Characterization of Ca2+ influx in human neutrophils using the patch clamp technique. Schweiz Med Wochenschr 125:1174–1178
Schrenzel J, Lew DP, Krause KH (1996) Proton currents in human eosinophils. Am J Phys 271:C1861–C1871
Schrenzel J, Serrander L, Banfi B, Nusse O, Fouyouzi R, Lew DP, Demaurex N, Krause KH (1998) Electron currents generated by the human phagocyte NADPH oxidase. Nature 392:734–737
Schwab A, Wulf A, Schulz C, Kessler W, Nechyporuk-Zloy V, Romer M, Reinhardt J, Weinhold D, Dieterich P, Stock C, Hebert SC (2006) Subcellular distribution of calcium-sensitive potassium channels (IK1) in migrating cells. J Cell Physiol 206:86–94
Schwab A, Fabian A, Hanley PJ, Stock C (2012) Role of ion channels and transporters in cell migration. Physiol Rev 92:1865–1913
Schwingshackl A, Moqbel R, Duszyk M (2000) Involvement of ion channels in human eosinophil respiratory burst. J Allergy Clin Immunol 106:272–279
Schwingshackl A, Moqbel R, Duszyk M (2002) Nitric oxide activates ATP-dependent K+ channels in human eosinophils. J Leukoc Biol 71:807–812
Sharma B, Nannuru KC, Varney ML, Singh RK (2015) Host Cxcr2-dependent regulation of mammary tumor growth and metastasis. Clin Exp Metastasis 32:65–72
Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J (2014) Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One 9:e98259
Shikano M, Ueda T, Kamiya T, Ishida Y, Yamada T, Mizushima T, Shimura T, Mizoshita T, Tanida S, Kataoka H, Shimada S, Ugawa S, Joh T (2011) Acid inhibits TRPV4-mediated Ca2+ influx in mouse esophageal epithelial cells. Neurogastroenterol Motil 23(1020–8):e497
Shimizu S, Takahashi N, Mori Y (2014) TRPs as chemosensors (ROS, RNS, RCS, gasotransmitters). Handb Exp Pharmacol 223:767–794
Sogkas G, Vogtle T, Rau E, Gewecke B, Stegner D, Schmidt RE, Nieswandt B, Gessner JE (2015) Orai1 controls C5a-induced neutrophil recruitment in inflammation. Eur J Immunol 45:2143–2153
Song S, Yamamura A, Yamamura H, Ayon RJ, Smith KA, Tang H, Makino A, Yuan JX (2014) Flow shear stress enhances intracellular Ca2+ signaling in pulmonary artery smooth muscle cells from patients with pulmonary arterial hypertension. Am J Physiol Cell Physiol 307:C373–C383
Starkus JG, Fleig A, Penner R (2010) The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification. J Physiol 588:1227–1240
Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, Eberlein C, Candido JB, Clarke M, Nixon C, Connelly J, Jamieson N, Carter CR, Balkwill F, Chang DK, Evans TRJ, Strathdee D, Biankin AV, Nibbs RJB, Barry ST, Sansom OJ, Morton JP (2016) CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 29:832–845
Steffen S, Abraham S, Herbig M, Schmidt F, Blau K, Meisterfeld S, Beissert S, Guck J, Gunther C (2018) Toll-like receptor-mediated upregulation of CXCL16 in psoriasis orchestrates neutrophil activation. J Investig Dermatol 138:344–354
Stoddard JS, Steinbach JH, Simchowitz L (1993) Whole cell Cl− currents in human neutrophils induced by cell swelling. Am J Phys 265:C156–C165
Storch U, Forst AL, Philipp M, Gudermann T, Mederos y Schnitzler M (2012) Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J Biol Chem 287:3530–3540
Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, Smith BL, Ferrone CR, Hornicek FJ, Boucher Y, Munn LL, Jain RK (2012) Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci U S A 109:15101–15108
Sumoza-Toledo A, Lange I, Cortado H, Bhagat H, Mori Y, Fleig A, Penner R, Partida-Sanchez S (2011) Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release. FASEB J 25:3529–3542
Tare M, Prestwich SA, Gordienko DV, Parveen S, Carver JE, Robinson C, Bolton TB (1998) Inwardly rectifying whole cell potassium current in human blood eosinophils. J Physiol 506:303–318
Tazzyman S, Barry ST, Ashton S, Wood P, Blakey D, Lewis CE, Murdoch C (2011) Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. Int J Cancer 129:847–858
Trevani AS, Andonegui G, Giordano M, Lopez DH, Gamberale R, Minucci F, Geffner JR (1999) Extracellular acidification induces human neutrophil activation. J Immunol 162:4849–4857
Tsai FC, Meyer T (2012) Ca2+ pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Curr Biol 22:837–842
Tsai FC, Seki A, Yang HW, Hayer A, Carrasco S, Malmersjo S, Meyer T (2014) A polarized Ca2+, diacylglycerol and STIM1 signalling system regulates directed cell migration. Nat Cell Biol 16:133–144
Tsurumaki H, Mogi C, Aoki-Saito H, Tobo M, Kamide Y, Yatomi M, Sato K, Dobashi K, Ishizuka T, Hisada T, Yamada M, Okajima F (2015) Protective role of proton-sensing TDAG8 in lipopolysaccharide-induced acute lung injury. Int J Mol Sci 16:28931–28942
Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS (2004) Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem 279:34643–34654
von Tscharner V, Prod'hom B, Baggiolini M, Reuter H (1986) Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. Nature 324:369–372
Wang CH, Rong MY, Wang L, Ren Z, Chen LN, Jia JF, Li XY, Wu ZB, Chen ZN, Zhu P (2014) CD147 up-regulates calcium-induced chemotaxis, adhesion ability and invasiveness of human neutrophils via a TRPM-7-mediated mechanism. Rheumatology (Oxford) 53:2288–2296
Wang G (2016) Chloride flux in phagocytes. Immunol Rev 273:219–231
Wang G, Cao L, Liu X, Sieracki NA, Di A, Wen X, Chen Y, Taylor S, Huang X, Tiruppathi C, Zhao YY, Song Y, Gao X, Jin T, Bai C, Malik AB, Xu J (2016) Oxidant sensing by TRPM2 inhibits neutrophil migration and mitigates inflammation. Dev Cell 38:453–462
Weber EW, Han F, Tauseef M, Birnbaumer L, Mehta D, Muller WA (2015) TRPC6 is the endothelial calcium channel that regulates leukocyte transendothelial migration during the inflammatory response. J Exp Med 212:1883–1899
Winterbourn CC, Kettle AJ (2013) Redox reactions and microbial killing in the neutrophil phagosome. Antioxid Redox Signal 18:642–660
Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y (2008) TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14:738–747
Yin J, Michalick L, Tang C, Tabuchi A, Goldenberg N, Dan Q, Awwad K, Wang L, Erfinanda L, Nouailles G, Witzenrath M, Vogelzang A, Lv L, Lee WL, Zhang H, Rotstein O, Kapus A, Szaszi K, Fleming I, Liedtke WB, Kuppe H, Kuebler WM (2016) Role of transient receptor potential vanilloid 4 in neutrophil activation and acute lung injury. Am J Respir Cell Mol Biol 54:370–383
Zhang Z, Cui P, Zhang K, Chen Q, Fang X (2017) Transient receptor potential melastatin 2 regulates phagosome maturation and is required for bacterial clearance in Escherichia coli sepsis. Anesthesiology 126:128–139
Zhu X, Chu PB, Peyton M, Birnbaumer L (1995) Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett 373:193–198
Acknowledgements
The authors wish to thank past and present members of their laboratories whose enthusiastic work contributed largely to developing the concepts described in this review.
Funding
K.N. is supported by a fellowship from the CIM-IMPRS graduate school. A.S. thanks the support from the Deutsche Forschungsgemeinschaft (DFG; SCHW 407/17-1), Cells-in-Motion Cluster of Excellence (EXC 1003-CiM), University of Münster, Germany, and IZKF Münster (Schw2/020/18). B.F. received support from Cells-in-Motion Cluster of Excellence (EXC 1003-CiM; PP 2016-12), and B.M. is supported by DFG grant MU 3574/4-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Najder, K., Musset, B., Lindemann, O. et al. The function of TRP channels in neutrophil granulocytes. Pflugers Arch - Eur J Physiol 470, 1017–1033 (2018). https://doi.org/10.1007/s00424-018-2146-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2146-8