Abstract
High salt (NaCl) intake promotes the development of vascular diseases independent of a rise in blood pressure, whereas reduction of salt consumption has beneficial effects for the arterial system. This article summarizes our current understanding of the molecular mechanisms of high salt-induced alterations of the endothelial phenotype, the impact of the individual endothelial genotype, and the overall vascular phenotype. We focus on the endothelial Na+ channel (EnNaC)-controlled nanomechanical properties of the endothelium, since high Na+ leads to an EnNaC-induced Na+-influx and subsequent stiffening of endothelial cells. The mechanical stiffness of the endothelial cell (i.e., the endothelial phenotype) plays a crucial role as it controls the production of the endothelium-derived vasodilator nitric oxide (NO) which directly affects the tone of the vascular smooth muscle cells. In contrast to soft endothelial cells, stiff endothelial cells release reduced amounts of NO, the hallmark of endothelial dysfunction. This endothelium-born process is followed by the development of arterial stiffness (i.e., the vascular phenotype), predicting the development of vascular end-organ damage such as myocardial infarction, stroke, and renal impairment. In this context, we outline the potential clinical implication of direct (amiloride) and indirect (spironolactone) EnNaC inhibition on vascular function. However, interindividual differences exist in the response to high salt intake which involves different endothelial genotypes. Thus, selected genes and genetic variants contributing to the development of salt-induced endothelial dysfunction and hypertension are discussed. In this review, we focus on the role of salt in endothelial and vascular (dys)function and the link between salt-induced changes of the endothelial and vascular phenotype and its clinical implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Salt and endothelial phenotype
Salt-induced endothelial stiffening
The daily moderate intake of salt (sodium chloride, NaCl) is essential for life in that it maintains a proper homeostasis of extracellular fluid volume and blood pressure. However, in clinical trials, it was demonstrated that the intake of 6 g salt leads to an acute increase in blood pressure that correlates with acute increases in plasma Na+ concentrations: a 1 mmol/l increase is associated with a 1.9 mmHg increase in systolic blood pressure [45, 123]. Thus, excessive salt intake may lead to the manifestation and aggravation of arterial hypertension. In addition, salt excess has harmful effects on the cardiovascular system (e.g., stroke, coronary heart disease, stiffening of conduit arteries, and thickening and narrowing of resistance arteries) which has also been observed independent of the rise in blood pressure [45, 75, 121].
During the last years, considerable effort has been made to reveal the underlying cellular mechanisms which are responsible for these deleterious systemic effects of salt excess. In this regard, the endothelial cortex, a functional region 50–150 nm beneath the plasma membrane, has been detected as important target of salt. A small rise in Na+ concentration (>140 mM) alters the nanomechanical properties of endothelial cells (i.e., the phenotype of the cell) in that the endothelial cortex stiffens. Stiffness is a mechanical property which reflects the physiological state of the endothelial cell. The degree of stiffness is important for physiological functions as cellular responses to biochemical and biophysical signals [96]. The mechanical properties of the endothelial cortex are mainly determined by actin which builds a dynamic network beneath the plasma membrane. This peripheral actin is composed of cross-linked actin filaments (F-actin), globular actin (G-actin), and embedded proteins [38, 98]. High Na+ apparently stabilizes F-actin by conformational changes, e.g., strengthening the inter-subunit contacts of the protein, a process which might explain the Na+-induced stiffening of the endothelial cortex [89]. Recently, Koltsova et al. [55] reported that an elevation of intracellular [Na+] affects the expression of many genes. This might contribute to salt-induced changes of the endothelial transcriptome and thus the endothelial phenotype.
The endothelial sodium channel (EnNaC) was recognized as mediator of the Na+-dependent cortical stiffening [59], and thus the phenotype of the endothelium. The term “EnNaC” was recently introduced by David Warnock [134] with the intention to distinguish it from the epithelial sodium channel (ENaC) which is well defined in epithelial tissues since its first description in 1995 by Canessa et al. [15]. By using genetically modified cell lines and state of the art mouse models, a positive correlation between EnNaC membrane abundance and mechanical stiffness of the cellular cortex has been found, i.e., the more EnNaC, the stiffer the endothelial cortex [48, 59, 135]. EnNaC is regulated by Na+ in that high extracellular Na+ concentrations increase the abundance of EnNaC compared to low Na+ concentrations, thereby enabling Na+ influx into the cell, followed by stiffening of the endothelial cortex. A mechanism called “feedforward activation” of EnNaC by Na+ [56] (Fig. 1). EnNaC-mediated endothelial stiffening likely occurs via interaction of the α-subunit C-terminus with F-actin in the cortical cytoskeleton [42, 73]. Na+-dependent EnNaC accumulation in the plasma membrane leads to an enhanced Na+ influx into the cell, stabilization of F-actin, and thus rigidity of the EnNaC-F-actin complexes.
High salt induces increased EnNaC membrane abundance and cortical stiffness. a Under low salt conditions, EnNaC membrane abundance is moderate and the endothelial cortex is soft. b In the case of high extracellular salt concentrations, the membrane abundance of EnNaC is increased and Na+ influx into the cell facilitated, leading to a stiff cortex. Thus, the number of EnNaC molecules in the plasma membrane of endothelial cells determines its mechanical properties. EnNaC endothelial Na+ channel
The mineralocorticoid receptor is a mediator of salt-induced endothelial stiffening
In vascular endothelial cells, the mineralocorticoid hormone aldosterone, the major regulator of ENaC and EnNaC [60, 112], modifies the endothelial phenotype similar to Na+: it swells and stiffens endothelial cells [86, 88]. This indicates a concerted action of both hormone and ion. Classically, aldosterone binds to its cytosolic mineralocorticoid receptor (MR) causing dissociation of chaperones and formation of MR dimers. These dimers then translocate into the nucleus and act as transcription factors to influence the expression of target genes. Several genes have been identified as being directly or indirectly regulated by aldosterone, for example Na+/K+-ATPase, serum and glucocorticoid-dependent kinase (SGK) 1, and the renal outer medullary potassium channel (ROMK), all of them concerned with electrolyte and volume regulation. SGK1 regulates ENaC in that it phosphorylates NEDD4-2 and prevents ubiquitylation and degradation of ENaC (for review, see [112]). The MR as well as NEDD4-2 could also be detected in non-epithelial cells indicating that major prerequisites for the regulation of EnNaC along this pathway are given in endothelial cells [16, 79, 139]. Interestingly, in the absence of aldosterone, high salt significantly increases the transcription of the MR [56] which is in agreement with the observation that salt loading decreases serum aldosterone, whereas MR activation in the target tissue is augmented [78]. In addition, the importance of the MR was demonstrated by the fact that aldosterone antagonism can prevent the effects of high Na+ [56, 87].
Mutations in ENaC which impair the MR-dependent regulation of the channel have been identified in some genetically defined forms of arterial hypertension [1]. One of the classical examples is Liddle’s syndrome [118], which results from mutations in the PY-motif of the ENaC β- or γ-subunit. These mutations prevent degradation of the ion channel by the ubiquitin ligase NEDD4-2, causing an increased channel surface density and thus augmented Na+ influx via ENaC in collecting duct epithelial cells resulting in severe hypertension [104, 105, 120]. Whether this mechanism alone is sufficient to explain the disease pathology was questioned: it has been proposed that dysregulation of the vascular tone contributes to the development and maintenance of hypertension, independent of actions resulting from increased blood pressure [79, 108]. By using an appropriate mouse model, it could be shown that known ENaC mutations causing Liddle’s syndrome also induce an increased EnNaC surface expression and a concomitantly elevated cortical stiffness in the endothelium, indicating that EnNaC dysregulation (increased Na+ influx) participates in the pathology of Liddle’s syndrome. Thus, a general contribution of EnNaC- and Na+-mediated changes of the endothelial phenotype to hypertension can be concluded. In addition to its extrarenal effects, aldosterone and MR regulate ion channel expression and Na+ homeostasis which might explain the vasoprotective effects of MR antagonism [33].
Salt-induced damage of the endothelial glycocalyx
The endothelial glycocalyx (eGC), a negatively charged biopolymer, is located on top of endothelial cells. The eGC mainly consists of proteoglycans, especially those of the syndecan family, to which glycosaminoglycans (GAG, mainly heparan and chondroitin sulfates) and hyaluronan are attached [100, 103]. The eGC has been identified as being an important shear stress sensor, in that changes in blood flow affects the conformation of the eGC with subsequent inward signal transduction through intracellular domains of the eGC to the cortical cytoskeleton. GAGs in particular have been extensively studied in regard to their ability to function as a mechanotransducer [119, 124]. Beyond its function as shear stress sensor, the eGC was recognized as effective Na+ buffer [83]. Due to the high charge density of Na+, the ion is attracted by the negative charges of the eGC surface and accumulates in the mesh-like structure [19, 84]. After exposure to excessive Na+ intake over time, the capacity of the eGC to buffer Na+ is found diminished, leading to conformational changes of the negatively charged heparan sulfate residues in the eGC [85]. Since the eGC is connected with proteins of the cortical web, changes of the eGC conformation influences the signal transduction into the interior of the cell and thus its function. In other words, high Na+ leads to collapse and stiffening of the eGC, a process which contributes to signal transduction processes into the cell and thus the overall Na+-induced changes of the mechanical properties of the endothelium [38] (Fig. 2). Hence, high Na+ not only changes the phenotype of the endothelial cortex, but also the phenotype of the eGC. Taken together, Na+ excess might lead over time to a damage of the eGC barrier, cortical stiffening, and might trigger endothelial dysfunction. Thus, the (Na+-dependent) endothelial phenotype reflects the functional status and quality of the vascular endothelium.
High salt deteriorates the endothelial glycocalyx. a Under low salt conditions, the endothelial cell is protected by a well-developed glycocalyx with an optimum of negatively charged proteoglycans and a low number of EnNaC in the plasma membrane. Thus, plasma Na+ is buffered and the access of Na+ to the respective channels of the endothelium is limited, resulting in a soft cortex. b In the case of high salt concentrations, a poorly-developed glycocalyx with a reduced number of negatively charged proteoglycans can be found while the number of EnNaC is increased. Thus, plasma Na+ has facilitated access to the sodium channels of the endothelium and cortical stiffening occurs. EnNaC endothelial Na+ channel, eGC endothelial glycocalyx
Salt intake affects endothelial function
One of the precursors leading to salt-induced cardiovascular pathologies is endothelial dysfunction, which can be defined as systemic pathological state of the endothelium derived from an imbalance between vasodilating and vasoconstricting substances released from the endothelium. More than 30 years ago, Furchgott and Zawadzki [39] introduced the term “endothelial dysfunction” upon identification of the endothelium-derived relaxing factor as being the vasodilating gas nitric oxide (NO). Endothelial cells are exposed to the shear forces of the streaming blood which deform the endothelial cell at the very surface. These rhythmical traction forces control the activity of the endothelial nitric oxide synthase (eNOS) and thus the synthesis and release of NO which diffuses to adjacent vascular smooth muscle cells where it triggers vasodilation via cGMP-dependent pathways [117]. Hence, endothelial cells control vascular function by yielding the release of NO. A reduction in NO is strongly associated with increased levels of reactive oxygen species (ROS) generated by NAD(P)H oxidase, xanthine oxidase, or uncoupled eNOS within the vascular wall, leading not only to scavenging of NO but also to disruption of some signaling pathways that mediate its production [11]. Thus, a reduced NO secretion is a hallmark of endothelial dysfunction.
Endothelial dysfunction, due to high salt intake, is related to disturbed NO synthesis by the endothelium [12, 80, 87]. As a consequence, endothelium-dependent vasodilation is impaired in Dahl-R rats fed with a high-salt diet, and it has been concluded that high salt intake compromises the responsiveness of endothelial cells to shear stress [9, 10, 70]. Studies in humans confirmed what has been learned from animal models: a high-Na+ diet (300–350 mmol/day) led to an increase in plasma [Na+] of 2–4 mmol/l and thus to a loss of NO-dependent vasodilation [32, 123].
These observations are linked to the mechanical properties of endothelial cells: cortical stiffness and NO release are tightly coupled in that a soft endothelial cell cortex is easily deformable by the streaming blood and thus the endothelial cell releases increased amounts of NO in contrast to a stiff cell cortex [37, 87]. Li et al. [66] reported that a small increase in Na+ concentration (from 137 to 142 mM) suppresses eNOS activity. An explanation might involve active EnNaC which is likely to inhibit eNOS phosphorylation via a blockade of the phosphoinositide 3-kinase (PI3K)/Akt pathway leading to reduced NO release and thus vasoconstriction [94]. Based on these data, the following sequence of events can be postulated: The Na+-dependent incorporation of EnNaC into the endothelial plasma membrane stiffens the cortex altering the release of NO and thus endothelial function. The amount of released NO in turn is responsible for either relaxing or contraction of the vascular smooth muscle cells and thus blood vessel function (Fig. 3). Hence, cortical stiffening, triggered by high-Na+ concentrations, results in a reduced NO release which is the hallmark of endothelial dysfunction, also termed “stiff endothelial cell syndrome” (SECS) [61]. This apparently local mechanism might contribute to pathophysiological conditions such as increased overall arterial stiffness, an early step in the pathogenesis of atherosclerosis and hypertension (Fig. 4).
High salt impairs endothelial function. a Under low salt conditions, the soft endothelial cortex is easily deformable, the amount of shear stress-induced NO release is high, and the vascular smooth muscle cells relaxed. b In the case of high salt-induced cortical stiffening, the NO release is reduced, leading to contracted smooth muscle cells and vasoconstriction. EnNaC endothelial Na+ channel, eGC endothelial glycocalyx, VSMC vascular smooth muscle cells
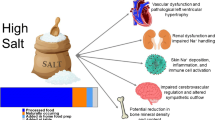
Link between salt intake, endothelial phenotype, and arterial phenotype Excess dietary salt intake increases the plasma Na+ concentration whereupon the phenotype of endothelial cells is shifted toward cortical stiffening. This in turn reduces the release of nitric oxide (NO), the hallmark for endothelial dysfunction. Since NO serves as a general vasodilator, a reduced NO release might be accompanied by peripheral vasoconstriction and arterial stiffening predicting the development of vascular end-organ damage such as myocardial infarction, stroke, and renal impairment. This process is modified by interindividual different genetic profiles
Beyond its pathophysiological implications, Na+-induced stiffening seems to trigger a “physiological” response in that it transiently reduces NO release which increases vascular smooth muscle tone and thus may prevent any deleterious decrease in arterial blood pressure. If, however, the rise in plasma Na+ persists, the stiffness of the endothelial cells remains increased and the release of NO is chronically reduced, a pathophysiological response which finally leads to a transition from endothelial function to endothelial dysfunction [61], accompanied by structural damage of the endothelium.
Salt and endothelial genotype/genetic aspects
Salt intake, blood pressure response, and individual genotype
The physiologic response to dietary salt in humans is heterogeneous. This has led to the classification that individuals are either salt-sensitive or salt-resistant. Most prominently, dietary salt restriction is not effective in reducing blood pressure of all hypertensive patients. First studies on the heritability of salt sensitivity including observations in genetically identical twins in the late 1980s revealed that genetic variance influences the response of the renin-angiotensin-aldosterone-system (RAAS), the sympathetic nervous system, and thus the glomerular filtration rate on salt intake [69]. Since then, several attempts have been made to identify genotype-phenotype associations in salt sensitivity and how genes and dietary Na+ interact. Initially, these investigations were driven by knowledge on physiological mechanisms and monogenic blood pressure disorders including animal models [35, 67, 75] but become strongly supported by the emerging field of -omics, the field of large-scale data-rich biology [13]. More recently, the vascular endothelium has been identified as primary target for deleterious salt effects [87], and subclinical endothelial salt sensitivity may lead to endothelial stiffness and dysfunction [109]. Accordingly, several studies suggest that the endothelial response to dietary salt is modulated by individual genetic predispositions.
Genetic associations with salt sensitivity
The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) Study, one of the largest family-based genome-wide linkage scan studies introduced to analyze the genetic determinants of salt sensitivity of blood pressure, identified a region on chromosome 2 (2p24.3–2p24.1) to be significantly associated with diastolic blood pressure (DBP) and mean arterial pressure (MAP) responses to high-Na+ interventions [74]. The identified genomic region had previously been reported in an analysis based on a genome-wide discovery and follow-up study in over 120,000 individuals by the International Consortium of Blood Pressure Genome-Wide Association Studies (ICBP-GWAS) to influence pulse pressure and MAP [133]. The locus harbors FIGN encoding fidgetin, a molecular chaperone of the AAA protein family [22]. Besides the fact that very little is known about the underlying mechanism of how the identified locus may affect pulse pressure and MAP, the independent and robust association with blood pressure phenotypes and blood pressure response to high Na+ interventions suggests that genetic determinants of both traits might include an overlap of genes.
Genes of the endothelial system
The same dataset based on GenSalt Study participants receiving a 7-day low-Na+ diet (51.3 mmol of sodium/day) followed by a 7-day high-Na+ diet (307.8 mmol of sodium/day) has been used to examine the association of endothelial genes with blood pressure response to Na+ intake [25]. The study identified genetic variants of the DDAH1, VWF, COL18A1, and SELE locus to be associated with systolic blood pressure (SBP), DBP, or MAP, respectively. The identification of the DDAH1 gene is of considerable interest as it encodes the dimethylarginine dimethylaminohydrolase 1 which is involved in the regulation of vascular NO levels [28] and has been suggested to be almost exclusively expressed in the vascular endothelium [46]. NO synthesis is inhibited by asymmetrical dimethylarginine (ADMA), an endogenous arginine analogue, and ADMA is eliminated from the body by renal excretion and DDAH-dependent hydrolysis [64]. Consistently, elevated ADMA levels have been observed in different disease states such as stroke, atherosclerosis, hypertension, renal disease, and endothelial dysfunction [64]. Notably, ADMA has been shown to increase after salt loading [127] and DDAH1 variants have independently been associated with circulating ADMA levels [64].
The GenSalt Study also identified the known hypertension susceptibility VWF locus to be associated with SBP, DBP, and MAP and dietary sodium intervention [25]. Endothelium-derived von Willebrand Factor (vWF) is released by damaged endothelial cells, promoting coagulation and platelet activation. Most recently, a direct link between production and secretion of vWF and high NaCl levels has been proposed [29]. High NaCl was shown to increase the expression of tonicity-regulated transcription factor NFAT5 and subsequent vWF promoter binding, suggesting the involvement of hypertonic signaling in vWF regulation [29]. Data from the Atherosclerosis Risk in Communities (ARIC) Study suggested that plasma Na+ is positively associated with circulating vWF levels in blood and an increased risk of stroke [29].
In addition, the reported association of COL18A1 with SBP and MAP responses to high-Na+ intake has linked plasma Na+ to extracellular matrix proteoglycans. COL18A1 is a major basement membrane heparan sulfate proteoglycan with anti-angiogenic effects [47]. Col18 deficiency leads to increased vascular permeability of small and large vessels and thickening of basement membranes in the heart and kidney [7]. Degradation of COL18A1 leads to endostatin, a C-terminal fragment of COL18A1 [47], with vasodilative and anti-angiogenic properties [7].
In correspondence to its association with salt sensitivity, the SELE locus has been reported in blood pressure regulation and hypertension. SELE encodes E-selectin, also called endothelial leukocyte adhesion molecule-1, which was originally detected on the surfaces of cytokine-activated endothelial cells [5]. Genetic variants of the SELE gene have also been associated with a higher risk for early severe atherosclerosis [137].
Data from Kelly et al. [51] supported a role for guanine nucleotide-binding protein beta polypeptide 3 (GNB3) in salt-sensitive hypertension as GNB3 rs1129649 was significantly predictive of MAP response to low-Na+ intervention. The study also investigated GNB3 rs2301339 which is in perfect linkage disequilibrium with the C825T polymorphism and identified an association with SBP response to high Na+. Although the rs2301339 variant of GNB3 did not retain significance after adjustment for multiple testing, the report establishes a link with other findings of the GNB3 C825T polymorphism [50]. The 825T allele has been shown to be associated with an increased intracellular signal transduction on stimulation of different G protein-coupled receptors [53]. Carriers of the 825T allele present enhanced vasoconstriction to endothelin-1, angiotensin II, and noradrenaline in the skin microcirculation [138], and hypertensive 825T allele carriers show large blood pressure reductions under thiazide diuretics [53]. In addition, the 825T allele has been associated with arterial stiffness in young healthy males as T allele carriers presented significantly higher values for pulse wave velocity (PWV) and augmentation index compared to homozygous C allele carriers [81]. Endothelial processes affected by high plasma Na+ which involve the large group of G protein-coupled receptors might also be affected in 825T allele carriers.
Salt sensitivity also appears to be modulated by genetic variants in the eNOS gene as an interaction between the eNOS T-786C variant and Na+ intake on blood pressure has been identified [76].
Genes of the aldosterone-MR axis
In 622 subjects from the European Project on Genes in Hypertension (EPOGH), Wojciechowska et al. [141] investigated whether the cytochrome P450 (CYP)11B2 C-344T variant influenced arterial wave reflections as a measure of vascular stiffness. CYP11B2 encodes for the aldosterone synthase which generates aldosterone mainly in the adrenal gland. However, aldosterone is also produced in endothelial cells with the potential to influence vascular structure and function locally [26, 57]. The peripheral and central augmentation indices were significantly higher in CYP11B2-344C allele carriers compared to CYP11B2-344T homozygotes. However, this effect of the CYP11B2 variant was only detected in subjects with a higher than median urinary sodium excretion (210 mmol/day), suggesting a salt-modifying effect in the CYP11B2-344C allele carriers.
As recent studies reported, the induction of SGK1 expression by Na+, the associations between SGK1, and SBP, DBP, and MAP responses to high Na+ is of considerable interest [65, 102]. As SGK1 is involved in the regulation of both, ENaC and EnNaC, a genetic interaction of variants of the SGK1 locus and E(n)NaC subunit genes may exist. Zhao et al. [142] identified six variants in the SCNN1G gene encoding the ENaC-gamma subunit to be associated with SBP response to low Na+. Synergistic interactions of SGK1 and ENaC variants in the endothelium might lead to enhanced ENaC expression and thus salt sensitivity leading to the “stiff endothelial cell syndrome.”
Alpha-adducin and actin-based cytoskeleton dynamics
Alpha-adducin (ADD1) is a substrate for protein kinase C (PKC) and Rho-associated kinase, promotes spectrin-actin interaction, and has modulatory effects on actin cytoskeleton dynamics [128, 129]. Carriers of the ADD1 460Trp (rs4961) amino acid residue showed higher rates of renal tubular Na+ reabsorption [113], and 460Trp has been associated with hypertension and salt sensitivity, while 460Trp carriers also show a better response to diuretics than wild-type homozygotes [23]. ADD1-associated blood pressure changes with Na+ have also been observed to interact synergistically with the ACE ID gene polymorphism on blood pressure, increasing with the Na+ load [4]. Interestingly, physiological interactions between ADD1 and the ubiquitin protein ligase NEDD4L have been reported, and synergistic effects of genetic variants in both genes may exist on renal Na+ handling [72]. The NEDD4L rs4149601 G > A substitution leads to an alternative splice site and a NEDD4L protein lacking the Ca2+-dependent lipid-binding domain. This isoform downregulates ENaC more potently, suggesting that carriers of the NEDD4L G allele have higher ENaC levels than carriers of the A allele [72], which could make the endothelium more sensitive for elevated salt concentrations. Besides the findings on ADD1 genetic variants on renal sodium handling and associated cardiovascular disease, ADD1 variants have also been linked directly to vascular phenotypes. Investigations of the EPOGH project provided evidence that peripheral and central pulse pressures were elevated in carriers of the ADD1Trp amino acid [24] while the association of brachial diameter was detected in a multiple-gene analyses of ADD1 wt homozygotes and the ADD3 G allele (rs3731566) [116]. The group of Cusi [95] reported for the first time that 460Trp was associated with blunted endothelium-dependent vasodilation in a group of untreated patients with essential hypertension.
Genes involved in Ca2+ homeostasis
Additional genes involved in salt-sensitive vasoconstriction/vasodilation have been identified using acute salt loading tests and a low-density genome-wide genotyping array [18]. The group identified PRKG1 (rs7897633), SLC24A3 (rs3790261), and SLC8A1 (rs11893826, rs434082), all involved in Ca2+ homeostasis, and their interaction to be associated with salt-dependent blood pressure changes. PRKG1 encodes for the type 1 cGMP-dependent protein kinase (cGKI), a vasodilator effector that has been shown to mediate vascular smooth muscle cell relaxation by lowering intracellular Ca2+ levels [136]. SLC24A3 encodes for the NCKX3, the potassium-dependent Na+/K+/Ca2+ exchanger type 3, an important regulator of intracellular calcium homeostasis while SLC8A1 encodes for the NCX1 transporter, which is involved in hypertension and Na+-sensitivity [8]. Altogether, the identified genes involved in Ca2+ signal transduction may modify endothelial stiffness, potentially by affecting Ca2+-dependent actin-binding proteins.
Is there a (cellular) salt memory?
Only recently, Oguchi et al. [90] reported long-lasting effects on blood pressure after the temporary exposure to a high-salt diet in Dahl salt-sensitive rats, a phenomenon which they termed “salt memory.” In their study, they explained the persisting rise in blood pressure by deleterious renal microvascular changes. Besides these obvious changes in the vasculature after temporary salt exposure, chromosomal alterations in form of epigenetic reprogramming might determine a cellular “salt memory.” During the last years, environmental and dietary factors have been shown to have epigenetic effects on gene expression with important impact on the manifestation and progression of cardiovascular diseases/hypertension [21, 43]. These epigenetic changes mostly occur via alterations of chromatin packaging and the accessibility of DNA for regulatory proteins [17]. Most importantly, epigenetic information can be inherited to the next generation. Some reports have proposed epigenetic mechanisms to exist also for dietary salt intake. Using the well-established model of Dahl salt-sensitive rats, the group of Liu et al. [68] observed broad changes of 5-methylcytosine in several hundred CpG dinucleotides throughout the genome when analyzing the renal outer medulla after exposure to a high-salt diet. This observation was associated with profound changes in mRNA levels suggesting a substantial and potentially long-lasting impact of salt consumption on gene expression [68]. An additional independent report identified the lysine-specific demethylase 1 (LSD-1), which regulates gene expression by histone methylation, to be involved in salt-sensitive hypertension [97]. In a subsequent animal study, mice on a high-salt diet showed decreased LSD-1 expression levels compared to mice with lower salt intake [140]. Similar to LSD-1+/− mice, African-American and Hispanic minor allele carriers of two LSD-1 SNPs displayed greater changes in systolic blood pressure in response to dietary salt change. The identified mechanisms may also be of relevance for salt-dependent epigenetic changes in the vascular endothelium.
Salt and vascular phenotype—what is the clinical relevance?
Salt-induced cardiovascular morbidity and mortality
Tuomilehto et al. [130] analyzed a large adult population of 1173 Finnish men and 1263 women with complete data on 24 h urinary sodium excretion and concluded that high salt intake predicted mortality and risk of coronary heart disease (CHD), independent of other cardiovascular risk factors, including blood pressure. In a long-term follow-up of two lifestyle intervention trials—the trials of hypertension prevention phase I (TOHP I) and II (TOHP II)—prehypertensive subjects assigned to a sodium reduction intervention had a 25–30 % lower risk of cardiovascular outcomes in the 10 to 15 years after the trial [20]. Using the Coronary Heart Disease (CHD) Policy Model, the potential health benefits of daily dietary salt reductions of up to 3 g (1.2 g sodium) were quantified, and the data allowed for an estimation of a principal reduction of the annual number of incidences with respect to CHD by 60,000 to 120,000, stroke by 32,000 to 66,000, and myocardial infarction by 54,000 to 99,000, which may correspond to an annual reduction of deaths from any cause by 44,000 to 92,000 [6]. In a very recent current global survey, Mozaffarian et al. [77] reported that approximately 1.65 million cardiovascular deaths in 2010 were due to a daily sodium consumption above a reference level of 2.0 g, which is of major relevance for global health authorities.
As current data reveal an average daily consumption of 3 to 6 g of sodium, corresponding to 7.5 to 15.0 g of salt across populations [99], mostly assigned to the use of processed foods [52], recent global and American guidelines on cardiovascular disease prevention recommend a maximum daily sodium intake of 1.5 to 2.4 g (3.75 to 6.0 g of salt) [34, 41, 58]. A daily salt restriction to 5–6 g is also recommended by the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) [71].
The mechanisms of how salt intake triggers the development of vascular disease phenotypes are manifold and go beyond its sole impact on blood pressure regulation (which is the subject of another review in this issue of Pflügers Archiv). To only refer to one landmark study in this field, Rakova et al. [101] performed a nutritional intervention study during space flight simulations in an enclosed habitat consisting of hermetically sealed interconnecting modules. In 12 healthy young men followed 105 or 520 days (Mars105/Mars520), blood pressure decreased when daily salt intake was reduced stepwise from 12 to 9 g to 6 g/day and increased with re-exposition to the 12 g/day salt diet. Significant positive association between sodium intake and pulse pressure [14, 31] provides further evidence for the current concept linking sodium to rise in blood pressure through stiffening processes of the arteries [106].
Arterial stiffness and impact of salt
Increased arterial stiffness has been documented to predict the development of cardiovascular disease (CVD) such as myocardial and cerebral infarction, heart failure, as well as renal impairment, and is an independent predictor of CVD mortality [107, 131, 132]. Several noninvasive methods are currently in clinical use to assess vascular stiffness [62]. Carotid–femoral pulse wave velocity (cfPWV) is considered the gold standard for measuring central arterial stiffness, and is an independent predictor of cardiovascular mortality and morbidity. Although the relationship between aortic stiffness and cardiovascular events is continuous, a PWV threshold of 10 m/s, above which there is an increased risk for cardiovascular events, has been proposed by a recent expert consensus statement [131] and the 2013 ESH/ESC Guidelines as an estimate of significant alterations of aortic function in middle-aged hypertensive patients [71]. The additive value of PWV for risk prediction above and beyond traditional cardiovascular risk factors has been quantified by several studies [114, 115].
Vascular stiffening is induced by alterations of the vessel wall [143, 63], which are amplified by “extrinsic factors” such as dietary salt consumption, and comorbidities such as hypertension, hyperlipidemia, diabetes mellitus, or the process of aging itself. Previous pathophysiological reports linked high dietary salt intake to arterial wall stiffness, in particular via vascular hypertrophy as well as increased collagen fibers and decreased elastin fibers and hyaluronan contents in the extracellular matrix [36, 92]. Recently, Sanders [109] summarized the pathophysiological link between high salt consumption, endothelial stiffness/dysfunction, and end-organ damage induced by altered vascular structure and function. High salt consumption induces endothelial dysfunction with altered endothelial NO production. NO has vasodilator properties and inhibits endothelial TGF-β1 production. In the pathophysiologic condition of endothelial dysfunction, TGF-β1 is not sufficiently blocked. TGF-β1-mediated fibrogenesis contributes to an increased arterial stiffness, and the vasoconstrictive effect of TGF-β1 promotes the development of hypertension, finally leading to clinical relevant vascular end-organ damage. Taking into account that NO release depends on the mechanical properties of endothelial cells, it can be concluded that salt-induced endothelial stiffening leads to arterial stiffening and if, however, the rise in plasma Na+ persists, to end-organ damage (Fig. 4).
High salt intake has been shown to aggravate age-related changes in the vasculature [3], and salt restriction has a beneficial effect in improving distensibility of the central aorta and large peripheral arteries, which is independent of its antihypertensive action [2, 40], demonstrating again that also pressure-independent mechanisms impact on the arterial wall (reviewed in [93]). Both short- and long-term sodium restrictions have been reported to increase arterial compliance [2, 40]. PWV decreased in African-Americans in response to salt reduction [44]. Other studies showed that salt reduction improved endothelium-dependent vasodilation in normotensive subjects independently, again suggesting additional vasoprotective effects of salt reduction beyond blood pressure reduction [27].
The exact mechanism of the progression from endothelial stiffness to arterial stiffness remains to be further investigated. One of the determinants might be that EnNaC and thus Na+ influx into the endothelial cell increases with age and induces mechanical stiffening. Older endothelial cells are stiffer and showed higher increments in stiffness due to Na+ load indicating an increase in salt sensitivity in the process of aging [91]. Since stiff endothelial cells release less NO than soft endothelial cells, old endothelial cells might reach a status of SECS and endothelial dysfunction which contributes to arterial stiffness. In vitro as well as ex vivo, it could be shown that direct EnNaC inhibition with amiloride as well as indirect EnNaC inhibition with the aldosterone receptor antagonist spironolactone prevented the salt-induced changes of the endothelial phenotype over time [30, 91].
Beneficial effects of mineralocorticoid receptor antagonists and amiloride on vascular function
For decades, the role of aldosterone was thought to be limited to salt and water homeostasis control [49]. This traditional view of its action restricted to Na+ reabsorption in epithelial tissues must be renewed. Clinical studies show that aldosterone, high salt intake, or both, could be responsible for the development of arterial hypertension but also for direct harmful effects on the cardiovascular system, even independent of any significant rise in blood pressure [75, 126]. Aldosterone interacts with mineralocorticoid receptors, promoting endothelial dysfunction and impaired vascular compliance, finally leading to end-organ damage in the vasculature, heart, and kidneys [122]. In this context, aldosterone is a mediator of the damaging effects of angiotensin II. From a clinical point of view, angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers are important vasoprotective drugs. Initially, they reduce aldosterone plasma levels, but “aldosterone escape” or “breakthrough” is well known during long-term therapy [110, 111]. Consequently, aldosterone blockade using mineralocorticoid receptor antagonists (MRAs) is required to reduce the risk of progressive end-organ damage. This may be achieved non-selectively with spironolactone or with the use of selective MRA eplerenone. While both drugs have been demonstrated to be effective antihypertensive agents, eplerenone may produce improved target organ protection without the antiandrogenic and progestational effects commonly observed with spironolactone. The search for novel generations of MRA with the ultimate goal of a more tissue-selective mode of action may require novel compounds that are differentiated with respect to the binding mode to the MR [54]. With respect to the vasoprotective potential of ENaC blockade [125, 134] besides the already used potassium-sparing amiloride, amiloride analogues are currently under investigation for clinical use [82].
Conclusion
As summarized in Fig. 4, high salt directly targets vascular endothelial cells which respond with mechanical stiffening and a reduced release of NO. This salt-induced condition of the endothelium is an important determinant of the vascular phenotype and might lead to end-organ damage such as myocardial infarction, stroke, and chronic kidney disease. The endothelial genotype has a high impact in these processes. Local events such as salt-induced EnNaC activation may result in considerable systemic effects. This pathophysiological, and thus one-sided view, requires an extension to physiological considerations. Taking into account that the salt-induced endothelial stiffening is a transient event due to a loss of extracellular volume and thus an increase of plasma Na+, this mechanism might be helpful for the stabilization of the blood pressure. When this persists over time, the above described pathophysiological processes are the result. Thus, early identification of gene-associated salt sensitivity and treatment with direct (amiloride) and indirect (spironolactone) EnNaC inhibitors might gain importance in the treatment and prevention of cardiovascular diseases.
References
Ambrosius WT, Bloem LJ, Zhou L, Rebhun JF, Snyder PM, Wagner MA, Guo C, Pratt JH (1999) Genetic variants in the epithelial sodium channel in relation to aldosterone and potassium excretion and risk for hypertension. Hypertension 34:631–637
Avolio AP, Clyde KM, Beard TC, Cooke HM, Ho KK, O'Rourke MF (1986) Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis 6:166–169
Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O'Rourke MF (1985) Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation 71:202–210
Barlassina C, Schork NJ, Manunta P, Citterio L, Sciarrone M, Lanella G, Bianchi G, Cusi D (2000) Synergistic effect of alpha-adducin and ACE genes causes blood pressure changes with body sodium and volume expansion. Kidney Int 57:1083–1090
Bevilacqua M, Butcher E, Furie B, Furie B, Gallatin M, Gimbrone M, Harlan J, Kishimoto K, Lasky L, McEver R (1991) Selectins: a family of adhesion receptors. Cell 67:233
Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L (2010) Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 362:590–599
Bishop JR, Passos-Bueno MR, Fong L, Stanford KI, Gonzales JC, Yeh E, Young SG, Bensadoun A, Witztum JL, Esko JD, Moulton KS (2010) Deletion of the basement membrane heparan sulfate proteoglycan type XVIII collagen causes hypertriglyceridemia in mice and humans. PLoS One 5:e13919
Blaustein MP, Zhang J, Chen L, Song H, Raina H, Kinsey SP, Izuka M, Iwamoto T, Kotlikoff MI, Lingrel JB, Philipson KD, Wier WG, Hamlyn JM (2009) The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension 53:291–298
Boegehold MA (1993) Effect of dietary salt on arteriolar nitric oxide in striated muscle of normotensive rats. Am J Physiol 264:H1810–H1816
Boegehold MA (1993) Microvascular changes associated with high salt intake and hypertension in Dahl rats. Int J Microcirc Clin Exp 12:143–156
Boegehold MA (2013) The effect of high salt intake on endothelial function: reduced vascular nitric oxide in the absence of hypertension. J Vasc Res 50:458–467
Bragulat E, de La SA, Antonio MT, Coca A (2001) Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension 37:444–448
Brand SM (2012) Genetics, genomics and other molecular approaches: example of salt-sensitive hypertension. J Hypertens 30:877–879
Buyck JF, Blacher J, Kesse-Guyot E, Castetbon K, Galan P, Safar M, Hercberg S, Czernichow S (2009) Differential associations of dietary sodium and potassium intake with blood pressure: a focus on pulse pressure. J Hypertens 27:1158–1164
Canessa CM, Horisberger J-D, Schild L, Rossier BC (1995) Expression cloning of the epithelial sodium channel. Kidney Int 48:950–955
Caprio M, Newfell BG, la Sala A, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ (2008) Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res 102:1359–1367
Chen T, Dent SY (2014) Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat Rev Genet 15:93–106
Citterio L, Simonini M, Zagato L, Salvi E, Delli CS, Lanzani C, Messaggio E, Casamassima N, Frau F, D'Avila F, Cusi D, Barlassina C, Manunta P (2011) Genes involved in vasoconstriction and vasodilation system affect salt-sensitive hypertension. PLoS One 6:e19620
Collins KD (1995) Sticky ions in biological systems. Proc Natl Acad Sci USA 92:5553–5557
Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK (2007) Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 334:885–888
Cowley AW Jr, Nadeau JH, Baccarelli A, Berecek K, Fornage M, Gibbons GH, Harrison DG, Liang M, Nathanielsz PW, O'Connor DT, Ordovas J, Peng W, Soares MB, Szyf M, Tolunay HE, Wood KC, Zhao K, Galis ZS (2012) Report of the National Heart, Lung, and Blood Institute Working Group on epigenetics and hypertension. Hypertension 59:899–905
Cox GA, Mahaffey CL, Nystuen A, Letts VA, Frankel WN (2000) The mouse fidgetin gene defines a new role for AAA family proteins in mammalian development. Nat Genet 26:198–202
Cusi D, Barlassina C, Azzani T, Casari G, Citterio L, Devoto M, Glorioso N, Lanzani C, Manunta P, Righetti M, Rivera R, Stella P, Troffa C, Zagato L, Bianchi G (1997) Polymorphisms of alpha-adducin and salt sensitivity in patients with essential hypertension. Lancet 349:1353–1357
Cwynar M, Staessen JA, Ticha M, Nawrot T, Citterio L, Kuznetsova T, Wojciechowska W, Stolarz K, Filipovsky J, Kawecka-Jaszcz K, Grodzicki T, Struijker-Boudier HA, Thijs L, Van Bortel LM, Bianchi G (2005) Epistatic interaction between alpha- and gamma-adducin influences peripheral and central pulse pressures in white Europeans. J Hypertens 23:961–969
Defago MD, Gu D, Hixson JE, Shimmin LC, Rice TK, Gu CC, Jaquish CE, Liu DP, He J, Kelly TN (2013) Common genetic variants in the endothelial system predict blood pressure response to sodium intake: the GenSalt study. Am J Hypertens 26:643–656
Delcayre C, Silvestre JS, Garnier A, Oubenaissa A, Cailmail S, Tatara E, Swynghedauw B, Robert V (2000) Cardiac aldosterone production and ventricular remodeling. Kidney Int 57:1346–1351
Dickinson KM, Keogh JB, Clifton PM (2009) Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr 89:485–490
Ding H, Wu B, Wang H, Lu Z, Yan J, Wang X, Shaffer JR, Hui R, Wang DW (2010) A novel loss-of-function DDAH1 promoter polymorphism is associated with increased susceptibility to thrombosis stroke and coronary heart disease. Circ Res 106:1145–1152
Dmitrieva NI, Burg MB (2014) Secretion of von Willebrand factor by endothelial cells links sodium to hypercoagulability and thrombosis. Proc Natl Acad Sci USA 111:6485–6490
Druppel V, Kusche-Vihrog K, Grossmann C, Gekle M, Kasprzak B, Brand E, Pavenstadt H, Oberleithner H, Kliche K (2013) Long-term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J 27:3652–3659
Du CG, Mimran A, Fesler P, Ribstein J, Blacher J, Safar ME (2004) Dietary sodium and pulse pressure in normotensive and essential hypertensive subjects. J Hypertens 22:697–703
Dupont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG (2012) High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens 31:530–536
Dupont JJ, Hill MA, Bender SB, Jaisser F, Jaffe IZ (2014) Aldosterone and vascular mineralocorticoid receptors: regulators of ion channels beyond the kidney. Hypertension 63:632–637
Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston MN, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF (2014) 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129:S76–S99
Ehret GB, Caulfield MJ (2013) Genes for blood pressure: an opportunity to understand hypertension. Eur Heart J 34:951–961
Et-Taouil K, Schiavi P, Levy BI, Plante GE (2001) Sodium intake, large artery stiffness, and proteoglycans in the spontaneously hypertensive rat. Hypertension 38:1172–1176
Fels J, Callies C, Kusche-Vihrog K, Oberleithner H (2010) Nitric oxide release follows endothelial nanomechanics and not vice versa. Pflugers Arch 460:915–923
Fels J, Jeggle P, Liashkovich I, Peters W, Oberleithner H (2014) Nanomechanics of vascular endothelium. Cell Tissue Res 355:727–737
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376
Gates PE, Tanaka H, Hiatt WR, Seals DR (2004) Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension 44:35–41
Geneva: World Health Organization (2012) WHO Guideline: Sodium Intake for Adults and Children
Gorelik J, Zhang Y, Sanchez D, Shevchuk A, Frolenkov G, Lab M, Klenerman D, Edwards C, Korchev Y (2005) Aldosterone acts via an ATP autocrine/paracrine system: the Edelman ATP hypothesis revisited. Proc Natl Acad Sci USA 102:15000–15005
Handy DE, Castro R, Loscalzo J (2011) Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation 123:2145–2156
He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA (2009) Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension 54:482–488
He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA (2005) Plasma sodium: ignored and underestimated. Hypertension 45:98–102
Hu X, Xu X, Zhu G, Atzler D, Kimoto M, Chen J, Schwedhelm E, Luneburg N, Boger RH, Zhang P, Chen Y (2009) Vascular endothelial-specific dimethylarginine dimethylaminohydrolase-1-deficient mice reveal that vascular endothelium plays an important role in removing asymmetric dimethylarginine. Circulation 120:2222–2229
Iozzo RV (2005) Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol 6:646–656
Jeggle P, Callies C, Tarjus A, Fassot C, Fels J, Oberleithner H, Jaisser F, Kusche-Vihrog K (2013) Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertension 61:1053–1059
Joffe HV, Adler GK (2005) Effect of aldosterone and mineralocorticoid receptor blockade on vascular inflammation. Heart Fail Rev 10:31–37
Kelly TN, He J (2012) Genomic epidemiology of blood pressure salt sensitivity. J Hypertens 30:861–873
Kelly TN, Rice TK, Gu D, Hixson JE, Chen J, Liu D, Jaquish CE, Bazzano LA, Hu D, Ma J, Gu CC, Huang J, Hamm LL, He J (2009) Novel genetic variants in the alpha-adducin and guanine nucleotide binding protein beta-polypeptide 3 genes and salt sensitivity of blood pressure. Am J Hypertens 22:985–992
Klaus D, Hoyer J, Middeke M (2010) Salt restriction for the prevention of cardiovascular disease. Dtsch Arztebl Int 107:457–462
Klenke S, Kussmann M, Siffert W (2011) The GNB3 C825T polymorphism as a pharmacogenetic marker in the treatment of hypertension, obesity, and depression. Pharmacogenet Genomics 21:594–606
Kolkhof P, Borden SA (2012) Molecular pharmacology of the mineralocorticoid receptor: prospects for novel therapeutics. Mol Cell Endocrinol 350:310–317
Koltsova SV, Trushina Y, Haloui M, Akimova OA, Tremblay J, Hamet P, Orlov SN (2012) Ubiquitous [Na+]i/[K+]i-sensitive transcriptome in mammalian cells: evidence for Ca(2+)i-independent excitation-transcription coupling. PLoS One 7:e38032
Korte S, Strater AS, Druppel V, Oberleithner H, Jeggle P, Grossmann C, Fobker M, Nofer JR, Brand E, Kusche-Vihrog K (2014) Feedforward activation of endothelial ENaC by high sodium. FASEB J 28:4015–4025
Korte S, Wiesinger A, Straeter AS, Peters W, Oberleithner H, Kusche-Vihrog K (2011) Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflugers Arch 463:269–278
Kotchen TA, Cowley AW Jr, Frohlich ED (2013) Salt in health and disease—a delicate balance. N Engl J Med 368:2531–2532
Kusche-Vihrog K, Jeggle P, Oberleithner H (2013) The role of ENaC in vascular endothelium. Pflugers Arch. doi:10.1007/s00424-013-1356-3
Kusche-Vihrog K, Sobczak K, Bangel N, Wilhelmi M, Nechyporuk-Zloy V, Schwab A, Schillers H, Oberleithner H (2008) Aldosterone and amiloride alter ENaC abundance in vascular endothelium. Pflugers Arch 455:849–857
Lang F (2011) Stiff endothelial cell syndrome in vascular inflammation and mineralocorticoid excess. Hypertension 57:146–147
Laurent S, Cockcroft J, Van BL, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H (2006) Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27:2588–2605
Lee HY, Oh BH (2010) Aging and arterial stiffness. Circ J 74:2257–2262
Leiper J, Nandi M (2011) The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov 10:277–291
Li C, Yang X, He J, Hixson JE, Gu D, Rao DC, Shimmin LC, Huang J, Gu CC, Chen J, Li J, Kelly TN (2014) A gene-based analysis of variants in the serum/glucocorticoid regulated kinase (SGK) genes with blood pressure responses to sodium intake: the GenSalt Study. PLoS One 9:e98432
Li J, White J, Guo L, Zhao X, Wang J, Smart EJ, Li XA (2009) Salt inactivates endothelial nitric oxide synthase in endothelial cells. J Nutr 139:447–451
Lifton RP (1996) Molecular genetics of human blood pressure variation. Science 272:676–680
Liu Y, Liu P, Yang C, Cowley AW Jr, Liang M (2014) Base-resolution maps of 5-methylcytosine and 5-hydroxymethylcytosine in Dahl S rats: effect of salt and genomic sequence. Hypertension 63:827–838
Luft FC, Miller JZ, Cohen SJ, Fineberg NS, Weinberger MH (1988) Heritable aspects of salt sensitivity. Am J Cardiol 61:1H–6H
Luscher TF, Raij L, Vanhoutte PM (1987) Endothelium-dependent vascular responses in normotensive and hypertensive Dahl rats. Hypertension 9:157–163
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De BG, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De BM, De GS, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34:2159–2219
Manunta P, Lavery G, Lanzani C, Braund PS, Simonini M, Bodycote C, Zagato L, Delli CS, Tantardini C, Brioni E, Bianchi G, Samani NJ (2008) Physiological interaction between alpha-adducin and WNK1-NEDD4L pathways on sodium-related blood pressure regulation. Hypertension 52:366–372
Mazzochi C, Bubien JK, Smith PR, Benos DJ (2006) The carboxyl terminus of the alpha-subunit of the amiloride-sensitive epithelial sodium channel binds to F-actin. J Biol Chem 281:6528–6538
Mei H, Gu D, Hixson JE, Rice TK, Chen J, Shimmin LC, Schwander K, Kelly TN, Liu DP, Chen S, Huang JF, Jaquish CE, Rao DC, He J (2012) Genome-wide linkage and positional association study of blood pressure response to dietary sodium intervention: the GenSalt Study. Am J Epidemiol 176(Suppl 7):S81–S90
Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA (2005) Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85:679–715
Miyaki K, Tohyama S, Murata M, Kikuchi H, Takei I, Watanabe K, Omae K (2005) Salt intake affects the relation between hypertension and the T-786C polymorphism in the endothelial nitric oxide synthase gene. Am J Hypertens 18:1556–1562
Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J (2014) Global sodium consumption and death from cardiovascular causes. N Engl J Med 371:624–634
Nagase M, Matsui H, Shibata S, Gotoda T, Fujita T (2007) Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension 50:877–883
Nguyen Dinh CA, Griol-Charhbili V, Loufrani L, Labat C, Benjamin L, Farman N, Lacolley P, Henrion D, Jaisser F (2010) The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J 24:2454–2463
Ni Z, Vaziri ND (2001) Effect of salt loading on nitric oxide synthase expression in normotensive rats. Am J Hypertens 14:155–163
Nurnberger J, Opazo SA, Mitchell A, Buhrmann S, Wenzel RR, Siffert W, Philipp T, Schafers RF (2004) The T-allele of the C825T polymorphism is associated with higher arterial stiffness in young healthy males. J Hum Hypertens 18:267–271
O'Riordan TG, Donn KH, Hodsman P, Ansede JH, Newcomb T, Lewis SA, Flitter WD, White VS, Johnson MR, Montgomery AB, Warnock DG, Boucher RC (2014) Acute hyperkalemia associated with inhalation of a potent ENaC antagonist: phase 1 trial of GS-9411. J Aerosol Med Pulm Drug Deliv 27:200–208
Oberleithner H (2012) Two barriers for sodium in vascular endothelium? Ann Med 44(Suppl 1):S143–S148
Oberleithner H (2014) Sodium selective erythrocyte glycocalyx and salt sensitivity in man. Pflugers Arch. doi:10.1007/s00424-014-1577-0
Oberleithner H, Peters W, Kusche-Vihrog K, Korte S, Schillers H, Kliche K, Oberleithner K (2011) Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch 462:519–528
Oberleithner H, Riethmuller C, Ludwig T, Hausberg M, Schillers H (2006) Aldosterone remodels human endothelium. Acta Physiol (Oxf) 187:305–312
Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M (2007) Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA 104:16281–16286
Oberleithner H, Schneider SW, Albermann L, Hillebrand U, Ludwig T, Riethmüller C, Shahin V, Schäfer C, Schillers H (2003) Endothelial cell swelling by aldosterone. J Membr Biol 196:163–172
Oda T, Makino K, Yamashita I, Namba K, Maeda Y (2001) Distinct structural changes detected by X-ray fiber diffraction in stabilization of F-actin by lowering pH and increasing ionic strength. Biophys J 80:841–851
Oguchi H, Sasamura H, Shinoda K, Morita S, Kono H, Nakagawa K, Ishiguro K, Hayashi K, Nakamura M, Azegami T, Oya M, Itoh H (2014) Renal arteriolar injury by salt intake contributes to salt memory for the development of hypertension. Hypertension 64:784–791
Paar M, Pavenstadt H, Kusche-Vihrog K, Druppel V, Oberleithner H, Kliche K (2014) Endothelial sodium channels trigger endothelial salt sensitivity with aging. Hypertension 64:391–396
Partovian C, Benetos A, Pommies JP, Mischler W, Safar ME (1998) Effects of a chronic high-salt diet on large artery structure: role of endogenous bradykinin. Am J Physiol 274:H1423–H1428
Pase MP, Grima NA, Sarris J (2011) The effects of dietary and nutrient interventions on arterial stiffness: a systematic review. Am J Clin Nutr 93:446–454
Perez FR, Venegas F, Gonzalez M, Andres S, Vallejos C, Riquelme G, Sierralta J, Michea L (2009) Endothelial epithelial sodium channel inhibition activates endothelial nitric oxide synthase via phosphoinositide 3-kinase/Akt in small-diameter mesenteric arteries. Hypertension 53:1000–1007
Perticone F, Sciacqua A, Barlassina C, Del VL, Signorello MC, Dal FC, Andreozzi F, Sesti G, Cusi D (2007) Gly460Trp alpha-adducin gene polymorphism and endothelial function in untreated hypertensive patients. J Hypertens 25:2234–2239
Pesen D, Hoh JH (2005) Micromechanical architecture of the endothelial cell cortex. Biophys J 88:670–679
Pojoga LH, Williams JS, Yao TM, Kumar A, Raffetto JD, do Nascimento GR, Reslan OM, Adler GK, Williams GH, Shi Y, Khalil RA (2011) Histone demethylase LSD1 deficiency during high-salt diet is associated with enhanced vascular contraction, altered NO-cGMP relaxation pathway, and hypertension. Am J Physiol Heart Circ Physiol 301:H1862-H1871
Pollard TD, Cooper JA (2009) Actin, a central player in cell shape and movement. Science 326:1208–1212
Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D (2013) Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 3:e003733
Pries AR, Secomb TW, Gaehtgens P (2000) The endothelial surface layer. Pflugers Arch 440:653–666
Rakova N, Juttner K, Dahlmann A, Schroder A, Linz P, Kopp C, Rauh M, Goller U, Beck L, Agureev A, Vassilieva G, Lenkova L, Johannes B, Wabel P, Moissl U, Vienken J, Gerzer R, Eckardt KU, Muller DN, Kirsch K, Morukov B, Luft FC, Titze J (2013) Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab 17:125–131
Rao AD, Sun B, Saxena A, Hopkins PN, Jeunemaitre X, Brown NJ, Adler GK, Williams JS (2013) Polymorphisms in the serum- and glucocorticoid-inducible kinase 1 gene are associated with blood pressure and renin response to dietary salt intake. J Hum Hypertens 27:176–180
Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG (2007) The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 454:345–359
Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, Boase NA, Perrier R, Maillard M, Yang B, Stokes JB, Koesters R, Kumar S, Hummler E, Loffing J, Staub O (2013) Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest 123:657–665
Rotin D, Staub O (2011) Role of the ubiquitin system in regulating ion transport. Pflugers Arch 461:1–21
Safar ME, Benetos A (2003) Factors influencing arterial stiffness in systolic hypertension in the elderly: role of sodium and the renin-angiotensin system. Am J Hypertens 16:249–258
Safar ME, Levy BI, Struijker-Boudier H (2003) Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 107:2864–2869
Safar ME, Temmar M, Kakou A, Lacolley P, Thornton SN (2009) Sodium intake and vascular stiffness in hypertension. Hypertension 54:203–209
Sanders PW (2009) Vascular consequences of dietary salt intake. Am J Physiol Renal Physiol 297:F237–F243
Sato A, Saruta T (2003) Aldosterone breakthrough during angiotensin-converting enzyme inhibitor therapy. Am J Hypertens 16:781–788
Sato A, Saruta T, Funder JW (2006) Combination therapy with aldosterone blockade and renin-angiotensin inhibitors confers organ protection. Hypertens Res 29:211–216
Schild L (2010) The epithelial sodium channel and the control of sodium balance. Biochimica Biophysica Acta 1802:1159–1165
Sciarrone MT, Stella P, Barlassina C, Manunta P, Lanzani C, Bianchi G, Cusi D (2003) ACE and alpha-adducin polymorphism as markers of individual response to diuretic therapy. Hypertension 41:398–403
Sehestedt T, Jeppesen J, Hansen TW, Rasmussen S, Wachtell K, Ibsen H, Torp-Pedersen C, Olsen MH (2012) Thresholds for pulse wave velocity, urine albumin creatinine ratio and left ventricular mass index using SCORE, Framingham and ESH/ESC risk charts. J Hypertens 30:1928–1936
Sehestedt T, Jeppesen J, Hansen TW, Wachtell K, Ibsen H, Torp-Pedersen C, Hildebrandt P, Olsen MH (2010) Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J 31:883–891
Seidlerova J, Staessen JA, Bochud M, Nawrot T, Casamassima N, Citterio L, Kuznetsova T, Jin Y, Manunta P, Richart T, Struijker-Boudier HA, Fagard R, Filipovsky J, Bianchi G (2009) Arterial properties in relation to genetic variations in the adducin subunits in a white population. Am J Hypertens 22:21–26
Sessa WC (2004) eNOS at a glance. J Cell Sci 117:2427–2429
Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR Jr, Ulick S, Milora RV, Findling JW (1994) Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79:407–414
Siegel G, Walter A, Kauschmann A, Malmsten M, Buddecke E (1996) Anionic biopolymers as blood flow sensors. Biosens Bioelectron 11:281–294
Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D (1996) WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na + channel deleted in Liddle's syndrome. EMBO J 15:2371–2380
Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP (2009) Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ 339:b4567
Struthers AD, MacDonald TM (2004) Review of aldosterone- and angiotensin II-induced target organ damage and prevention. Cardiovasc Res 61:663–670
Suckling RJ, He FJ, Markandu ND, MacGregor GA (2012) Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney Int 81:407–411
Tarbell JM, Ebong EE (2008) The endothelial glycocalyx: a mechano-sensor and -transducer. Sci Signal 1:t8
Teiwes J, Toto RD (2007) Epithelial sodium channel inhibition in cardiovascular disease. A potential role for amiloride. Am J Hypertens 20:109–117
Titze J, Ritz E (2009) Salt and its effect on blood pressure and target organ damage: new pieces in an old puzzle. J Nephrol 22:177–189
Toda N, Arakawa K (2011) Salt-induced hemodynamic regulation mediated by nitric oxide. J Hypertens 29:415–424
Torielli L, Tivodar S, Montella RC, Iacone R, Padoani G, Tarsini P, Russo O, Sarnataro D, Strazzullo P, Ferrari P, Bianchi G, Zurzolo C (2008) alpha-Adducin mutations increase Na/K pump activity in renal cells by affecting constitutive endocytosis: implications for tubular Na reabsorption. Am J Physiol Renal Physiol 295:F478–F487
Tripodi G, Valtorta F, Torielli L, Chieregatti E, Salardi S, Trusolino L, Menegon A, Ferrari P, Marchisio PC, Bianchi G (1996) Hypertension-associated point mutations in the adducin alpha and beta subunits affect actin cytoskeleton and ion transport. J Clin Invest 97:2815–2822
Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P, Nissinen A (2001) Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet 357:848–851
Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De BT, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T (2012) Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 30:445–448
Vlachopoulos C, Aznaouridis K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55:1318–1327
Wain LV, Verwoert GC, O'Reilly PF, Shi G, Johnson T, Johnson AD, Bochud M, Rice KM, Henneman P, Smith AV, Ehret GB, Amin N, Larson MG, Mooser V, Hadley D, Dorr M, Bis JC, Aspelund T, Esko T, Janssens AC, Zhao JH, Heath S, Laan M, Fu J, Pistis G, Luan J, Arora P, Lucas G, Pirastu N, Pichler I, Jackson AU, Webster RJ, Zhang F, Peden JF, Schmidt H, Tanaka T, Campbell H, Igl W, Milaneschi Y, Hottenga JJ, Vitart V, Chasman DI, Trompet S, Bragg-Gresham JL, Alizadeh BZ, Chambers JC, Guo X, Lehtimaki T, Kuhnel B, Lopez LM, Polasek O, Boban M, Nelson CP, Morrison AC, Pihur V, Ganesh SK, Hofman A, Kundu S, Mattace-Raso FU, Rivadeneira F, Sijbrands EJ, Uitterlinden AG, Hwang SJ, Vasan RS, Wang TJ, Bergmann S, Vollenweider P, Waeber G, Laitinen J, Pouta A, Zitting P, McArdle WL, Kroemer HK, Volker U, Volzke H, Glazer NL, Taylor KD, Harris TB, Alavere H, Haller T, Keis A, Tammesoo ML, Aulchenko Y, Barroso I, Khaw KT, Galan P, Hercberg S, Lathrop M, Eyheramendy S, Org E, Sober S, Lu X, Nolte IM, Penninx BW, Corre T, Masciullo C, Sala C, Groop L, Voight BF, Melander O, O’Donnell CJ, Salomaa V, D'Adamo AP, Fabretto A, Faletra F, Ulivi S, Del GF, Facheris M, Collins FS, Bergman RN, Beilby JP, Hung J, Musk AW, Mangino M, Shin SY, Soranzo N, Watkins H, Goel A, Hamsten A, Gider P, Loitfelder M, Zeginigg M, Hernandez D, Najjar SS, Navarro P, Wild SH, Corsi AM, Singleton A, de Geus EJ, Willemsen G, Parker AN, Rose LM, Buckley B, Stott D, Orru M, Uda M, van der Klauw MM, Zhang W, Li X, Scott J, Chen YD, Burke GL, Kahonen M, Viikari J, Doring A, Meitinger T, Davies G, Starr JM, Emilsson V, Plump A, Lindeman JH, Hoen PA, Konig IR, Felix JF, Clarke R, Hopewell JC, Ongen H, Breteler M, Debette S, Destefano AL, Fornage M, Mitchell GF, Smith NL, Holm H, Stefansson K, Thorleifsson G, Thorsteinsdottir U, Samani NJ, Preuss M, Rudan I, Hayward C, Deary IJ, Wichmann HE, Raitakari OT, Palmas W, Kooner JS, Stolk RP, Jukema JW, Wright AF, Boomsma DI, Bandinelli S, Gyllensten UB, Wilson JF, Ferrucci L, Schmidt R, Farrall M, Spector TD, Palmer LJ, Tuomilehto J, Pfeufer A, Gasparini P, Siscovick D, Altshuler D, Loos RJ, Toniolo D, Snieder H, Gieger C, Meneton P, Wareham NJ, Oostra BA, Metspalu A, Launer L, Rettig R, Strachan DP, Beckmann JS, Witteman JC, Erdmann J, van Dijk KW, Boerwinkle E, Boehnke M, Ridker PM, Jarvelin MR, Chakravarti A, Abecasis GR, Gudnason V, Newton-Cheh C, Levy D, Munroe PB, Psaty BM, Caulfield MJ, Rao DC, Tobin MD, Elliott P, van Duijn CM (2011) Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet 43:1005–1011
Warnock DG (2013) The amiloride-sensitive endothelial sodium channel and vascular tone. Hypertension 61:952–954
Warnock DG, Kusche-Vihrog K, Tarjus A, Sheng S, Oberleithner H, Kleyman TR, Jaisser F (2013) Blood pressure and amiloride-sensitive sodium channels in vascular and renal cells. Nat Rev Nephrol. doi:10.1038/nrneph.2013.275
Weber S, Bernhard D, Lukowski R, Weinmeister P, Worner R, Wegener JW, Valtcheva N, Feil S, Schlossmann J, Hofmann F, Feil R (2007) Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ Res 101:1096–1103
Wenzel K, Felix S, Kleber FX, Brachold R, Menke T, Schattke S, Schulte KL, Glaser C, Rohde K, Baumann G (1994) E-selectin polymorphism and atherosclerosis: an association study. Hum Mol Genet 3:1935–1937
Wenzel RR, Siffert W, Bruck H, Philipp T, Schafers RF (2002) Enhanced vasoconstriction to endothelin-1, angiotensin II and noradrenaline in carriers of the GNB3 825T allele in the skin microcirculation. Pharmacogenetics 12:489–495
Wildling L, Hinterdorfer P, Kusche-Vihrog K, Treffner Y, Oberleithner H (2009) Aldosterone receptor sites on plasma membrane of human vascular endothelium detected by a mechanical nanosensor. Pflugers Arch 458:223–230
Williams JS, Chamarthi B, Goodarzi MO, Pojoga LH, Sun B, Garza AE, Raby BA, Adler GK, Hopkins PN, Brown NJ, Jeunemaitre X, Ferri C, Fang R, Leonor T, Cui J, Guo X, Taylor KD, Ida Chen YD, Xiang A, Raffel LJ, Buchanan TA, Rotter JI, Williams GH, Shi Y (2012) Lysine-specific demethylase 1: an epigenetic regulator of salt-sensitive hypertension. Am J Hypertens 25:812–817
Wojciechowska W, Staessen JA, Stolarz K, Nawrot T, Filipovsky J, Ticha M, Bianchi G, Brand E, Cwynar M, Grodzicki T, Kuznetsova T, Struijker-Boudier HA, Svobodova V, Thijs L, Van Bortel LM, Kawecka-Jaszcz K (2004) Association of peripheral and central arterial wave reflections with the CYP11B2–344C allele and sodium excretion. J Hypertens 22:2311–2319
Zhao Q, Gu D, Hixson JE, Liu DP, Rao DC, Jaquish CE, Kelly TN, Lu F, Ma J, Mu J, Shimmin LC, Chen J, Mei H, Hamm LL, He J (2011) Common variants in epithelial sodium channel genes contribute to salt sensitivity of blood pressure: The GenSalt study. Circ Cardiovasc Genet 4:375–380
Zieman SJ, Melenovsky V, Kass DA (2005) Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25:932–943
Acknowledgments
This work was supported by grants of the Deutsche Forschungsgemeinschaft (Koselleck OB 63/18, KU 1496/7-1), the Else-Kröner-Fresenius Stiftung (2010_A116), “Innovative Medical Research” (IMF) of the University of Münster (KU 120808), and by the Centre of Excellence (Cells in Motion; CIM), University of Münster. EB was supported by a Heisenberg professorship from the Deutsche Forschungsgemeinschaft (Br1589/8-2). The authors would also thank COST Action TD1002 and COST Action BM 1301 for supporting their networking activities.
Conflict of interests
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kusche-Vihrog, K., Schmitz, B. & Brand, E. Salt controls endothelial and vascular phenotype. Pflugers Arch - Eur J Physiol 467, 499–512 (2015). https://doi.org/10.1007/s00424-014-1657-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1657-1