Abstract
Intestinal epithelial electrolyte secretion is activated by increase in intracellular cAMP or Ca2+ and opening of apical Cl− channels. In infants and young animals, but not in adults, Ca2+-activated chloride channels may cause secretory diarrhea during rotavirus infection. While detailed knowledge exists concerning the contribution of cAMP-activated cystic fibrosis transmembrane conductance regulator (CFTR) channels, analysis of the role of Ca2+-dependent Cl− channels became possible through identification of the anoctamin (TMEM16) family of proteins. We demonstrate expression of several anoctamin paralogues in mouse small and large intestines. Using intestinal-specific mouse knockout models for anoctamin 1 (Ano1) and anoctamin 10 (Ano10) and a conventional knockout model for anoctamin 6 (Ano6), we demonstrate the role of anoctamins for Ca2+-dependent Cl− secretion induced by the muscarinic agonist carbachol (CCH). Ano1 is preferentially expressed in the ileum and large intestine, where it supports Ca2+-activated Cl− secretion. In contrast, Ano10 is essential for Ca2+-dependent Cl− secretion in jejunum, where expression of Ano1 was not detected. Although broadly expressed, Ano6 has no role in intestinal cholinergic Cl− secretion. Ano1 is located in a basolateral compartment/membrane rather than in the apical membrane, where it supports CCH-induced Ca2+ increase, while the essential and possibly only apical Cl− channel is CFTR. These results define a new role of Ano1 for intestinal Ca2+-dependent Cl− secretion and demonstrate for the first time a contribution of Ano10 to intestinal transport.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Electrolyte secretion in the intestine requires Cl− channels in the apical membrane of epithelial cells. Electrolyte secretion is controlled by a number of hormones leading to increase in either intracellular cAMP or Ca2+, with consecutive activation of cystic fibrosis transmembrane conductance regulator (CFTR) and Ca2+-activated Cl− channels (CaCC), respectively [14, 24]. While the contribution of CFTR to intestinal Cl− secretion is well defined, controversial results have been reported for CaCC, which has been identified recently as anoctamin 1 (TMEM16A; Ano1) and which was detected in the basolateral rather than in the apical membrane of adult intestinal epithelial cells [6, 16, 22, 38, 44, 55]. The role of Ano1 for intestinal Cl− secretion, and also whether Ano1 is the only relevant anoctamin in the intestine, is therefore unclear.
We earlier reported that neonatal mice lacking expression of Ano1 do not show Ca2+-dependent Cl− secretion in airways, salivary glands, and distal colon (37). However, total knockout of Ano1 led to severely ill animals, which died within 3 days after birth. We therefore could not completely rule out the possibility that lack of colonic Ca2+-dependent Cl− secretion is caused by secondary effects occurring in these diseased animals. In the present report, we examined the effects of tissue-specific knockouts for Ano1 and Ano10 in intestinal epithelial cells and examined the effects of a conventional Ano6 knockout on mouse Ca2+-dependent intestinal Cl− secretion. The results establish a clear role of Ano1 for Ca2+-dependent Cl− secretion in the large intestine, while Ano10 controls Ca2+-dependent Cl− secretion in the small intestine. However, the role of Ano1 is to support proper intracellular Ca2+ signaling rather than acting as a luminal secretory Cl− channel.
Materials and methods
Generation of knockout models
Generation of the Ano1fl allele Ano1Tm2JRR has been described in a previous publication [12]. In brief, to produce the Ano1fl allele Ano1Tm2JRR, a portion of BAC bMQ-379H21 (129S7/SvEv Brd-Hprt b-m2, AB2.2 embryonic stem (ES) cell DNA) was subcloned. A LoxP site was inserted 161 bp upstream of exon 12 (the same exon replaced in Ano1tm1Bdh). A PGK-neo cassette flanked by FRT sites for positive selection in ES cells was inserted downstream of exon 12, followed by a second LoxP site. The construct was linearized and electroporated into 129S6/SvEvTac ES cells by the Duke University Medical Center Transgenic Mouse Facility. Correctly targeted clones were identified by Southern blot and were injected into C57BL/6 blastocysts, which were transferred into the uteri of foster female mice. Cre transgenic mice containing a Cre-expression cassette under the control of the epithelial-specific villin promoter were crossed with Ano1fl/fl animals.
The Ano10 targeting construct (pTMEM16K_targ.) was designed as follows. The 5.6-kb right flanking region containing exons 8 and 9 and intronic sequences was PCR-amplified and subcloned. A 1.0-kb left flanking region containing intron 6 genomic sequences and a 0.3-kb exon 7 genomic region together with intronic sequences were PCR-amplified and subcloned. The exon 7 flanking LoxP site was introduced by PCR. All individual clones were verified by sequencing and assembled into the final targeting construct (Supplementary Fig. S1). The pBluescript-based backbone together with the negative selection marker (thymidine kinase cassette and diphtheria toxin gene) was added to the left flanking region. The positive selection marker (neomycin cassette flanked by two FRT sites and one LoxP site) was cloned as EcoRI – BamHI DNA fragment between left flanking region and 0.3-kb exon 7 genomic PCR clone. Positively targeted ES cell clones were analyzed using Southern blots. Positively targeted ES cells were identified and injected into B6D2F1 blastocysts and transferred into the uteri of 2.5-day pseudopregnant CD-1 foster mice. Chimeras were identified by their agouti coat color contribution. For the germ line transmission, high-percentage male chimeras were crossed to the C57BL/6 J female mice and heterozygous offsprings were confirmed by Southern blotting (Supplementary Fig. S2). All mouse procedures were performed in compliance with the guidelines for the welfare of experimental animals issued by the Federal Government of Germany. The mouse line was established by breeding male with female C57Bl/6 J mice to produce heterozygous mice.
Generation of Ano6 (TMEM16F) knockout mice has been described earlier [10]. These mice were kindly provided by Prof. Dr. A. Vortkamp (Department of Developmental Biology, University of Essen, Germany). Bleeding tests were performed as described in Elvers et al. [11].
Ussing chamber
Mice were killed after exposure to CO2, and the jejunum, ileum, and proximal and distal colon were removed. Stripped intestinal sections were put into ice-cold Ringer bath solution (in mM; NaCl 145, KH2PO4 0.4, K2HPO4 1.6, D-glucose 6, MgCl2 1, Ca-gluconate 1.3, pH 7.4) containing indomethacin (10 μM). Tissues were mounted into a micro-perfused Ussing chamber with a circular aperture of 0.785 mm2. Luminal and basolateral sides of the epithelium were perfused continuously at a rate of 5 ml/min. Bath solutions were heated to 37 °C, using a water jacket. Experiments were carried out under open circuit conditions. Data were collected continuously using PowerLab (AD Instruments, Australia). Values for transepithelial voltages (V te) were referred to the serosal side of the epithelium. Transepithelial resistance (R te) was determined by applying short (1 s) current pulses (ΔI = 0.5 μA). R te and equivalent short circuit currents (I′SC) were calculated according to Ohm’s law (R te = ΔVte/ΔI, I′SC = V te/R te).
RT-PCR
Crypts and villi were isolated in Ca2+-free Ringer solution. Total RNA (2 μg) was reverse-transcribed, and multiplex reverse transcription PCR (RT-PCR) was performed using 0.5 μM primers [43].
Western blot of Ano1
Lysates were prepared from isolated crypts and villi using lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 100 mM DTT, 1 % NP-40) and 1 % protease inhibitor cocktail (Roche). Proteins were separated on 5 or 7.5 % sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (GE Healthcare) by wet electroblotting (BioRad). Membranes were incubated overnight at 4 °C with a polyclonal rabbit anti-mouse Ano1 antibody (kindly provided by Dr. B. Harfe, University of Florida, Gainesville, USA). Proteins were visualized using a horseradish-peroxidase-conjugated secondary antibody and Super Signal west pico (Thermo Scientific).
Immunohistochemistry
Affinity-purified polyclonal antiserum against mouse or human Ano1 was produced in rabbits immunized with (mouse) NHSPTTHPEAGDGSPVPSYE (aa957-976, C-terminus) coupled to keyhole limpet hemocyanin (Davids Biotechnologie, Regensburg, Germany). Mouse intestine was fixed by perfusion with 4 % paraformaldehyde (PFA) and post-fixed in 0.5 mol/l sucrose, 4 % PFA solution. Cryosections of 5 μm were incubated in 0.1 % SDS for 5 min, washed with PBS, and blocked with 5 % bovine serum albumin (BSA) and 0.04 % Triton X-100 in PBS for 30 min. Sections were incubated with primary antibodies in 0.5 % BSA and 0.04 % Triton X-100 overnight at 4 °C and with Alexa Fluor 488 labeled donkey anti rabbit IgG (Invitrogen). Sections were counterstained with Hoe33342 (Sigma-Aldrich). Immunofluorescence was detected using an Axiovert 200 microscope equipped with ApoTome and AxioVision (Zeiss, Germany).
Intracellular Ca2+ concentrations and organoid cultures
Intracellular Ca2+ concentrations have been measured on isolated colonic crypts using Fura 2 as described earlier [21]. Mouse intestinal epithelial organoid and measurement of rapid carbachol (CCH)-induced swelling of organoids has been adopted from Dekkers et al. [8].
Results
Intestinal expression of anoctamins and Ca2+-dependent Cl− secretion
We analyzed expression of all ten anoctamin paralogues in isolated epithelial cells of mouse small (jejunum and ileum) and large (proximal and distal colon) intestine (Fig. 1a). Ano1 was clearly detected in the colon, showed weak expression in the ileum, and was absent in the jejunum. Transcripts for a number of other anoctamin paralogues were detected in the small and large intestines, including Ano6 and Ano10. Ca2+-dependent ion transport was measured in stripped intestinal mucosa under open circuit conditions. Stimulation of basolateral muscarinic receptors with 100 μM carbachol (CCH) induced transient negative voltage deflections in all intestinal tissues, and activation of an equivalent short circuit current in the small and large intestines, which is due to activation of apical Cl− channels and electrolyte secretion (Fig. 1b, c) [24, 33].
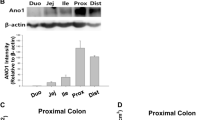
Expression of anoctamins in intestinal epithelial cells. a RT-PCR analysis of all ten anoctamins in freshly isolated epithelial cells from mouse small and large intestine. b Original recordings of the transepithelial voltage in intestinal mucosa obtained in micro-Ussing chambers under open circuit conditions. Basolateral application of CCH (100 μM) induced negative voltage deflections, indicating Ca2+-dependent activation of luminal Cl− channels. c Calculated equivalent short circuit currents activated by CCH in mouse small and large intestinal mucosa. Mean ± SEM; number of animals is enclosed in parentheses
Knockout of Ano1 eliminates intestinal Ca2+-dependent Cl− secretion
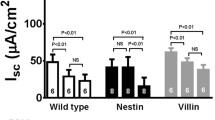
Transgenic mice containing a Cre-expression cassette under the control of the epithelial-specific villin promoter were crossed with floxed Ano1fl/fl animals to eliminate Exon12 (LoxP/LoxP-Crevil) and abolish expression of Ano1 specifically in intestinal epithelial cells (Fig. 2a, b). Immunohistochemistry, RT-PCR, and Western blotting indicated successful knockdown of Ano1 in LoxP/LoxP-Crevil animals (Fig. 2c–f). Immunocytochemistry indicated that Ano1 is primarily expressed in the basolateral compartment and/or membrane of adult intestinal epithelial cells (Fig. 2c and Supplementary Fig. S3). Expression of Ano1 is more pronounced in the distal when compared to the proximal colon. In the distal colon, we find expression particularly in the mid-crypt region and upper part of the crypts, less in the basal part (Fig. S3). Using open circuit Ussing chamber recordings, we examined CCH-induced Cl− secretion in wild-type controls, which was inhibited by niflumic acid (NFA; 100 μM). The corresponding transepithelial resistances under control and after stimulation with CCH are summarized in Supplementary Table S1. In contrast, Ca2+-activated Cl− secretion and effects of NFA were almost completely absent in the ileum and proximal and distal colon of mice lacking intestinal Ano1 expression. These results clearly indicate the role of Ano1 for Ca2+-dependent Cl− secretion in the ileum and large intestine (Fig. 3). In the jejunum, which did not reveal any expression of Ano1 in intestinal epithelial cells (Fig. 1), no changes in CCH-induced Cl− secretion was observed in LoxP/LoxP-Crevil mice (Fig. 3b). It is therefore concluded that Ano1 accounts for most of the Ca2+-induced Cl− secretion in the large intestine and distal parts of the small intestine, while other anoctamins may be important for Cl− secretion in the jejunum.
Knockout of Ano1 expression in mouse intestinal epithelial cells. a Cleavage of loxP sites to delete exon 12 within the Ano1 locus in mouse intestinal epithelial cells, using Cre-recombinase under the control of a villin promoter. b Genomic PCR from intestinal epithelial cells to identify epithelial specific knockdown of Ano1. c Immunohistochemistry of Ano1 expressed in large intestine of Ano1+/+animals (lox/lox). d Immunohistochemistry of Ano1 expressed in large intestine of Ano1−/− animals (lox/lox-Crevil). e RT-PCR analysis of Ano1-expression in intestinal epithelial cells of Ano1+/+and Ano1−/− animals. f Western blot analysis of Ano1 expression in intestinal epithelial cells of Ano1+/+and Ano1−/− animals. Bar indicates 50 μm
No Ca2+-dependent Cl− secretion in the absence of Ano1. a Original recordings of transepithelial voltages in proximal colon from Ano1+/+mice and mice with intestinal epithelial knockout of Ano1. CCH (100 μM) was unable to induce negative voltage deflections and activation of chloride secretion in the intestine of Ano1−/− knockout animals. b–e Calculated equivalent short circuit currents in Ano1+/+and Ano1−/− animals indicate largely reduced Ca2+-activated Cl− secretion in ileum and colon of Ano1−/− animals, but not in jejunum. Ca2+-dependent Cl− secretion was inhibited by niflumic acid (NFA, 10 μM). Mean ± SEM. Asterisk indicates significant inhibition by NFA (paired t test). Number sign indicates significant difference when compared to Ano1+/+ (unpaired t test). Number of animals is enclosed in parentheses
Basolateral Ano1 supports CCH-induced Ca2+ signaling and secretion via luminal CFTR
We asked how basolateral expression of Ano1 may support Ca2+-dependent Cl− secretion. To that end, we also applied NFA (and another inhibitor of anoctamin, tannic acid) also from the basolateral site of the epithelium and found that both inhibitors potently inhibited CCH-induced Cl− secretion in the proximal colon of wild-type (wt) animals but had much less inhibitory effect in the proximal colon of Ano1−/− animals (Fig. 4a). It is entirely possible that both inhibitors pass the membrane and also inhibit ion currents from the cytosolic side of the membrane. We examined if Ano1 affects CCH-induced intracellular Ca2+ signals, using Fura-2-loaded isolated crypts from the large intestine (Fig. 4b). Remarkably, CCH-induced (100 μM) Ca2+ peak and plateau were significantly reduced in crypts from Ano1−/− animals (Fig. 4c, d). These data support the concept that Ano1 expressed in or close to the basolateral membrane supports Cl− secretion by supporting intracellular Ca2+ increase. Ca2+ is likely to activate basolateral K+ channels and to augment the driving force for apical Cl− secretion, which may happen substantially or even exclusively through CFTR. This has been shown earlier for human intestine [29]. To further examine the contribution of apical CFTR to CCH-activated Cl− secretion, we made use of the specific CFTR inhibitors CFTRinh-172 and GlyH101. However, from earlier studies, we knew that both inhibitors do not work very well in naïve intestinal tissues in Ussing chamber experiments. We therefore adopted the novel intestinal organoid technique, which allows generation of small intestinal organoids grown in a matrigel that allow direct measurement of fluid transport induced by Ca2+ agonists or by increase in intracellular cAMP (Fig. 4e) [8]. Stimulation with 100 μM carbachol induced secretion into the lumen of intestinal organoids, which expanded their lumen and increased the luminal area. Thus, increase in area can be used as a measure for secretion [8]. Notably, a brief incubation with the inhibitors CFTRinh172 and glyH101 (both 20 μM) completely inhibited secretion and thus expansion of the area, suggesting that in the mouse large intestine like in human rectal epithelium, the luminal exit pathway for Ca2+-activated Cl− secretion is CFTR [29] (Fig. 4f).
Basolateral Ano1 supports CCH-induced Ca2+ signaling and secretion via luminal CFTR. a Summary of basolateral application of niflumic acid (NFA; 100 μM) on CCH-induced equivalent short circuit currents in wt and Ano1−/− animals. b Isolated colonic crypt after 30-min incubation with Fura 2 (fluorescence). b Summary time course of intracellular Ca2+ concentrations from 12 experiments showing CCH (100 μM) induced Ca2+ increase which was largely attenuated in crypts from Ano1−/− animals. d Summary of intracellular Ca2+ concentrations and effects of CCH measured in isolated crypts from wt and Ano1−/− animals. e Intestinal organoid grown in matrigel demonstrating luminal expansion upon stimulation with 100 μM CCH. f Summary of organoid luminal area increase induced by CCH and inhibition by 5-min pre-incubation with the specific CFTR inhibitors CFTRinh-172 and GlyH101 (both 20 μM). Number sign indicates significant difference when compared to wt or absence of blockers (unpaired t test). Number of animals, crypts, cells, or organoids is enclosed in parentheses. Asterisk indicates significant inhibition by NFA or TA and stimulation by CCH (paired t test)
Ano6 does not contribute to intestinal Ca2+-dependent Cl− secretion
Ano6 is a broadly expressed anoctamin with relatively high levels of mRNA expression in most mouse tissues and all mammalian cell lines [23, 43]. We made use of conventional Ano6 knockout mice that were shown earlier to have a decreased mineral deposition in skeletal tissues [10]. Ano6 operates as a Ca2+-dependent phospholipid scramblase that is essential for platelet function and proper blood coagulation [17]. Ano6 is defective in the rare Scott syndrome, which is a bleeding disorder based on defective Ano6-mediated scrambling of membrane phospholipids [21]. We analyzed Ano6 mRNA in the small and large intestines and found Ano6 expression throughout the whole intestine of Ano6+/+animals, which was not detectable in Ano6−/− animals (Fig. 1 and Supplementary Fig. S4a). To obtain independent evidence for abolished Ano6 function in Ano6−/− animals, we examined bleeding times and found that they were significantly enhanced in Ano6−/− when compared to heterozygous or Ano6+/+animals (Fig. S4b). We examined CCH-induced Cl− secretion in the ileum and large intestine and found that it was not different between Ano6+/+and Ano6−/− animals. It is therefore unlikely that Ano6 contributes to intestinal Ca2+-dependent Cl− secretion (Fig. 5).
Lack of Ano6-expression does not compromise intestinal Ca2+-dependent Cl− secretion. a Original recordings of transepithelial voltages in proximal colon from Ano6+/+mice and mice with intestinal epithelial knockout of Ano6. CCH (100 μM) induced Cl− secretion was unaffected in Ano6−/− animals. b Equivalent short circuit currents activated by CCH (100 μM) were indistinguishable in ileum and colon of Ano6+/+and Ano6−/− animals. Mean ± SEM. Asterisk indicates significant inhibition by NFA (paired t test). Number of animals is enclosed in parentheses
Ano10 is required for Ca2+-dependent Cl− secretion in jejunum
We recently found evidence that anoctamins other than Ano1, Ano2, or Ano6 produce Cl− currents through receptor-mediated increase in intracellular Ca2+ [49]. Because this was also demonstrated for Ano10, we made use of mice with a tissue-specific knockdown of Ano10 expression in intestinal epithelial cells. To that end, Ano10fl/fl animals were bred with mice containing a Cre-expression cassette under the control of the epithelial-specific villin promoter Exon12 (lox/lox-Crevil) (see “Materials and methods” section). Ano10−/− mice did not express Ano10 mRNA or protein in intestinal epithelial cells (Fig. 6). Remarkably and in contrast to wt littermates, Ca2+-induced Cl− secretion was not detectable in the jejunum of Ano10−/− animals, while the CCH-activated transport was not affected in the large intestine of Ano10−/− animals (Fig. 6c, d). These results support the concept of Ano10 being a Ca2+-dependent Cl− channel and demonstrate for the first time a role of Ano10 for Ca2+-dependent Cl− secretion in the small intestine.
Lack of Ano10 expression eliminates Ca2+-dependent Cl− secretion in jejunum. a RT-PCR analysis of expression of all anoctamins in intestinal epithelial cells of Ano10+/+and Ano10−/− animals. b Western blot analysis of Ano10 expression in jejunal epithelial cells of Ano10+/+and Ano10−/− animals. c Original recordings of transepithelial voltages in jejunum from Ano10+/+mice and mice with intestinal epithelial knockout of Ano10. CCH (100 μM) induced Cl− secretion was abolished in Ano10−/− animals. d Equivalent short circuit currents activated by CCH (100 μM) were indistinguishable in colon and ileum of Ano10+/+and Ano10−/− animals but were abolished in jejunum of Ano10−/− mice. Mean ± SEM. Number sign indicates significant difference when compared to Ano10+/+animals (unpaired t test). Number of animals is enclosed in parentheses
Discussion
Anoctamins
Molecular insight into Ca2+-dependent Cl− secretion has become possible after identification of Ano1 (TMEM16A) as Ca2+-activated Cl− channel [6, 44, 55]. Subsequent reports identified the closest relative of Ano1, Ano2, also as Ca2+-activated Cl− channel, with a lower affinity for Ca2+ [5, 39, 47]. Analysis of the other anoctamin family members showed that they all produce Ca2+-activated whole-cell Cl− and cation currents, when coexpressed with G-protein-coupled receptors (P2Y2) in HEK293 cells, including Ano10 [49].
Ano6 (TMEM16F) is probably the most broadly expressed anoctamin. Compared to the other nine anoctamin paralogues, it shows relatively high transcript levels in mouse tissues [43]. Ano6 has attracted large attention due to its properties as a Ca2+-activated phospholipid scramblase and Ca2+-activated Cl− channel (for review see Kunzelmann et al. [26]). Despite earlier controversies arguing against the role of Ano6 as a Cl− permeable ion channel [54], it has now been clearly shown by several independent groups, to produce a large conductance Cl− permeable channel activated by strong (≥10 μM) increase in intracellular Ca2+ [15, 21, 30, 31, 46, 49]. Moreover, Ano6 has been shown to be activated during cellular volume regulation and apoptotic cell death [1, 20, 21, 31]. In contrast to Ano1 and Ano6, little is known about the role of Ano10 (TMEM16K), apart from its association with cerebellar ataxia [7, 32, 41, 50]. However, previous work suggested that also Ano10 is able to produce Ca2+-activated Cl− currents [43, 49, 51]. The present data indicate expression of a number of anoctamin paralogues in mouse intestine, with Ano1 and Ano6 being upregulated in Ano10−/− intestine (Supplementary Fig. S5).
Role of Ano1 for intestinal Cl− secretion
Ca2+-dependent Cl− secretion was detected in rat and mouse naïve colonic epithelium, in contrast to the human large intestine, which does not express anoctamin 1 [18, 24, 29, 40]. It is noteworthy that analysis of anoctamin expression and Ca2+-dependent transport should take place in naïve intestinal mucosa rather than in cultured epithelial cells, since cultured cells behave differently, even when grown under polarized conditions [24]. At any rate, expression and localization in apical or basolateral membranes of Ano1 appear to be age dependent, with more apical expression in the colon of mice aging 14 days and younger, while expression is shifted to the basolateral membrane in older animals, which have also been examined in the present study [27, 38]. Basolateral expression of Ano1 has also been found in adult guinea pig colon [16]. We demonstrated earlier that Ca2+-mediated Cl− secretion is absent in the colon of Ano1−/− pups [37]. Moreover, in the present study, selective knockout of Ano1 in intestinal epithelial cells also abolished Ca2+-activated Cl− secretion in the adult (8 weeks and older) mouse colon and ileum.
However, according to the present data, Ano1 does not form an apical secretory Cl− channel but rather a basolateral channel, which may also be localized in the endoplasmic reticulum close to the basolateral membrane. The data suggest that Ano1 controls intracellular Ca2+ signaling triggered by stimulation of Gq-coupled receptors such as muscarinic M3 receptors.
Thus, Ano1 supports apical Ca2+-dependent Cl− secretion by maintaining the driving force due to activation of basolateral Ca2+-activated K+ channels. How does Ano1 support Ca2+ signaling? Several mechanisms are possible: (i) Ano1 may be ER-localized and facilitate Ca2+ release by IP3 receptors by operating as a counter ion channel. This concept has been demonstrated earlier for bestrophin 1 [3, 35, 48]. (ii) Ano1 could tether basolateral ER to the plasma membrane and thereby facilitate activation of basolateral Ca2+-activated KCNN4 K+ channels. Remarkably, the yeast homologue of Ano1, Ist2, recruits the endoplasmic reticulum to the plasma membrane [53]. (iii) Also, Ca2+ influx may be controlled by Ano1 either indirectly by supporting Ca2+ store emptying or again, as Cl− bypass channel (Fig. 7). A bypass channel function has been described for Ano1 in renal proximal tubular cells, where electrogenic transport by the proton pump (V-ATPase) is supported by Cl− transport through Ano1 [12].
Basolateral anoctamin 1 supports intestinal Cl− secretion. Transport model for intestinal Ca2+-activated Cl− secretion. (1) Basolateral Ano1 may be localized in the endoplasmic reticulum and facilitate Ca2+ release by IP3 receptors, possibly by operating as a counter ion channel. (2) Ca2+ influx may be controlled by Ano1 either indirectly by supporting Ca2+ store emptying or again, as Cl− bypass channel. (3) Ano1 could tether basolateral ER to the plasma membrane and thereby facilitate activation of basolateral Ca2+-activated KCNN4 K+ channels
CFTR and anoctamins
Ano1 has also gained importance through its role in rotaviral diarrhea. Although the cAMP-regulated Cl− channel cystic fibrosis transmembrane conductance regulator (CFTR) is central to bacterial diarrhea [52], Ca2+-activated Cl− channels seem to play a central role during rotaviral diarrhea [2, 9, 22, 38]. Secretory diarrhea is a major health problem worldwide with rotavirus being the most common cause for severe secretory diarrhea in infants and young children [22]. We reported earlier the expression of Ano1 in both apical and basolateral membranes of colonic epithelial cells of young mice [38]. Evidence was further provided for a role of Ano1 in secretory diarrhea induced by the rotavirus toxin NSP4, which acts through increase in intracellular Ca2+. As mice grow older, expression of Ano1 appears to shift from the luminal toward the basolateral membrane, and cholinergic Ca2+-dependent Cl− secretion was found to be reduced in the older animals [27]. A recent report analyzed rotavirus-induced diarrhea in vivo and in vitro and nicely demonstrates inhibition of Cl− secretion and diarrhea by different blockers of anoctamins [22]. These studies demonstrate the large medical relevance of intestinal anoctamins and may trigger subsequent clinical trials.
Cl− secretion though anoctamins and CFTR
The available data clearly indicate the role of anoctamins for intestinal Ca2+-dependent Cl− secretion and rotavirus-induced diarrhea in younger animals [22, 27, 38]. It was suggested from experiments in Xenopus oocytes coexpressing CFTR and P2Y2 receptors, and also from measurements in human airway epithelial cells, that Ca2+-dependent Cl− secretion elicited through stimulation of purinergic P2Y receptors is due to activation of CFTR rather than anoctamin 1 [13, 34, 42]. Thus, during Ca2+-dependent stimulation, a substantial portion of Cl− may actually move through CFTR rather than anoctamins. Our present data fully support this concept as CFTR inhibitors completely blocked CCH-induced secretion in intestinal organoids (Fig. 4). Moreover, despite the presence of two seemingly independent anion conductances that are selectively activated by cAMP or Ca2+, a considerable overlap exists between both intracellular pathways, as discussed recently in several reports. Thus, intracellular Ca2+ signals not only stimulate basolateral K+ channels and supply additional driving force for apical Cl− secretion but also activate CFTR through inhibition of phosphatases and increase of protein kinase C activity (Fig. 7) [4, 25, 28, 42]. Furthermore, a recent report demonstrates that CFTR and Ano1 are separate but functionally related Cl− channels [36]. It will be crucial to determine in future the fractions of Cl− that move through anoctamins and CFTR during Ca2+-dependent stimulation of airway epithelial cells.
Anoctamins as compensatory channels for CFTR
Understanding the correlation between CFTR and anoctamins is essential because pharmacological stimulation of Ca2+-activated Cl− conductance in human airways has been proposed as a therapeutic strategy to compensate for the defective CFTR function. This, however, only has a chance to succeed if CFTR is not a substantial fraction of the Ca2+-activated Cl− current. Although CaCC appears slightly enhanced in cystic fibrosis, it is nevertheless not able to compensate for defective CFTR in mice. Notably, Ca2+-stimulated HCO3 − secretion is also largely reduced in the intestine of mice lacking CFTR expression cftr null mice [19, 45]. Thus, it will be important to determine the amount of Cl− ions truly moving through apical anoctamin channels in human airway cells.
References
Almaca J, Tian Y, AlDehni F, Ousingsawat J, Kongsuphol P, Rock JR, Harfe BD, Schreiber R, Kunzelmann K (2009) TMEM16 proteins produce volume regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem 284:28571–28578
Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK (1996) Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101–104
Barro Soria R, AlDehni F, Almaca J, Witzgall R, Schreiber R, Kunzelmann K (2009) ER localized bestrophin1 acts as a counter-ion channel to activate Ca2+ dependent ion channels TMEM16A and SK4. Pflugers Arch 459:485–497
Billet A, Hanrahan JW (2013) The secret life of CFTR as a calcium-activated chloride channel. J Physiol 591(21):5273–5278
Billig GM, Pál B, Fidzinski P, Jentsch TJ (2011) Ca2+−activated Cl− currents are dispensable for olfaction. Nat Neurosci 14:763–769
Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322:590–594
Chamova T, Florez L, Guergueltcheva V, Raycheva M, Kaneva R, Lochmuller H, Kalaydjieva L, Tournev I (2012) ANO10 c.1150_1151del is a founder mutation causing autosomal recessive cerebellar ataxia in Roma/Gypsies. J Neurol 259:906–911
Dekkers JF, Wiegerinck CL, De Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19:939–945
Dong Y, Zeng CQ, Ball JM, Estes MK, Morris AP (1997) The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5- trisphosphate production. Proc Natl Acad Sci U S A 94:3960–3965
Ehlen HW, Chinenkova M, Moser M, Munter HM, Krause Y, Gross S, Brachvogel B, Wuelling M, Kornak U, Vortkamp A (2012) Inactivation of Anoctamin-6/Tmem16f, a regulator of phosphatidylserine scrambling in osteoblasts, leads to decreased mineral deposition in skeletal tissues. J Bone Miner Res 28:246–259
Elvers M, Stegner D, Hagedorn I, Kleinschnitz C, Braun A, Kuijpers ME, Boesl M, Chen Q, Heemskerk JW, Stoll G, Frohman MA, Nieswandt B (2010) Impaired alpha(IIb)beta(3) integrin activation and shear-dependent thrombus formation in mice lacking phospholipase D1. Sci Signal 3:ra1
Faria D, Schlatter E, Witzgall R, Grahammer F, Bandulik S, Schweda F, Bierer S, Rock JR, Heitzmann D, Kunzelmann K, Schreiber R (2013) The calcium activated chloride channel Anoctamin 1 contributes to the regulation of renal function. Kindey Int 85(6):1369–1381
Faria D, Schreiber R, Kunzelmann K (2009) CFTR is activated through stimulation of purinergic P2Y2 receptors. Pflugers Arch 457:1373–1380
Frizzell RA, Hanrahan JW (2012) Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med 2:a009563
Grubb S, Poulsen KA, Juul CA, Kyed T, Klausen TK, Larsen EH, Hoffmann EK (2013) TMEM16F (Anoctamin 6), an anion channel of delayed Ca2+ activation. J Gen Physiol 141:585–600
He Q, Halm ST, Zhang J, Halm DR (2011) Activation of the basolateral membrane Cl conductance essential for electrogenic K secretion suppresses electrogenic Cl secretion. Exp Physiol 96:305–316
Heemskerk JW, Bevers EM, Lindhout T (2002) Platelet activation and blood coagulation. Thromb Haemost 88:186–193
Hennig B, Schultheiss G, Kunzelmann K, Diener M (2008) Ca(2+ )-induced Cl (−) efflux at rat distal colonic epithelium. J Membr Biol 221:61–72
Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA (1997) CFTR mediates cAMP- and Ca2+−activated duodenal epithelial HCO3- secretion. Am J Physiol 272:G872–G878
Juul CA, Grubb S, Poulsen KA, Kyed T, Hashem N, Lambert IH, Larsen EH, Hoffmann EK (2014) Anoctamin 6 differs from VRAC and VSOAC but is involved in apoptosis and supports volume regulation in the presence of Ca. Pflugers Arch [Epub ahead of print]
Kmit A, van Kruchten R, Ousingsawat J, Mattheij NJ, Senden-Gijsbers B, Heemskerk JW, Bevers EM, Kunzelmann K (2013) Calcium-activated and apoptotic phospholipid scrambling induced by Ano6 can occur independently of Ano6 ion currents. Cell Death Dis 4:e611
Ko EA, Jin BJ, Namkung W, Ma T, Thiagarajah JR, and Verkman AS (2013) Chloride channel inhibition by a red wine extract and a synthetic small molecule prevents rotaviral secretory diarrhoea in neonatal mice. Gut 63(7):1120–1129
Kunzelmann K, Kongsuphol P, AlDehni F, Tian Y, Ousingsawat J, Warth R, Schreiber R (2009) Bestrophin and TMEM16—Ca2+ activated Cl− channels with different functions. Cell Calcium 46:233–241
Kunzelmann K, Mall M (2002) Electrolyte transport in the colon: mechanisms and implications for disease. Physiol Rev 82:245–289
Kunzelmann K, Mehta A (2013) CFTR: a hub for kinases and cross-talk of cAMP and Ca. FEBS J 280:4417–4429
Kunzelmann K, Nilius B, Owsianik G, Schreiber R, Ousingsawat J, Sirianant L, Wanitchakool P, Bevers EM, Heemskerk JW (2013) Molecular functions of anoctamin 6 (TMEM16F): A chloride channel, cation channel or phospholipid scramblase? Pflügers Arch 466(3):407–14
Kunzelmann K, Tian Y, Martins JR, Faria D, Kongsuphol P, Ousingsawat J, Thevenod F, Roussa E, Rock JR, Schreiber R (2011) Anoctamins. Pflugers Arch 462:195–208
Kunzelmann K, Tian Y, Martins JR, Faria D, Kongsuphol P, Ousingsawat J, Wolf L, Schreiber R (2012) Cells in focus: airway epithelial cells-Functional links between CFTR and anoctamin dependent Cl(−) secretion. Int J Biochem Cell Biol 44:1897–1900
Mall M, Bleich M, Greger R, Schürlein M, Kühr J, Seydewitz HH, Brandis M, Kunzelmann K (1998) Cholinergic ion secretion in human colon requires co-activation by cAMP. Am J Physiol 275:G1274–G1281
Malvezzi M, Chalat M, Janjusevic R, Picollo A, Terashima H, Menon AK, Accardi A (2013) Ca(2+)-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nat Commun 4:2367
Martins JR, Faria D, Kongsuphol P, Reisch B, Schreiber R, Kunzelmann K (2011) Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc Natl Acad Sci U S A 108:18168–18172
Maruyama H, Morino H, Miyamoto R, Murakami N, Hamano T, Kawakami H (2013) Exome sequencing reveals a novel ANO10 mutation in a Japanese patient with autosomal recessive spinocerebellar ataxia. Clin Genet 85(3):296–7
Murek M, Kopic S, Geibel J (2010) Evidence for intestinal chloride secretion. Exp Physiol 95:471–478
Namkung W, Finkbeiner WE, Verkman AS (2010) CFTR-Adenylyl Cyclase I association is responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell 21:2639–2648
Neussert R, Muller C, Milenkovic VM, Strauss O (2010) The presence of bestrophin-1 modulates the Ca(2+) recruitment from Ca (2+) stores in the ER. Pflugers Arch 460:163–175
Ousingsawat J, Kongsuphol P, Schreiber R, Kunzelmann K (2011) CFTR and TMEM16A are separate but functionally related Cl channels. Cell Physiol Biochem 28:715–724
Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K (2009) Loss of TMEM16A causes a defect in epithelial Ca2+ dependent chloride transport. J Biol Chem 284:28698–28703
Ousingsawat J, Tian Y, AlDehni F, Roussa E, Schreiber R, Mirza M, Cook DI, Kunzelmann K (2011) Rotavirus toxin NSP4 activates the calcium dependent chloride channel TMEM16A and inhibits absorptive Na+transport. Pflugers Arch 461:579–589
Pifferi S, Dibattista M, Menini A (2009) TMEM16B induces chloride currents activated by calcium in mammalian cells. Pflugers Arch 458:1023–1038
Puntheeranurak S, Schreiber R, Spitzner M, Ousingsawat J, Krishnamra N, Kunzelmann K (2007) Control of ion transport in mouse proximal and distal colon by prolactin. Cell Physiol Biochem 19:77–88
Sailer A, Houlden H (2012) Recent advances in the genetics of cerebellar ataxias. Curr Neurol Neurosci Rep 12:227–236
Schreiber R, Kunzelmann K (2005) Purinergic P2Y6 receptors induce Ca2+ and CFTR dependent Cl− secretion in mouse trachea. Cell Physiol Biochem 16:99–108
Schreiber R, Uliyakina I, Kongsuphol P, Warth R, Mirza M, Martins JR, Kunzelmann K (2010) Expression and function of epithelial anoctamins. J Biol Chem 285:7838–7845
Schroeder BC, Cheng T, Jan YN, Jan LY (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134:1019–1029
Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M (1997) A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca(2+)-dependent HCO3- secretion. J Physiol 505:411–423
Shimizu T, Lehara T, Sato K, Fujii T, Sakai H, Okada Y (2013) TMEM16F is a component of a Ca2+−activated Cl− channel but not a volume-sensitive outwardly rectifying Cl− channel. Am J Physiol Cell Physiol 304:C748–C759
Stohr H, Heisig JB, Benz PM, Schoberl S, Milenkovic VM, Strauss O, Aartsen WM, Wijnholds J, Weber BH, Schulz HL (2009) TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci 29:6809–6818
Strauss O, Muller C, Reichhart N, Tamm ER, Gomez NM (2014) The role of bestrophin-1 in intracellular ca(2+) signaling. Adv Exp Med Biol 801:113–119
Tian Y, Schreiber R, Kunzelmann K (2012) Anoctamins are a family of Ca2+ activated Cl− channels. J Cell Sci 125:4991–4998
Vermeer S, Hoischen A, Meijer RP, Gilissen C, Neveling K, Wieskamp N, de Brouwer A, Koenig M, Anheim M, Assoum M, Drouot N, Todorovic S, Milic-Rasic V, Lochmuller H, Stevanin G, Goizet C, David A, Durr A, Brice A, Kremer B, van de Warrenburg BP, Schijvenaars MM, Heister A, Kwint M, Arts P, van der Wijst J, Veltman J, Kamsteeg EJ, Scheffer H, Knoers N (2010) Targeted next-generation sequencing of a 12.5 Mb homozygous region reveals ANO10 mutations in patients with autosomal-recessive cerebellar ataxia. Am J Hum Genet 87:813–819
Viitanen T, Sukumaran P, Lof C, Tornquist K (2012) Functional coupling of TRPC2 cation channels and the calcium-activated anion channels in rat thyroid cells: implications for iodide homeostasis. J Cell Physiol 228(4):814–823
Watt SA, Kular G, Fleming IN, Downes CP, Lucocq JM (2002) Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C delta1. Biochem J 363(Pt.3):657–666
Wolf W, Kilic A, Schrul B, Lorenz H, Schwappach B, Seedorf M (2012) Yeast Ist2 recruits the endoplasmic reticulum to the plasma membrane and creates a ribosome-free membrane microcompartment. PLoS ONE 7:e39703
Yang H, Kim A, David T, Palmer D, Jin T, Tien J, Huang F, Cheng T, Coughlin SR, Jan YN, Jan LY (2012) TMEM16F forms a Ca(2+)-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 151:111–122
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455:1210–1215
Acknowledgments
This study was supported by DFG SFB699A7 and Wilhelm-Sander Stiftung Ano6 and Deutsche Krebshilfe Projekt 109438. We gratefully acknowledge the generous supply of the Ano6−/− mice by Prof. Dr. A. Vortkamp (Department Entwicklungsbiologie, University of Essen, Essen, Germany) and Ano-1 antibodies by Prof. Dr. Brian Harfe (University of Florida at Gainesville, Gainesville, USA).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table S1
(DOCX 15 kb)
Supplementary Fig. S1
(PDF 75 kb)
Supplementary Fig. S2
(PDF 499 kb)
Supplementary Fig. S3
(PDF 575 kb)
Supplementary Fig. S4
(PDF 107 kb)
Supplementary Fig. S5
(PDF 55 kb)
Rights and permissions
About this article
Cite this article
Schreiber, R., Faria, D., Skryabin, B.V. et al. Anoctamins support calcium-dependent chloride secretion by facilitating calcium signaling in adult mouse intestine. Pflugers Arch - Eur J Physiol 467, 1203–1213 (2015). https://doi.org/10.1007/s00424-014-1559-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1559-2